Significance
Myelin sheaths insulate the neuronal axons and speed the transmission of electrical impulses. The demyelination process occurs in many human diseases, including multiple sclerosis (MS). Using multiple mouse models of MS, we demonstrate in this study the role of MLKL, a protein known to function in a form of regulated necrosis (necroptosis), in facilitating demyelination in a necroptosis-independent manner in the central nervous system. An RIP1 kinase inhibitor was shown to block the progression of MS in an immune-induced demyelination model, and it does so at the stage after antibody generation but before breakdown of the blood–brain barrier, at a likely step of monocyte elevation. These findings provide insights into the demyelination process and potential new therapeutic agents for MS.
Keywords: RIP1 kinase, myelin, demyelination, multiple sclerosis, MLKL
Abstract
Demyelination in the central nervous system (CNS) underlies many human diseases, including multiple sclerosis (MS). We report here the findings of our study of the CNS demyelination process using immune-induced [experimental autoimmune encephalomyelitis (EAE)] and chemical-induced [cuprizone (CPZ)] mouse models of demyelination. We found that necroptosis, a receptor-interacting protein 3 (RIP3) kinase and its substrate mixed lineage kinase domain-like protein (MLKL)-dependent cell death program, played no role in the demyelination process, whereas the MLKL-dependent, RIP3-independent function of MLKL in the demyelination process initially discovered in the peripheral nervous system in response to nerve injury, also functions in demyelination in the CNS in these models. Moreover, a receptor-interacting protein 1 (RIP1) kinase inhibitor, RIPA-56, blocked disease progression in the EAE-induced model but showed no effect in the CPZ-induced model. It does so most likely at a step of monocyte elevation downstream of T cell activation and myelin-specific antibody generation, although upstream of breakdown of the blood–brain barrier. RIP1-kinase dead knock-in mice shared a similar result as mice treated with the RIP1 inhibitor. These results indicate that RIP1 kinase inhibitor is a potential therapeutic agent for immune-mediated demyelination diseases that works by prevention of monocyte elevation, a function previously unknown for RIP1 kinase.
Myelin is a lipid-rich (fatty) substance formed by glial cells called oligodendrocytes in the central nervous system (CNS) and by Schwann cells in the peripheral nervous system (PNS) that protects and nourishes neuron axons by wrapping the axons (1). Physiologically, the myelin structure speeds the transmission of electrical impulses called action potentials along myelinated axons by insulating the axon and reducing axonal membrane capacitance (2). The demyelination process occurs during nerve injury and happens in many human diseases, including, for example, in the PNS, Charcot–Marie–Tooth disease (3) and abdominal numbness brought about by diabetes (4), and in the CNS, multiple sclerosis (MS), during which the insulating covers of nerve cells in the brain and spinal cord are damaged by infiltrated immune cells, such as T cells and monocytes (5, 6). Patients with MS are paralyzed gradually from the lower body to the upper body and eventually die; most of these patients are young adults ranging in age from their 20s–40s (7).
The molecular mechanisms that underlie these demyelination diseases are largely unknown. A recent study indicated that preventing oligodendrocytes from necroptosis, a regulated form of necrotic cell death induced by ligands of the tumor necrosis factor (TNF) receptor family or Toll-like receptors, as well as certain viral infections, could decrease disease symptoms in MS disease models of experimental autoimmune encephalomyelitis (EAE) and a cuprizone (CPZ)-induced demyelination model (8). The TNF receptor family-mediated cell necroptosis signaling pathway requires the activation of receptor-interacting protein 1 (RIP1) kinase, which then binds and phosphorylates the downstream receptor-interacting protein 3 (RIP3) kinase to form necrosomes in which RIP3 is activated (9). Actived RIP3 then phosphorylates its substrate the mixed lineage kinase domain-like pseudokinase (MLKL) (10). The phosphorylation of MLKL by RIP3 drives the monomeric MLKL toward oligomerization and translocation from the cytoplasm to the plasma membrane, where MLKL disrupts the membrane integrity, resulting in necroptosis (11). Activation markers of necroptosis such as the phosphorylated form of RIP3 and MLKL were detected in the brain of patients with MS and in the spinal cord white matter of EAE mice (8). More recently, MLKL was found to accelerate the demyelination process in a mouse sciatic nerve injury model independent of its role in necroptosis (12). This function of MLKL requires phosphorylation of MLKL at the serine 441 (mouse origin) site by an unknown kinase activated in Schwann cells after nerve injury.
To further illustrate the role of necroptosis and the necroptosis-independent role of MLKL in the demyelination process in MS, we used Rip3−/− and Mlkl−/− mice, as well as RIP1 kinase-dead knock-in mice, to generate EAE to study this demyelination disease. Additionally, we orally fed EAE mice of wild-type (WT) background a specific RIP1 inhibitor, RIPA-56 (13), added to chow to systematically study multiple disease-related parameters during EAE disease progression. Our results validated the necroptosis-independent role of RIP1 and MLKL in the demyelination process of the CNS and pinpointed elevated monocytes as the main point of interference for the RIP1 inhibitor to prevent EAE disease progression.
