Abstract
Background:
Autologous hematopoietic cell transplantation (AHCT) is curative for 60% of patients with relapsed or refractory Hodgkin lymphoma (R/R HL). A more precise depth of remission assessment prior to AHCT may help to identify patients likely to benefit from AHCT.
Aim:
To determine whether PET-based assessment of total metabolic tumor volume (TMTV), total lesion glycolysis (TLG), and maximal standardized uptake volume (SUVmax) predicts progression-free survival (PFS) after AHCT.
Methods:
Pre-transplant PET/CT images of 96 consecutive patients with R/R HL were analyzed to determine the quantitative parameters TMTV, TLG, SUVmax and semi-quantitative Deauville (D) 5-point score.
Results:
Median TMTV, TLG, and SUVmax were 7.97 cm3 (range 1.3-102.1cm3), 23.7 (4.0-813.1), and 5.23 (2.7-23.2). Two-year PFS in patients with high metabolic tumor volume TMTVhigh (>median; n=17) was only 12% (95%CI 1-38%) compared to 53% (95%CI 28-73%; P=0.05) in patients with TMTVlow (≤median; n=17) and 63% (95%CI; 50-74%) in 61 patients without metabolically active tumor (TMTV0; P>0.01). In concordance, high TLG (>19) and SUVmax (>4.9) predicted inferior 2-year PFS. In multivariate analysis, patients with TMTVhigh had 3.5-fold higher risk of treatment failure compared to TMTV0/TMTVlow (Hazard ratio 3.49 (1.75-6.93; p<0.01). D-score 4,5 associated with significantly worse PFS than D 1-3 (HR 3.73 (95%CI, 1.92-7.28; p<0.01). TMTV and D-score were disconcordant in 12 subjects.
Conclusion:
Patients with residual disease with low TMTV, TLG, and SUVmax prior to AHCT have similar outcomes to patients without metabolic active disease. High TMTV can identify very poor AHCT responders.
Keywords: Hodgkin lymphoma, Positron Emission Tomography Computed Tomography, autologous transplantation, prognosis
CONDENSED ABSTRACT:
High metabolic tumor volume may identify very poor AHCT responders and patients with low metabolic volume have favorable PFS.
INTRODUCTION
Despite the relatively high curability of classic type Hodgkin lymphoma (cHL), about 30% of patients with advanced disease relapse or become refractory to front-line therapy and about 15% succumb to their illness.1 The overall outcome of patients with relapsed or refractory (R/R) cHL who proceed with high dose therapy and autologous hematopoietic cell transplantation (AHCT) remains unsatisfactory.2–4 About 30-40% of transplant recipients relapse and need further treatment.5,6 The need to identify patients with unfavorable prognosis and poor response to transplant has become increasingly relevant, given the opportunity to use novel drugs and immune modulators earlier in the disease course.
Hodgkin lymphoma is 18-fluorodeoxyglucose (18FDG)-avid in virtually all cases. Positron emission tomography (PET)/computed tomography (CT) is used routinely for cHL to stage, evaluate response to therapy and more recently to adapt the therapeutic interventions.7,8 Moskowitz et al. demonstrated that patients undergoing AHCT with negative FDG-PET had significantly better event-free survival of >80% versus 29% for patients with positive PET scan.2,9,10 Other studies suggested pre-transplant FDG-PET has value in predicting outcome in R/R cHL nevertheless about a third of PET positive patients remain disease free and 1 of 4 PET negative patients develop disease after AHCT.5,11–14
Revised response criteria for malignant lymphoma apply a Deauville grading score to the lesion with the most intense FDG uptake as a key parameter for determining remission quality15,16. Deauville score takes two reference points, the mediastinum and the liver of the individual patient, which have relatively constant uptake on serial imaging. The scale ranges from 1 to 5, where 1 is best and 5 is the worst. However, Deauville score is semi-quantitative approach to assess the depth of remission and does not reflect the size of residual disease and variability of SUV uptake in individual lesions. Therefore, a new approach that encompasses the entirety of disease burden is needed to increase the precision in determining disease status and to evaluate the prognostic value in patients considering AHCT. Novel quantitative and volumetric parameters—such as total metabolic tumor volume (TMTV), total lesion glycolysis (TLG), or maximal standardized uptake volume (SUVmax)—have been recently developed to more precisely estimate depth of response to therapy and tumor burden.17–19 The value of quantitative metabolic parameters in patients with R/R cHL treated with AHCT is unknown. We hypothesized that quantitative tumor parameters will improve the predictive power of pre-transplant PET and help to select candidates for whom novel treatment approaches are needed.
