Abstract
Objective:
Tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) are postoperative urinary biomarkers of renal stress and AKI. We conducted this study to test the hypothesis that intraoperative concentrations of urinary [TIMP-2]•[IGFBP7] are associated with postoperative AKI.
Methods:
We measured urinary [TIMP-2]•[IGFBP7] at eight perioperative timepoints in 400 patients who participated in a RCT of statins for AKI in cardiac surgery. We compared [TIMP-2]•[IGFBP7] between subjects who did and did not develop KDIGO stage 2–3 AKI within 48 hours of surgery, adjusted for AKI risk factors.
Results:
Fourteen patients (3.5%) met the primary endpoint of stage 2–3 AKI within 48 hours of surgery, and an additional 77 patients (19.3%) developed stage 1 AKI. Patients who developed stage 2–3 AKI displayed bimodal elevations of [TIMP-2]•[IGFBP7], with a first elevation (median 0.45 [ng/mL]2/1000) intraoperatively and the second peak (1.45 [ng/mL]2/1000) six hours postoperatively. Patients who did not develop AKI did not have any elevations in [TIMP-2]•[IGFBP7]. Each 10-fold increase in intraoperative [TIMP-2]•[IGFBP7] was independently associated with a 290% increase in the odds of stage 2–3 AKI (P=0.01), and each 10-fold increase in six hours postoperative [TIMP-2]•[IGFBP7] a 650% increase (P<0.001). The maximum [TIMP-2]•[IGFBP7] between these two timepoints provided an AUROC of 0.82 (95% CI: 0.73–0.90), 100% sensitivity, and 100% negative predictive value using the >0.3 cutoff to predict stage 2–3 AKI.
Conclusion:
Intraoperative elevations of [TIMP-2]•[IGFBP7] can predict moderate-severe AKI and could provide opportunity to alter postoperative management to prevent kidney injury.
Graphical Abstract
Perspective Statement
This study provides first evidence that AKI following cardiac surgery can be predicted with intraoperative urine biomarker measurements and that patients who develop moderate-severe AKI experience both intraoperative and early postoperative renal stress. These markers provided 100% sensitivity to predict AKI and 100% negative predictive value to identify patients who would not develop AKI.
INTRODUCTION
Acute kidney injury (AKI) is one of the most serious and common complications of cardiac surgery. A recent systematic review and meta-analysis reported that cardiac surgery associated-acute kidney injury (CSA-AKI) affects 22.3% of patients, roughly 450,000 of the 2 million patients who receive cardiac surgery annually,1, 2 and the Society of Thoracic Surgery National Database reports that 2.3% of cardiac surgery patients develop renal failure (stage 3 AKI), defined as a 200% increase in serum creatinine, creatinine exceeding 4.0 mg/dl with minimum increase of 0.5 mg/dl, or dialysis.3 CSA-AKI is independently associated with both postoperative morbidity and mortality, with severe AKI being independently associated with up to an eight-fold increase in perioperative mortality, a prolonged length of stay, and increased cost.4, 5 The mechanisms of CSA-AKI include renal ischemia-reperfusion, inflammation, hemolysis, oxidative damage, and nephrotoxin exposure.4, 6
Current diagnostic criteria for AKI rely on changes in serum creatinine (SCr) or urine output, which reflect kidney function, as a surrogate for injury. Single values of SCr during acute evolution of renal dysfunction will underestimate the degree of dysfunction.7, 8 Thus, diagnosis of AKI is typically time-delayed from the actual renal insult. Furthermore, hemodilution during cardiopulmonary bypass (CPB), volume resuscitation, and mannitol administration may confound the use of these criteria to diagnose AKI in cardiac surgery patients.4
Identification of biomarkers reflective of kidney injury and stress might allow clinicians to identify renal injury earlier, thus providing opportunities for intervention. The biomarkers neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and cystatin C have been studied for this purpose. The area under the receiver operating characteristic curve (AUROC) for these markers to predict CSA-AKI, however, remains modest (0.67 for NGAL, 0.65 for KIM-1, and 0.71 for cystatin C).9, 10
Recently, urine tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) have been identified as early biomarkers associated with AKI.11 During cellular stress caused by conditions such as ischemia or sepsis, renal epithelial cells undergo G1 cell-cycle arrest to prevent potential damage to DNA during replication.12 TIMP-2 and IGFBP7 are two proteins expressed during cell-cycle arrest. In the past several years, the product of these markers, [TIMP-2]•[IGFBP7], has been validated to predict the development of Kidney Disease: Improving Global Outcomes (KDIGO)13 stage 2–3 (moderate-severe) AKI in cardiac surgery,14, 15 non-cardiac surgery,16, 17 and intensive care unit patient populations.11, 18 In fact, studies have shown that measurement of [TIMP-2]•[IGFBP7] as early as intensive care unit (ICU) admission can discriminate between patients who will and will not develop KDIGO stage 2–3 AKI following cardiac surgery.14, 15 These studies have shown [TIMP-2]•[IGFBP7] to be a moderate to good predictor of AKI with an AUROC between 0.80 and 0.90.
It remains unclear, however, if urinary [TIMP-2]•[IGFBP7] rises even earlier in the perioperative course, such as during surgery, among patients who subsequently develop AKI. Many of the insults responsible for CSA-AKI, such as ischemia reperfusion, hemolysis, and oxidative damage occur intraoperatively.2, 4, 6, 19, 20 Intraoperative identification of patients with a high likelihood of developing AKI might provide physicians an early opportunity to initiate monitoring and treatment regimens to reduce AKI.
We conducted this study to test the hypothesis that intraoperative concentrations of urinary [TIMP-2]•[IGFBP7] are associated with postoperative AKI and to elucidate the perioperative expression of urinary [TIMP-2]•[IGFBP7] in patients who do and do not develop AKI.