Results
An RIP1 Inhibitor Reduces EAE-Induced MS Symptoms Independent of Necroptosis.
In addition to its role in necroptosis signaling, RIP1 kinase is known to be a pleiotropic protein that functions in apoptosis (14). To validate the previous claim that blocking necroptosis slows down MS, we first sought to test if the RIP1 inhibitor RIPA-56 could decrease or block EAE disease progression in a similar manner as Nec-1, a structurally different RIP1 kinase inhibitor used previously to block MS-like symptoms in EAE (8). To further differentiate the necroptosis-dependent or necroptosis-independent contribution to the disease, we tested RIPA-56 in the disease model by inducing EAE in WT and in Rip3−/− mice. Recall that RIP3 is an essential protein of the necroptosis pathway (9). Using a standard method for quantifying EAE paralysis based on disease progression curves of clinical scores (15), we found that whereas WT EAE and Rip3−/− EAE mice all had aggressive disease progression, treatment of both WT and Rip3−/− mice with RIPA-56 from the first day of EAE induction can strongly block the development of clinical EAE symptoms (Fig. 1A). We conducted a detailed analysis of the clinical scores of all mice in the experiment at day 21 and found that while there was no significant difference in the clinical scores between WT EAE and Rip3−/− EAE mice, there were significant differences between WT EAE and WT EAE+RIPA-56 mice and between Rip3−/− EAE and Rip3−/− EAE+RIPA-56 mice (Fig. 1B).
Fig. 1.
RIP1 inhibitor reduces EAE-induced MS symptoms independent of necroptosis. (A) EAE model of disease progression. (B) Clinical score at day 21 (mean ± SEM; ***P < 0.001 measured by one-way ANOVA followed by Tukey’s multiple comparison test). n.s., not significant. (C) Spinal cord immunofluorescence analysis of the EAE-induced demyelination process of the spinal cord compared with naive and EAE+RIPA-56–treated WT or Rip3−/− mice. Inflammation signals in the lesion and demyelination regions were analyzed using the myelin marker MBP (to test demyelination of white matter) and the monocyte and microglia marker F4/80 (to test the inflammation region). (Upper and Middle) Individual channels for MBP (red) and F4/80 (purple). (Lower) Confocal view of the MBP and F4/80 with enlarged views for the dotted box regions. These cross-sections represent transverse views of the thoracic part of the spinal cord and are 6 μm thick. (Scale bars: Upper and Middle, 500 μm; Lower, 50 μm.)
The extent of EAE-induced paralysis is well known to correlate strongly with the amount of infiltrated peripheral immune cells in spinal cord white matter (16), and this process is known to trigger demyelination and inflammation in spinal cord white matter. We therefore used immunofluorescence methods to analyze both demyelination and inflammation in the spinal cord white matter of WT and Rip3−/− mice. Demyelination was monitored via an antibody against the myelin-specific marker MBP, and inflammation was monitored via an antibody against the monocyte and microglia marker F4/80. The MBP signal showed that whereas the naive mice and both the WT EAE+RIPA-56 and Rip3−/− EAE+RIPA-56 mice exhibited intact spinal cord white matter, the WT EAE and Rip3−/− EAE mice had obvious dark regions indicative of damaged myelin. Thus, RIPA-56 can block demyelination in EAE mice (Fig. 1C). Consistently, the F4/80 signal showed that the WT EAE and Rip3−/− EAE mice, but not the naive WT, WT EAE+RIPA-56, or Rip3−/− EAE+RIPA-56 mice, had obvious inflammation in their spinal cord white matter (Fig. 1C).
Importantly, the regions showing weak MBP signals were colocalized with the F4/80-positive regions in the EAE group (Fig. 1C), which indicates that RIPA-56 not only blocks demyelination but also apparently decreases the extent of inflammation in EAE mice. Staining against another monocyte and microglia marker, Iba-1, revealed similar patterns of inflammation regions (SI Appendix, Fig. S1A). Moreover, transmission electron microscopy showed that compared with the normal myelin sheath morphology of the WT naive or WT EAE+RIPA-56 mice (sheath intact and wrapped tightly around axons), WT EAE mice had myelin sheaths that were thinner and obviously loosened (SI Appendix, Fig. S1B). These results establish that RIPA-56 treatment protects myelin structure by blocking the demyelination and inflammation of spinal cord white matter that occurs in EAE mice and, together with our results showing that RIPA-56 can block EAE paralysis, demonstrates that this small-molecule inhibitor of RIP1 dramatically reduces EAE-induced disease symptoms. However, unlike the previous report (8), it apparently does so through a necroptosis-independent process since the key necroptosis signaling kinase, RIP3, did not have any effect in the process.
The Blood–Brain Barrier Remains Intact in RIP1-Inhibitor–Treated Animals Following EAE Induction.