PATIENTS AND METHODS
Study Design
The study population included patients ≥18 years old with R/R cHL who were consecutively enrolled on a clinical trial of AHCT (NCT00345865) between January 2000 and December 2014. The study received Institutional Review Board (IRB) approval, and all patients signed the informed consent. We also obtained IRB approval to retrospectively review all imaging studies. Patient data prospectively collected in the University of Minnesota Transplant Database were supplemented with data from individual medical records and imaging files. Pretransplant disease burden after salvage chemotherapy was determined by PET/CT scan obtained within 4 weeks before AHCT and evaluated according to criteria described by Cheson et al.20 Treatment consisted of cyclophosphamide (CY) 1.5 g/m2 daily for 4 days, carmustine 300 mg/m2 for 1 day, and etoposide 150 mg/m2 I.V. × 6 doses (CBV) or BEAM (carmustine 300 mg/m2 IV once on day −6, etoposide 100 mg/m2 IV twice daily on days −5 to −2, cytarabine 100 mg/m2 IV twice daily on days −5 to −2, and melphalan 140 mg/m2 IV once on day −1). All patients received standard supportive care and were monitored uniformly by CT scan at months 1, 6, 12, and 24 post-transplant and PET/CT scan at day 100.21 Bone marrow was obtained at these time-points in patients with prior marrow involvement. Relapsed disease was generally confirmed by biopsy of PET-avid disease site.
Imaging analyses
Patients underwent whole body imaging by PET/CT within 4 weeks before AHCT, and all PET/CT scans were retrospectively re-reviewed by two nuclear medicine radiologists (RG, ZC) who were blinded to clinical outcomes. Majority of scans were performed on a Siemens Biograph 16 PET or Siemens Biograph 64 scanner with High Definition (HD) detector system. For some patients, scans were performed at scanners with similar specifications at outside institutions and were uploaded to picture archiving and communication system (PACS). Image analysis was provided using PACS and Syngo.Via software (Siemens Healthcare). Treatment response was scored visually using the semi-quantitative Deauville scale (D) per the criteria proposed by the Imaging Subcommittee for International Harmonization Project for lymphoma.22 Each FDG-avid lesion was rated independently as follows: D1, no uptake or no residual uptake; D2, slight uptake, but below the mediastinum SUV; D3, uptake above mediastinal, but below or equal to uptake in the liver; D4, uptake slightly to moderately higher than liver; and D5, markedly increased uptake or any new lesion.
In addition, we performed quantitative image analysis in all patients with a measurable PET-positive lesion of any size except one patient whose imaging series lacked needed software parameters. Region-of-interest (ROI) areas were manually selected in each imaging study defined as voxels with ≥41% of the SUVmax activity of the given lesion as previously reported.23 SUVmax, SUVpeak and metabolic tumor volume (MTV) for each ROI were determined by software. The lymphoma lesion in each patient with the highest SUV was defined as the SUVmax. Total MTV (TMTV) was calculated as the sum of all individual lesion volumes for each pre-transplant PET/CT study and reported in cm3. In patients with multiple lesions, a total of six largest lesions were selected for analysis. Total lesion glycolysis (TLG) was calculated as the sum of products of SUVpeak (for each ROI) and MTV in all lesions. Syngo.via image analytic software was used.
Statistical methods
Baseline patient and transplant characteristics, post-transplantation complications, and outcomes were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized collection procedures. Demographic and transplant characteristics were summarized by standard descriptive statistical methods. Statistical comparison of categorical variables was performed by Chi-square test and Kruskal-Wallis (Willcoxon) rank-sum test was used for comparison of continuous variables between D-score groups and TMTV groups.
The Kaplan-Meier method was used to estimate the probabilities of progression-free survival (PFS) through 2 years after AHCT and the log-rank test was used for univariate comparisons.24 Cox proportional hazard regression model was used to estimate the adjusted survival curves.25 Cumulative incidence estimator was used to calculate the probabilities of relapse reflecting the nonevent deaths as a competing risk. The cumulative incidence of TRM was also calculated reflecting relapse or progression as a competing risk.26
Positive and negative predictive values of PET were calculated both for categorized quantitative (TMTV, TLG, SUVmax) and semi-quantitative (Deauville system) variables separately. The Cox regression model for PFS was applied for the entire cohort using the Deauville score (optimal cut-off: D1-3: PETneg and D4-5: PETpos) cohorts as the main effect. Second Cox regression model for PFS used TMTV group (TMTV0 and TMTVlow vs TMTVhigh. TMTV cutpoint were defined by median. Contal and O´Quigley’s method was used to determine the optimal cut off based on 2-year-PFS for TLG and SUVmax.27 Other factors that were considered in the models included age at transplant, time from diagnosis to transplant (<1 year vs. ≥1 year), pre-transplant radiotherapy (yes vs. no), B symptoms (yes vs. no), extranodal disease (yes vs. no), bone marrow involvement (yes vs. no), HL subtype (nodular sclerosis vs. mixed cellularity vs. other), LDH at AHCT (< upper laboratory limit vs. ≥ upper laboratory limit), albumin level at AHCT (≤3.5 g/dL vs. >3.5g/dL), hemoglobin level at AHCT (<10 g/dL vs. ≥10 g/dL), absolute lymphocyte count at AHCT (<1×103/mL vs. ≥1×103/mL), collected graft CD34+ cells count (<4.985 vs. ≥4.985×106/kg), plerixafor administration (yes vs. no). Factors with clinical meaning or with univariate p-value less than 0.15 were used for multi variable analysis. Prognostic factor models for all clinical outcomes were built by backward selection method (P value < 0.10 was considered significant for remaining in the model).