METHODS
Study participants
This was a prospective cohort study using urine specimens collected during a previously published trial of statin therapy for prevention of AKI in cardiac surgery.21 The Statin AKI Cardiac Surgery RCT was a randomized, double-blinded, placebo-controlled trial that enrolled patients between November 2009 and December 2014 to test the efficacy of perioperative atorvastatin administration for reducing CSA-AKI. All adult patients undergoing elective coronary bypass grafting, valvular heart surgery, or ascending aortic surgery at Vanderbilt University Medical Center (VUMC) were eligible for inclusion. Patients with prior statin intolerance, acute coronary syndrome, liver dysfunction, current use of CYP3A4 inhibitors, current use of cyclosporine, current use of renal replacement therapy, history of kidney transplant, urgent or emergent surgery, or pregnancy were excluded from participation. The study was approved by the VUMC institutional review board, conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent.
Procedures
All patients received surgical, anesthetic, and critical care management according to the standard of care of the medical center, as previously described21. Fresh urine specimens were obtained after induction of anesthesia (baseline), 30 minutes following initiation of CPB or off-pump coronary artery grafting (OpCAB), immediately following the completion of CPB or OpCAB, upon ICU admission, six hours after ICU admission, and at 9:00 on postoperative days 1, 2, and 3. Samples were collected on ice, centrifuged at 1000g for 15 minutes, and the supernatant frozen at −80 degrees Celsius until thawing for measurement. [TIMP-2]•[IGFBP7] was measured using the commercially available Astute Medical NephroCheck® Test by laboratory personnel blinded to all subject characteristics.
Outcomes
The primary endpoint was stage 2–3 (moderate-severe) AKI occurring within 48 hours of surgery, defined by changes in serum creatinine according to KDIGO criteria. KDIGO criteria define stage 1 AKI as a 50% or 0.3 mg/dl increase in serum creatinine from baseline within 48 hours of surgery; stage 2 AKI as a 100% increase in serum creatinine; and stage 3 as a 200% serum creatinine increase, a serum creatinine > 4.0 mg/dl with an acute rise of 0.5 mg/dL, or initiation of renal replacement therapy.13 Secondary endpoints were incidence of renal replacement therapy, ICU and hospital length of stay, ICU readmission, and death.
Statistical Analysis
To compare the distributions of [TIMP-2]•[IGFBP7] among groups with no AKI, stage1 AKI, and stage 2–3 AKI at each timepoint, a Kruskal-Wallis test was first performed to test for the global difference among these three groups. If there was a significant difference, a post hoc Conover test (R package “PMCMR”) for pairwise multiple comparisons was used to identify which group or groups were significantly different.22 The P values for pairwise multiple comparisons were adjusted by the Holm method.
We then used multivariable logistic regression to measure the association between [TIMP-2]•[IGFBP7] and stage 2–3 AKI independent from clinical factors associated with AKI. We used LASSO regression to select a subset of 18 candidate covariates (Table S1) in order to prevent overfitting and enhance model interpretation. The data for these 18 candidate variables were power transformed by the Box-Cox procedure to better approximate normal distributions before entering them into the LASSO regression. Body mass index, Thakar score, and baseline hematocrit constituted the final set of model covariates.23 The exploratory analysis comparing [TIMP-2]•[IGFBP7] levels with death or dialysis was performed with Fisher’s exact test.
The 95% CIs for AUROC metrics and the interquartile ranges of the group medians of [TIMP-2]•[IGFBP7] were estimated by bootstrap. The AUROCs of the baseline clinical model were compared to the AUROCs of the final model (clinical factors in addition to [TIMP-2]•[IGFBP7]) with the “roc.test” function for comparing two paired AUROCs in R package “pROC”.24 The continuous Net Reclassification Improvements associated with adding [TIMP-2]•[IGFBP7] to the clinical model was calculated with the “improveProb” function in R package “Hmisc”.25 We performed similar analyses in the subgroup of patients who received cardiopulmonary bypass during surgery. Categorical baseline variables were analyzed using Fisher’s exact test or chi-squared test, and continuous variables were analyzed using Wilcoxon rank-sum test. Statistical analyses were performed using SAS 9.3 and R3.0.26 Two-sided P-values <0.05 were considered statistically significant.
RESULTS
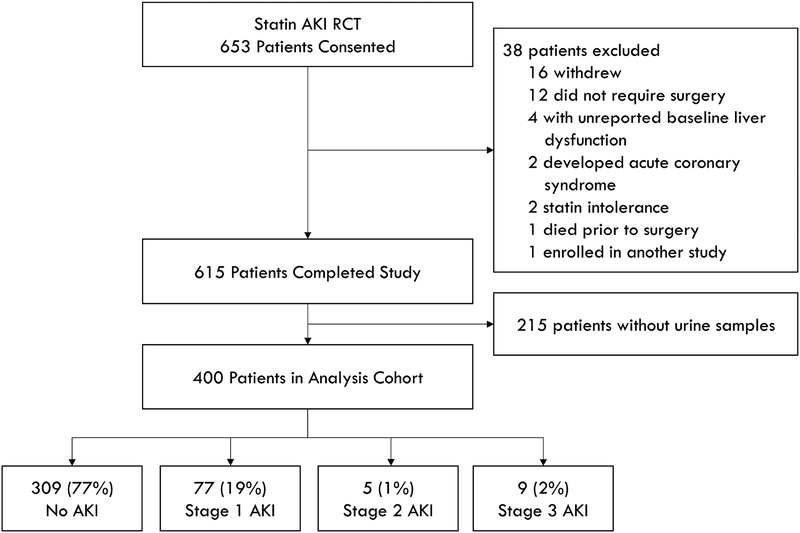
Four hundred sequential Statin AKI Cardiac Surgery Randomized Clinical Trial (RCT) patients comprised the cohort. Fourteen of these patients (3.5%) developed stage 2–3 (moderate-severe) AKI within 48 hours of surgery, 77 patients (19.3%) developed stage 1 AKI, and 309 patients (77.3%) did not develop AKI (Figure 1). Median age of the cohort was 67 years, 67.3 % of patients were men, median estimated glomerular filtration rate (eGFR) was 73.2 ml/min/1.73m2, and 70.8 % of patients had surgery with CPB (Table 1). Advanced age, higher Thakar scores, and lower baseline hematocrit were associated with increased stage 2–3 AKI (Table S1).
