Abstract
The endoplasmic reticulum-Golgi intermediate compartment protein-53 (ERGIC-53, aka LMAN1), which cycles between the endoplasmic reticulum (ER) and Golgi, is a known cargo receptor for a number of soluble proteins. However, whether LMAN1 plays a role as a trafficking factor in the central nervous system is largely unknown. Here, we determined the role of LMAN1 on endogenous protein levels of the Cys-loop superfamily of neuroreceptors, including gamma-aminobutyric acid type A receptors (GABAARs), 5-hydroxytryptamine (serotonin) type 3 (5-HT3) receptors, and nicotinic acetylcholine receptors (nAChRs). Knockdown of LMAN1 reduces the surface trafficking of endogenous β3 subunits of GABAARs in mouse hypothalamic GT1-7 neurons. Furthermore, Western blot analysis of brain homogenates from LMAN1 knockout mice demonstrated that loss of LMAN1 decreases the total protein levels of 5HT3A receptors and γ2 subunits of GABAARs. LMAN1 knockout regulates the ER proteostasis network by upregulating ERP44 without changing calnexin levels. Interestingly, despite the critical role of the glycan-binding function of LMAN1 in its other known cargo clients, LMAN1 interacts with GABAARs in a glycan-independent manner. In summary, LMAN1 is a trafficking factor for certain neuroreceptors in the central nervous system. This is the first report of LMAN1 function in membrane protein trafficking.
Keywords: LMAN1, GABAARs, Cys-loop receptors, ERP44
1. Introduction
The anterograde transport from the endoplasmic reticulum (ER) to the Golgi apparatus is critical in guaranteeing properly folded and assembled proteins in eukaryotic cells to reach their destinations in order to perform their functions [1]. The ER export process of such cargo proteins (secretory and membrane proteins) begins from their package into the COPII-coated vesicles. To bridge the soluble cargo proteins that are processed in the ER lumen and COPII coats that are in the cytosolic side of the ER membrane, cargo receptors that span the ER membrane are required to interact simultaneously with cargo proteins and coat subunits. Although in principle integral membrane proteins can directly bind COPII subunits if they contain COPII binding motifs, cargo receptors for membrane proteins have been demonstrated, such as Erv14p in yeast [2]. Probably the best-characterized cargo receptor in mammals is the ER-Golgi intermediate compartment protein-53 (ERGIC-53, aka LMAN1). It cycles between the ER and the Golgi through COPII and COPI dependent pathways [3]. LMAN1 is a known cargo receptor for a number of soluble proteins, including blood clotting factor V and factor VIII, cathepsin C, cathepsin Z, α1-antitrypsin, and matrix metalloproteinase-9 (MMP9) [1,3–6]. Mutations in LMAN1 lead to the genetic bleeding disorder combined deficiency of FV and FVIII [7,8]. As a type I transmembrane protein, LMAN1 recognizes and binds to the high mannose structure on the glycoprotein substrates through its ER luminal carbohydrate recognition domain (CRD) [9–12]. Its cytosolic C-terminus contains a diphenylalanine motif that binds to the COPII coat, which facilitates the anterograde transport, and a dilysine motif that binds to the COPI coat, which mediates the retrograde transport [7].
We focused on proteostasis maintenance of neuroreceptors, specifically the pentameric Cys-loop receptors [13]. They play an essential role in the nervous system and include γ-aminobutyric acid type A receptors (GABAARs), nicotinic acetylcholine receptors (nAChRs), 5-hydroxytryptamine type-3 receptors (5HT3Rs), and glycine receptors (GlyRs) [14]. To reach their final destination, individual subunits undergo synthesis, folding and assembly into pentameric receptors in the ER and then go through the ER-to-Golgi transport, post Golgi transport and surface membrane insertion [15–20]. Disturbing any biogenesis step of these neuroreceptors could influence their surface expression level and the functional synaptic transmission, and impairment of the neurotransmission process can lead to related neurological diseases [13]. For example, for GABAARs, several proteins are known to be critical in their post-Golgi trafficking onto the plasma membrane, including GABARAP (GABAAR-associated protein), Hap1 (Huntingtin-associated protein 1), KIF5A, multidomain protein Muskelin [16,17]. Although the post-Golgi trafficking pathway of GABAARs is relatively well-understood [21], little is known about their regulatory factors that control the ER-to-Golgi transport.
Our previous proteomics study identified LMAN1 as an interacting protein for GABAARs [22]. To better understand the role of LMAN1 in the ER-to-Golgi trafficking of GABAARs and other Cys-loop receptors, we determined the influence of genetic manipulation of LMAN1 on the endogenous protein levels of these neuroreceptors. Furthermore, we reported how LMAN1 knockout regulated the endogenous proteostasis network and the glycan-independent interaction between LMAN1 and GABAARs.
