Abstract
Neuroinflammation is a physiologic response aimed at protecting the central nervous system during injury. However, unresolved and chronic neuroinflammation can lead to long term damage and eventually neurologic disease including Parkinson’s disease, Alzheimer’s disease and dementia. Recently, enhancing the concentration of epoxyeicosatrienoic acids (EETs) through blocking their hydrolytic degradation by soluble epoxide hydrolase (sEH) has been applied towards reducing the long-term damage associated with central neurologic insults. Evidence suggests this protective effect is mediated, at least in part, through polarization of microglia to an anti-inflammatory phenotype that blocks the inflammatory actions of prostaglandins and promotes wound repair. This mini-review overviews the epidemiologic basis for using sEH inhibition towards neuroinflammatory disease and pharmacologic studies testing sEH inhibition in several neurologic diseases. Additionally, the combination of sEH inhibition with other eicosanoid signaling pathways is considered as an enhanced approach for developing potent neuroprotectants.
Keywords: epoxide hydrolase, epoxyeicosatrienoic acids, Alzheimer disease, Parkinson’s disease, dementia
1. Neuroinflammation in the Context of Disease
Neuroinflammation is a subset of inflammatory responses aimed at protecting the central nervous system (CNS) from injury. It can be triggered by a variety of insults ranging from mechanical stress, xenobiotic exposure or bacterial/viral infection. Compared to a standard inflammatory response that is primarily mediated by circulating cell types in the blood (including monocytes and lymphocytes), neuroinflammatory responses are primarily mediated by resident cell types found in the CNS including microglia and astrocytes. Like inflammation, excess of neuroinflammation in intensity and/or duration can lead to irreversible damage and long-term effects. Thus, in the context of disease, controlling neuroinflammation can be essential for reducing long term damage from acute catastrophic insults (including stroke, hemorrhage or seizures) and for reducing disease associated from sustained chronic insults (including Parkinson’s disease, dementia and Alzheimer’s disease). In this mini-review, the recent literature related to the role of epoxy-fatty acids (EpFA) in mediating and controlling neuroinflammatory responses is summarized. A recent review described the possible cellular mechanisms that EpFAs modulate to mediate these effects [1]; thus, we focus primarily on the practical therapeutic applications including how genetic changes in EpFA signaling affect neuroinflammatory disease and how to modulate EpFA signaling pharmacologically.
EpFAs are one component of a broad network of signaling lipids that generally regulate inflammatory disease. They are generated through epoxidation of their parent fatty acid by cytochrome P450 (CYP450) and are primarily degraded by epoxide hydrolases including microsomal epoxide hydrolase (mEH) and soluble epoxide hydrolase (sEH). Due to its high catalytic efficiency, inhibition of sEH is a generally effective approach for increasing endogenous EpFA concentrations in vivo. However, mEH expression is higher in neurons, suggesting a possible role for both enzymes in the metabolism of EpFAs in the brain that is cell-type and function dependent [2]. Genetic knockout studies have shown sEH knockout is sufficient for enhancing blood levels of EpFA, including 14,15-epoxyeicosatrienoic acid (14,15-EET) and 12,13-epoxyoctadecaenoic acid (12,13-EpOME) [3]. Other EpFA, including 11,12-EET and 19,20-epoxydocosapentaenoic acid (19,20-EDP), require double sEH/mEH knockout to maximize blood levels [3]. The 14,15-epoxyeicosatrienoic acid (14,15-EET) is one of the most commonly studied EpFA. However, EpFAs broadly exist as a mixture of regioisomers and stereoisomers and can exist on the backbone of a variety of parent polyunsaturated fatty acids (PUFAs) (Figure 1). Although many of these metabolites have overlapping functions, individual lipids can have discreet and at times opposite function. For example, EETs are angiogenic and promote tumor growth [4] while 19,20-EDP is anti-angiogenic and blocks tumor growth [5]. Thus, the biological consequence of increasing EpFA levels, through either increasing CYP450 synthesis or blocking EH degradation, is the combination of many metabolites.
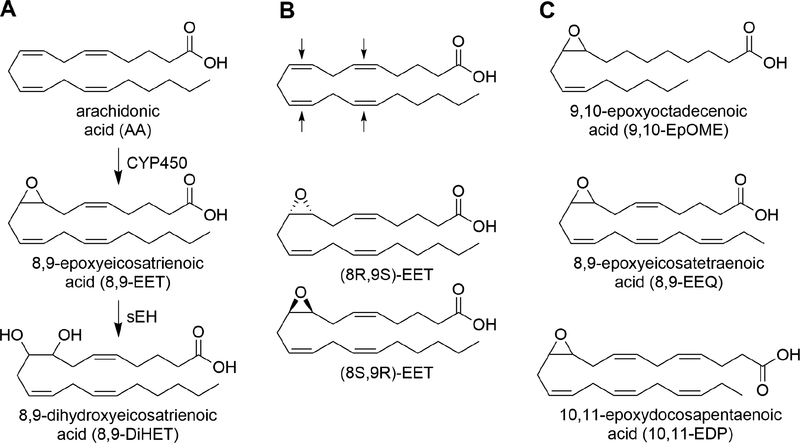
Figure 1.