Fig 1.
Consort diagram. AKI status is the maximum KDIGO stage reached within 48 hours of surgery.
Table 1.
Baseline and intraoperative patient characteristics for all patients and separated into patients who developed no or stage 1 AKI and stage 2 or 3 AKI within 48 hours of surgery. Continuous variables are represented as median (25th–75th percentiles) and categorical variables as number (percent).
| All (n=400) | No AKI or Stage 1 (n=386) | Stage 2 or 3 AKI (n=14) | P-value | |
|---|---|---|---|---|
| Male | 269 (67.3%) | 261 (67.6%) | 8 (57.1%) | 0.40 |
| Age (Years) | 67 (58–75) | 67 (58–75) | 59 (50–71) | 0.04 |
| BMI (kg/m2) | 27.4 (24.7–31.1) | 27.3 (24.7–31.0) | 31.7 (23.0–39.5) | 0.10 |
| Race | 0.59 | |||
| Black or African American | 19 (4.8%) | 18 (4.7%) | 1 (7.1%) | |
| Other | 5 (1.3%) | 5 (1.3%) | 0 (0%) | |
| White | 376 (94.0%) | 363 (94.0%) | 13 (92.9%) | |
| Ethnicity | 1.00 | |||
| Hispanic or Latino | 6 (1.5%) | 6 (1.6%) | 0 (0%) | |
| Not Hispanic or Latino | 394 (98.5%) | 380 (98.4%) | 14 (100%) | |
| Medical History | ||||
| Atrial Fibrillation | 100 (25.0%) | 96 (24.9%) | 4 (28.6%) | 0.76 |
| Coronary Artery Disease | 267 (66.8%) | 258 (66.8%) | 9 (64.3%) | 1.00 |
| Heart Failure | 163 (40.8%) | 156 (40.4%) | 7 (50.0%) | 0.58 |
| Cardiac Surgery | 74 (18.5%) | 69 (17.9%) | 5 (35.7%) | 0.15 |
| CABG | 37 (9.3%) | 36 (9.3%) | 1 (7.1%) | 1.00 |
| Valve Surgery | 40 (10.0%) | 36 (9.3%) | 4 (28.6%) | 0.04 |
| Diabetes | 123 (30.8%) | 120 (31.1%) | 3 (21.4%) | 0.56 |
| Hypertension | 348 (87.0%) | 335 (86.8%) | 13 (92.9%) | 1.00 |
| Peripheral Vascular Disease | 113 (28.3%) | 109 (28.2%) | 4 (28.6%) | 1.00 |
| Stroke (CVA) | 22 (5.5%) | 20 (5.2%) | 2 (14.3%) | 0.18 |
| TIA | 13 (3.3%) | 12 (3.1%) | 1 (7.1%) | 0.38 |
| Dementia | 5 (1.3%) | 5 (1.3%) | 0 (0%) | 1.00 |
| COPD | 47 (11.8%) | 46 (11.9%) | 1 (7.1%) | 1.00 |
| Obstructive Sleep Apnea | 57 (14.3%) | 53 (13.7%) | 4 (28.6%) | 0.12 |
| Current Smoker | 67 (16.8%) | 66 (17.1%) | 1 (7.1%) | 0.48 |
| Charlson Index | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 4.0 (1.0–5.0) | 0.12 |
| LV Ejection Fraction baseline (%) | 60.0 (50.0–60.0) | 60.0 (50.0–60.0) | 55.0 (50.0–60.0) | 0.43 |
| ACE inhibitor use | 125 (31.3%) | 120 (31.1%) | 5 (35.7%) | 0.77 |
| Atorvastatin treatment | 204 (51.0%) | 197 (51.0%) | 7 (50.0%) | 1.00 |
| eGFR (ml/min/1.73m2) | 72.3 (53.6–87.9) | 72.9 (54.3–87.9) | 45.8 (19.9–84.6) | 0.08 |
| CKD stage | 0.002 | |||
| 1 (eGFR > 90 ml/min/1.73m2) | 80 (20.0%) | 77 (19.9%) | 3 (21.4%) | |
| 2 (eGFR 60–90 ml/min/1.73m2) | 188 (47.0%) | 185 (47.9%) | 3 (21.4%) | |
| 3 (eGFR 30–60 ml/min/1.73m2) | 116 (29.0%) | 112 (29.0%) | 4 (28.6%) | |
| 4 (eGFR 15–30 ml/min/1.73m2) | 16 (4.0%) | 12 (3.1%) | 4 (28.6%) | |