2. Materials and methods
2.1. Plasmids, siRNAs, and antibodies
The pCMV6 plasmids containing human GABAA receptor α1 (Uniprot no. P14867-1), β2 (isoform 2, Uniprot no. P47870-1), and γ2 (isoform 2, Uniprot no. P18507-2) subunits and the pCMV6 Entry Vector plasmid (pCMV6-EV) were obtained from Origene. The Flag-tagged wild type (WT), ΔCRD, ΔHM, N156A, D181A, KKAA, ΔHelix, Δβ1, Δβ2, Δβ3 and Δβ4 LMAN1 plasmids were constructed as previously described [23]. Mouse LMAN1 siRNA-1 (J-050981-11-0005) and siRNA-2 (J-050981-09-0005) and non-targeting siRNA (D-001810-01-20) were obtained from Dharmacon. The mouse monoclonal anti-α1 (clone BD24) antibody came from Millipore (MAB339). The mouse monoclonal anti-β-actin (A1978) and anti-FLAG M2 peroxidase (A8592) antibodies were from Sigma. The mouse anti-GABAAR β3 antibody was from Neuromab (75149). The rabbit monoclonal anti-LMAN1 (ab125006), rabbit monoclonal anti-Na,K-ATPase (ab76020), rabbit polyclonal anti-5HT3AR (ab13897), and rabbit polyclonal anti-nAChR α4 (ab88239) antibodies were from Abcam. The rabbit polyclonal anti-GABAAR γ2 antibody was from Synaptic Systems (224003). The rabbit polyclonal anti-BiP (AP50016), rabbit polyclonal anti-P4HB (AP2911B-EV20), and rabbit polyclonal anti-Sec13 (AP10738CS) antibodies were from Abgent. The rat monoclonal anti-GRP94 (ADI-SPA-850), rabbit polyclonal anti-calnexin (ADI-SPA-860-F), and rabbit polyclonal anti-HSP70 (ADI-SPA-812-F) antibodies were from Enzo Life Science. The rabbit polyclonal anti-ERP44 antibody was from GeneTex (106636).
2.2. Cell culture and transfection
Human HEK293T cells (ATCC) and mouse GT1-7 cells (Professor Pamela Mellon, UCSD) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone) with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich) and 1% Penicillin-Streptomycin (Hyclone) at 37 °C in 5% CO2. Cells were transfected using TransIT-2020 (Mirus). The HEK293T cell line that stably expressing α1β2γ2 GABAARs was generated by transient transfection with α1:β2:γ2 (1:1:1) plasmids and selected using G-418. For siRNA transfections, cells were treated with 50 nM LMAN1 siRNA or non-targeting (NT) siRNA using HiPerfect transfection reagent (Qiagen).
2.3. Western blot analysis and immunoprecipitation
Cells were rinsed with ice-cold Dulbecco’s Phosphate-Bufferd Saline (DPBS) twice before harvested with Co-IP buffer (50 mM Tris, pH 7.5, 150 mM NaCl, and 1% Triton X-100) supplemented with Roche complete protease inhibitor cocktail. The lysates were then rotated for an hour at 4 °C and subject to centrifugation (16,000×g, 15 min, 4 °C) to remove cell debris and nucleus. The supernatant was collected as the total cellular protein. Protein concentrations were determined by the MicroBCA assay (Pierce). Western blot analysis was performed using appropriate antibodies. Band intensities were quantified using the Image J software from the NIH.
Cell lysates (500 μg) were precleared with 30 μL of protein A/G Plus-agarose beads (Santa Cruz Biotechnology) and 1.0 μg of normal mouse IgG for 1 hat 4°C to remove nonspecific binding proteins. The precleared cell lysates were incubated with 30 μL mouse anti-Flag M2 magnetic beads (M8823, Sigma Aldrich) or normal protein A/G plus agarose beads (negative control for nonspecific binding) overnight at 4 °C. The magnetic beads were collected using a magnetic separation stand (Promega) and washed three times with Co-IP buffer. Flag-tagged proteins were eluted by incubation with 30 μL of SDS loading buffer in the presence of β-mercaptoethanol. The immunopurified eluents were separated in an 8% tris-glycine gel, and Western blot analysis was performed.
2.4. Biotinylation of cell surface proteins
GT1-7 cells were plated in 10-cm dishes for surface biotinylation experiments according to published procedure [24]. Then, intact cells were rinsed gently twice with ice-cold PBS and incubated with the membrane-impermeable biotinylation reagent Sulfo-NHS SS-Biotin (0.5 mg/mL; Pierce) in PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS + CM) for 30 min at 4 °C to label surface membrane proteins. The reaction was quenched by incubating the cells with 10 mM glycine in ice-cold PBS + CM for 5 min at 4 °C. Sulfhydryl groups were blocked by incubating the cells with 5 nM N-ethylmaleimide (NEM) in PBS for 15 min at room temperature. Cells were solubilized for 1 h at 4 °C in lysis buffer (Triton X-100,1%; SDS 0.1%, Tris–HCl, 50 mM; NaCl, 150 mM; and EDTA, 5 mM; pH 7.5) supplemented with Roche complete protease inhibitor cocktail and 5 mM NEM. The lysates were cleared by centrifugation (16,000×g, 15 min at 4 °C) to pellet cellular debris. The supernatant were kept as it contained the biotinylated surface proteins. MicroBCA assay (Pierce) was then performed to measure the concentration of the supernatant. Biotinylated surface proteins were affinity-purified from the above supernatant by incubating for 1 h at 4 °C with 30–50 μL of immobilized neutravidin-conjugated agarose bead slurry (Pierce). The beads were washed with Co-IP buffer (50 mM Tris, pH 7.5,150 mM NaCl, 1% Triton X-100) twice followed by Co-IP buffer without Triton X-100 twice. Surface proteins were eluted by boiling for 5 min with 30–60 μL of LSB / Urea buffer (2× Laemmli sample buffer (LSB) with 100 mM DTT and 6 M urea; pH 6.8) for SDS-PAGE and Western blotting analysis.