A. Arachidonic acid is oxidized by cytochrome P450s (CYP450s) to epoxyeicosatrienoic acids (EETs) which are further hydrolyzed by soluble epoxide hydrolase (sEH) to dihydroxyeicosatrienoic acids (DiHETs). B. CYP450s can generate epoxides on any of the available unsaturated bonds of arachidonic acid and the resulting epoxides can exist in either the (R,S) or (S,R) enantiomeric forms (example given for the 8,9-double bond only). Activity of EETs are typically reported as one of the four regioisomers; however, 8 separate molecules exist and each may have independent activities. C. CYP450s can generate epoxy-fatty acids from a variety of polyunsaturated fatty acids (PUFAs) including linoleic acid to EpOME, eicosapentaenoic acid (EPA) to EEQ and docosahexaenoic acid (DHA) to EDPs.
2. Epidemiologic evidence for the role of EpFA in neurologic disease
Evidence supporting a role for EpFA in regulating neuroinflammation comes from epidemiologic studies investigating the effects of variants of CYP450 and EH genes on the prevalence of disease. The rs890293 variant of CYP2J2, associated with reduced enzymatic function, has been associated with late onset Alzheimer’s disease in the Chinese Han Population [6, 7]. CYP2J2 is one of the major isoforms of CYP450 responsible for the production of EpFA; thus, these single nucleotide polymorphisms (SNPs) indicate EpFA may provide a neuroprotective role that reduces the risk of Alzheimer’s.
There are several known SNPs of sEH that include the K55R, R103C, C254Y and R287Q. Of these, the R287Q variant has substantially reduced catalytic efficiency (14-fold reduced kcat/KM compared to WT) on 14,15-EET [8]. Somewhat unexpectedly, the R287Q variant has been associated with increased white matter hyper-intensity in human subjects, an indicator of white matter lesions brought by vascular cognitive impairment [9]. The association between the R287Q variant and prevalence of Parkinson’s disease has been investigated; however, there was no statistically significant association between the two [10].
Several studies have tested the relationship between sEH polymorphisms and the prevalence of cerebrovascular events with varying results [11]. A study in Swedes found that the K55R variant resulted in increased blood pressure in males but not females and an increase in the incidence of strokes [12]. While some studies investigating the effects of the R287Q variant have shown both a protective effect in ischemic stroke [13, 14], others have shown no clear association [15, 16]. It should be noted that the reduced incidence of stroke reflects a reduction in hypertension and cardiovascular disease. After the ischemic event occurs, there is also evidence that differences in sEH activity due to SNPs may affect the subsequent inflammatory response. Koerner et al. (2007) demonstrated the SNP with reduced activity, R287Q, had reduced neuronal cytotoxicity in cell culture models [17]. Additionally, polymorphisms associated with increased EETs hydrolysis has been associated with worse outcomes after ischemia [18, 19].
Several associations between mEH and neuroinflammatory diseases have also been reported. The reduced function Y113H polymorphism is associated with an increased prevalence of Parkinson’s disease [20], although this association was not reproducible in other populations [10]. Furthermore, mEH expression is increased in Alzheimer’s patients and in experimental Alzheimer’s models [21]. It should be noted that mEH is also involved in xenobiotic metabolism and these associations may be due to altered xenobiotic rather than EETs transformation. Since the currently reported mEH inhibitors are unsuitable for experimental in vivo studies [22], there have been limited tools to study these associations experimentally.
3. Possible therapeutic application of sEH inhibitors and other EpFA inhibitors in reducing neuroinflammation
The anti-inflammatory properties of EETs have been thoroughly tested in numerous disease contexts using sEH inhibitors, including inflammatory pain and cardiovascular disease [23]. These effects have been ascribed to their ability inactivate NF-κB signaling and their ability to directly counter-act the pro-inflammatory effects of PGE2 (Figure 2) [24, 25].
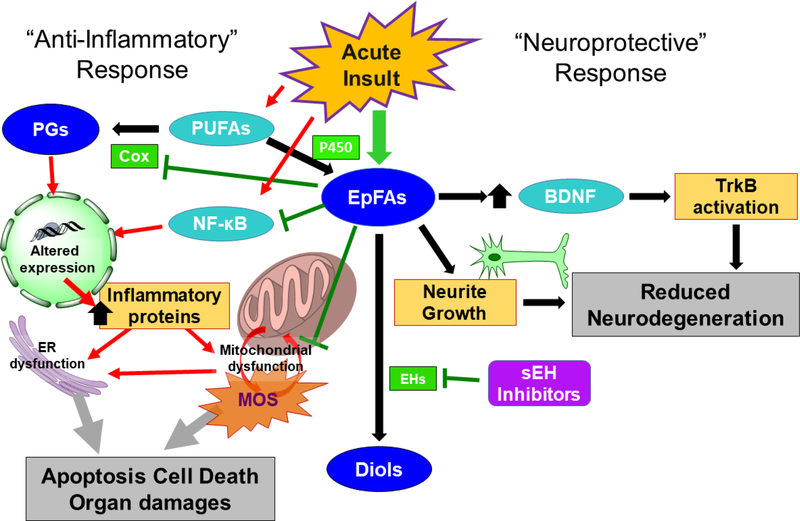
Figure 2.
Cellular action of epoxy-fatty acids (EpFAs). Acute CNS injury can stimulate the release of polyunsaturated fatty acids (PUFAs) from the membrane that are converted by cyclooxygenase (COX) to prostaglandins (PGs). PGs promote production of inflammatory proteins leading to mitochondrial dysfunction, endoplasmic reticulum (ER) dysfunction and eventually apoptosis. EpFAs, produced from PUFAs by cytochrome P450 (P450) and degraded by epoxide hydrolases (EHs), reduce inflammation by blocking NF-κB signaling, by reducing COX activity and through other poorly understood mechanisms. In addition, EpFA can promote neuroprotection by promoting BDNF signaling in astrocytes and stimulate neurite growth in neurons.