| Thakar score | <0.001 | |||
| 1 | 233 (58.3%) | 230 (59.6%) | 3 (21.4%) | |
| 2 | 19 (4.8%) | 19 (4.9%) | 0 (0%) | |
| 3 | 117 (29.3%) | 112 (29.0%) | 5 (35.7%) | |
| 4 | 15 (3.8%) | 14 (3.6%) | 1 (7.1%) | |
| >=5 | 16 (4.0%) | 11 (2.8%) | 5 (35.7%) | |
| Procedure characteristics | ||||
| Isolated CABG | 131 (32.8%) | 127 (32.9%) | 4 (28.6%) | 0.74 |
| Isolated valve | 168 (42.0%) | 165 (42.7%) | 3 (1.4%) | 0.11 |
| CABG/valve | 61 (15.3%) | 58 (15.0%) | 3 (21.4%) | 0.51 |
| Aorta surgery | 40 (10.0%) | 36 (9.3%) | 4 (28.5%) | 0.04 |
| Cardiopulmonary bypass | 283 (70.8%) | 272 (70.4%) | 11 (78.5%) | 0.77 |
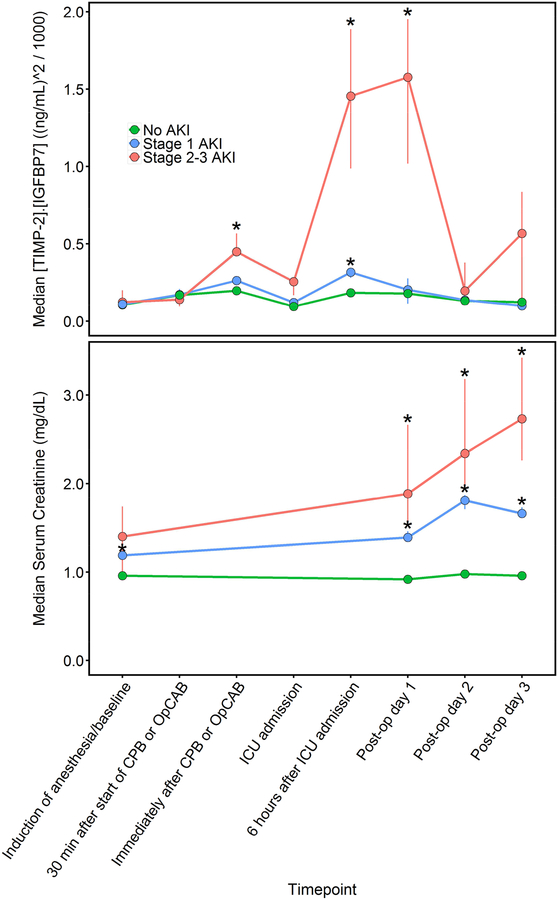
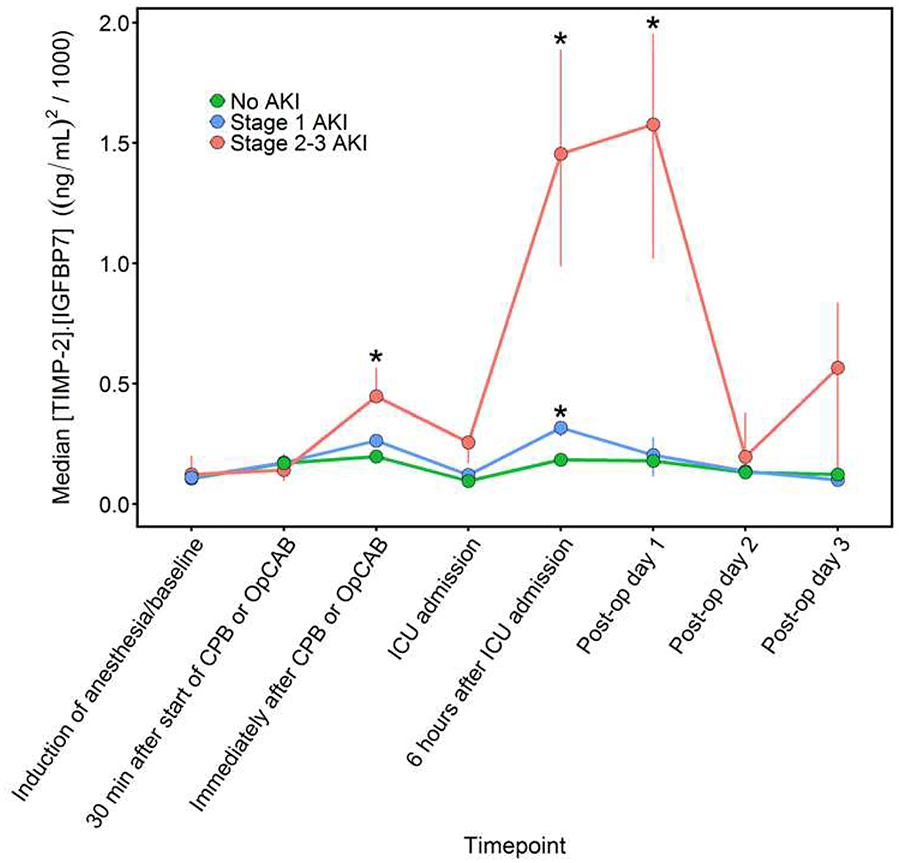
Median urinary [TIMP-2]•[IGFBP7] was 0.11 (ng/mL)2/1000 at baseline (10th, 90th percentile: 0.02, 0.64), increased to 0.17 (0.02, 0.93) during CPB/OpCAB, peaked at 0.22 (0.04, 1.58) following CPB/OpCAB, decreased to 0.10 (0.02, 0.67) at ICU admission, increased again to 0.21 (0.04, 0.84) 6-hours after ICU admission, remained elevated at 0.19 (0.02, 1.34) on postoperative day 1, and decreased back towards baseline by postoperative day 2. Patients who developed stage 2–3 AKI displayed a bimodal time course. Specifically, [TIMP-2]•[IGFBP7] increased significantly immediately after the end of CPB or OpCAB, returned towards baseline at ICU admission, and then subsequently increased considerably more six hours after ICU admission and remained elevated into postoperative day 1 before decreasing towards baseline on postoperative day 2 in stage 2–3 AKI patients compared to patients with stage 1 or no AKI (Figure 2). Both of these [TIMP-2]•[IGFBP7] elevations occurred before serum creatinine concentrations had risen above baseline. Median [TIMP-2]•[IGFBP7] values in the stage 2–3 AKI patients were 0.12, 0.45, 0.26, 1.45, and 1.58 (ng/mL)2/1000 at baseline, immediately after CPB/OpCAB, ICU admission, six hours after ICU admission, and postoperative day 1, respectively. Patients who developed stage 1 AKI also had significantly elevated [TIMP-2]•[IGFBP7] six hours after ICU admission, a median concentration of 0.32 (ng/mL)2/1000, compared to non-AKI patients and also demonstrated intraoperative postoperative bi-modal urinary [TIMP-2]•[IGFBP7] peaks but significantly less so than patients who developed stage 23 AKI. Minimal change was observed in [TIMP-2]•[IGFBP7] during and after surgery in patients who did not develop any AKI, with median [TIMP-2]•[IGFBP7] at or below 0.20 (ng/mL)2/1000 throughout the perioperative period (Table S2). Urinary [TIMP-2]•[IGFBP7] was unaffected by perioperative atorvastatin treatment, the intervention in the randomized trial (Table S3).