2.5. Mouse whole brain sample preparation
Whole mouse brains were collected on ice and snap frozen in liquid nitrogen and stored at −80 °C. On the day of experiments, tissues were briefly thawed on ice and homogenized in homogenization buffer (25 mM Tris-HCl pH 7.6,150 mM NaCl, 1 mM EDTA, 2% Triton-X-100 supplemented with Roche protease inhibitors) using a plastic micro tissue homogenizer. Homogenates were centrifuged at 800 g for 10 min at 4 °C and supernatants were collected. Additional homogenization buffer was added to the pellet and homogenizing procedure was repeated. Supernatants were combined and rotated at 4 °C for 2–4 h. Debris in tissue lysates was removed by centrifugation at 13500g for 20 min and 18400 g for 30 min at 4 °C. The animal studies followed the guidelines of the Institutional Animal Care and Use Committees (IACUC) at Case Western Reserve University and Cleveland Clinic Institutional Review Board.
2.6. Statistical analysis
All data were presented as mean ± SEM. Statistical significance was evaluated using two-tailed Student’s t-Test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. LMAN1 positively regulates the surface expression of endogenous GABAAR subunits
Because our previous tandem mass spectrometry-based proteomics analysis identified LMAN1 as an interactor for GABAARs [22] and LMAN1 is a trafficking factor from the ER to Golgi, we tested whether LMAN1 regulates the receptors’ surface trafficking. To assess endogenous GABAARs, we used mouse GT1-7 hypothalamic GnRH neuronal cells, which express endogenous α1 and β3 subunits of GABAARs [25,26]. If LMAN1 plays a role in the anterograde transport of GABAARs from the ER to the Golgi, knocking down LMAN1 would interfere with the ER-to-Golgi trafficking process, and as a result, surface level of endogenous GABAARs will be decreased. As expected, surface biotinylation experiments demonstrated that the surface level of β3 subunits was reduced significantly (Fig. 1Aand B) after knockdown of LMAN1 by treating GT1-7 neurons with LMAN1 siRNA-1 (Fig. 1Cand E). Such a reduction of the surface β3 subunits was also significant (Fig. 1Fand G) by using LMAN1 siRNA-2 (Fig. 1Hand J). LMAN1 siRNA-2 treatment to GT1-7 neurons also decreased the total intracellular level of β3 subunits significantly (Fig. 1Hand I); LMAN1 siRNA-1 treatment to GT1-7 neurons decreased total intracellular level of β3 subunits in 6 out of 7 groups, but not significantly (Fig. 1Cand D). However, we did not find good anti-α1 antibody to detect the surface level of endogenous α1 subunits in GT1-7 neurons. Nonetheless, the β3 subunit result indicated that reducing the endogenous LMAN1 level attenuates the surface trafficking of endogenous GABAAR subunits.
Fig. 1. Transient knockdown of LMAN1 affects the total and surface expression of endogenous GABAARs subunits.
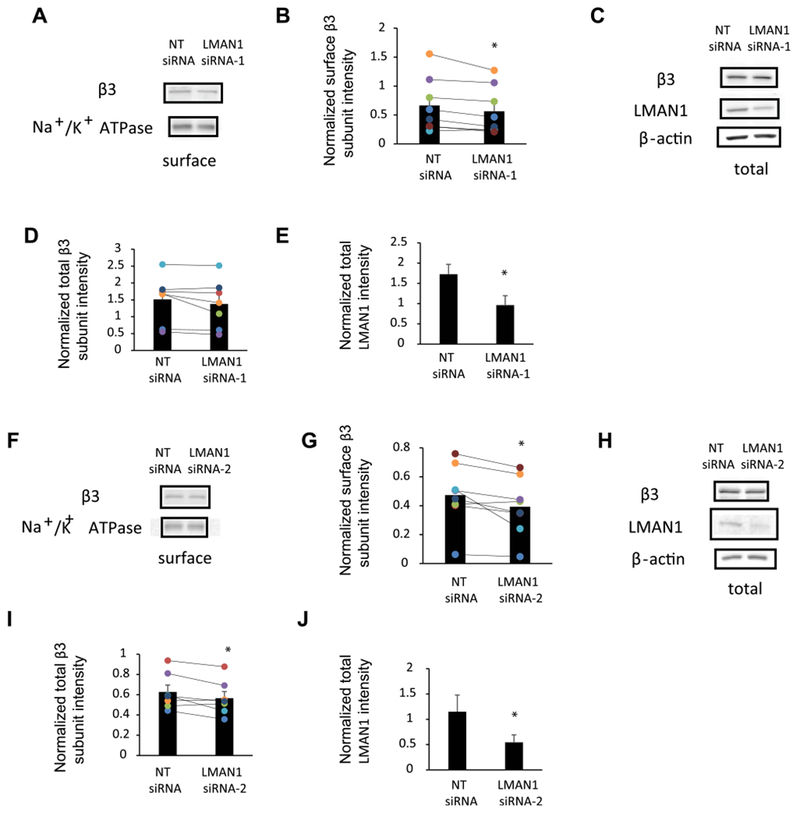
LMAN1 siRNA and non-targeting (NT) siRNA were applied to mouse hypothalamic GT1-7 neurons. Sixty-eight hours post transfection, cells were harvested, and protein analysis was performed. Knockdown of endogenous LMAN1 using LMAN1 siRNA-1 (A) or LMAN1 siRNA-2 (F) reduces the surface level of β3 subunits. Cell surface proteins were labeled with membrane-impermeable biotinylation reagent sulfo-NHS SS-Biotin. Biotinylated surface proteins were affinity-purified using neutravidin-conjugated beads and then subjected to SDS-PAGE and Western blot analysis. The Na+/K+-ATPase serves as a surface protein loading control. Quantification of normalized surface β3 protein levels to the Na+/K+-ATPase controls is shown in (B) (n = 8) and (G) (n = 8). Influence of knockdown of endogenous LMAN1 using LMAN1 siRNA-1 (C) or LMAN1 siRNA-2 (H) on the total protein level of β3 subunits by using SDS-PAGE and Western blot analysis. Quantification of normalized total β3 protein levels to β-actin loading controls is shown in(D) (n = 7) and (I) (n = 7). Quantification of the LMAN1 knockdown efficiency is shown in (E) (n = 7) and (J) (n = 7). *, p < 0.05, paired student t-test.