3.1. Neuro-inflammation Induced by Acute Injury
One promising therapeutic application of EpFAs is for mitigating long term damage caused by neuroinflammation from acute episodes including ischemic strokes, hemorrhagic strokes, traumatic brain injury and seizures. These insults can result in neuronal loss that leads to activation of resident immune cells including microglia and astrocytes. The recruited immune cells are necessary to promote repair of the injured tissue; however, uncontrolled activation without resolution leads to long term damage as seen in other organ systems [26]. A key characteristic of this resolution is the shift towards alternatively activated macrophages (M2) that is promoted by sEH inhibition [27, 28]. In the context of the CNS, this is observed as a shift from pro-inflammatory polarized microglia that express iNOS and Iba1 and release pro-inflammatory cytokines to microglia that primarily release anti-inflammatory cytokines [29–31]. Subsequently, sEH inhibitor treatment is associated with reduced activation of NF-κB, a reduction of localized and circulating pro-inflammatory cytokines such as TNF-α, and an increase in anti-inflammatory cytokines including IL-10 [29, 30].
In addition to the role of microglia in the activity of sEH inhibitors, in vitro culture experiments have shown that 14,15-EET cause astrocytes to secrete neuronal growth factors including vascular endothelial growth factor (VEGF) and brain derived neurotrophic factor (BDNF) [32, 33]. BDNF acts through its receptor tropomysin receptor kinase B (TrkB) to promote growth and differentiation of nerve cells and to elicit neuroprotection of existing neurons. Consequently, blockade of BDNF-TrkB signaling ablates the protective effect of sEH genetic deletion in middle cerebral arterial occlusion (MCAO) models of stroke [31, 32]. In addition to the protective effects of EETs through their actions on auxiliary cells, EETs are directly able to elicit effects in neurons including growth of neurites [34, 35]. These effects suggest sEH inhibition may reduce CNS injury both by reducing the inflammatory response in resident immune cells and by direct neuro-protective effects on the neurons.
Ischemic stroke, constituting loss of blood flow due to a variety of etiologies, has been thoroughly explored in the context of sEH inhibition. Occlusions, blockage of blood vessels, are the primary causes of stroke and constitute 87% of an estimated 795,000 stroke cases [36]. Despite extensive efforts to produce better therapies for stroke, stroke remains particularly difficult to treat because of the relatively short time window to administer treatment before neuronal loss starts. The primary current treatment for ischemic stroke is thrombolysis with tissue plasminogen activator (tPA), which needs be given within the first several hours of the patient exhibiting symptoms. While tPA can be effective for those patients with ischemic stroke, it can exacerbate hemorrhagic strokes which have many clinical symptoms that are similar to ischemia [37]. sEH inhibition has been proposed as a potential intervention for stroke patients based on the vasodilatory, anti-inflammatory and angiogenic properties of EETs. Numerous studies have investigated the effects of either genetic sEH deletion or chemical sEH inhibition [38]. Broadly, sEH inhibition appears to be effective at reducing infarct volume, functional deficits associated with occlusion and a reduction in the associated neuroinflammation. Aside from cerebral ischemia caused by occlusion, similar neuroinflammation and functional deficits may be caused by the cerebral ischemia following cardiac arrest. In these models, sEH inhibitors (including 4-phenylchalcone oxide and AS2586114; Figure 3) have similarly reduced neuronal loss and enhances anti-inflammatory microglial activation in cardiac arrest/cardiopulmonary resuscitation models [29, 30].
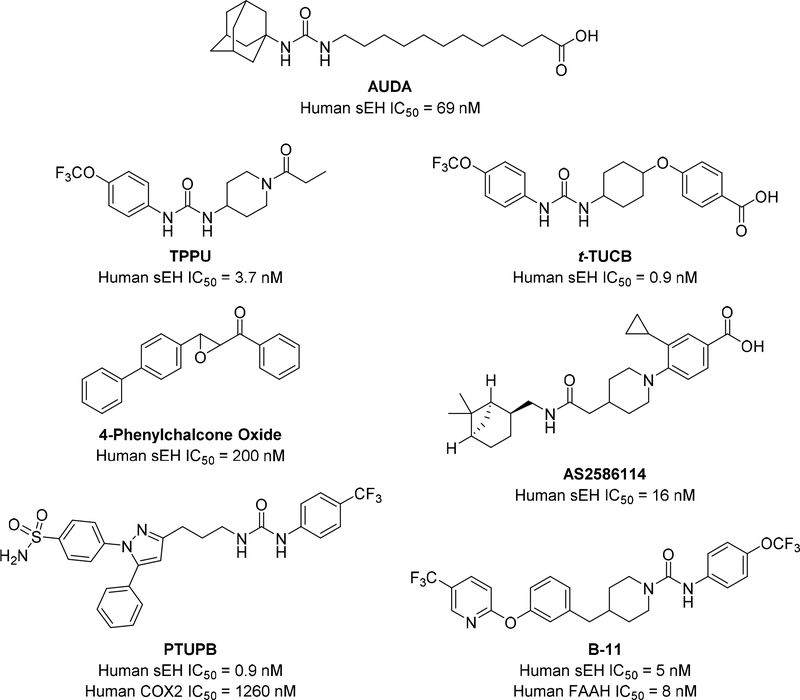
Figure 3.