Fig 2.
Median perioperative urinary [TIMP-2]•[IGFBP7] and serum creatinine concentrations in patients with No AKI (triangles), Stage 1 AKI (squares), and Stage 2–3 AKI (circles). Vertical lines through the symbols show the interquartile range of bootstrap medians for the [TIMP-2]•[IGFBP7] and creatinine concentrations. *P<0.05 for comparing the distributions of [TIMP-2]•[IGFBP7] or creatinine values to the distributions among patients without AKI.
Increased concentrations of intraoperative and early postoperative [TIMP-2]•[IGFBP7] were independently associated with increased AKI. The adjusted OR for [TIMP-2]•[IGFBP7] to predict stage 2–3 AKI (95% CI) was 3.9 (1.3–11.1) post CPB/OpCAB, 2.5 (1.1–5.6) at ICU admission, 7.5 (2.0–28.0) six hours after ICU admission, and 4.6 (1.7–12.7) on postoperative day 1 (Table 2). The reported OR indicate the odds increase for developing stage 2–3 AKI for each log10([TIMP-2]•[IGFBP7]) (ng/mL)2/1000 increase. Body mass index, Thakar score, and baseline hematocrit were also independently associated with stage 2–3 AKI in these models. There were no significant interactions among these clinical factors and the association between increasing [TIMP-2]•[IGFBP7] and AKI (e.g., increasing or decreasing Thakar score did not modify the effect of [TIMP-2]•[IGFBP7] on AKI).
Table 2.
Unadjusted and adjusted odds ratios for development of stage 2–3 AKI using perioperative measurements of [TIMP-2]•[IGFBP7]. P-values reflect association between [TIMP-2]•[IGFBP7] and odds of AKI at each timepoint.
| Timepoint | Unadjusted OR (95% CI) |
P-value | Adjusted OR* (95% CI) |
P-value |
|---|---|---|---|---|
| Post CPB or OpCAB | 3.3 (1.3–8.3) | 0.01 | 3.9 (1.3–11.1) | 0.01 |
| ICU admission | 2.7 (1.2–5.7) | 0.01 | 2.5 (1.1–5.6) | 0.03 |
| Six hours after ICU admission | 11.5 (3.6–37.2) | <0.001 | 7.5 (2.0–28.1) | 0.003 |
| Post-operative day 1 | 5.4 (2.2–15.1) | <0.001 | 4.6 (1.7–12.7) | 0.003 |
| Sum of Post CPB/OpCAB and six hours after ICU admission | 10.8 (3.5–33.0) | <0.001 | 9.4 (2.6–34.2) | <0.001 |
| Product of Post CPB/OpCAB and six hours after ICU admission | 9.9 (3.3–30.0) | <0.001 | 8.2 (2.3–30.0) | 0.001 |
| Max of Post CPB/OpCAB and six hours after ICU admission | 9.6 (3.2–28.7) | <0.001 | 8.5 (2.4–30.0) | <0.001 |
| Sum of all timepoints | 15.4 (4.6–51.6) | <0.001 | 12.6 (3.2–50.0) | <0.001 |
| Max of all timepoints | 13.4 (4.2–42.3) | <0.001 | 10.9 (3.0–39.3) | <0.001 |
Adjusted for body mass index, Thakar score, and baseline hematocrit. [TIMP-2]•[IGFBP7] values were log-transformed in all models. As such, OR values represent the change in odds of AKI per log10([TIMP-2]•[IGFBP7]ng/mL)2/1000 increase.
The AUROC (95% CI) values for prediction of stage 2–3 AKI were 0.71 (0.58–0.82), 0.68 (0.54–0.81), 0.78 (0.58–0.93), and 0.75 (0.59–0.90), respectively, for [TIMP-2]•[IGFBP7] samples collected immediately after the end of CPB/OpCAB, at ICU admission, six hours after ICU admission, and on post-operative day 1. These results indicate that [TIMP-2]•[IGFBP7] both during and after surgery is informative for assessing risk of postoperative stage 2–3 AKI.
To explore AKI predictive capabilities using the breadth of the perioperative [TIMP-2]•[IGFBP7] data, we combined data at different timepoints using addition, multiplication, and selecting maximum values of timepoints. These different methods yielded similar results to prediction using single timepoints, with AUROC estimates ranging from 0.81 to 0.82 (Table 3). The AUROC to predict stage 2–3 AKI using the maximum value of the [TIMP-2]•[IGFBP7] collected immediately after the end of CPB/OpCAB and the [TIMP-2]•[IGFBP7] collected six hours after ICU admission yielded the highest AUROC, a value of 0.82 (95% CI: 0.73–0.90), equivalent to that for the maximum of all eight perioperative [TIMP-2]•[IGFBP7] measurements, 0.82 (0.72–0.91). When combined with the clinical factors used in the baseline model, addition of the maximum [TIMP-2]•[IGFBP7] between the post CPB/OpCAB and the six-hours after ICU admission timepoints increased the AUROC from 0.86 (0.77–0.94) to 0.90 (0.82–0.96), P=0.07 (Figure S1) and led to a continuous net reclassification improvement of 0.91 (0.38–1.45), P<0.001.