3.2. LMAN1 has a more general role for the Cys-loop neuroreceptors
Because GABAARs belong to the Cys-loop superfamily neuroreceptors [13,14], we continued to determine whether LMAN1 has a general role within this superfamily, which also includes nAChRs and 5-HT3Rs. We utilized the LMAN1 knockout mice, which were previously shown to result in combined deficiency of plasma factor V and factor VIII [27]. Five whole mouse brains were collected to access endogenous neuroreceptors: two were wild type (WT) controls (Lman1 +/+), and three were LMAN1 knockouts (Lman1 −/−). Knockout of LMAN1 in the brain was confirmed by Western Blot analysis (Fig. 2A, cf. lanes 2, 4, and 5 to lanes 1 and 3). Depleting LMAN1 decreased the total protein level of γ2 subunits of GABAARs significantly (Fig. 2B, cf. lanes 2, 4, and 5 to lanes 1 and 3; quantification shown in Fig. 2C). Due to the unavailability of proper anti-GABAAR β2 or β3 antibodies for mouse brain tissues, we could not evaluate the β subunits. Interestingly, LMAN1 knockout also significantly decreased the total protein level of 5-HT3A subunits (Fig. 2D, cf. lanes 2, 4, and 5 to lanes 1 and 3; quantification shown in Fig. 2E), whereas LMAN1 depletion did not seem to influence the protein level of the α4 subunits of nAChRs significantly (Fig. 2F, cf. lanes 2, 4, and 5 to lanes 1 and 3; quantification shown in Fig. 2G). Collectively, these results indicated that LMAN1 has a more general role for endogenous Cys-loop receptor subunits.
Fig. 2. Knockout of LMAN1 in mouse leads to decreased total expression level of GABAARs subunits and other Cys-loop family protein 5HT3A receptor subunits.
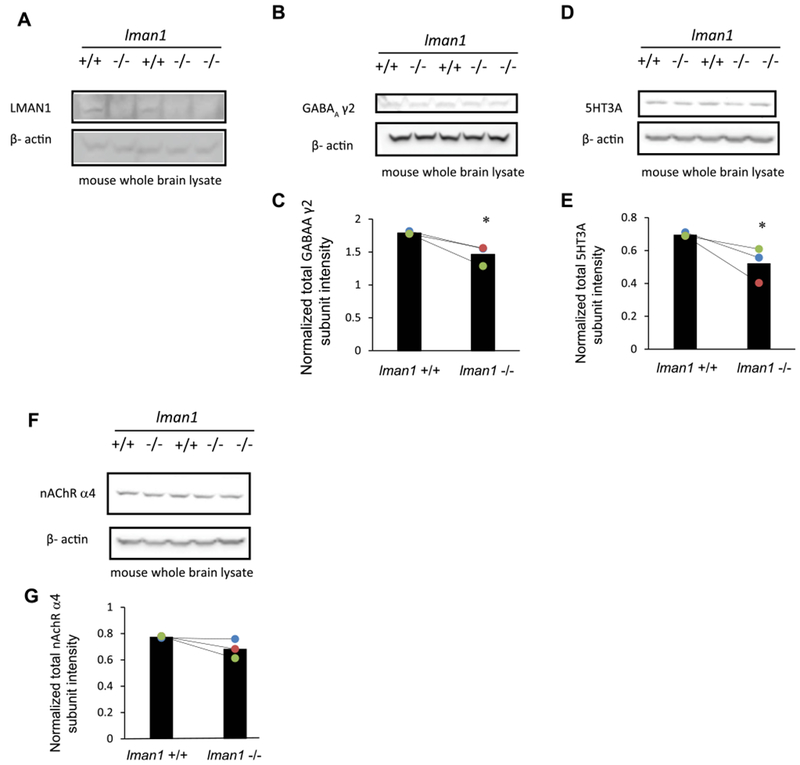
Whole brain lysates from two WT (LMAN1+/+) and three LMAN1 knockout (LMAN1−/−) mice were subject to SDS-PAGE. Western blot results of LMAN1, γ2 subunits of GABAARs, 5HT3A subunits, and nAChR α4 subunits are shown in (A), (B), (D), and (F). Corresponding band intensity quantification results are shown in (C), (E), and (G) (n = 3). *, p < 0.05.
