Abstract
Meningioma in vivo research is hampered by the difficulty of establishing an easy and reproducible orthotopic model able to mimic the characteristics of a human meningioma. Moreover, leptomeningeal dissemination and high mortality are often associated with such orthotopical models, making them useless for clinical translation studies. An optimized method for inducing meningiomas in nude mice at two different sites is described in this paper and the high reproducibility and low mortality of the models are demonstrated. Skull base meningiomas were induced in the auditory meatus and convexity meningiomas were induced on the brain surface of 23 and 24 nude mice, respectively. Both models led to the development of a mass easily observable by imaging methods. Dynamic contrast enhanced MRI was used as a tool to monitor and characterize the pathology onset and progression. At the end of the study, histology was performed to confirm the neoplastic origin of the diseased mass.
Keywords: animal models, neuroscience, pharmaceutical development, preclinical imaging, solid tumors
1. INTRODUCTION
This study focuses on the induction of two orthotopic meningioma models: a skull base meningioma of the auditory meatus, and a convexity meningioma. The models were selected as examples of meningioma tumors in mice, to be used in preclinical studies for translational research, being examples of brain tumors with the highest incidence. Despite the relatively low mortality characterizing these human meningiomas, the development of early diagnostic tools and/or effective therapies is extremely important to improve the outcome of the medical treatment of this pathology.1, 2
Both models were induced using the CH157MN cell line, isolated in 1977 from a 41‐year‐old woman.3 This line was selected because of its ability to reproduce histological, immunohistochemical and structural features of human meningioma. A CH157MN model has been described by Ragel et al4, 5 in 2007 and 2008, but these papers focused on characterization of the model via luciferase rather than on a description of the induction or characterization by MRI (tumors were imaged post mortem by MRI, but only to calculate tumor volume, rather than to give a precise localization and description of the model in real time). Similarly, Giogigeni et al6 and Karsy et al6, 7 adopted the model, but they used bioluminescence imaging to monitor tumor growth and response to treatment. Using this methodology, it was possible to approximate the effectiveness of treatment by observing a reduction in tumor mass. However, since bioluminescence imaging lacks resolution, it was not possible to determine the precise location and characteristics of the tumors because their burden, depth, and localization could not be accurately measured. In our study, 47 athymic female nude mice (5‐6 weeks old) were inoculated with CH157MN cells and imaged weekly on a 1 T scanner, before and after administration of a commercial gadolinium based contrast agent (GBCA). MRI provided a non‐invasive method of accurately evaluating of tumor growth, vascularization and GBCA perfusion/permeability.
2. PROTOCOL
All the procedures involving animals were conducted according to national and international laws for the care and use of laboratory animals (L.D. 26/2014; Directive 2010/63/EU). This experimental protocol was approved by the Italian Ministry of Health with Authorization 724/2017 PR.
CH157MN cells were cultured in DMEM F12 with 7% FBS. For tumor induction, they were resuspended in 3‐8 μL of serum‐free DMEM F12.
2.1. Tumor induction
Mice were subcutaneously injected with carprofen (5 mg/kg) 1 hour before the surgery. Anesthesia was induced with sevoflurane gas and then maintained systemically with Rompun® (5 mg/kg) and Zoletil® (40 mg/kg). Each mouse was then mounted on the stereotaxic apparatus and its temperature was continuously monitored and maintained in the range 36.5‐38.5°C. After cleaning the skin with a disinfectant (iodopovidone), a local anesthesia was administered (lidocaine) at a dose of 3 mg/kg. Bregma was then exposed and the induction sites identified using the following coordinates: 3 mm anterior, 2 mm lateral to bregma and 2 mm under the frontal bone for the convexity meningioma, and at the skull base (13‐14 mm) for Skull Base Meningioma. At each site, a small hole was drilled manually, and 3‐8 μL of cell suspension, containing 5 × 104‐5 × 105 CH157MN cells for the skull base site and 105‐106 cells for the convexity site, was injected manually at a rate of approximately 1 μL/3 min using a Hamilton syringe with 25G needle. The hole was closed using either bone wax or surgical glue after removing the needle, and the mouse was removed from the stereotaxic apparatus. The wound was sutured with surgical glue or silk thread. Carprofen (5 mg/kg) was subcutaneously administered for 3 days after surgery. For more information, see Supporting Information.