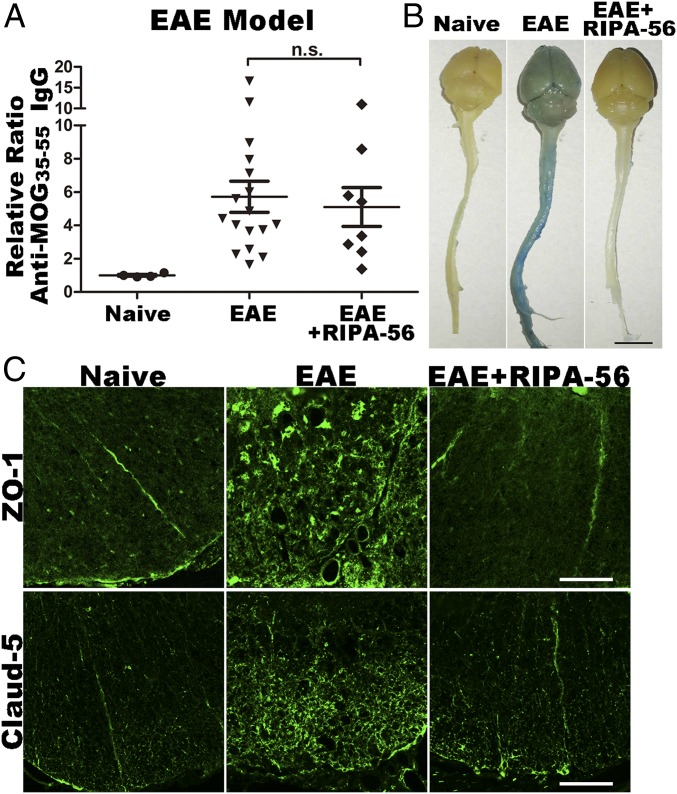
A variety of antigens can be targeted in studies using the EAE experimental model (17). We chose to immunize EAE animals with the well-characterized MOG35–55 antigen, which is a peptide derived from the myelin component MOG (18). Exposure to MOG35–55 trains animals’ immune system to recognize myelin as an antigen, and to subsequently trigger an immune response specifically targeting myelin in the CNS (19). We first analyzed if RIPA-56 influences the antibody generation process in EAE mice with ELISAs monitoring serum anti-MOG35–55 IgG concentrations (Fig. 2A). These assays were conducted with WT mice, and they showed that both the EAE and EAE+RIPA-56 mice had increased antibody titers compared with the naive mice; they also showed that there was no significant difference between the two EAE sample groups. Thus, the RIP1 inhibitor RIPA-56 does not significantly affect the extent of antibody production in EAE mice.
Fig. 2.
BBB is preserved in RIP1 inhibitor-treated EAE animals. (A) ELISA analysis of antibody concentration of serum from naive, EAE, and EAE+RIPA-56–treated WT mice (mean ± SEM). n.s, not significant. (B) Tail i.v. injection of Evans blue showing BBB integrity of naive, EAE, and EAE+RIPA-56 WT mice. (Scale bar: 1 cm.) (C) Immunofluorescence analysis of BBB integrity in cross-sections of white matter of the spinal cords of naive, EAE, and EAE+RIPA-56–treated WT mice. Anti–ZO-1 (Upper) and anti–Claud-5 (Lower) are shown; both are markers of BBB integrity. These cross-sections represent transverse views of the thoracic part of the spinal cord and are 6 μm thick. (Scale bars: 100 μm.)
Extensive previous research has established that EAE progression occurs in three consecutive steps: The first step of the disease is a peripheral immune response (generation of antimyelin antibodies and activation of monocytes, T cells, and B cells); the second step is the breakdown of the blood–brain barrier (BBB) and immune cell infiltration from the PNS to the CNS; and the third step is demyelination of nerve fibers (20). Since the antibody generation seemed to be the same with or without RIPA-56, this RIP1 kinase inhibitor must work at a downstream step. To pinpoint the step at which our RIP1 inhibitor blocks EAE progression, we first tested the integrity of the BBB. In doing so, we used a tail i.v. injection assay with Evans blue, a dye that is widely used to assess the permeability of the BBB to macromolecules (21). We first collected and examined the peripheral tissues to confirm that i.v. injection of Evans blue, but not control saline solution, resulted in the successful blue staining of the livers and kidneys of all mice tested (SI Appendix, Fig. S2A).
Examination of brain and spinal cord organs revealed obvious blue staining in WT EAE mice that was not present in either WT naive or WT EAE+RIPA-56 mice (Fig. 2B), a result suggesting that RIPA-56 treatment of EAE mice preserved the integrity of the BBB. To confirm this observation, we next prepared spinal cord white matter transverse sections from these mice and performed immunostaining against ZO-1 and Claud-5, two well-established markers of BBB integrity (22, 23). In both WT and Rip3−/− mice, the naive or EAE+RIPA-56 mice had obviously different staining patterns than the EAE mice (Fig. 2C and SI Appendix, Fig. S2B). Specifically, whereas the naive and EAE+RIPA-56 mice had ZO-1 and Claud-5 signals that appeared as well-ordered discrete lines at a few places in the sections, the WT EAE and Rip3−/− EAE mice had widespread ZO-1 and Claud-5 signals distributed throughout the sections.