3.3. Influence of LMAN1 knockout on the proteostasis network in the central nervous system
Because the proteostasis network (including chaperones, degradation factors, and trafficking factors) plays an essential role in controlling the biogenesis of membrane proteins, we evaluated how loss of LMAN1 affects the proteostasis network in the central nervous system [28,29]. It was previously reported that in Lman1−/− mouse liver, the total protein level of GRP78 increased substantially without significant induction of the unfolded protein response (UPR) genes, including Grp78, Grp94, Xbp1, Chop and Atf4 [27]. However, it is unknown how the proteostasis network was influenced in Lman1−/− mice brains. We evaluated the protein expression levels of major chaperones in the ER (including GRP78, GRP94, and calnexin), folding enzymes in the ER (including P4HB and ERP44), Hsp70 in the cytosol, and a COPII subunit in the cytosol (Sec13a) in Lman1+/+ and Lman1−/− mice brain homogenates (Fig. 3A–N). Western blotting analysis demonstrated that among these proteins, only the ERP44 level was increased significantly in LMAN1 knockout brain (Fig. 3E and F). These results indicated that loss of LMAN1 leads to limited changes in the proteostasis network in the brain.
Fig. 3. Influence of LMAN1 knockout on the proteostasis network in the central nervous system.
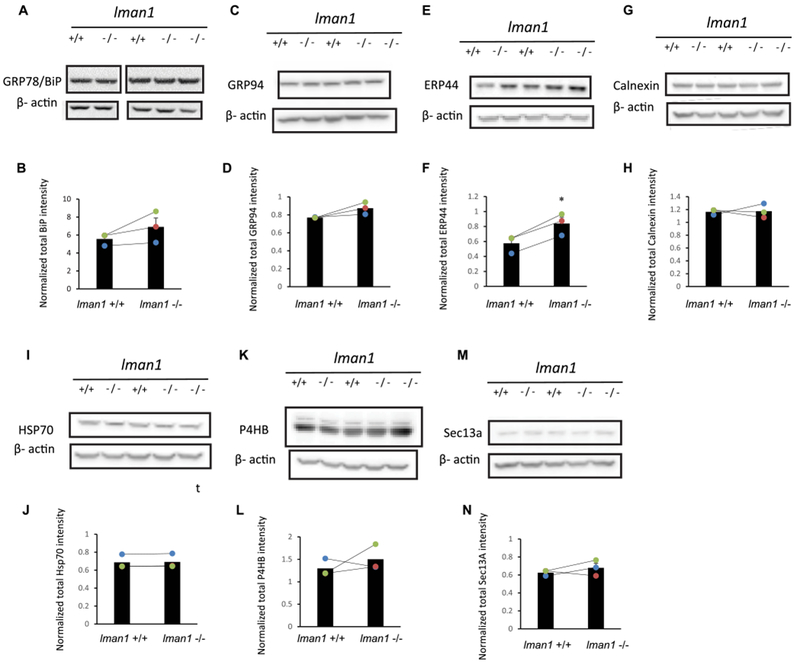
Western blot of whole brain lysates from two WT (LMAN1+/+) and three LMAN1 knockout (LMAN1−/−) mice. Results of GRP78, GRP94, ERP44, Calnexin, HSP70, P4HB and Sec13a are shown in (A), (C), (E), (G), (I), (K), and (M). Corresponding band intensity quantification results are shown in (B), (D), (F), (H), (J), (L), and (N) (n = 3). *, p < 0.05.
3.4. LMAN1 interacts with GABAARs in a glycan-independent manner
We next evaluated whether LMAN1 interacts with neuroreceptors to act as their cargo receptors. We focused on GABAAR subunits because our previous proteomics study revealed that LMAN1 binds GABAARs [22]. Indeed, co-immunoprecipitation experiments demonstrated that pulling down endogenous LMAN1 leads to the detection of α1 subunits in HEK293T cells transiently expressing α1β2γ2 GABAARs (Fig. 4A, lane 3), indicating that LMAN1 acts as a cargo receptor for GABAARs. For its soluble cargo glycoproteins, LMAN1 is known to interact with them through their mannose-rich glycans. To evaluate whether LMAN1 also binds to the glycans installed on the α1 subunits, we mutated the two N-glycosylation sites (Asn 38 and Asn 138) into glutamine to generate the N38Q/N138Q α1 subunits, which cannot be glycosylated in the ER lumen. Intriguingly, co-immunoprecipitation experiments demonstrated that such mutant α1 subunits interacted with endogenous LMAN1 (Fig. 4A, lane 4), and the interaction in the mutant α1 form was much stronger than that in the WT α1 form (Fig. 4A, cf. lane 4 to 3), indicating that LMAN1 binds GABAAR α1 subunits independent of the glycan structure.