2.2. Imaging protocol
Mice were imaged once or twice a week using a 1 T Icon (Bruker Biospin, Ettlingen, Germany) scanner. After preliminary anatomical scans, MSME sequences were acquired before and after the intravenous administration of the GBCA. The following parameters were set: matrix size = 128 × 128, field of view = 1.6 × 1.6 cm, slice thickness 1.2 mm, TE (echo time) = 9.2 milliseconds, TR (repetition time) = 350 milliseconds, acquisition time = 90 seconds, NA = 2.
3. RESULTS
CH157MN meningiomas were induced in 47 nude mice, according to the experimental protocols summarized in Table 1. The induction was successful in 35/47 animals. Specifically:
15/24 mice developed skull base meningiomas (four mice did not survive the systemic anesthesia and one showed clinical signs immediately after the surgery; four mice did not develop any tumors).
20/23 mice developed convexity meningiomas (one mouse did not survive the systemic anesthesia; two mice did not develop any tumors).
Table 1.
Experimental scheme and results
| Group | Total animals | Cell number/suture procedure | Site | Stereotaxic coordinates | Intraoperatory mortality | Tumor take rate | Average tumor volume (mm3) | Average maximum enhancement (%) | Clinical signs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 5 × 104 suspended in 3 μL, surgical glue to seal/suture both the skull hole and the wound | Skull base | Ant. 3 mm, Lat. 2 mm, Vent. 12‐13 mm and needle with tip; Ant. 3 mm, Lat. 2 mm, Vent. at skull and needle without tip | 1/6 | 4/5 | 55 | 80 | Neurological damage, unsteady gait, tumbling, apathy |
| 2 | 6 | 5 × 105 suspended in 3 μL, surgical glue to seal/suture the skull hole and the wound | Skull base | Ant. 3 mm, Lat. 2 mm, Vent. at skull and needle without tip | 1/6 | 3/5 | 85 | 70 | No observations |
| 3 | 6 | 5 × 104 suspended in 3 μL, silk thread (5.0) to suture the wound | Skull base | Ant. 3 mm, Lat. 2 mm, Vent. at skull and needle without tip | 1/6 | 2/5 | 45 | 70 | Loss body weight <8%, dyspnea, isolation from the group, dehydration, closed eye lids, unsteady gait |
| 4 | 6 | 5 × 105 suspended in 5 μL, silk thread (5.0) to suture the wound | Skull base | Ant. 3 mm, Lat. 2 mm, Vent. at skull and needle without tip | 2/6 | 4/4 | 50 | 55 | Tachipnea, isolation from the group, immobility, closed lid eye, loss of coordination and equilibrium |
| 5 | 6 | 105 suspended in 3 μL, surgical glue to seal/suture the skull hole and the wound | Convexity | Ant. 3 mm, Lat. 2 mm, Vent. 2 mm and needle without tip | 1/6 | 5/5 | 62 | 70 | Domed head |
| 6 | 5 | 106 suspended in 8 μL, surgical glue to seal/suture the skull hole and the wound | Convexity | Ant. 3 mm, Lat. 2 mm, Vent. 2 mm and needle without tip | 0/5 | 4/5 | 50 | 70 | No observations |
| 7 | 6 | 105 suspended in 3 μL, bone wax to seal the skull hole and silk thread (5.0) to suture the wound | Convexity | Ant. 3 mm, Lat. 2 mm, Vent. 2 mm and needle without tip | 0/6 | 5/6 | 40 | 80 | Domed head and retracted eyes |
| 8 | 6 | 106 suspended in a 6 μL, bone wax to seal the skull hole and silk thread (5.0) to suture the wound | Convexity | Ant. 3 mm, Lat. 2 mm, Vent. 2 mm and needle without tip | 0/6 | 5/6 | 50 | 60 | Domed head and retracted eyes |
As detailed in Table 1, once the inoculation site had been set, different experimental conditions were tested (ie the cell number and the method adopted to suture the surgical wound) to optimize the protocol.
The convexity meningioma model was generally more successful than the skull base model, in terms of both surgical procedure and animal welfare. Specifically, the intra‐operative mortality was lower for three out of four groups inoculated superficially, indicating, as expected, a minor surgery risk, since the needle stops at the surface rather than passing through the brain to reach the auditory meatus. Even after surgery, the occurrence of clinical signs was more severe in the skull base model, with the appearance of unsteady gait, domed head and loss of equilibrium. Loss of equilibrium was probably due to the specific location of the tumor in the auditory meatus. A further advantage of the convexity model was that the tumor take rate was superior. Tumor volume, growth rate and maximum enhancement after GBCA administration were comparable between the two sites. All the different procedures to close the skull hole adopted for both the skull base and convexity meningioma models were effective at avoiding the appearance of any extra‐cranial mass. Surgical glue is preferred to silk thread for suturing the wound, to prevent the animal from opening the wound by over‐grooming.