This widespread distribution is typical of a damaged BBB and reflects the translocation of the ZO-1 and Claud-5 proteins from the membrane to the cytosol of brain endothelial cells. As our Evans blue assays of whole organs and our immunostaining for BBB integrity markers both demonstrated that RIPA-56 treatment can prevent BBB breakdown in EAE mice, we can conclude that RIPA-56 acts to prevent EAE progression via a mechanism that occurs outside the CNS before the integrity of the BBB is compromised during disease progression.
Inhibition of RIP1 Reduces the Number of Monocytes and Decreases Monocyte Infiltration Following EAE Induction.
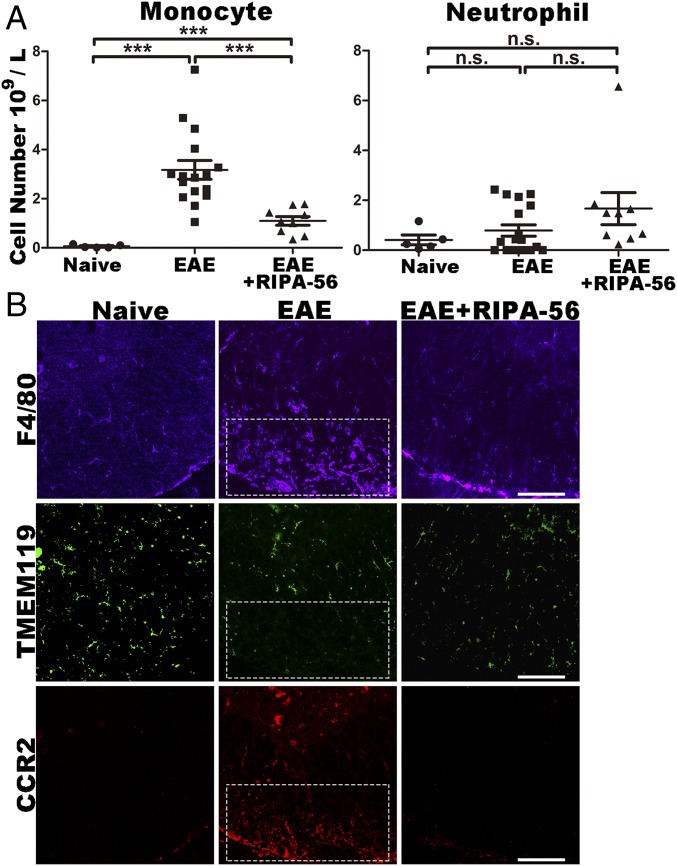
Considering that we observed no differences between the EAE and EAE+RIPA-56 mice in antibody production, which is a process known to be strongly dependent on the activity of B cells (24), our data suggested that rather than functioning as a global immune suppressor, RIPA-56 may specifically target a particular immune cell subtype. We therefore used peripheral blood cell composition analysis to identify potential targets of RIPA-56 in the immune system. There were no significant differences in the numbers of lymphocytes (SI Appendix, Fig. S3A) or neutrophils (Fig. 3A) between WT EAE and WT EAE+RIPA-56 mice. However, WT EAE+RIPA-56 mice had significantly fewer monocytes than WT EAE mice (a 66% reduction; Fig. 3A), suggesting that RIPA-56 might block EAE disease progression by specifically hindering the elevation of peripheral monocytes.
Fig. 3.
Inhibition of RIP1 reduces the number of monocytes and decreases CNS monocyte infiltration following EAE induction. (A) Blood cell composition analysis of naive, EAE, and EAE+RIPA-56–treated WT mice: monocytes (Left) and neutrophils (Right) (mean ± SEM; ***P < 0.001 measured by one-way ANOVA followed by Tukey’s multiple comparison test). n.s, not significant. (B) Immunofluorescence analysis of the cell types in the white matter of spinal cords of naive, EAE, and EAE+RIPA-56–treated WT mice. (Upper) F4/80, a monocyte and microglia marker for inflammation. (Middle) TMEM119, a marker for CNS local microglia. (Lower) CCR2, a marker for invaded monocytes. These cross-sections represent transverse views of the thoracic part of the spinal cord and are 6 μm thick. (Scale bars: 100 μm.)
The particular cytokines generated by different immune cells or tissues are markers for the activation and/or function of specific cell types (25). Thus, seeking to characterize the specific effects of RIPA-56 in greater detail, we performed assays for 23 cytokines in WT naive, WT EAE, and WT EAE+RIPA-56 mice. Granulocyte (G)-CSF, which is produced by monocytes (26, 27), was the only cytokine with a significantly different concentration between WT EAE and WT EAE+RIPA-56 mice (SI Appendix, Fig. S3B). These results further support the conclusion that RIPA-56 blocks EAE disease progression via a peripheral monocyte-specific mechanism.