All statistical analyses were implemented using Statistical Analysis System statistical software version 9.3 (SAS Institute Inc., Cary, NC). The cut-off significance level for all P values was 0.05.
RESULTS
Patient and Transplant Characteristics
Patient, disease, and conditioning characteristics are reported in Table 1. Median age at transplant was 33.1 years (range 18.0-71.3); 51% of patients were males. Patients had limited (60%) or advanced (40%) stage disease. Advanced stage disease commonly involved extranodal (32%) and bone marrow (11%) sites. Nodular sclerosis (86%) was the most common histology. Most patients (69%) had less <1 year duration of first remission or were refractory to front-line therapy. All patients were chemosensitive. The most frequent conditioning regimen used was CBV (n=88, 92%), followed by BEAM and cytoxan, mitoxantrone, ara-C, and dexamethasone. Graft sources were peripheral blood (92%) or bone marrow in 11% patients. Per protocol, patients with persistent nodal masses ≥2 cm or sites suspicious for residual disease on day +28 post-transplant imaging were referred for consideration of consolidative radiotherapy. A total of 33 patients (35%) received involved field radiotherapy (XRT) at the discretion of the treating radiation oncologist. The median dose delivered was 30.6 Gy (range: 16-44 Gy) and all radiotherapy was completed within 6 months of transplantation (median: 84 days).
Table 1.
Patient, Disease, and Transplant Characteristics
| All Groups |
D1-3 |
D4-5 |
P-value (for D score) |
TMTV0 |
TMTVlow |
TMTVhigh |
P-value (for TMTV) | |
|---|---|---|---|---|---|---|---|---|
| N | 96 | 73 | 23 | 61 | 17 | 17 | ||
| Median Age (range) | 33 (18-71) | 34 (18-66) | 30 (18-71) | 0.64 | 33.1(18-66) | 36 (20-71) | 29 (18-67) | 0.89 |
| Gender | 0.55 | 0.76 | ||||||
| Male | 49 (51%) | 36 (49%) | 13(56%) | 30 (48%) | 10 (58.8%) | 8 (47.1%) | ||
| Female | 47 (49%) | 37 (51%) | 10 (44%) | 31 (52%) | 7 (41.2%) | 9 (52.9%) | ||
| KPS | 0.08 | 0.10 | ||||||
| <80% | 1 (1.0%) | 0 | 1 (4.3%) | 0 | 0 | 1 (5.9%) | ||
| ≥80% | 93 (96.9%) | 71 (97.3%) | 22 (95.7%) | 60 (95%) | 17 (100.0%) | 16 (94.1%) | ||
| Duration of 1st remission | 0.12 | 0.43 | ||||||
| ≥1 year | 29 (30%) | 25 (34%) | 4 (17%) | 20 (30%) | 3 (17.6%) | 5 (29.4%) | ||
| <1 year | 67 (70%) | 48 (66%) | 19 (83%) | 41(70%) | 14 (82.4%) | 12 (70.6%) | ||
| Prior XRT | 0.04 | 0.04 | ||||||
| No | 62 (65%) | 43 (59%) | 19 (83%) | 35 (57%) | 15 (88.2%) | 12 (70.6%) | ||
| Yes | 34 (35%) | 30 (41%) | 4 (17%) | 26 (43%) | 2 (11.8%) | 5 (29.4%) | ||
| Ann Arbor Stage | 0.67 | 0.15 | ||||||
| I/II | 37 (38%) | 29 (40%) | 8 (35%) | 26 (40%) | 3 (17.6%) | 7 (41.2%) | ||
| III/IV | 59 (62%) | 44 (60%) | 15 (65%) | 35 (60%) | 14 (82.4%) | 10 (58.8%) | ||
| B symptoms | 0.83 | 0.57 | ||||||
| No | 44 (46%) | 33 (45%) | 11 (48%) | 28 (44%) | 9 (52.9%) | 6 (35.3%) | ||
| Yes | 52 (54%) | 40 (55%) | 12 (52%) | 33 (56%) | 8 (47.1%) | 11 (64.7%) | ||
| Extranodal ds | 0.42 | <0.01 | ||||||
| No | 65 (68%) | 51 (70%) | 14 (61%) | 47 (75%) | 5 (29.4%) | 12 (70.6%) | ||
| Yes | 31 (32%) | 22 (30%) | 9 (39%) | 14 (25%) | 12 (70.6%) | 5 (29.4%) | ||
| BM involvement | 0.63 | 0.21 | ||||||
| No | 85 (88%) | 64 (88%) | 21 (91%) | 55 (90%) | 13 (76.5%) | 16 (94.1%) | ||
| Yes | 11 (12%) | 9 (12%) | 2 (9%) | 6 (10%) | 4 (23.5%) | 1 (5.9%) | ||
| Histology | 0.28 | 0.28 | ||||||
| Nodular Sclerosis | 83 (86%) | 62 (85%) | 21 (91%) | 52 (84%) | 16 (94.1%) | 15 (88.2%) | ||
| Mixed Cellularity | 6 (6%) | 4 (5%) | 2 (9%) | 3(4%) | 1 (5.9%) | 2 (11.8%) | ||
| Other | 7 (8%) | 7 (10%) | 0 | 6 (12%) | 0 | 0 | ||