Table 3.
AUROCs for development of stage 2–3 AKI at different perioperative measurements of [TIMP-2]•[IGFBP7] alone, [TIMP-2]•[IGFBP7] in addition to baseline clinical model factors, and the continuous net classification improvement (NRI) to predict stage 2–3 AKI by adding [TIMP-2]•[IGFBP7] to the baseline model.
| Timepoint | AUROC[TIMP-2]•[IGFBP7] | AUROC [TIMP-2]•[IGFBP7] + Baseline factors | P-value* | Continuous NRI | P-value |
|---|---|---|---|---|---|
| Post CPB or OpCAB | 0.71 (0.58–0.82) | 0.86 (0.75–0.95) | 0.93 | 0.83 (0.27–1.38) | 0.003 |
| ICU admission | 0.68 (0.54–0.81) | 0.88 (0.79–0.95) | 0.26 | 0.39 (0.17–0.94) | 0.17 |
| Six hours after ICU admission | 0.78 (0.58–0.93) | 0.86 (0.72–0.98) | 0.76 | 0.87 (0.27–1.47) | 0.005 |
| Post-operative day 1 | 0.75 (0.59–0.90) | 0.92 (0.88–0.96) | 0.19 | 0.74 (0.17–1.32) | 0.01 |
| Sum of Post CPB/OpCAB and six hours after ICU admission | 0.81 (0.74–0.90) | 0.90 (0.82–0.96) | 0.07 | 0.91 (0.38–1.45) | 0.001 |
| Product of Post CPB/OpCAB and six hours after ICU admission | 0.81 (0.71–0.89) | 0.86 (0.78–0.96) | 0.20 | 0.83 (0.29–1.36) | 0.002 |
| Maximum of Post CPB/OpCAB and six hours after ICU admission | 0.82 (0.73–0.90) | 0.90 (0.82–0.96) | 0.07 | 0.91 (0.38–1.45) | 0.001 |
| Sum of all timepoints | 0.82 (0.71–0.91) | 0.90 (0.79–0.97) | 0.17 | 1.04 (0.50–1.57) | <0.001 |
| Max of all timepoints | 0.82 (0.72–0.91) | 0.92 (0.80–0.98) | 0.05 | 1.14 (0.61–1.68) | <0.001 |
Represent the differences between the AUROCs (95% CI) of the baseline model [0.86 (0.77–0.94)] and the model that includes [TIMP-2]•[IGFBP7] and baseline factors.
When stratified by the 0.3 and 2.0 (ng/mL)2/1000 clinical thresholds established in other cohorts for the prediction of moderate-severe AKI, increased baseline urinary [TIMP-2]•[IGFBP7] was associated with in-hospital dialysis and with death (Table S4). Patients with elevated urinary [TIMP-2]•[IGFBP7] six hours after ICU admission also had more in-hospital dialysis (P<0.001) and a longer ICU length of stay (P=0.006). [TIMP-2]•[IGFBP7] immediately after the end of CPB or OpCAB and six hours after ICU admission was predictive of stage 2–3 AKI, consistent with the agnostic approach that did not use 0.3 or 2.0 clinical thresholds. In addition, use of the maximum of the post CPB/OpCAB measurement and the six-hour ICU measurement with the 0.3 clinical threshold yielded 100% sensitivity for prediction of AKI, compared to 85% and 73% sensitivity using the individual post CPB/OpCAB and the six-hour ICU individual timepoints, respectively (Table S5). The incidence of not having AKI with a [TIMP-2]•[IGFBP7] less than the 0.3 threshold was 99% at both the post CPB/OpCAB and the six-hour ICU timepoints and 100% when using the maximum of these two timepoints (100% negative predictive value). The specificity of [TIMP-2]•[IGFBP7] to predict AKI using the 0.3 threshold was lower (62% when using the value post CPB/OpCAB and 64% six hours after ICU) but 93% and 98%, respectively, when using the 2.0 threshold.
Analysis of the 283-patient subgroup who received surgery with CPB (70.8% of the total cohort) yielded similar results to the analysis of the total cohort. Eleven of the 283 patients (3.9%) developed stage 2–3 (moderate-severe) AKI. These patients displayed bimodal elevations of [TIMP-2]•[IGFBP7], with a first elevation (median 0.57 [ng/mL]2/1000) occurring intraoperatively following CPB and the second elevation (1.22 [ng/mL]2/1000) six hours postoperatively. CPB patients who did not develop AKI did not have any elevations in [TIMP-2]•[IGFBP7]. Each 10-fold increase in intraoperative [TIMP-2]•[IGFBP7] was independently associated with a 433% increase in the odds of stage 2–3 AKI (P = 0.01), and each 10-fold increase in six hours postoperative [TIMP-2]•[IGFBP7], a 319% increase in the odds of stage 2–3 AKI (P = 0.07). The maximum [TIMP-2]•[IGFBP7] between the intraoperative and the six-hour postoperative timepoints provided an AUROC of 0.81 (95% CI: 0.71–0.90), 100% sensitivity, and 100% negative predictive value using the >0.3 cutoff to predict stage 2–3 AKI, similar to the 0.82 (95% CI: 0.72–0.90) AUROC, 100% sensitivity, and 100% NPV metrics from the entire cohort.
