Animal migrations, which can span hundreds to thousands of kilometers and require journeys lasting weeks to months, are fascinating biological phenomena. Across systems and species, key questions focus on the behavioral mechanisms that facilitate successful migrations. Perception, information sharing among individuals, and various forms of memory separate migration from other forms of animal movement, such as range residency and nomadism (1–3). However, almost all explorations of these navigation factors have been in terrestrial systems (4–8). Understanding when, and in what kinds of resource landscapes, different navigation factors provide value remains a core challenge. In PNAS, Abrahms et al. (9) combine a decade’s worth of satellite tracking and remote sensing data to analyze migration patterns of blue whales (Balaenoptera musculus), discovering evidence for memory-based movement that allows the whales to match their distributions to reliable resource peaks.
For some migratory species, the phenology (biological timing) of arrival at a particular destination, such as a nesting site or calving grounds, is paramount. In other species, particularly those for which migrations are of long duration, the timing of events along the entire route is critical, such as when migration facilitates regular access to fleetingly available resources (10, 11). Systems in which the timing matters throughout the migratory journey, such as the whales studied by Abrahms et al. (9), present excellent opportunities for testing the relative importance of different movement mechanisms.
Migration is favored as an evolutionary strategy if the net fitness benefits that accrue from recurrent movement exceed those from remaining resident in one region (3, 12). Among mammal species, such fitness benefits hinge on spatial variation in resource availability, physiological constraints, or escape from natural enemies (13). Migration is selected for when seasonality, rather than patchiness, dominates resource availability (3), and memory-based movement mechanisms will be favored when resources are predictably available (2, 14).
Analyzing the movement records of 60 satellite-tagged blue whales in the eastern North Pacific, Abrahms et al. (9) demonstrate that the whales’ migrations closely tracked the long-term average concentration of chlorophyll-a, rather than contemporaneous oceanic conditions (Fig. 1). Chlorophyll-a concentration, which can be mapped by satellite, is a standard index for the density of marine phytoplankton, which varies seasonally at the ocean basin scale as a consequence of variation in water temperature and the availability of current-derived nutrients.
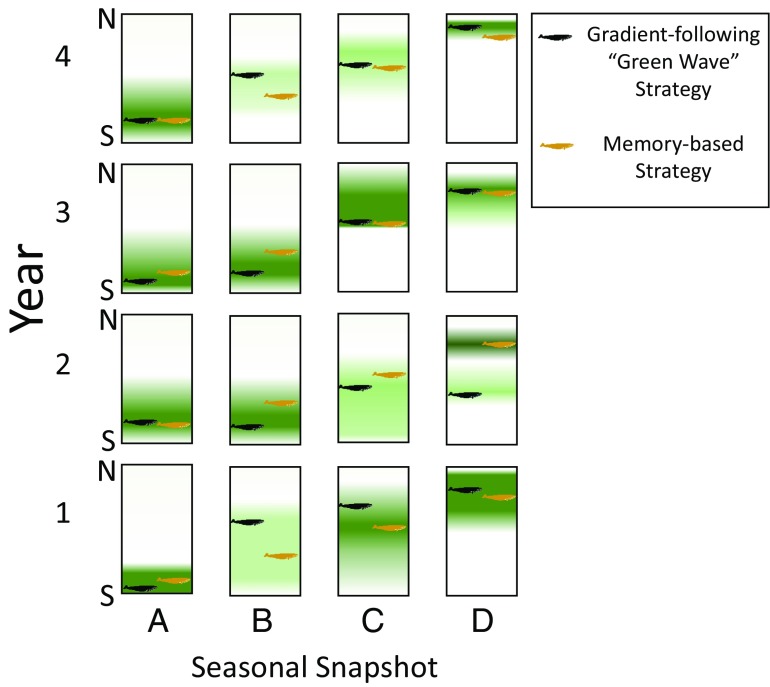
Fig. 1.