| LDH | 0.82 | 0.77 | ||||||
| <ULN | 85 (88.5%) | 64 (87.7%) | 21 (91.3%) | 54(87%) | 15 (88.2%) | 16 (94.1%) | ||
| ≥ULN | 7 (7.3%) | 5 (6.8%) | 2 (8.7%) | 4(6%) | 2 (11.8%) | 1 (5.9%) | ||
| Albumin | 0.63 | |||||||
| <3.5 g/dL | 86 (89.6%) | 65 (89.0%) | 21 (91.3%) | 55(89%) | 16 (94.1%) | 15 (88.2%) | 0.62 | |
| ≥3.5 g/dL | 6 (6.3%) | 4 (5.5%) | 2 (8.7%) | 3(5%) | 1 (5.9%) | 2 (11.8%) | ||
| ALC | 0.03 | 0.30 | ||||||
| Missing | 4 (4.2%) | 4 (5.5%) | 0 | 3 | ||||
| <1×103/mL | 42 (43.8%) | 27 (37.0%) | 15 (65.2%) | 45(73%) | 9 (52.9%) | 10 (58.8%) | ||
| ≥1×103/mL | 50 (52.1%) | 42 (57.5%) | 8 (34.8%) | 13(21%) | 8 (47.1%) | 7 (41.2%) | ||
| Stem cell source | 0.46 | 0.78 | ||||||
| Marrow | 1 (1.0%) | 1 (1.4%) | 0 | 16 (94.1%) | 16 (94.1%) | |||
| PBSC | 85 (88.5%) | 63 (86.3%) | 22 (95.7%) | 4(6%) | 1 (5.9%) | 1 (5.9%) | ||
| Marrow+PBSC | 10 (10.4%) | 9 (12.3%) | 1 (4.3%) | 22 (35%) | 0 | 0 | ||
| 35(59%) | ||||||||
|
Median CD34 cell dose in 106/kg
(range) |
5.0 (0.3-24.3) | 4.9 (0.3-24.3) | 5.6 (1.3-19.0) | 0.27 | 4.5(0.3-24.3) | 5.2 (1.8-19.0) | 5.8 (1.3-17.8) | 0.23 |
| Plerixafor | 0.07 | 0.09 | ||||||
| Missing | 3 (3.1%) | 2 (2.7%) | 1 (4.3%) | 1 | 1 (5.9%) | 1 (5.9%) | ||
| No | 79 (82.3%) | 63 (86.3%) | 16 (69.6%) | 53(85.5%) | 13 (76.5%) | 11 (64.7%) | ||
| Yes | 14 (14.6%) | 8 (11.0%) | 6 (26.1%) | 8(13%) | 3 (17.6%) | 5 (29.4%) | ||
| >2 chemo regimen | 0.03 | 0.04 | ||||||
| NO | 25 (26.0%) | 15 (20.5%) | 10 (43.5%) | 11 (20%) | 7 (41.2%) | 7 (41.2%) | ||
| YES | 71 (74.0%) | 58 (79.5%) | 13 (56.5%) | 50 (80) | 10 (58.8%) | 10 (58.8%) | ||
| Deauville score pre-AHCT | <0.01 | <0.01 | ||||||
| 1 | 57 (59.4%) | 57 (78.1%) | 0 | 57(92%) | 0 | 0 | ||
| 2 | 4 (4.2%) | 4 (5.5%) | 0 | 4(7%) | 0 | 0 | ||
| 3 | 12 (12.5%) | 12 (16.4%) | 0 | 0 | 8 (47.1%) | 3 (17.6%) | ||
| 4 | 19 (19.8%) | 0 | 19 (82.6%) | 0 | 9 (52.9%) | 10 (58.8%) | ||
| 5 | 4 (4.2%) | 0 | 4 (17.4%) | 0 | 0 | 4 (23.5%) | ||
| Consolidation XRT | 0.58 | 0.45 | ||||||
| Yes | 33 (34%) | 24 (32.9%) | 9 (39.1%) | 19 (34%) | 8 (47%) | 6 (35%) | ||
| No | 63 (66%) | 49 (67.1%) | 14 (60.9%) | 42 (66%) | 9 (53%) | 11 (65%) |
Abbreviations: AHCT, autologous hematopoietic cell transplant; ALC, absolute lymphocyte count; BM, bone marrow; CR1, first complete remission; ds, disease; D, Deauville score category; KPS, Karnofsky performance status; TMTV; total metabolic tumor volume; ULN, upper laboratory limit; XRT, radiotherapy. PBSC peripheral blood stem cells
Pre-transplant PET/CT
Using integrated PET/CT prior to AHCT, 61 patients (64%) attained complete metabolic remission without metabolically active tumor (TMTV0). In 34 patients with measurable metabolic tumor volume, quantitative FDG measures TMTV, TLG, and SUVmax were collected. The median TMTV was 7.97 cm3 (range 1.3-102.1cm3), the median TLG was 23.7 (range 4.0-813.1), and the median SUVmax was 5.23 (range 2.7-23.2). The median long-axis diameter of involved nodes was 2.2cm (range 0.8-9.8 cm). The cut-off points for predicting 2-year PFS using ROC analysis were median for TMTV, and optimal cut-off 4.6 for SUVmax and 19.9 for TLG. Using Deauville scores, patient were assigned score of D1 (n=57), D2 (n=4), D3 (n=12), D4 (n=19) and D5 (n=4). Concordance of TMTVhigh (≥ median), TMTVlow (< median) and TMTV0 (no metabolically active tumor volume) with D score was variable and D3 distributed to both TMTVhigh and TMTVlow groups. (Figure 1). Baseline patients and transplant characteristics by TMTV and D score are summarized in Table 1. One patient from D3 group was not evaluable for metabolic volume assessment.