Multiple soluble epoxide hydrolase (sEH) inhibitors have been developed with varying potencies, water solubility and pharmacokinetic properties. AUDA, TPPU and t-TUCB have been used extensively for studying sEH inhibition in disease [23]. 4-Phenylchalcone Oxide and AS2586114 were used to study cerebral ischemia after cardiac arrest [29, 30]. PTUPB and B-11 were developed as dual inhibitors for COX-2 and FAAH, respectively [53, 64].
The differences in sEH expression between sexes is well reported in the literature, with reduced expression in female than male mice [39–41]. This disparity in sEH expression results in functional differences on infarct size after middle cerebral arterial occlusion that disappears in sEH KO mice [40]. The clinical relevance of these sex differences remains to be explored – however, they highlight the caution required for possibly translating sEH inhibitors to the clinic for stroke. It should be noted that incidence of stroke rises with menopause; thus, the risk for stroke may be directly correlated with estrogen levels and sEH inhibition may be useful in post-menopausal patients. To test this hypothesis, Zuloaga et al. (2015) used reproductively senescent female mice to show that older sEH KO mice have reduced infarct volume relative to wild-type mice while the corresponding young mice had no difference in infarct size [42]. Interestingly, the reproductively senescent mice did not have significantly reduced levels of estradiol or sEH, indicating these effects may be through a different mechanism than the loss of estrogen.
As mentioned previously, one of the challenges in treating stroke is determining between ischemic and hemorrhagic strokes. Treating with anti-thrombolytic agents such as tPA can mitigate the damage caused by ischemic strokes but will enhance the damage caused by hemorrhagic strokes. Thus, new agents that treat both types of strokes are attractive as novel therapeutic approaches. In a collagenase model of intracerebral brain hemorrhage, genetic deletion or pharmacologic inhibition was successful at reducing the post-insult inflammation and the subsequent functional losses associated with injury [43]. In a model of subarachnoid hemorrhage, sEH KO reduced edema, the VCAM-1 marker for inflammation and behavioral deficits without affecting the intracranial pressure [44]. Thus, sEH inhibitors are beneficial across multiple experimental stroke models of different etiology, likely owing to their ability to target and resolve inflammation rather than modify the initial insult.
Compared to stroke, which is primarily triggered by a vascular event leading to aberrant blood flow, the same acute neuronal toxicity events can be observed in seizures and traumatic brain injury. EpFA regulated by sEH have been implicated in both the convulsing and subsequent inflammation aspects of seizures. The anticonvulsant effects of EpFA were initially tested based on the hypothesis that EpFA have GABA-ergic activity that mediate non-inflammatory analgesia [45]. In support of this hypothesis, genetic knockout and/or pharmacologic inhibition of sEH was able to delay the onset of seizures from GABA receptor antagonists (tetramethylene-disulfotetramine, pentylenetetrazole and picrotoxin) but not from a potassium channel blocker (4-aminopyridine) [45, 46]. It is unlikely sEH inhibitors can be used as direct anti-convulsant agents since they need to be administered prior to the seizure initiation [46]. However, sEH inhibitors are also able to reduce the neuroinflammation associated with seizures with a diverse set of stimuli and may be effective adjuvants with other anti-convulsant agents [46, 47]. Similar reductions in neuroinflammation has been observed when sEH inhibitors have been tested in a controlled cortical impact injury model of traumatic brain injury [48].
3.2. Diseases of Chronic Neuro-inflammation
In addition to neuroinflammation associated with acute events, sEH inhibition is a proposed therapeutic approach for reducing the severity of several diseases associated with chronic neuroinflammation.
Parkinson’s Disease (PD) is a neurodegenerative disease that is characterized by neuronal death in the substantia nigra that results in the characteristic loss of controlled motor function. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is typically used as a chemical inducer of PD in experimental models due to its’ selective toxicity towards dopaminergic neurons leading to PD-like symptoms. In MPTP models of PD, the progression of the disease is associated with increasing sEH expression, which suggests sEH is involved in the pathology of the disease [49, 50]. In support of this observation, expression of tyrosine hydroxylase, a biomarker of dopaminergic neurons, is negatively correlated with sEH expression in dementia patients [49]. When blocking sEH through chemical inhibition or genetic knockout, these dopaminergic neurons are protected from neurotoxicity after dosing with MPTP [49, 50]. Interestingly, genetic loss of mEH also reduces the loss of dopaminergic neurons in the MPTP model of PD [51]. This effect was presumed to be related to the role of mEH as a Phase I detoxifying enzyme; however, these results may also be mediated through changes in EpFA metabolism. These observations further highlight that both EHs may be important for regulating brain levels of EpFAs.
The effects of sEH inhibitors have not yet been robustly studied in Alzheimer’s Disease; however, sEH inhibitors have been tested in a mouse model of scrapie [52]. This infectious disease is caused by pathogenic forms of prion protein that catalyze misfolding of native prion proteins leading to neuronal loss and eventual death. This disease shares several characteristics with Alzheimer’s as similar protein misfolding leads to microglial activation and neuroinflammation. In a model where scrapie was initiated by i.p. injection, treating with sEH inhibitors significantly increased survival times in mice [52]. It should be noted this increase was relatively modest and the lack of a robust effect may reflect the general aggressiveness of scrapie.