A standard needle was used during cell inoculation in only three animals. For all the other experimental groups the tip of the needle was cut, since only one animal out of the three inoculated using a standard needle developed an observable tumor mass; most likely the standard tip prevented release of tumor cells.
From 1 week after tumor induction to the end of the observation period (ie, 30 days after tumor induction) or the occurrence of one of the end‐points (ie, tumor mass larger than 0.15 cm3, weight loss ≥20%), animals without significant or prolonged clinical signs were imaged weekly (or more frequently) using MRI and administered with GBCA in the presence of a clearly visible tumor mass. In the case of skull base meningiomas, euthanasia after the occurrence of severe or prolonged clinical signs was required for 10 animals: two animals just after the surgical procedure, one animal 1 week post tumor induction and seven animals 2 weeks post tumor induction. In the case of the convexity meningiomas, euthanasia was required for four animals: two animals 1 week post tumor induction and two animals 2 weeks post tumor induction.
A tumor mass was generally visible 1 week‐10 days after induction at both inoculation sites, as shown for representative animals in all geometrical sections of T2‐weighted images (Figure 11A and 1B). T1‐weighted images (pre‐ and post‐contrast) together with signal enhancement vs time quantification are shown in panels A and B of Figure 2 for skull base and convexity meningiomas, respectively. In pre‐contrast images, brain tissue and tumor mass gave the same signal, but after GBCA administration the diseased tissue gave an enhanced signal and was clearly distinguishable from the contralateral region, ie auditory meatus for skull base meningioma and healthy brain for convexity meningioma. The tumor mass was enhanced by between 50% and 120% in all images with two exceptions, immediately after induction and when the tumor mass was not yet well developed. Finally, meningioma identity and tissue vascularization were assessed by histological analysis. Both CH157MN orthotopic tumors exhibited histological analogies with human grade III meningioma tumor, such as nuclear polymorphism, large cells with eccentric nuclei and abundant cytoplasm and mitosis (Figure 3). The tumors displayed infiltrative growth, widespread vascularization and, often, the presence of hemorrhages.
Figure 1.
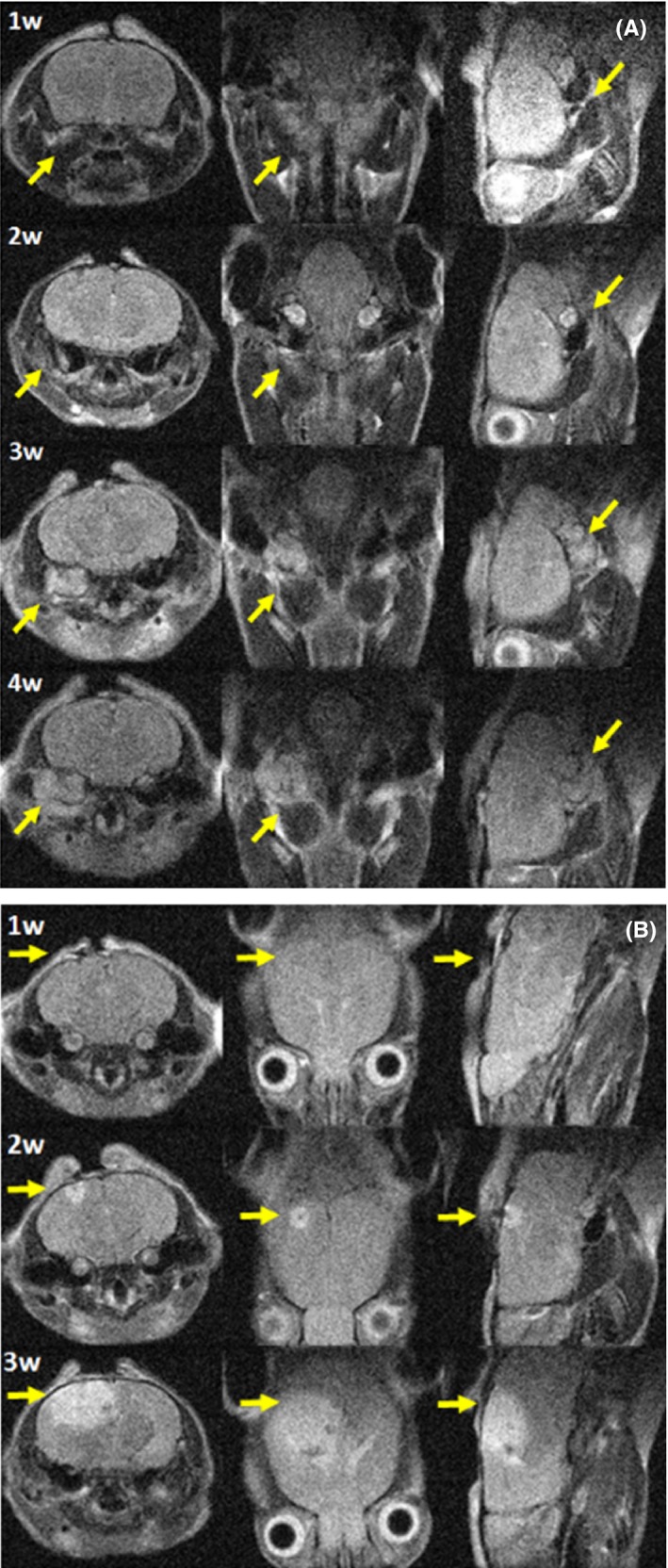
Representative anatomical T2‐weighted images at different weeks post tumor induction in all geometrical sections (from left to right axial, coronal and sagittal). A, skull base meningioma and B, convexity meningioma
Figure 2.
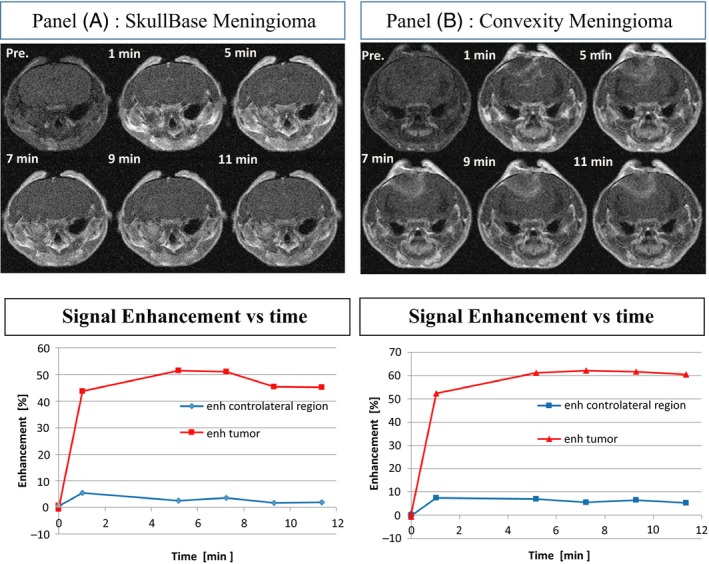
A, Skull Base and B, Convexity Meningioma Representative contrast enhanced T1‐weighted images and MSME signal enhancement as a function of time for healthy contralateral tissue (blue) and tumor mass (red)
Figure 3.