Figure 1.
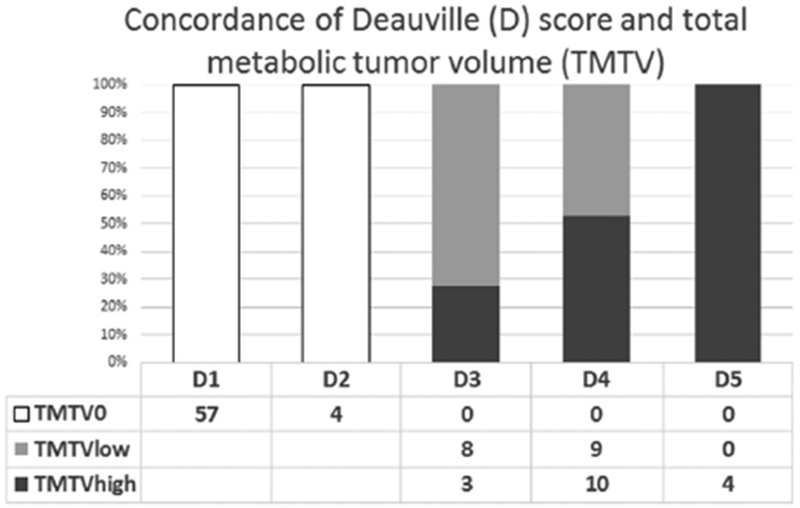
Concordance of TMTV and D score
Outcomes after AHCT
The median follow-up was 39.9 months (3.3 years; range: 1.0-10.3 years). At that time, 11 patients had progressed or relapsed and 13 patients had died (10 from recurrent HL, one from complications of subsequent allogeneic HCT and 2 from recurrent HL and therapy related myelodysplastic syndrome). There were no transplant related deaths in this cohort. The 2-year PFS for all patients was 53.3% (95% CI 0.43-0.64), and OS was 92.2% (95% CI 0.87-0.98).
To discriminate the prognostic impact of quantitative PET parameters, we compared patients with metabolically active tumor TMTVhigh (>median) to TMTVlow (≤median) and TMTV0 (no metabolic activity). The TMTVhigh group (n=17) less often had pre-transplant extranodal (29.4% vs 70%) and bone marrow disease (5.9% vs 23.5%) and more often received XRT prior to AHCT as compared to the TMTVlow group (Table 1). Other characteristics were similar among the groups including the proportion receiving post-transplant XRT (34% (TMTV0) vs 35% (TMTVhigh) vs 47% (TMTVlow)). Unadjusted 2-year PFS in TMTV0 group was 63% (95%CI 50-74%). Patients with TMTVhigh experienced a remarkably poor 2-year PFS of 12% (95%CI 1-38%) as compared to 53% (95%CI 28-73%) TMTVlow group (P=0.01; Figure 2). Consistently, patients with TLG > 19 (optimal cut-off) had worse unadjusted 2-year PFS: TLG high vs low was 12% vs 57% (P=0.04). Parameter of SUVmaxhigh (optimal cut-off 4.6) on pre-transplant PET scan yield inferior 2-years PFS (17%) than SUVmaxlow (62%; P=0.03). All three parameters TMTV, TLG and SUVmax were concordant. In Cox regression analysis adjusting for all other factors, TMTVhigh was associated with significantly worse PFS at 2 years (vs TMTV0/TMTVlow HR 3.49; 95%CI 1.75-6.93; p<0.01). Other factors independently affecting PFS were presence of B-symptoms (HR 2.13; 95%CI 1.10-4.13; p=0.02) and >1 year duration of remission (HR 0.24; 95%CI 0.10-0.58; p<0.01) (Table 2 and 3)
Figure 2:

2-year PFS for PET positive patients by TMTV groups
Table 2.