4. Polypharmacology as a possible approach in sEH and neuroinflammation
The action of EpFAs, especially EETs, through sEH inhibition is a promising approach towards clinically limiting neuroinflammation in several CNS diseases. In addition to the design of small molecule inhibitors that are intended to selectively target sEH, our laboratory has moved towards the development of novel small molecules that concurrently target sEH and other anti-inflammatory targets. One such inhibitor is PTUPB (Figure 3), a dual sEH/cyclooxygenase-2 (COX-2) inhibitor that has enhanced efficacy towards reducing inflammatory pain relative to either an sEH or COX-2 inhibitor alone [53]. As shown on Figure 2, both COX and sEH are implied in the pathology of inflammation and tissues damage. While sEH negative regulates beneficial EpFAs through their hydrolysis, COX-2 positively regulates the pro-inflammatory PGE2 to promote inflammation. Inhibition of both enzymes has synergetic effects in reducing inflammation in various models. PTUTB is effective at improving fasting blood glucose in a Zucker diabetic rat [54] and has been highly efficacious in numerous cancer models [55–57]. Based on their potent anti-inflammatory properties, these may be powerful therapies to be explored in future studies of neurodegenerative disease. It should be noted that the anti-tumorigenic effects of dual sEH/COX-2 inhibition were associated with a reduction in tumor angiogenesis [55, 56]. These anti-angiogenic effects should be considered with caution as angiogenesis is one component of EET activity that may promote recovery from ischemic events.
Separately, combining sEH and fatty acid amide hydrolase (FAAH) inhibition has been similarly effective at synergistically reducing models of neuropathic and inflammatory pain [58]. FAAH hydrolyzes, and thus negatively regulates, N-acylethanolamides including the PPAR-α agonists palmitoylethanolamide and oleoylethanolamide and the endocannabinoid arachidonoylethanolamide. Similar to sEH, FAAH is neuroprotective in a number of models of brain injury partially through modulation of the microglia polarization [59, 60]. These effects could be attributed primarily to activation of the immunomodulatory cannabinoid receptor 2 (CB2R) rather than the CB1R. Epoxide metabolites of AEA, called epoxy-fatty-ethanolamides (EpFEAs), are common metabolites that correspond to both sEH and FAAH signaling that have higher potency on the CB2R relative to the CB1R [61, 62]. The combination of sEH and FAAH inhibition has yet to be tested in any neuroinflammatory models; however, this approach is likely to be effective for neuroprotection. In support of the neuroprotective role of EpFEA, these metabolites are reported to shift the polarization in BV-2 microglial cells to an anti-inflammatory phenotype through their action on the CB2R [61]. To harness the dual sEH/FAAH synergy and to further explore the biological activity of EpFEAs, several dual sEH/FAAH inhibitors were recently described [63, 64]. Additionally, dual COX/FAAH inhibition is similarly synergistic in pain models and the combination of sEH inhibitors with dual COX/FAAH inhibitors could further be effective at reducing neuroinflammation [65, 66].
5. Conclusions and future directions
Our current knowledge suggests a strong role of lipid mediators, especially EpFAs, in the regulation of neuroinflammation and the resulting brain damage that can lead to neurologic diseases. Thus, pharmacologic inhibition of sEH is a novel approach to maintain high EpFA concentrations to reduce neuroinflammation and the subsequent damage [67].
However, several fundamental questions need to be answered before pursuing this route toward a possible treatment. To our knowledge, it is not clear what are the relative roles of mEH and sEH in the metabolism of EpFA in the brain. As noted, mEH is highly expressed in neurons which suggests a specific function related to signaling between cell types. Also, it is not clear whether the neuro-protective effects of sEH is through a centrally or peripherally mediated mechanism. Pharmacologically, there is no indication that a sEH inhibitor needs to pass the blood brain barrier to be efficacious in neuronal diseases. Although some of the biological effects associated with EpFAs are likely mediated locally, the systemic effects of EpFAs have not been robustly investigated. Addressing these questions should assist with future application of sEH inhibition in neuroinflammatory disease.
Highlights.
Epoxy-fatty acids reduce neuroinflammation and neurodegeneration
Soluble epoxide hydrolase (sEH) inhibition increase epoxy-fatty acid levels and could reduce the onset of Parkinson’s disease, Alzheimer’s disease and dementia
Co-inhibition of sEH and either COX or FAAH could enhance efficacy in neuroinflammatory disease
Acknowledgements:
This work was supported by the National Institute of Environmental Health Sciences R01-ES002710 and P42-ES004699.
Abbreviations and definitions:
- CB2
cannabinoid receptor 2
- CNS
central nervous system
- COX-2
cyclooxygenase-2
- CYP450
cytochrome P450
- EDPs
epoxydocosapentaenoic acids
- EETs
epoxyeicosatrienoic acids
- EpFA
epoxy-fatty acid
- EpFEA
epoxy-fatty-ethanolamides
- EpOMEs
epoxyoctadecaenoic acids
- FAAH
fatty acid amide hydrolase
- mEH
microsomal epoxide hydrolase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s Disease
- sEH
soluble epoxide hydrolase
- SNPs
single nucleotide polymorphisms
- tPA
tissue plasminogen activator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests’ declaration: C. Morisseau and S. Kodani are inventors on University of California patents on soluble epoxide hydrolase inhibitors.