DISCUSSION
In this study we observed that increased intraoperative concentrations and increased early postoperative concentrations of [TIMP-2]•[IGFBP7] are independently associated with development of moderate-severe AKI and that perioperative [TIMP-2]•[IGFBP7] is a highly sensitive predictor of postoperative AKI. During surgery, patients at high risk for AKI can be identified and therefore can be given specific treatment or selected for testing novel interventions.
The current study also provides the most granular characterization of the time-course of [TIMP-2]•[IGFBP7] in patients at typical risk of AKI after heart surgery – all comers. We report an initial urinary [TIMP-2]•[IGFBP7] increase during surgery followed by a decrease at ICU admission and then a sharp increase within six hours in patients later diagnosed with stage 2–3 AKI. This bimodal elevation of [TIMP-2]•[IGFBP7], also reported in a pilot study be Mayer et al,27 may be important. It is consistent with the concept that CSA-AKI results from both intraoperative and postoperative insults. This transient reduction at ICU admission in AKI patients could be a reflection of decreased kidney stress following termination of CPB, post-CPB resuscitation, and hemodynamic optimization prior to further renal insults occurring in the early postoperative period or could be a reflection of changes in glomerular filtration that alter urine creatinine or urine dilution. From an AKI prediction standpoint, the sensitivity of intraoperative [TIMP-2]•[IGFBP7] to predict AKI was limited by misclassification of patients who suffer a renal insult later in surgery as low risk. A test cannot detect an insult that has not yet occurred. Conversely, the use of only postoperative data misclassified different patients – those who had suffered intraoperative renal injury – as low risk. When using the maximum of the post CPB/OpCAB intraoperative measurement and the six hours after ICU measurement, however, both intraoperative and/or early postoperative renal stress has occurred, and in the current study, all patients with AKI were identified (100% sensitivity). These data suggest that elevated intraoperative and early postoperative urinary [TIMP-2]•[IGFBP7] may effectively identify patients undergoing acute kidney stress and may provide opportunities for intervention. We examined the perioperative course of the patients who developed moderate-severe AKI to identify additional clinical characteristics that could account for the bimodal [TIMP-2]•[IGFBP7] peak in these patients. We did not discover a specific pattern in patients who developed moderate-severe AKI, although these patients had a lower hematocrit post CPB/OpCAB, received more packed red blood cell transfusions during surgery, and received higher doses of norepinephrine in the early postoperative period than patients who did not develop moderate-severe AKI. Use and duration of aortic cross-clamp, intraoperative cardiac output, intraoperative blood pressure, and intraoperative doses of norepinephrine, however, were similar between patients who did and did not develop moderate-severe AKI.
Meersch et al. randomized patients identified as high-risk for CSA-AKI, defined as [TIMP-2]•[IGFBP7] >0.3 (ng/mL)2/1000 four hours after termination of CPB, to early intervention with the KDIGO bundle, primarily hemodynamic and volume optimization and avoidance of toxins or renal stressors, versus standard care and showed a significant decrease in the subsequent rates of moderate to severe AKI.28 Gocze et al., in a similar study of major non-cardiac surgery patients, showed that early application of the KDIGO bundle in patients with [TIMP-2]•[IGFBP7] >0.3 also resulted in decreased rates of moderate-severe AKI, decreased ICU and hospital length of stay, and decreased cost.16
Use of [TIMP-2]•[IGFBP7] in clinical practice remains somewhat limited, in part due to uncertainty about how and when to use the test. As initially demonstrated by Hoste et al., a 0.3 (ng/mL)2/1000 cutoff for [TIMP-2]•[IGFBP7] provides early recognition with high sensitivity for development of AKI in adult ICU patients.18 This 0.3 cutoff was further validated in another cohort of ICU patients and also among adults undergoing cardiac surgery.14, 15, 27, 29, 30 Given the time-course of [TIMP-2]•[IGFBP7] seen in our study, we propose measuring [TIMP-2]•[IGFBP7] immediately after termination of CPB and, if < 0.3, again at 4–6 hours after ICU admission. If either the intraoperative or the early postoperative [TIMP-2]•[IGFBP7] is >0.3, we recommend instituting renal supportive measures such as the KDIGO bundle, as demonstrated by Meersch et al, and additional vigilance to patient monitoring.15 If neither the intraoperative nor the early postoperative test is positive (>0.3), the treating clinician may be confident the patients will not develop moderate-severe AKI (the “double-negative” [TIMP-2]•[IGFBP7]). Indeed, the negative predictive value using this sampling strategy in the current study was 100%. Measurement at additional perioperative timepoints did not confer additional benefit for predicting AKI, nor did combining the amplitude of the intraoperative and early postoperative measurements by computing their sum or product. This sampling strategy is consistent with studies demonstrating that the maximum value of [TIMP-2]•[IGFBP7] during the first 24 hours outperforms individual timepoint data values.14 [TIMP-2]•[IGFBP7] post CPB/OpCAB and six hours after ICU admission identified all patients who would subsequently develop moderate-severe AKI. An intraoperative and early postoperative sampling scheme may therefore adequately capture relevant renal stress, provide good opportunity for early intervention, and allow clinicians to identify low risk patients, thereby maximizing resources and attention.
We acknowledge several limitations to this study. Foremost, although consistent with many cardiac surgery cohorts, the rate of stage 2–3 AKI was low. The use of urine output criteria to identify additional patients who may have developed stage 2–3 AKI could have added additional valuable data. However, the use of urine output to diagnose AKI following cardiac surgery is controversial due to confounding by intravascular hypovolemia and diuretic administration, and the separation of [TIMP-2]•[IGFBP7] between patients who developed stage 2–3 AKI and those who developed stage 1 or no AKI using creatinine data was striking. The study was completed at a single center and results could be different elsewhere. More data from additional medical centers and patient populations are needed. Nonetheless, this is the largest and most granular urinary [TIMP-2]•[IGFBP7] cardiac surgery study to date, and is further strengthened by the rigorous phenotyping of the Statin AKI Cardiac Surgery RCT cohort.