Contrasting predictions for whale phenology and spatial distribution from a perception-based green wave gradient-following strategy and a memory-based strategy. In a green wave framework, migratory animals attempt to spatially track transient peaks in resource availability. In a memory strategy, animals rely on a record of past experiences to position themselves in areas where resources will be most reliably available, despite year-to-year and seasonal variation. A memory strategy may also allow migratory animals to avoid being trapped by a local peak in the resource distribution (year 2, seasonal snapshot D). Rectangles are the identical spatial region, portraying interyear variation (vertical sequences) and intrayear variation (horizontal sequences). Density of the green shading is proportional to resource availability. Neither the historical basis for the memory strategy nor any interyear updating of memories is represented. Image of blue whale silhouette courtesy of Pixabay/OpenClipart-Vectors.
Phytoplankton density reflects marine productivity and is routinely used as a surrogate for the density of krill, the shrimplike arthropods on which blue whales and many other marine predators specialize. Over the whole dataset, the timing of whale arrival at a location correlated strongly with the timing of the seasonal peak in chlorophyll-a concentration at that location averaged over a 10-y period. The phenological match was strongest when chlorophyll-a data were time-lagged by 30 d to accommodate the trophic delay arising because krill eat phytoplankton.
Abrahms et al. (9) use simulated migration tracks—carefully built from the statistical properties of real migration tracks—as a benchmark for comparisons. Unlike the real data, arrival times of simulated whales at locations along artificially generated tracks did not parallel the 10-y average times of peak chlorophyll-a concentration along those tracks; if anything, the opposite pattern held. Actual whale arrival times also correlated with the onset of appropriately warm water conditions, but the same was true for the simulated migration data, suggesting that the temperature relationship was a general consequence of seasonal variation rather than an indicator of navigational decision making.
Importantly, this matching between whale movement patterns and long-term average resource conditions held for arrival timing over the entire course of the whales’ migrations, not just for arrival at the summer feeding grounds or other migratory waypoints. This is a key advance because previous studies of cetacean migratory phenology have involved local-scale analyses.
Interestingly, Abrahms et al. (9) do not find evidence to support a contemporaneous green wave hypothesis, under which migrating animals time their movements to track each year’s pulse of spring green-up as it moves poleward along a continent or up a mountainside (15). This kind of gradient-following movement, which aptly characterizes the migratory pathways of diverse species (10, 11, 16), is a form of oriented movement based on perception and response to contemporaneous conditions. In mathematical models of animal movement, gradient-following (advective) mechanisms like this can greatly facilitate resource acquisition in heterogeneous landscapes compared with nonoriented strategies (17, 18). If the green wave framework were operating for the whales, we would expect to see them consistently located in areas (and at times) featuring high concentrations of contemporaneous chlorophyll-a, moving onward as conditions improved elsewhere. But this is not the case. Abrahms et al. (9) find that whales are not shifting among resource peaks as conditions changed, nor are they “surfing” along a mobile peak of resources.
Instead, something entirely different appears to be at work. The whales undertook migratory journeys by which they could exploit those areas and times that featured, over a long historical average, both reliably high mean chlorophyll-a concentrations and consistently low variation in those concentrations. This strategy of focusing on resource dependability proved profitable. Chlorophyll-a concentrations for the distribution of ∼2,400 sites/times at which whales were recorded were, on average, more than double the concentrations for a distribution of alternative sites/times that the whales could just as easily have visited based on the simulated migration tracks.
The memory-based strategy was not perfect, however, and the whales were occasionally located in areas with very low contemporaneous levels of chlorophyll-a. Nevertheless, the visited sites/times were those where, over the years, chlorophyll-a concentrations were predictably high shortly before the whales arrived. Given the time-lagged relationship between phytoplankton and krill, such migratory timing would correspond to a reliable supply of krill for the whales upon arrival. This is an impressive achievement considering that the whales had to navigate across thousands of kilometers of ocean and contend with substantial interannual variability in broad-scale environmental conditions.