References:
- [1].Wang X, Yu T, Liao X, Yang C, Han C, Zhu G, Huang K, Yu L, Qin W, Su H, Liu X, Peng T, The prognostic value of CYP2C subfamily genes in hepatocellular carcinoma, Cancer Med, 7 (2018) 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M, Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism, Neuroscience, 163 (2009) 646–661. [DOI] [PubMed] [Google Scholar]
- [3].Edin ML, Hamedani BG, Gruzdev A, Graves JP, Lih FB, Arbes SJ 3rd, Singh R, Orjuela Leon AC, Bradbury JA, DeGraff LM, Hoopes SL, Arand M, Zeldin DC, Epoxide hydrolase 1 (EPHX1) hydrolyzes epoxyeicosanoids and impairs cardiac recovery after ischemia, The Journal of biological chemistry, 293 (2018) 3281–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu J, Fromel T, Fleming I, Angiogenesis and vascular stability in eicosanoids and cancer, Cancer Metastasis Rev, 37 (2018) 425–438. [DOI] [PubMed] [Google Scholar]
- [5].Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD, Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis, Proc Natl Acad Sci U S A, 110 (2013) 6530–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Geng S, Wang Y, Sun Y, Li J, Yin H, Zeng Z, Yang X, Zhang Y, Wang Y, Gene- gene interaction between CYP2J2 and PPAR -gamma gene on late-onset Alzheimer’s disease in the eastern Chinese Han population, Behav Brain Res, 322 (2017) 362–367. [DOI] [PubMed] [Google Scholar]
- [7].Yan H, Kong Y, He B, Huang M, Li J, Zheng J, Liang L, Bi J, Zhao S, Shi L, CYP2J2 rs890293 polymorphism is associated with susceptibility to Alzheimer’s disease in the Chinese Han population, Neurosci Lett, 593 (2015) 56–60. [DOI] [PubMed] [Google Scholar]
- [8].Morisseau C, Wecksler AT, Deng C, Dong H, Yang J, Lee KS, Kodani SD, Hammock BD, Effect of soluble epoxide hydrolase polymorphism on substrate and inhibitor selectivity and dimer formation, Journal of lipid research, 55 (2014) 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, Silbert LC, Grafe MR, Alkayed NJ, Role of soluble epoxide hydrolase in age-related vascular cognitive decline, Prostaglandins & other lipid mediators, 113–115 (2014) 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farin FM, Janssen P, Quigley S, Abbott D, Hassett C, Smith-Weller T, Franklin GM, Swanson PD, Longstreth WT Jr., Omiecinski CJ, Checkoway H, Genetic polymorphisms of microsomal and soluble epoxide hydrolase and the risk of Parkinson’s disease, Pharmacogenetics, 11 (2001) 703–708. [DOI] [PubMed] [Google Scholar]
- [11].Harris TR, Hammock BD, Soluble epoxide hydrolase: gene structure, expression and deletion, Gene, 526 (2013) 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fava C, Montagnana M, Danese E, Almgren P, Hedblad B, Engstrom G, Berglund G, Minuz P, Melander O, Homozygosity for the EPHX2 K55R polymorphism increases the long-term risk of ischemic stroke in men: a study in Swedes, Pharmacogenet Genomics, 20 (2010) 94–103. [DOI] [PubMed] [Google Scholar]
- [13].Ma L, Jiang Y, Kong X, Yan M, Zhao T, Zhao H, Liu Q, Zhang H, Cao Y, Li P, Synergistic Effect of the MTHFR C677T and EPHX2 G860A Polymorphism on the Increased Risk of Ischemic Stroke in Chinese Type 2 Diabetic Patients, J Diabetes Res, 2017 (2017) 6216205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ, Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia, Stroke, 39 (2008) 2073–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gonzalez-Giraldo Y, Barreto GE, Fava C, Forero DA, Ischemic Stroke and Six Genetic Variants in CRP, EPHX2, FGA, and NOTCH3 Genes: A Meta-Analysis, J Stroke Cerebrovasc Dis, 25 (2016) 2284–2289. [DOI] [PubMed] [Google Scholar]
- [16].Lee J, Dahl M, Grande P, Tybjaerg-Hansen A, Nordestgaard BG, Genetically reduced soluble epoxide hydrolase activity and risk of stroke and other cardiovascular disease, Stroke, 41 (2010) 27–33. [DOI] [PubMed] [Google Scholar]
- [17].Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ, Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury, J Neurosci, 27 (2007) 4642–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Martini RP, Ward J, Siler DA, Eastman JM, Nelson JW, Borkar RN, Alkayed NJ, Dogan A, Cetas JS, Genetic variation in soluble epoxide hydrolase: association with outcome after aneurysmal subarachnoid hemorrhage, J Neurosurg, 121 (2014) 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yi X, Lin J, Li J, Zhou Q, Han Z, Epoxyeicosatrienoic Acids are Mediated by EPHX2 Variants and may be a Predictor of Early Neurological Deterioration in Acute Minor Ischemic Stroke, J Atheroscler Thromb, 24 (2017) 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmadi A, Fredrikson M, Jerregard H, Akerback A, Fall PA, Rannug A, Axelson O, Soderkvist P, GSTM1 and mEPHX polymorphisms in Parkinson’s disease and age of onset, Biochem Biophys Res Commun, 269 (2000) 676–680. [DOI] [PubMed] [Google Scholar]
- [21].Liu M, Sun A, Shin EJ, Liu X, Kim SG, Runyons CR, Markesbery W, Kim HC, Bing G, Expression of microsomal epoxide hydrolase is elevated in Alzheimer’s hippocampus and induced by exogenous beta-amyloid and trimethyl-tin, Eur J Neurosci, 23 (2006) 2027–2034. [DOI] [PubMed] [Google Scholar]
- [22].Morisseau C, Newman JW, Wheelock CE, Hill Iii T, Morin D, Buckpitt AR, Hammock BD, Development of metabolically stable inhibitors of Mammalian microsomal epoxide hydrolase, Chemical research in toxicology, 21 (2008) 951–957. [DOI] [PubMed] [Google Scholar]