We also tested if RIPA-56 blocks the infiltration of monocytes into spinal cord white matter using immunofluorescence staining. To this end, we monitored the immunofluorescence of antibodies against the monocyte and microglia marker F4/80, the CNS local microglia marker TMEM119, and the invaded monocyte marker CCR2 in this region where demyelination happens. Confocal microscopy analysis of spinal cord white matter in the demyelination region clearly showed that strong signals of both F4/80 and CCR2 and a weak signal for TMEM119 were present there (Fig. 3B). These colocalized signals for F4/80 and CCR2 demonstrate that in demyelination regions of spinal cord white matter the cell type of these F4/80 positive cells are peripheral monocyte derived macrophages other than CNS derived microglia. These experiments also showed that treatment with RIPA-56 obviously blocks the infiltration of monocytes into spinal cord white matter. Moreover, we found that RIPA-56 treatment did not influence the distribution of microglia: The TMEM119 signals were not obviously different across the three experimental groups (Fig. 3B).
RIP1 Kinase and MLKL Function in the Demyelination Process at Different Steps.
We recently discovered that MLKL, a protein known to rupture cell membranes during necroptotic cell death (9), is induced following sciatic nerve injury and targets the myelin sheath membrane of Schwann cells to promote myelin breakdown (12). We showed that unlike the situation in necroptosis, during which MLKL must be activated by phosphorylation mediated by RIP3, the function of MLKL in disrupting the myelin sheath does not depend on RIP3. Specifically, mice with Mlkl, but not Rip3, knocked out showed delayed myelin sheath breakdown and reduced nerve regeneration following nerve injury (12). We therefore investigated whether MLKL may also be involved in RIPA-56’s protective effects against demyelination in EAE mice.
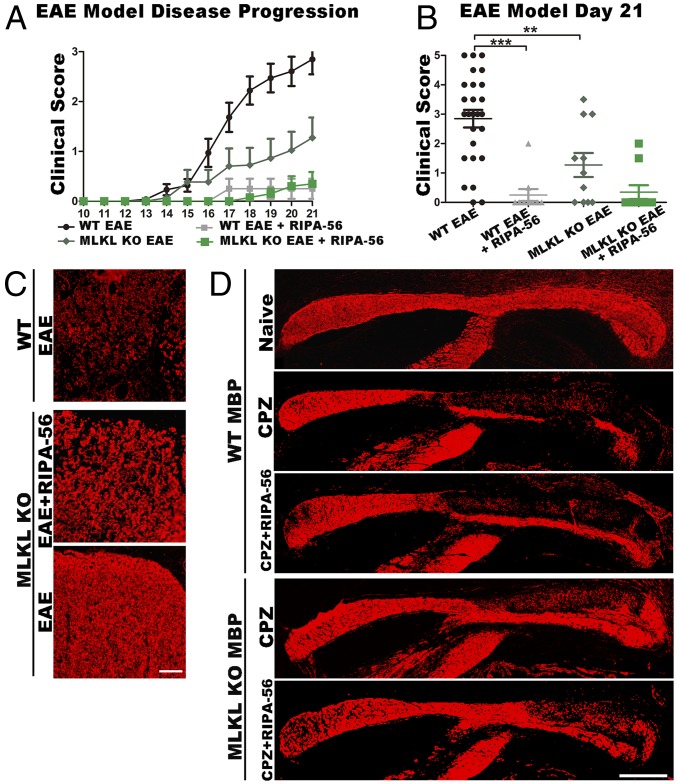
We conducted EAE induction experiments with and without RIPA-56 in Mlkl−/− mice. Beyond clearly establishing that that the loss of Mlkl function does not influence RIPA-56’s ability to block demyelination in EAE mice (Fig. 4A), these experiments revealed that Mlkl−/− mice have significantly reduced clinical EAE disease scores compared with WT EAE mice (Fig. 4B). This finding suggests the interesting notion that MLKL, beyond its RIP3-independent role in demyelination-related functions following sciatic nerve injury, also functions in demyelination during EAE disease progression, again independent of RIP3. This idea was also supported by immunofluorescence staining against the myelin-specific marker MBP in the spinal cords of WT and Mlkl−/− mice. The data clearly showed that reduction of the myelin signal following EAE induction was delayed in Mlkl−/− mice (Fig. 4C). These findings in EAE mice lend further support to an RIP3-independent role of MLKL in demyelination that was initially discovered in a sciatic nerve injury experimental model. Interestingly, in Mlkl−/− EAE mice, the percentage of monocytes among the blood leukocytes showed no significant difference compared with that in Mlkl+/− EAE mice (SI Appendix, Fig. S4A), indicating that MLKL did not influence the function of monocytes after EAE challenge. Moreover, serum G-CSF analysis showed no significant difference between WT EAE and Mlkl−/− EAE mice (SI Appendix, Fig. S4B). However, feeding Mlkl−/− mice with RIPA-56 still significantly decreased the serum G-CSF level (SI Appendix, Fig. S4B), indicating that RIPA-56 still reduced the percentage of blood monocytes in Mlkl−/− mice. Therefore, RIP1 inhibition and MLKL ablation impact demyelination in the EAE model at discrete steps.