In conclusion intraoperative elevations of [TIMP-2]•[IGFBP7] were independently associated with development of stage 2–3 CSA-AKI and an intraoperative and early postoperative two-measurement sampling scheme provided excellent ability to detect both patients who would and those who would not develop CSA-AKI. Larger trials should be conducted to characterize any reductions in major adverse kidney events or costs from measuring [TIMP-2]•[IGFBP7] to identify patients who will and will not develop CSA-AKI.
Supplementary Material
Video Legend.
Dr. Jared J. Cummings discusses the rationale for the project, the principal findings, and the relevance of these findings to readers of the Journal.
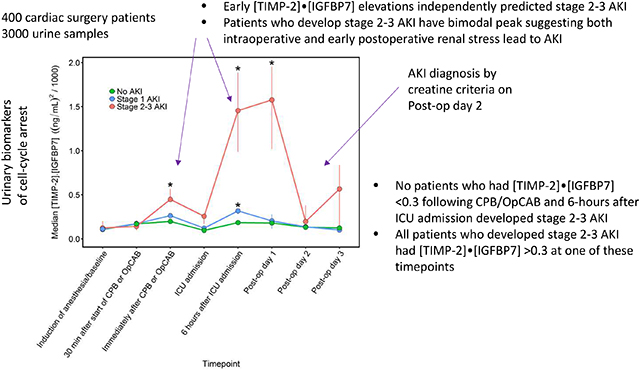
Central Picture Legend: Perioperative urinary [TIMP-2]•[IGFBP7].
Intraoperative elevations of [TIMP-2]•[IGFBP7] can predict moderate-severe AKI and could provide opportunity to alter postoperative management to prevent kidney injury.
Acknowledgments
We acknowledge Patty Hendricks, R.N., for nursing support and Astute Medical for blinded measurements of [TIMP-2]•[IGFBP7].
Source of Funding:
This work was supported by T32GM108554, K23GM102676, R01GM112871, and UL1TR000445 from the National Institutes of Health, the Foundation for Anesthesia Education and Research, and the Vanderbilt University Medical Center Department of Anesthesiology.
Glossary of Abbreviations
- AUROC
area under the receiver operating characteristic curve
- CPB
cardiopulmonary bypass
- CSA-AKI
cardiac surgery-associated acute kidney injury
- ICU
intensive care unit
- IGFBP7
insulin-like growth factor-binding protein 7
- KDIGO
kidney disease: improving global outcomes
- KIM-1
kidney injury molecule 1
- NGAL
neutrophil gelatinase-associated lipocalin
- OpCAB
off-pump coronary artery bypass
- RCT
randomized clinical trial
- TIMP-2
tissue inhibitor of metalloproteinase 2
- VUMC
Vanderbilt University Medical Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Dr. Shaw and Dr. Shi are consultants for Astute Medical, which developed the [TIMP-2]•[IGFBP7] NephroCheck assay and performed urinary [TIMP-2]•[IGFBP7] measurements blinded to all subject characteristics. Astute Medical did not participate in the design of the study or decision to submit the findings for publication. Dr. Shi completed the statistical analysis. Dr. Cummings, Shaw, Shi, and Billings wrote the manuscript. The authors declare no other conflict of interest.
References
- 1.Hu J, Chen R, Liu S, Yu X, Zou J, Ding X. Global Incidence and Outcomes of Adult Patients With Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth. 2016;30:82–89. [DOI] [PubMed] [Google Scholar]
- 2.Fuhrman DY, Kellum JA. Epidemiology and pathophysiology of cardiac surgery-associated acute kidney injury. Curr Opin Anaesthesiol. 2017;30:60–65. [DOI] [PubMed] [Google Scholar]
- 3.D’Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg. 2018;105:15–23. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Zakkar M, Bruno VD, Guida G, Angelini GD, Chivasso P, Suleiman MS, et al. Postoperative acute kidney injury defined by RIFLE criteria predicts early health outcome and long-term survival in patients undergoing redo coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2016;152:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neal JB, Shaw AD, Billings FTt. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–37. [DOI] [PubMed] [Google Scholar]
- 8.Albert C, Albert A, Kube J, Bellomo R, Wettersten N, Kuppe H, et al. Urinary biomarkers may provide prognostic information for subclinical acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:2441–2452 e2413. [DOI] [PubMed] [Google Scholar]
- 9.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxí). 2017;219:554–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group KW. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2. [Google Scholar]
- 14.Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS, et al. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care. 2015;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gocze I, Jauch D, Gotz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One.2015;10:e0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29:2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billings FTt, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, et al. Obesity and oxidative stress predict AKI after cardiac surgery. JAm Soc Nephrol. 2012;23:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billings FTt, Ball SK, Roberts LJ 2nd, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings FTt, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, et al. High-Dose Perioperative Atorvastatin and Acute Kidney Injury Following Cardiac Surgery: A Randomized Clinical Trial. JAMA. 2016;315:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pohlert T The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). 2014;R package.
- 23.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. [DOI] [PubMed] [Google Scholar]
- 24.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell Jr FD C; et al. Hmisc: Harrell Miscellaneous. 4.1–1 ed2018.
- 26.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2014. [Google Scholar]
- 27.Mayer T, Bolliger D, Scholz M, Reuthebuch O, Gregor M, Meier P, et al. Urine Biomarkers of Tubular Renal Cell Damage for the Prediction of Acute Kidney Injury After Cardiac Surgery-A Pilot Study. J Cardiothorac Vasc Anesth. 2017. [DOI] [PubMed] [Google Scholar]
- 28.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Zou Z, Jin J, Teng J, Xu J, Shen B, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017; 18:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am JRespir Crit Care Med. 2014;189:932–939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.