- [23].Kodani SD, Hammock BD, The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: drug metabolism to therapeutics for chronic pain, Drug Metab Dispos, 43 (2015) 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK, Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids, Science (New York, N.Y.), 285 (1999) 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD, Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP, Proc Natl Acad Sci U S A, 108 (2011) 5093–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature, 510 (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dai M, Wu L, Wang P, Wen Z, Xu X, Wang DW, CYP2J2 and Its Metabolites EETs Attenuate Insulin Resistance via Regulating Macrophage Polarization in Adipose Tissue, Sci Rep, 7 (2017) 46743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gilroy DW, Edin ML, De Maeyer RP, Bystrom J, Newson J, Lih FB, Stables M, Zeldin DC, Bishop-Bailey D, CYP450-derived oxylipins mediate inflammatory resolution, Proc Natl Acad Sci U S A, 113 (2016) E3240–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taguchi N, Nakayama S, Tanaka M, Single administration of soluble epoxide hydrolase inhibitor suppresses neuroinflammation and improves neuronal damage after cardiac arrest in mice, Neurosci Res, 111 (2016) 56–63. [DOI] [PubMed] [Google Scholar]
- [30].Wang J, Fujiyoshi T, Kosaka Y, Raybuck JD, Lattal KM, Ikeda M, Herson PS, Koerner IP, Inhibition of soluble epoxide hydrolase after cardiac arrest/cardiopulmonary resuscitation induces a neuroprotective phenotype in activated microglia and improves neuronal survival, J Cereb Blood Flow Metab, 33 (2013) 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chang LH, Lin HC, Huang SS, Chen IC, Chu KW, Chih CL, Liang YW, Lee YC, Chen YY, Lee YH, Lee IH, Blockade of soluble epoxide hydrolase attenuates post-ischemic neuronal hyperexcitation and confers resilience against stroke with TrkB activation, Sci Rep, 8 (2018) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yuan L, Liu J, Dong R, Zhu J, Tao C, Zheng R, Zhu S, 14,15-epoxyeicosatrienoic acid promotes production of brain derived neurotrophic factor from astrocytes and exerts neuroprotective effects during ischaemic injury, Neuropathol Appl Neurobiol, 42 (2016) 607–620. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Y, Hong G, Lee KS, Hammock BD, Gebremedhin D, Harder DR, Koehler RC, Sapirstein A, Inhibition of soluble epoxide hydrolase augments astrocyte release of vascular endothelial growth factor and neuronal recovery after oxygen-glucose deprivation, J Neurochem, 140 (2017) 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Abdu E, Bruun DA, Yang D, Yang J, Inceoglu B, Hammock BD, Alkayed NJ, Lein PJ, Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures, J Neurochem, 117 (2011) 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oguro A, Inoue T, Kudoh SN, Imaoka S, 14,15-epoxyeicosatrienoic acid produced by cytochrome P450s enhances neurite outgrowth of PC12 and rat hippocampal neuronal cells, Pharmacol Res Perspect, 6 (2018) e00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American C Heart Association Statistics, S. Stroke Statistics, Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association, Circulation, 133 (2016) e38–360. [DOI] [PubMed] [Google Scholar]
- [37].Nor AM, Ford GA, Misdiagnosis of stroke, Expert review of neurotherapeutics, 7 (2007) 989–1001. [DOI] [PubMed] [Google Scholar]
- [38].Davis CM, Liu X, Alkayed NJ, Cytochrome P450 eicosanoids in cerebrovascular function and disease, Pharmacol Ther, 179 (2017) 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ, Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia, Frontiers in bioscience : a journal and virtual library, 13 (2008) 2833–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ, Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia, J Cereb Blood Flow Metab, 29 (2009) 1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wagner K, Gilda J, Yang J, Wan D, Morisseau C, Gomes AV, Hammock BD, Soluble epoxide hydrolase inhibition alleviates neuropathy in Akita (Ins2 Akita) mice, Behav Brain Res, 326 (2017) 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zuloaga KL, Zhang W, Roese NE, Alkayed NJ, Soluble epoxide hydrolase gene deletion improves blood flow and reduces infarct size after cerebral ischemia in reproductively senescent female mice, Front Pharmacol, 5 (2014) 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wu CH, Shyue SK, Hung TH, Wen S, Lin CC, Chang CF, Chen SF, Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase reduces brain damage and attenuates neuroinflammation after intracerebral hemorrhage, J Neuroinflammation, 14 (2017) 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Siler DA, Berlow YA, Kukino A, Davis CM, Nelson JW, Grafe MR, Ono H, Cetas JS, Pike M, Alkayed NJ, Soluble Epoxide Hydrolase in Hydrocephalus, Cerebral Edema, and Vascular Inflammation After Subarachnoid Hemorrhage, Stroke, 46 (2015) 1916–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, Hwang SH, Lee KS, Rogawski MA, Morisseau C, Hammock BD, Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures, PLoS One, 8 (2013) e80922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vito ST, Austin AT, Banks CN, Inceoglu B, Bruun DA, Zolkowska D, Tancredi DJ, Rogawski MA, Hammock BD, Lein PJ, Post-exposure administration of diazepam combined with soluble epoxide hydrolase inhibition stops seizures and modulates neuroinflammation in a murine model of acute TETS intoxication, Toxicol Appl Pharmacol, 281 (2014) 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hung YW, Hung SW, Wu YC, Wong LK, Lai MT, Shih YH, Lee TS, Lin YY, Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy, Brain Behav Immun, 43 (2015) 118–129. [DOI] [PubMed] [Google Scholar]