Fig. 4.
RIP1 kinase and MLKL function in the demyelination process at different steps. (A) EAE model of disease progression using WT and Mlkl knockout (KO) mice. (B) Clinical score at day 21 (mean ± SEM; **P < 0.01, ***P < 0.001 measured by one-way ANOVA followed by Tukey’s multiple comparison test). (C) Spinal cord immunofluorescence analysis of the EAE-induced demyelination process of the spinal cord, by using MBP (red), compared with EAE or EAE+RIPA-56–treated Mlkl KO mice and EAE WT mice. These cross-sections are transverse views of the thoracic part of the spinal cord and are 6 μm thick. (Scale bar: 50 μm.) (D) Immunofluorescence analysis of brain sections from the CPZ demyelination model in WT (naive, CPZ, and CPZ+RIPA56) and Mlkl KO (CPZ and CPZ+RIPA-56) mice. MBP labels the corpus callosum, a myelin-rich brain region; these cross-sections are sagittal views of the brain corpus callosum and are 6 μm thick. (Scale bar: 500 μm.)
Beyond the EAE, we also tested the effects of RIPA-56 using two additional experimental demyelination mouse models: CPZ- and lysophosphatidylcholine (LPC)-induced demyelination in mice (28, 29). Since the demyelination process in these two models is hardly affected by the immune system, we used these two models to test whether RIPA-56 also affected the demyelinating process. Staining of the corpus callosum region in the brains of WT and Mlkl−/− mice with the myelin-specific marker MBP showed that RIPA-56 did not affect demyelination in CPZ model (Fig. 4D), a feature in striking contrast to the EAE model. RIPA-56 also did not affect demyelination in the sciatic nerves of the LPC disease model mice (SI Appendix, Fig. S4 C and D). Interestingly, our CPZ experiments revealed that MBP staining in the caudal corpus callosum regions of Mlkl−/− mice had much less demyelination than that in WT mice (Fig. 4D), further supporting an RIP1- and RIP3-independent function of MLKL in demyelination during CPZ-induced disease progression.
EAE Disease Progression Is Dependent on RIP1 Kinase Activity.
Our results clearly established that RIPA-56 can significantly block EAE disease progression. The most likely culprit of disease progression, a step at which our RIP1 kinase inhibitor exerts its effect, is at the level of monocytes in blood. This finding is puzzling since there has been no direct link identified to date between RIP1 kinase activity and the number of monocytes. On the other hand, RIP2, another related receptor-interacting kinase, has already been reported to influence EAE progression (30, 31). To correctly interpret the data generated from RIPA-56, we needed to determine the specificity of RIPA-56 toward other RIP family proteins. Further, given our demonstration that RIPA-56 is an RIP1 kinase activity inhibitor, we also wanted to confirm that a kinase-activity dead mutant would exhibit similar phenotypes as mice treated with RIPA-56. To test whether RIPA-56 inhibits the kinase activity of other RIP family proteins, we conducted in vitro kinase activity assays to determine the IC50 values for RIPA-56 with five RIP family proteins. These experiments showed that only the kinase activity of RIP1, but not RIP2–RIP5, decreased significantly as the concentration of RIPA-56 increased (SI Appendix, Fig. S5A). This result highlights the specificity of RIPA-56 for RIP1, and additionally raises the implication that RIP1 kinase activity is specifically responsible for EAE disease progression.
It is known that knockout of RIP1 is lethal in mice (32), so we needed an alternative genetic model to test whether EAE disease progression is dependent on RIP1 kinase activity. To this end, we used kinase activity-dead RIP1K45A knock-in mutant mice (33). These mice have been shown to develop normally, without any obvious phenotypic differences from WT mice; however, when we induced EAE, we observed that the RIP1K45A mice had significantly reduced clinical scores compared with WT mice by day 16 (SI Appendix, Fig. S5B). This result clearly suggested that RIP1 kinase activity is essential for EAE disease progression.
We next used fluorescence-activated cell sorting (FACS) to examine monocyte function in the RIP1K45A mice and found that, after induction of EAE, the number of monocytes was strikingly and significantly reduced in the blood of RIP1K45A mice compared with WT mice (SI Appendix, Fig. S5C), confirming our earlier results from the RIPA-56 experiments about the pathomechanistic contribution of monocyte activity in EAE. Note that there were no significant differences in the number of neutrophils between EAE WT and RIP1K45A EAE mice (SI Appendix, Fig. S5D). Additionally, given that induction of EAE in mice has been shown to cause splenomegaly (8), an immunity-induced abnormal enlargement of the spleen, we conducted experiments showing that, compared with naive WT mice and naive WT mice fed RIPA-56, the induction of EAE resulted in significantly enlarged spleens and significantly increased numbers of splenocytes in WT, RIPA-56–treated WT, and RIP1K45A mice (SI Appendix, Fig. S5 E and F). This result shows that general immune function is not compromised by RIPA-56 treatment or by loss of RIP1 kinase function.