Fig. 4. LMAN1 interacts with GABAARs in HEK293T cells in a glycan-independent manner.
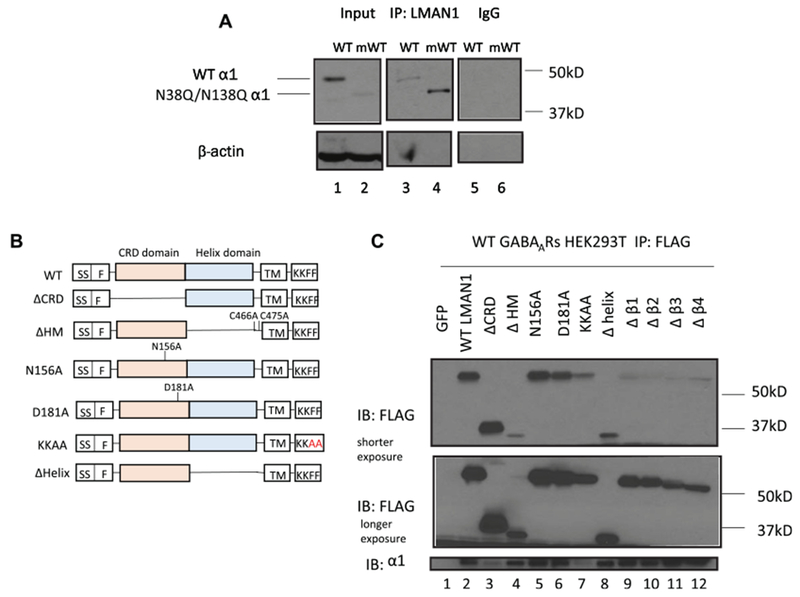
(A) WT or N38Q/N138Q α1 subunits together with β2 and γ2 subunits were overexpressed into HEK293T cells. Endogenous LMAN1 was co-immunoprecipitated using anti-LMAN1 antibody. The western blot result shows that both WT and N38Q/N138Q α1 subunits are detected (lanes 3 and 4). IgG control results are shown in lanes 5 and 6. (B) Cartoon figure for LMAN1 WT and mutants. For Δβ1 LMAN1 mutant, H43-Q59 in CRD domains is deleted. For Δβ2 LMAN1 mutant, H43-N72 in CRD domains is deleted. For Δβ3 LMAN1 mutant, H43-S76 in CRD domains is deleted. For Δβ4 LMAN1 mutant, H43-A83 in CRD domains is deleted. (C) Flag-tagged WT or mutant LMAN1 was overexpressed in HEK293T cells stably expressing WT GABAARs. WT or mutant LMAN1 was co-immunoprecipitated using anti-Flag antibody. Only LMAN1 without CRD domain and LMAN1 without ER exit signal (diphenylalanine to di-alanine mutation) abolish the interaction between LMAN1 and α1 subunits of GABAARs.
Furthermore, we evaluated which domain of LMAN1 plays an important role in its interaction with the α1 subunits. The LMAN1 mutations constructs were displayed in Fig. 4B. Co-immunoprecipitation experiments were used to determine how mutations in LMAN1 affected its interaction with α1 subunits. It was previously reported that N156A or D181A mutation in LMAN1 disrupts its binding ability to mannose structure on its soluble substrates. Interestingly, the N156A or D181A mutation in LMAN1 did not affect the interaction between LMAN1 and α1 subunits (Fig. 4C, cf. lanes 5, 6 to lane 2), consistent with their glycan-independent binding (Fig. 4A). The deletion of the complete CRD domain disrupted the interaction between LMAN1 and α1 subunits (Fig. 4C, cf. lane 3 to lane 2), possibly because deletion of such a domain induced global protein conformational changes. In addition, neither four β-sheets in the CRD domain nor the helix domain were required for α1 subunits binding (Fig. 4C, cf. lane 8–12 to lane 2). Oligomerization status of LMAN1 did not affect the α1 subunits binding because the ΔHM mutant, in which helix domain was deleted and both cysteine at 466 and 475 position were mutated to disrupt the formation of the LMAN1 hexamer through disulfide bonds, also interacted with α1 subunits (Fig. 4C, cf. lane 4 to lane 2). However, when the FF ER exit sorting signal was substituted for AA in LMAN1, this ER-retaining KKAA mutation interfered with the interaction between LMAN1 and α1 subunits (Fig. 4C, cf. lane 7 to lane 2). In conclusion, LMAN1 interacts with GABAARs independent of their glycosylation status.
4. Discussion
In this study we demonstrated that LMAN1 interacts with membrane protein GABAARs in a glycan-independent manner. Furthermore, LMAN1 positively affects the surface level of GABAARs. These results indicated that LMAN1 also serves as a trafficking factor for transmembrane proteins that are targeted to cell surface such as GABAARs, in addition to its known role of transporting soluble proteins. Since knockout of LMAN1 also affects the intracellular level of 5HT3A receptors, LMAN1 should have a more general role in the anterograde transport of neuroreceptors.