Univariate analysis of relevant prognostic factors for 2 year Progression-free Survival.
| Survival Estimate | Log Rank Test | ||||
|---|---|---|---|---|---|
| Factor | Strata | Total N | Estimate | CI 95% | P-value |
| Age (years) | 18-20 | 11 | 55% | 23- 78% | 0.48 |
| 20, 30 | 27 | 42% | 23- 60% | ||
| 30, 40 | 25 | 59% | 38- 76% | ||
| 40, 50 | 12 | 67% | 34- 86% | ||
| 50, 60 | 14 | 55% | 26- 77% | ||
| 60,70 | 6 | 33% | 5- 68% | ||
| Prior XRT | YES | 34 | 59% | 40- 73% | 0.61 |
| NO | 62 | 50% | 37- 62% | ||
| Stage | I/II | 37 | 61% | 43- 75% | 0.21 |
| III/IV | 59 | 49% | 35- 61% | ||
| B symptoms | YES | 52 | 42% | 28- 55% | 0.01 |
| NO | 44 | 67% | 51- 79% | ||
| Extra-nodal disease | YES | 31 | 45% | 27- 61% | 0.08 |
| NO | 65 | 57% | 44- 68% | ||
| Bone Marrow ds | YES | 11 | 55% | 23- 78% | 0.88 |
| NO | 85 | 53% | 42- 63% | ||
| Response to salvage therapy | CR | 56 | 62% | 48- 73% | 0.03 |
| PIF sensitive | 16 | 30% | 10- 53% | ||
| PR | 24 | 49% | 27- 67% | ||
| Histology | NS | 83 | 51% | 39- 61% | 0.53 |
| MC | 6 | 67% | 19- 90% | ||
| Other | 7 | 71% | 26- 92% | ||
| LDH@ AHCT | < ULN | 85 | 52% | 41- 62% | 0.78 |
| > ULN | 7 | 57% | 17- 84% | ||
| Albumin @ AHCT | <= 3.5g/dl | 86 | 52% | 41- 63% | 0.76 |
| > 3.5g/dl | 6 | 50% | 11- 80% | ||
| Hemoglobin @AHCT | < 10g/dl | 69 | 49% | 37- 61% | 0.57 |
| >= 10g/dl | 23 | 61% | 38- 77% | ||
| ALC @AHCT | < 1×10^3/ml | 42 | 49% | 32- 64% | 0.77 |
| >= 1×10^3/ml | 50 | 54% | 39- 67% | ||
| CD34 count/µL | <4.985 | 47 | 57% | 42- 70% | 0.52 |
| >=4.985 | 47 | 52% | 37- 66% | ||
| Plerixafor | YES | 14 | 29% | 8- 56% | 0.30 |
| NO | 79 | 57% | 45- 67% | ||
| Chemotherapy regimen | <3 | 71 | 56% | 43- 66% | 0.43 |
| ≥3 | 25 | 48% | 28- 66% | ||
| Graft source | Marrow | 1 | 0% | 0.62 | |
| PBSC | 85 | 53% | 42- 63% | ||
| Marrow+PBSC | 10 | 58% | 23- 82% | ||
| Duration of 1st remission | ≥1yr | 78 | 58% | 46- 68% | 0.02 |
| <1yr | 18 | 33% | 14- 55% | ||
Table 3.
Multivariate analysis of PFS at 2 years by TMTV and D-score
| 2 year Progression-free survival | |||
|---|---|---|---|
| Parameter | Class | Hazard Ratio CI 95% | P-value |
| TMTV group | TMTV0/TMTVlow | <0.01 | |
| TMTVhigh | 3.73 (1.92-7.28) | ||
| Duration of 1st remission | ≤1 year | 0.02 | |
| >1 year | 0.24 (0.10-0.58) | ||
| B-symptoms | no | ||
| yes | 2.13 (1.10-4.13) | <0.01 | |
| 2 year Progression-free survival | |||
| Parameter | Class | Hazard Ratio CI 95% | P-value |
| Deauville group | 1-3 | <0.01 | |
| 4-5 | 3.73 (1.92-7.28) | ||
| Duration of 1st remission | >1 year | <0.01 | |
| ≤1 year | 2.73 (1.34-5.55) | ||
PFS by D 1-5 score was D1: 63%, 95%CI 49-74%; D2: 75%, 95% CI 13-96%; D3: 64%, 95% CI 30-85%; D4: 22%, 95% CI 6-44% and D5: 20%, 95% CI 1-58%;). Using D3 as optimal cut-off point (D3 was favorable), baseline characteristics of D1-3 patients with D4,5 group differed in number of cycles of chemotherapy received and pre-AHCT absolute lymphocyte count (Table 1). Patients with pre-transplant D1-3 experienced significantly better 2-year PFS (63%; 95%CI 51-73%) compared to D4-5 score (20%; 95%CI 6-40%; p=0.01; Figure 3). In Cox regression analysis, D4-5 conferred a 3.7-fold increased risk of treatment failure as compared to D1-3 (HR 3.73 (95%CI, 1.92-7.28; Table 3). Twelve patients were disconcordant between D-score and TMTV categories (Figure 1).