- [48].Hung TH, Shyue SK, Wu CH, Chen CC, Lin CC, Chang CF, Chen SF, Deletion or inhibition of soluble epoxide hydrolase protects against brain damage and reduces microglia-mediated neuroinflammation in traumatic brain injury, Oncotarget, 8 (2017) 103236–103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ren Q, Ma M, Yang J, Nonaka R, Yamaguchi A, Ishikawa KI, Kobayashi K, Murayama S, Hwang SH, Saiki S, Akamatsu W, Hattori N, Hammock BD, Hashimoto K, Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson’s disease, Proc Natl Acad Sci U S A, 115 (2018) E5815–E5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Qin X, Wu Q, Lin L, Sun A, Liu S, Li X, Cao X, Gao T, Luo P, Zhu X, Wang X, Soluble Epoxide Hydrolase Deficiency or Inhibition Attenuates MPTP-Induced Parkinsonism, Mol Neurobiol, 52 (2015) 187–195. [DOI] [PubMed] [Google Scholar]
- [51].Liu M, Hunter R, Nguyen XV, Kim HC, Bing G, Microsomal epoxide hydrolase deletion enhances tyrosine hydroxylase phosphorylation in mice after MPTP treatment, J Neurosci Res, 86 (2008) 2792–2801. [DOI] [PubMed] [Google Scholar]
- [52].Poli G, Corda E, Martino PA, Dall’ara P, Bareggi SR, Bondiolotti G, Iulini B, Mazza M, Casalone C, Hwang SH, Hammock BD, Inceoglu B, Therapeutic activity of inhibition of the soluble epoxide hydrolase in a mouse model of scrapie, Life Sci, 92 (2013) 1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD, Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase, J Med Chem, 54 (2011) 3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hye Khan MA, Hwang SH, Sharma A, Corbett JA, Hammock BD, Imig JD, A dual COX-2/sEH inhibitor improves the metabolic profile and reduces kidney injury in Zucker diabetic fatty rat, Prostaglandins & other lipid mediators, 125 (2016) 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang G, Panigrahy D, Hwang SH, Yang J, Mahakian LM, Wettersten HI, Liu JY, Wang Y, Ingham ES, Tam S, Kieran MW, Weiss RH, Ferrara KW, Hammock BD, Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis, Proc Natl Acad Sci U S A, 111 (2014) 11127–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang F, Zhang H, Ma AH, Yu W, Zimmermann M, Yang J, Hwang SH, Zhu D, Lin TY, Malfatti M, Turteltaub KW, Henderson PT, Airhart S, Hammock BD, Yuan J, de Vere White RW, Pan CX, COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin, Mol Cancer Ther, 17 (2018) 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li J, Zhou Y, Wang H, Gao Y, Li L, Hwang SH, Ji X, Hammock BD, COX-2/sEH dual inhibitor PTUPB suppresses glioblastoma growth by targeting epidermal growth factor receptor and hyaluronan mediated motility receptor, Oncotarget, 8 (2017) 87353–87363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sasso O, Wagner K, Morisseau C, Inceoglu B, Hammock BD, Piomelli D, Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive, Pharmacological research, 97 (2015) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hermes DJ, Xu C, Poklis JL, Niphakis MJ, Cravatt BF, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S, Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS, Neuropharmacology, 141 (2018) 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tchantchou F, Tucker LB, Fu AH, Bluett RJ, McCabe JT, Patel S, Zhang Y, The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury, Neuropharmacology, 85 (2014) 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McDougle DR, Watson JE, Abdeen AA, Adili R, Caputo MP, Krapf JE, Johnson RW, Kilian KA, Holinstat M, Das A, Anti-inflammatory omega-3 endocannabinoid epoxides, Proc Natl Acad Sci U S A, 114 (2017) E6034–E6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Snider NT, Nast JA, Tesmer LA, Hollenberg PF, A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist, Mol Pharmacol, 75 (2009) 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kodani SD, Bhakta S, Hwang SH, Pakhomova S, Newcomer ME, Morisseau C, Hammock BD, Identification and optimization of soluble epoxide hydrolase inhibitors with dual potency towards fatty acid amide hydrolase, Bioorg Med Chem Lett, 28 (2018) 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kodani SD, Wan D, Wagner KM, Hwang SH, Morisseau C, Hammock BD, Design and Potency of Dual Soluble Epoxide Hydrolase/Fatty Acid Amide Hydrolase Inhibitors, ACS Omega, 3 (2018) 14076–14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Migliore M, Habrant D, Sasso O, Albani C, Bertozzi SM, Armirotti A, Piomelli D, Scarpelli R, Potent multitarget FAAH-COX inhibitors: Design and structure-activity relationship studies, Eur J Med Chem, 109 (2016) 216–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Naidu PS, Booker L, Cravatt BF, Lichtman AH, Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception, The Journal of pharmacology and experimental therapeutics, 329 (2009) 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zarriello S, Tuazon J, Corey S, Schimmel S, Rajani M, Gorsky A, Incontri D, Hammock BD, Borlongan CV, Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders, Progress in neurobiology, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]