Because we demonstrated that the glycan structure on the membrane proteins in the ER lumen is not necessary for the LMAN1 interaction, LMAN1 must use other signals to detect trafficking-competent GABAARs. Previously, it was shown that the interaction between FVIII and LMAN1 is calcium dependent but is not dependent on the glycosylation status of FVIII [30]. How LMAN1 interacts with transmembrane proteins like GABAARs needs to be further elucidated. One intriguing possibility is that LMAN1 binds the transmembrane domain of its cargo protein within the lipid bilayer. Erv14p, another cargo receptor in yeast, was demonstrated to interact with its cargo through the intra-membrane interactions [31 ].
Knockout of LMAN1 in mice increases the intracellular level of an important ER chaperone ERP44. The upregulated ERP44 level upon LMAN1 depletion could have at least two possible effects. First, ERP44, also known as PDIA10, belongs to the protein disulfide isomerase (PDI) family, which catalyzes the formation of disulfide bond to assist protein folding and assembly. Therefore, ERP44 could be upregulated to counter the influence of loss of LMAN1 to handle the membrane proteins that were retained in the ER. Such an effect is also consistent with the role of ERP44 as a downstream target of the UPR pathway [32] because the reduced trafficking from the ER to Golgi could cause the ER to sense an increased burden of proteins retaining in the ER, leading to the activation of the UPR and its downstream chaperones. Future experiments are required to evaluate whether ERP44 upregulation is through UPR activation. Second, ERP44 cycles between the ER and the cis-Golgi in a pH-dependent manner, and the pH gradient among ER, ERGIC, and cis-Golgi regulates the conformational changes of ERP44 for its binding with the clients [33,34]. Therefore, ERP44 could transport the neuroreceptors from the ER to the Golgi independent of LMAN1. Collectively, these results indicated that loss of LMAN1 can influence the anterograde transport of neuroreceptors from the ER to the Golgi by regulating the proteostasis network.
It was previously shown that LMAN1 knockout mice do not show significant phenotypes with the two exceptions: 1. LMAN1 knockout mice on C57BL6/J background have a higher mortality rate compared to controls; 2. There are partially reduced FV, FVIII and α1-antitrypsin levels in plasma, and the FV level in platelets in LMAN1 knockout mice [6,27]. This further indicated that there are other important trafficking pathways and factors available for ER exit sorting and that elevated chaperone levels may help maintain cell homeostasis.
Acknowledgements
This work was supported by the NIH (R01NS105789 to TM; R01HL094505 and R03CA202131 to BZ).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- [1].Gomez-Navarro N, Miller E, Protein sorting at the ER-Golgi interface, J. Cell Biol 215 (2016) 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nakanishi H, Suda Y, Neiman AM, Erv14 family cargo receptors are necessary for ER exit during sporulation in Saccharomyces cerevisiae, J. Cell Sci 120 (2007) 908–916. [DOI] [PubMed] [Google Scholar]
- [3].Hauri HP, Kappeler F, Andersson H, Appenzeller C, ERGIC-53 and traffic in the secretory pathway, J. Cell Sci 113 (Pt 4) (2000) 587–596. [DOI] [PubMed] [Google Scholar]
- [4].Duellman T, Burnett J, Shin A, Yang J, LMAN1 (ERGIC-53) is a potential carrier protein for matrix metalloproteinase-9 glycoprotein secretion, Biochem. Biophys. Res. Commun 464 (2015) 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang B, Cunningham MA, Nichols WC, Bernat JA, Seligsohn U, Pipe SW, McVey JH, Schulte-Overberg U, de Bosch NB, Ruiz-Saez A, White GC, Tuddenham EG, Kaufman RJ, Ginsburg D, Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex, Nat. Genet 34 (2003) 220–225. [DOI] [PubMed] [Google Scholar]
- [6].Zhu M, Zheng C, Wei W, Everett L, Ginsburg D, Zhang B, Analysis of MCFD2- and LMAN1-deficient mice demonstrates distinct functions in vivo, Blood Adv 2 (2018) 1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Khoriaty R, Vasievich MP, Ginsburg D, The COPII pathway and hematologic disease, Blood 120 (2012) 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ogawa Y, Yanagisawa K, Uchiyama Y, Matsumoto A, Inoue M, Toyama K, Miyazawa Y, Matsumoto N, Handa H, [Congenital factor V and factor VIII deficiency discovered in an elderly patient with abnormal bleeding after trauma], Rinsho Ketsueki 59 (2018) 383–388. [DOI] [PubMed] [Google Scholar]
- [9].Satoh T, Suzuki K, Yamaguchi T, Kato K, Structural basis for disparate sugarbinding specificities in the homologous cargo receptors ERGIC-53 and VIP36, PLoS One 9 (2014), e87963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zheng C, Liu HH, Yuan S, Zhou J, Zhang B, Molecular basis of LMAN1 in coordinating LMAN1-MCFD2 cargo receptor formation and ER-to-Golgi transport of FV/FVIII, Blood 116 (2010) 5698–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng C, Page RC, Das V, Nix JC, Wigren E, Misra S, Zhang B, Structural characterization of carbohydrate binding by LMAN1 protein provides new insight into the endoplasmic reticulum export of factors V (FV) and VIII (FVIII), J. Biol. Chem 288 (2013) 20499–20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zheng C, Zhang B, Combined deficiency of coagulation factors V and VIII: an update, Semin. Thromb. Hemost 39 (2013) 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fu YL, Wang YJ, Mu TW, Proteostasis maintenance of Cys-loop receptors, Adv Protein Chem Struct Biol 103 (2016) 1–23. [DOI] [PubMed] [Google Scholar]