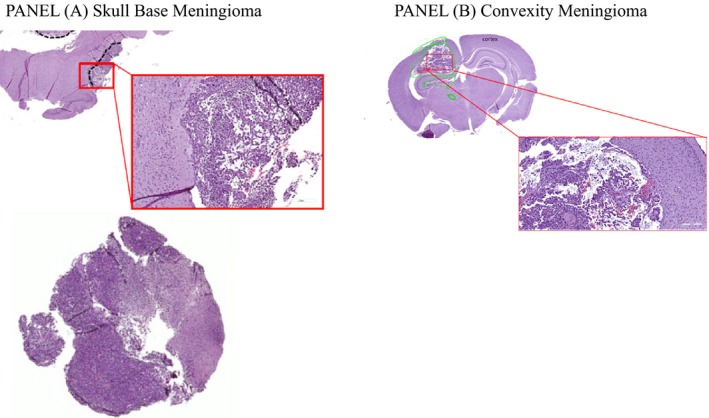
Representative examples of histological images of A, skull base meningioma, and B, convexity
4. DISCUSSION
Meningiomas have a 30% of incidence rate in humans. Even though they are mostly benign tumors, their intracranial location makes it difficult to remove them surgically, often leading to serious and potentially lethal outcomes. There is a need in preclinical brain tumor research for a reliable model of preclinical meningioma that is reproducible and well characterized. Here we describe and characterize two models of meningioma: skull base and convexity meningiomas in nude mice. Skull base meningiomas were successfully induced in the auditory meatus of female nude mice. There are some critical steps in the induction of this model that should be performed for the correct induction of the tumor. In particular, an optimal ventral coordinate of 14 mm from the dura mater to the skull base site was identified based on the fact that the needle encountered resistance when moved further. This value is correct if the mouse used is the same strain, sex and age as we describe. Another important step for a correct induction is the rate of cellular release into the auditory meatus. A large volume of medium is needed to keep cells in suspension, so it is important that the release happens slowly and carefully. An accumulation of fluid in the auditory meatus could cause severe clinical symptoms such as loss of equilibrium at the end of the procedure. Convexity meningiomas were easier to induce and overall caused fewer clinical symptoms and required less troubleshooting. However, the dura mater should be preserved for a successful induction. In particular, it should not be damaged when drilling through the skull: the hole should be drilled manually and the operator should use magnifying glasses. Drilling should stop when the drill encounters less resistance.
Non‐invasive MRI was used from early in the development of the tumors to characterize in real time the evolution of the cerebral pathologies. The onset of the pathologies was monitored at different time points, allowing the operator to monitor the appearance and growth of the disease, as well as the permeability properties, by injection of GBCA.
The feasibility and high reproducibility of these pathological models indicates their suitability for GBCA validation MRI studies. The precise location of the meningioma tumors, as seen with MRI, has been shown to mimic the complex situations in human patients.8 The enhanced signals for tumor masses obtained after GBCA administration in the investigated orthotopic meningiomas shows that these models could be used to “bridge” the gap between clinical and preclinical efficacy studies.
ACKNOWLEDGEMENTS
Human meningioma cells (CH157MN) were a kind gift from Dr Yancey Gillespie, University of Alabama.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
FLC, LM, AFM, PI, SCS, CC, ADV conceived and designed the study. FLC, LM, PI and ADV focused on surgery setup and cellular culture; AFM and SCS optimized the MRI experimental protocol and carried out data analysis; CB and AC performed histologies. All authors contributed to revising the manuscript and gave their final approval for manuscript publication.
Supporting information
La Cava F, Fringuello Mingo A, Irrera P, et al. Orthotopic induction of CH157MN convexity and skull base meningiomas into nude mice using stereotactic surgery and MRI characterization. Animal Model Exp Med. 2019;2:58–63. 10.1002/ame2.12050
REFERENCES
- 1. McCutcheon IE, Friend KE, Gerdes TM, Zhang BM, Wildrick DM, Fuller GN. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg. 2000;92:306‐314. [DOI] [PubMed] [Google Scholar]
- 2. van Furth WR, Laughlin S, Taylor MD, et al. Imaging of murine brain tumors using a 1.5 Tesla clinical MRI system. Can J Neurol Sci. 2003;30:326‐332. [DOI] [PubMed] [Google Scholar]
- 3. Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF‐BB, and bFGF. J Neurosurg. 1995;82:864‐873. [DOI] [PubMed] [Google Scholar]
- 4. Ragel BT, Jensen RL, Gillespie DL, Prescott SM, Couldwell WT. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. Cancer. 2007;109:588‐597. [DOI] [PubMed] [Google Scholar]
- 5. Ragel BT, Elam IL, Gillespie DL, et al. A novel model of intracranial meningioma in mice using luciferase‐expressing meningioma cells. J Neurosurg. 2008;108:304‐310. [DOI] [PubMed] [Google Scholar]
- 6. Gogigeni VR, Nalla AK, Gupta R, Dinh DH, Klopfenstein JD, Rao JS. Chk2‐mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011;313:64‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karsy M, Hoang N, Barth T, et al. Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: human and orthotopic xenograft studies. World Neurosurg. 2016;86:210‐219. [DOI] [PubMed] [Google Scholar]
- 8. Campbell BA, Jhamb A, Maguire JA, Toyota B, Ma R. Meningiomas in 2009: controversies and future challenges. Am J Clin Oncol. 2009;32:73‐85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