Abrahms et al. combine a decade’s worth of satellite tracking and remote sensing data to analyze migration patterns of blue whales (Balaenoptera musculus), discovering evidence for memory-based movement that allows the whales to match their distributions to reliable resource peaks.
This emphasis on reliability, rather than on perception-based tracking of current conditions, is a key discovery, suggesting that the timing and space use of migrating whales reflect a form of memory (19, 20). Memory-based frameworks for movement are fundamentally different from green wave ideas: They propose that an animal can anticipate the presence of a resource by remembering its location or conditions in years past without the benefit of an immediate cue. Although many experimental studies have demonstrated that animals store and act on memories, it has proved extremely difficult to demonstrate effects of memory for wild animals moving over large distances. Examples of memory-based movements include elephants tracking water resources (21) and wolves remembering prey locations (22). The importance of long-term averages in the whale system particularly echoes findings for zebra in which memory of historic resource availability, rather than perception of current conditions in the surrounding landscape, explained migration directionality (5).
Compared with other vertebrates, blue whales are very long-lived, and their ∼90-y natural life spans would afford them significant opportunities for the kind of long-term environmental averaging that underscores some kinds of spatial memory (19, 20). Abrahms et al. (9) raise the specter of climate change as a threat to continued successful blue whale migrations in the eastern North Pacific. Changing baselines and increased variability in ocean temperature and current dynamics could shift the timing and location of phytoplankton blooms, reducing the utility of memories of long-term averages as reliable predictors of resource availability.
As advantageous as memory can be, memory can be downright harmful if it repeatedly fails to represent the current reality. Indeed, decreases in landscape predictability greatly reduce the benefits of memory as a navigation mechanism (14). It is an intriguing issue of scale dependence that variability can drive the emergence of migratory movements, but predictability is essential to their long-term maintenance.
How blue whales will respond to changing conditions is unknown. Social exchange of information could play a role in shaping those responses. Such information could be passed vertically via spatial learning in persistent cow–calf pairs or via vocalizations. For example, humpback whales, whose well-known “song” vocalizations appear to play a role in sexual selection, also produce a diverse variety of nonsong sounds, some of which may be used in intergroup communication to convey location (23). Because migrating individuals may shift to rely on social information as historical information becomes inaccurate (3), a system like whales, whereby sound-based communication may span many tens of kilometers, affords intriguing opportunities to study how communication may enhance foraging success (24).
The ultimate analysis and results underpinning conclusions about memory-driven movement in whales are deceptively simple, but the data-intensive process to get there underscores just how much integration is necessary to make progress in cognitive movement ecology. Whales are most definitely not laboratory mice, and continued studies of their migrations will require creative investigations such as Abrahms et al.’s (9) that synthesize long-term tracking and environmental data.
Acknowledgments
W.F.F.’s research is supported by the NSF Grant ABI-1458748.
Footnotes
The author declares no conflict of interest.
See companion article on page 5582.