Discussion
Using several genetic modified mouse models defective in their necroptosis pathway and a small-molecule RIP1 inhibitor, RIPA-56, we intended to validate the previous reported role of necroptosis in EAE disease progression. To our surprise, we did not observe any difference in clinical scores and pathological loss of myelin in the spinal cord white matter transverse sections between WT and Rip3 knockout mice. Thus, the effect of the RIP1 inhibitor in blocking EAE disease progression is not mainly through preventing necroptosis as previously reported (8). However, since MS is a chronic disease with potentially multiple triggering factors, it is not surprising that a necroptosis marker, such as RIP3-mediated phosphorylation of MLKL, has been detected in the diseased tissues, as reported previously (8). Complicating the issue is the fact that MLKL also has a nonnecroptosis function in demyelination (12). The eventual clarification of this issue may have to wait for the development of a demyelination-specific biomarker of MLKL activation (i.e., a specific antibody against the phosphorylated serine 441 site).
In addition to ruling out the direct involvement of necroptosis in EAE disease progression, we confirmed the necroptosis-independent role of MLKL in the demyelination process of the CNS in different animal models. For example, in the CPZ-induced demyelination model, the demyelination process in the corpus callosum of WT mice cannot be rescued by RIPA-56, indicating that unlike EAE, in which inflammation serves as the trigger for demyelination, RIP1 kinase activity has no effect in demyelination induced by CPZ. In contrast, lack of MLKL protein delayed demyelination in CPZ-induced demyelination, indicating that even in this chemically induced disease model, MLKL is involved in the demyelination process, as it is in sciatic nerve injury as previously reported. This result is consistent with a previous study from our laboratory that RIPA-56 cannot block the MLKL-mediated PNS demyelination process induced by injury (12).
Combined with the observation that the RIPA-56 RIP1 inhibitor specifically prevents monocyte elevation in EAE and the finding that anti-MOG35–55 IgG was expressed normally and did not show any significant differences in EAE + RIPA-56–treated mice compared with EAE WT mice, the RIP1 inhibitor thus functions at the step after antibody generation, before BBB breakdown, and most likely at the step of monocyte activation.
The monocyte is an essential cell type in EAE disease progression. Based on a previous study, monocytes trigger EAE progression, and inhibition of chemokine receptor-dependent recruitment of monocytes to the CNS blocked EAE progression, suggesting that these infiltrating cells are essential for pathogenesis (16). Another study showed that peripheral administration of micro-RNA (miR-124) in EAE caused systemic deactivation of monocyte-derived macrophages (34), reduced activation of myelin-specific T cells, and marked suppression of disease. Since monocytes show a predominant function in EAE, the question arises of whether inhibiting the kinase activity of RIP1 directly influences the differentiation or proliferation of monocytes. We still do not have any mechanistic insight how RIP1 kinase participates in monocyte elevation in EAE. Since the research on the regulation of monocyte differentiation is still at a rather rudimentary stage (35), the finding that inhibition of RIP1 kinase activity by RIPA-56 arrests disease progression at the stage of monocyte activation should provide an interesting new direction for the study of this apparently important process of autoimmune response.
Methods
A complete description of the methods used in this study is available in SI Appendix, Methods.
General Reagents.
The reagents for EAE, the CPZ-induced demyelination model, immunofluorescence, serum cytokine analysis, and other experiments mentioned in this paper are discussed in SI Appendix, Methods.
Mice.
All animal experiments were conducted following the Chinese Ministry of Health national guidelines for the housing and care of laboratory animals and were performed in accordance with institutional regulations after review and approval by the Institutional Animal Care and Use Committee of the National Institute of Biological Sciences. C57BL/6 mice were purchased from Vital River Laboratory Animal Technology Co. The Rip3−/−, Mlkl−/−, and RIP1K45A mice were generated in previous studies.
Surgical Procedure.
Details of the surgical procedures for the EAE and CPZ demyelination models are provided in SI Appendix, Methods.
Immunohistochemistry.
The immunohistochemistry procedures for spinal cord sections in EAE and brain sections in the CPZ demyelination model are discussed in SI Appendix, Methods.
Histochemistry.
The Luxol fast blue-H&E staining procedure for spinal cord sections in EAE and brain sections in the CPZ-induced demyelination model is discussed in SI Appendix, Methods.
Electron Microscopy and Immunoelectron Microscopy.
The procedure for the transmission electron microscopy in EAE is discussed in SI Appendix, Methods.
ELISA.
The experimental procedure for ELISA is discussed in SI Appendix, Methods.
Mouse Serum Cytokine Analysis.
To test the level of mouse serum cytokines, we used the commercial Bio-Plex Pro Mouse Cytokine 23-plex Assay kit. The data were analyzed with the Bio-Plex 200 system.
In Vitro Kinase Activity Assay.
The kinase activity assay was performed by Reaction Biology Corp. More details can be found at www.reactionbiology.com/webapps/site/.