Figure 3:
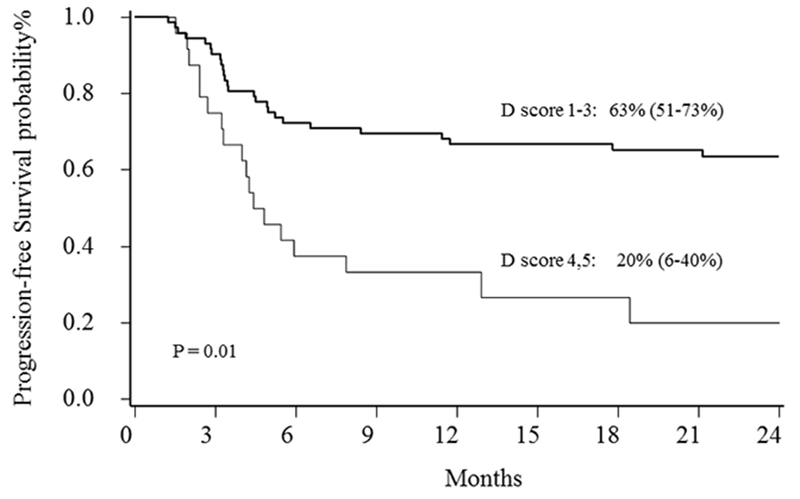
2-year PFS for patients by Deauville groups
DISCUSSION
PET/CT is currently used in routine clinical setting to assess the quality of remission after salvage therapy with the goal to achieve negative PET scan before AHCT. Positive PET scan heralds higher relapse risk and worse survival in patients with R/R HL treated with AHCT.9 While the Deauville score was developed to overcome inconsistency and variability in imaging technique and operator experience, routine semi-quantitative D score does not reflect the size of active residual lymphoma and maximum SUV uptake in individual lesions. Our data showed that quantitative PET parameters that combine metabolic activity and tumor volume in patients with R/R cHL undergoing AHCT have a favorable prognostic accuracy in predicting post-transplant survival. We found that in some patients, disconcordance exists between TMTV and D-score. Particularly, patients with score of D3 had either high or low TMTV, however the PFS for entire D3 group was favorable. In comparison, AHCT yielded very poor long-term PFS for patients with high TMTV above 7.97 cm3 and this group was heterogenous for D score and contained patients with D3, D4 and D5. Our results thus suggest that PET/CT evaluation using TMTV assessment can be particularly useful to identify patients in whom AHCT is associated with exceedingly high failure rate (12% for TMTVhigh vs 20% for D4,5). This conclusion extends on data from recent studies in front-line setting that showed TMTV and TLG to be superior predictors as compared to semi-quantitative PET assessment in patients with untreated HL and primary mediastinal large B-cell lymphoma (PMBCL).17,18,23,28,29 Kanoun et. al. showed that a TMTV determined at the time of diagnosis is more relevant than tumor bulk for predicting the PFS in patients with HL.17 In addition, reduction of SUVmax between baseline and interim PET remained independent predictor for relapse or progression.30 Similar results were obtained in PMBCL patients.28 Data from the prospective IELSG26 (International Extranodal Lymphoma Study Group) trial demonstrated that metabolic volume and TLG predict the outcomes after R-CHOP or R-CHOP like chemotherapy in PMBCL.31
Quantitative and volumetric metabolic parameters more precisely estimate depth of response to therapy and disease burden and have the potential to inform the clinical practice. TMTV method of using metabolic parameters is relatively simple and reproducible across different machines.19 Most clinical-grade PET scanners have built-in software to assess metabolic parameters and assign metabolic values.32 TMTV is a function of ROI and SUVmax, where ROIs have to be determined manually by radiologist as the major determinant of TMTV and SUV is estimated by software. Metabolic parameters are clinically interpretable and have a potential to be applied in decision making in the future.29
Previous studies reported the prognostic value of PET assessment pre-AHCT, yet more granular differences in outcomes among PETpos patients can be achieved by using quantitative methods of TMTV and TLG. In our study, patients with TMTVlow disease, which reflects low SUVmax and smaller total tumor bulk, experienced favorable outcomes similar to patients without metabolically active tumor, even though half of the patients in this cohort had D4 scores and 70% had extranodal involvement. This finding suggests that some PETpos patients with low tumor burden can have excellent outcomes after AHCT.
We collected post-AHCT radiation therapy data on all patients and found no difference in use of consolidation radiation therapy by TMTV. Given the time-frame of our study, only two patients received brentuximab vedotin (BV) as post-transplant consolidation. Recently, the randomized AETHERA trial demonstrated 20% improvement in PFS for chemosensitive cHL patients with high-risk features, including disease refractory to front-line therapy, relapse within 1 year after front-line therapy, extra-nodal disease or B-symptoms at relapse and ≥ 2 prior salvage therapies. Post-AHCT BV maintenance is now standard of care for high-risk cHL, and a recent multivariate analysis suggests significant benefit even for patients who were in CR but have >2 high-risk features.6 It is important to note that the AETHERA trial did not use pre-AHCT PET for risk stratification. In our series, TMTVhigh patients were heterogeneous for clinical high-risk features and only 12% achieved PFS post-AHCT, suggesting that novel therapeutic approaches are needed for this subset. We also acknowledge the limited sample size in our study and the need to validate our data in a larger cohort. Future prospective studies for R/R cHL using PET/CT scan should incorporate quantitative FDG parameters to allow for more precise depth of remission assessment.