References
- 1.Mueller T, Fagan WF. Search and navigation in dynamic environments–From individual behaviors to population distributions. Oikos. 2008;117:654–664. [Google Scholar]
- 2.Berbert JM, Fagan WF. How the interplay between individual spatial memory and landscape persistence can generate population distribution patterns. Ecol Complex. 2012;12:1–12. [Google Scholar]
- 3.Shaw AK, Couzin ID. Migration or residency? The evolution of movement behavior and information usage in seasonal environments. Am Nat. 2013;181:114–124. doi: 10.1086/668600. [DOI] [PubMed] [Google Scholar]
- 4.Bartlam-Brooks HLA, Bonyongo MC, Harris S. Will reconnecting ecosystems allow long-distance mammal migrations to resume? A case study of zebra Equus burchelli migration in Botswana. Oryx. 2011;45:210–216. [Google Scholar]
- 5.Bracis C, Mueller T. Memory, not just perception, plays an important role in terrestrial mammalian migration. Proc Biol Sci. 2017;284:20170449. doi: 10.1098/rspb.2017.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holdo RM, Holt RD, Fryxell JM. Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti. Am Nat. 2009;173:431–445. doi: 10.1086/597229. [DOI] [PubMed] [Google Scholar]
- 7.Flack A, Nagy M, Fiedler W, Couzin ID, Wikelski M. From local collective behavior to global migratory patterns in white storks. Science. 2018;360:911–914. doi: 10.1126/science.aap7781. [DOI] [PubMed] [Google Scholar]
- 8.Jesmer BR, et al. Is ungulate migration culturally transmitted? Evidence of social learning from translocated animals. Science. 2018;361:1023–1025. doi: 10.1126/science.aat0985. [DOI] [PubMed] [Google Scholar]
- 9.Abrahms B, et al. Memory and resource tracking drive blue whale migrations. Proc Natl Acad Sci USA. 2019;116:5582–5587. doi: 10.1073/pnas.1819031116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merkle JA, et al. Large herbivores surf waves of green-up during spring. Proc Biol Sci. 2016;283:20160456. doi: 10.1098/rspb.2016.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorup K, et al. Resource tracking within and across continents in long-distance bird migrants. Sci Adv. 2017;3:e1601360. doi: 10.1126/sciadv.1601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryxell JM, Holt RD. Environmental change and the evolution of migration. Ecology. 2013;94:1274–1279. doi: 10.1890/12-0668.1. [DOI] [PubMed] [Google Scholar]
- 13.Avgar T, Street G, Fryxell JM. On the adaptive benefits of mammal migration. Can J Zool. 2013;92:481–490. [Google Scholar]
- 14.Mueller T, Fagan WF, Grimm V. Integrating individual search and navigation behaviors in mechanistic movement models. Theor Ecol. 2011;4:341–355. [Google Scholar]
- 15.Van der Graaf AJ, Stahl J, Klimkowska A, Bakker JP, Drent RH. Surfing on a green wave—How plant growth drives spring migration in the Barnacle Goose Branta leucopsis. Ardea. 2006;94:567–577. [Google Scholar]
- 16.Aikens EO, et al. The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecol Lett. 2017;20:741–750. doi: 10.1111/ele.12772. [DOI] [PubMed] [Google Scholar]
- 17.Lam KY, Lou Y. Evolution of conditional dispersal: Evolutionarily stable strategies in spatial models. J Math Biol. 2014;68:851–877. doi: 10.1007/s00285-013-0650-1. [DOI] [PubMed] [Google Scholar]
- 18.Fagan WF, et al. Perceptual ranges, information gathering, and foraging success in dynamic landscapes. Am Nat. 2017;189:474–489. doi: 10.1086/691099. [DOI] [PubMed] [Google Scholar]
- 19.Fagan WF, et al. Spatial memory and animal movement. Ecol Lett. 2013;16:1316–1329. doi: 10.1111/ele.12165. [DOI] [PubMed] [Google Scholar]
- 20.Bracis C, Gurarie E, Van Moorter B, Goodwin RA. Memory effects on movement behavior in animal foraging. PLoS One. 2015;10:e0136057. doi: 10.1371/journal.pone.0136057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polansky L, Kilian W, Wittemyer G. Elucidating the significance of spatial memory on movement decisions by African savannah elephants using state-space models. Proc Biol Sci. 2015;282:20143042. doi: 10.1098/rspb.2014.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlägel UE, Merrill EH, Lewis MA. Territory surveillance and prey management: Wolves keep track of space and time. Ecol Evol. 2017;7:8388–8405. doi: 10.1002/ece3.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlop RA, Cato DH, Noad MJ. Non‐song acoustic communication in migrating humpback whales (Megaptera novaeangliae) Mar Mamm Sci. 2008;24:613–629. [Google Scholar]
- 24.Martínez-García R, Calabrese JM, Mueller T, Olson KA, López C. Optimizing the search for resources by sharing information: Mongolian gazelles as a case study. Phys Rev Lett. 2013;110:248106. doi: 10.1103/PhysRevLett.110.248106. [DOI] [PubMed] [Google Scholar]