FACS.
The FACS procedure in EAE is discussed in SI Appendix, Methods.
Supplementary Material
Acknowledgments
We thank Dr. John Hugh Snyder for critically reading the manuscript. We express our gratitude to Dr. Haibing Zhang (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for generously providing the RIP1K45A mice. This work was supported by an institutional grant from the Chinese Ministry of Science and Technology and the Science and Technology Bureau of Beijing Municipal Government.
Footnotes
Conflict of interest statement: X.W. and Zhiyuan Zhang are cofounders of a start-up company working on developing medicine for demyelination diseases.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819917116/-/DCSupplemental.
References
- 1.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 2.Bradl M, Lassmann H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soykan I, McCallum RW. Gastrointestinal involvement in neurologic disorders: Stiff-man and Charcot-Marie-Tooth syndromes. Am J Med Sci. 1997;313:70–73. doi: 10.1097/00000441-199701000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Yagihashi S, Kamijo M, Watanabe K. Reduced myelinated fiber size correlates with loss of axonal neurofilaments in peripheral nerve of chronically streptozotocin diabetic rats. Am J Pathol. 1990;136:1365–1373. [PMC free article] [PubMed] [Google Scholar]
- 5.Sørensen TL, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brück W, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg MM. Multiple sclerosis review. P T. 2012;37:175–184. [PMC free article] [PubMed] [Google Scholar]
- 8.Ofengeim D, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Ying Z, et al. Mixed lineage kinase domain-like protein MLKL breaks down myelin following nerve injury. Mol Cell. 2018;72:457–468.e5. doi: 10.1016/j.molcel.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Ren Y, et al. Discovery of a highly potent, selective, and metabolically stable inhibitor of receptor-interacting protein 1 (RIP1) for the treatment of systemic inflammatory response syndrome. J Med Chem. 2017;60:972–986. doi: 10.1021/acs.jmedchem.6b01196. [DOI] [PubMed] [Google Scholar]
- 14.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 15.Miller SD, Karpus WJ. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2007;77:15.1.1–15.1.18. doi: 10.1002/0471142735.im1501s77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 17.Lalive PH, Molnarfi N, Benkhoucha M, Weber MS, Santiago-Raber ML. Antibody response in MOG(35-55) induced EAE. J Neuroimmunol. 2011;240–241:28–33. doi: 10.1016/j.jneuroim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Denic A, et al. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerschensteiner M, et al. Targeting experimental autoimmune encephalomyelitis lesions to a predetermined axonal tract system allows for refined behavioral testing in an animal model of multiple sclerosis. Am J Pathol. 2004;164:1455–1469. doi: 10.1016/S0002-9440(10)63232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett J, et al. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229:180–191. doi: 10.1016/j.jneuroim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Saunders NR, Dziegielewska KM, Møllgård K, Habgood MD. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci. 2015;9:385. doi: 10.3389/fnins.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han S, et al. ImKTx88, a novel selective Kv1.3 channel blocker derived from the scorpion Isometrus maculates. Toxicon. 2011;57:348–355. doi: 10.1016/j.toxicon.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos D, Scheiner-Bobis G. Dehydroepiandrosterone sulfate augments blood-brain barrier and tight junction protein expression in brain endothelial cells. Biochim Biophys Acta Mol Cell Res. 2017;1864:1382–1392. doi: 10.1016/j.bbamcr.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eng LF, Ghirnikar RS, Lee YL. Inflammation in EAE: Role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem Res. 1996;21:511–525. doi: 10.1007/BF02527717. [DOI] [PubMed] [Google Scholar]
- 26.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 27.Zavala F, et al. G-CSF therapy of ongoing experimental allergic encephalomyelitis via chemokine- and cytokine-based immune deviation. J Immunol. 2002;168:2011–2019. doi: 10.4049/jimmunol.168.4.2011. [DOI] [PubMed] [Google Scholar]
- 28.Foster R, et al. Sciatic nerve injury induces functional pro-nociceptive chemokine receptors in bladder-associated primary afferent neurons in the rat. Neuroscience. 2011;183:230–237. doi: 10.1016/j.neuroscience.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipp M, Clarner T, Dang J, Copray S, Beyer C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009;118:723–736. doi: 10.1007/s00401-009-0591-3. [DOI] [PubMed] [Google Scholar]
- 30.He S, Wang X. RIP kinases as modulators of inflammation and immunity. Nat Immunol. 2018;19:912–922. doi: 10.1038/s41590-018-0188-x. [DOI] [PubMed] [Google Scholar]
- 31.Shaw PJ, et al. Signaling via the RIP2 adaptor protein in central nervous system-infiltrating dendritic cells promotes inflammation and autoimmunity. Immunity. 2011;34:75–84. doi: 10.1016/j.immuni.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, et al. RIP1 kinase activity-dependent roles in embryonic development of Fadd-deficient mice. Cell Death Differ. 2017;24:1459–1469. doi: 10.1038/cdd.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger SB, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hettinger J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.