- [14].Nemecz A, Prevost MS, Menny A, Corringer PJ, Emerging molecular mechanisms of signal transduction in pentameric ligand-gated ion Channels, Neuron 90 (2016) 452–470. [DOI] [PubMed] [Google Scholar]
- [15].Ge Y, Kang Y, Cassidy RM, Moon KM, Lewis R, Wong ROL, Foster LJ, Craig AM, Clptm1 limits forward trafficking of GABAA receptors to scale inhibitory synaptic strength, Neuron 97 (2018) 596–610, e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kittler JT, McAinsh K, Moss SJ, Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission, Mol. Neurobiol 26 (2002) 251–268. [DOI] [PubMed] [Google Scholar]
- [17].Lorenz-Guertin JM, Jacob TC, GABA type a receptor trafficking and the architecture of synaptic inhibition, Dev Neurobiol 78 (2018) 238–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Martenson JS, Yamasaki T, Chaudhury NH, Albrecht D, Tomita S, Assembly rules for GABAA receptor complexes in the brain, Elife 6 (2017), e30826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mele M, Leal G, Duarte CB, Role of GABAA R trafficking in the plasticity of inhibitory synapses, J. Neurochem 139 (2016) 997–1018. [DOI] [PubMed] [Google Scholar]
- [20].Wanamaker CP, Christianson JC, Green WN, Regulation of nicotinic acetylcholine receptor assembly, Ann. N. Y. Acad. Sci 998 (2003) 66–80. [DOI] [PubMed] [Google Scholar]
- [21].Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B, GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses, J. Neurosci 26 (2006) 12758–12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang YJ, Han DY, Tabib T, Yates JR 3rd, Mu TW, Identification of GABA(C) receptor protein homeostasis network components from three tandem mass spectrometry proteomics approaches, J. Proteome Res 12 (2013) 5570–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Klaus JP, Eisenhauer P, Russo J, Mason AB, Do D, King B, Taatjes D, Cornillez-Ty C, Boyson JE, Thali M, Zheng C, Liao L, Yates JR 3rd, Zhang B, Ballif BA, Botten JW, The intracellular cargo receptor ERGIC-53 is required for the production of infectious arenavirus, coronavirus, and filovirus particles, Cell Host Microbe 14 (2013) 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lachance-Touchette P, Brown P, Meloche C, Kinirons P, Lapointe L, Lacasse H, Lortie A, Carmant L, Bedford F, Bowie D, Cossette P, Novel alpha1 and gamma2 GABAA receptor subunit mutations in families with idiopathic generalized epilepsy, Eur. J. Neurosci 34 (2011) 237–249. [DOI] [PubMed] [Google Scholar]
- [25].Hales TG, Kim H, Longoni B, Olsen RW, Tobin AJ, Immortalized hypothalamic GT1–7 neurons express functional gamma-aminobutyric acid type A receptors, Mol. Pharmacol 42 (1992) 197–202. [PubMed] [Google Scholar]
- [26].Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI, Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis, Neuron 5 (1990) 1–10. [DOI] [PubMed] [Google Scholar]
- [27].Zhang B, Zheng C, Zhu M, Tao J, Vasievich MP, Baines A, Kim J, Schekman R, Kaufman RJ, Ginsburg D, Mice deficient in LMAN1 exhibit FV and FVIII deficiencies and liver accumulation of alpha1-antitrypsin, Blood 118 (2011) 3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balch WE, Morimoto RI, Dillin A, Kelly JW, Adapting proteostasis for disease intervention, Science 319 (2008) 916–919. [DOI] [PubMed] [Google Scholar]
- [29].Martinez G, Khatiwada S, Costa-Mattioli M, Hetz C, ER proteostasis control of neuronal physiology and synaptic function, Trends Neurosci 41 (2018) 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang B, Kaufman RJ, Ginsburg D, LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway, J. Biol. Chem 280 (2005) 25881–25886. [DOI] [PubMed] [Google Scholar]
- [31].Pagant S, Wu A, Edwards S, Diehl F, Miller EA, Sec24 is a coincidence detector that simultaneously binds two signals to drive ER export, Curr. Biol 25 (2015)403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Anelli T, Alessio M, Mezghrani A, Simmen T, Talamo F, Bachi A, Sitia R, ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family, EMBO J 21 (2002) 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vavassori S, Cortini M, Masui S, Sannino S, Anelli T, Fagioli C, Mossuto MF, Fornili A, van Anken E, Degano M, Inaba K, Sitia R, A pH-regulated quality control cycle for surveillance of secretory protein assembly, Mol. Cell 50 (2013) 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Watanabe S, Harayama M, Kanemura S, Sitia R, Inaba K, Structural basis of pH-dependent client binding by ERp44, a key regulator of protein secretion at the ER-Golgi interface, Proc. Natl. Acad. Sci. U. S. A 114 (2017) E3224–E3232. [DOI] [PMC free article] [PubMed] [Google Scholar]