Acknowledgments
FUNDING SUPPORT: Vít Procházka gratefully acknowledges the support of the J. W. Fulbright Commission (http://www.fulbright.cz) and University of Minnesota Proshek Scholar Fund. This study was kindly supported by IGA-LF-2017-007 and RVO: 61989592 grants. Research reported in this publication was supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core and the Translational Therapy Laboratory shared resources of the Masonic Cancer Center, University of Minnesota and by the by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. This work was also supported by American Society of Hematology Scholar Award (VB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: Authors declare no conflict of interests.
REFERENCES
- (1).Canellos GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 2014;32:163–168. [DOI] [PubMed] [Google Scholar]
- (2).Josting A, Rudolph C, Mapara M, et al. Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG). Ann Oncol. 2005;16:116–123. [DOI] [PubMed] [Google Scholar]
- (3).Rancea M, Monsef I, von Tresckow B, Engert A, Skoetz N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev. 2013;6:CD009411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–1545. [DOI] [PubMed] [Google Scholar]
- (5).Mocikova H, Pytlik R, Markova J, et al. Pre-transplant positron emission tomography in patients with relapsed Hodgkin lymphoma. Leuk Lymphoma. 2011;52:1668–1674. [DOI] [PubMed] [Google Scholar]
- (6).Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–1862. [DOI] [PubMed] [Google Scholar]
- (7).Johnson P, Federico M, Kirkwood A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N Engl J Med. 2016;374:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. [DOI] [PubMed] [Google Scholar]
- (9).Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. [DOI] [PubMed] [Google Scholar]
- (11).Kletter K, Kalhs P. (18)F-deoxyglucose PET: useful in the management of patients with stem cell transplantation for lymphoma? Expert Rev Hematol. 2010;3:405–410. [DOI] [PubMed] [Google Scholar]
- (12).Poulou LS, Thanos L, Ziakas PD. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging. 2010;37:156–162. [DOI] [PubMed] [Google Scholar]
- (13).Connors JM. Positron emission tomography in the management of Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:317–322. [DOI] [PubMed] [Google Scholar]
- (14).Moskowitz CH, Yahalom J, Zelenetz AD, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kanoun S, Rossi C, Berriolo-Riedinger A, et al. Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1735–1743. [DOI] [PubMed] [Google Scholar]
- (18).Kobe C, Kuhnert G, Kahraman D, et al. Assessment of tumor size reduction improves outcome prediction of positron emission tomography/computed tomography after chemotherapy in advanced-stage Hodgkin lymphoma. J Clin Oncol. 2014;32:1776–1781. [DOI] [PubMed] [Google Scholar]
- (19).Meignan M, Sasanelli M, Casasnovas RO, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014;41:1113–1122. [DOI] [PubMed] [Google Scholar]
- (20).Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- (21).Lerner RE, Thomas W, Defor TE, Weisdorf DJ, Burns LJ. The International Prognostic Index assessed at relapse predicts outcomes of autologous transplantation for diffuse large-cell non-Hodgkin’s lymphoma in second complete or partial remission. Biol Blood Marrow Transplant. 2007;13:486–492. [DOI] [PubMed] [Google Scholar]
- (22).Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. [DOI] [PubMed] [Google Scholar]
- (23).Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR. 2010;31:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kaplan EL MP. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- (25).Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- (26).Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. [DOI] [PubMed] [Google Scholar]
- (27).Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Statistics in Medicine. 1996;15:2203–2213. [DOI] [PubMed] [Google Scholar]
- (28).Ceriani L, Martelli M, Zinzani PL, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 2015. August 20;126(8):950–956. [DOI] [PubMed] [Google Scholar]
- (29).Meignan M, Gallamini A, Haioun C, et al. Report on the 5th International Workshop on Positron Emission Tomography in Lymphoma held in Menton, France, 19-20 September 2014. Leuk Lymphoma. 2015;56:1229–1232. [DOI] [PubMed] [Google Scholar]
- (30).Kanoun S, Tal I, Berriolo-Riedinger A, et al. Influence of Software Tool and Methodological Aspects of Total Metabolic Tumor Volume Calculation on Baseline [18F]FDG PET to Predict Survival in Hodgkin Lymphoma. PLoS One. 2015;10:e0140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ceriani L, Martelli M, Gospodarowicz MK, et al. Positron Emission Tomography/Computed Tomography Assessment After Immunochemotherapy and Irradiation Using the Lugano Classification Criteria in the IELSG-26 Study of Primary Mediastinal B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2017;97:42–49. [DOI] [PubMed] [Google Scholar]
- (32).Kanoun S, Tal I, Berriolo-Riedinger A, et al. Influence of Software Tool and Methodological Aspects of Total Metabolic Tumor Volume Calculation on Baseline [18F]FDG PET to Predict Survival in Hodgkin Lymphoma. PLoS One. 2015;10:e0140830. [DOI] [PMC free article] [PubMed] [Google Scholar]


