Abstract
Objective:
Papillary muscle (PM) displacement contributes to ischemic/functional mitral regurgitation (IMR/FMR). The displaced PMs pull the mitral leaflets into the left ventricle (i.e. towards the apex) thus hampering leaflet coaptation. Intuitively apical leaflet tethering results from apical PM displacement. The three-dimensional directions of PM displacement are, however, incompletely characterized.
Methods:
Data from in-vivo ovine models of IMR (6 to 8 weeks of postero-lateral infarction, n=12) and FMR (9–21 days of rapid LV pacing, n=11) were analyzed. All sheep had radiopaque markers implanted on the anterior (APM) and posterior PPM) PM tips, around the mitral annulus, and on left ventricular apex. In order to explore three-dimensional PM displacement directions, differences in marker coordinates were calculated at end-systole before and during IMR/FMR using a right-handed coordinate system centered on the mitral annular “saddle horn” with the y-axis passing through the apical marker.
Results:
No apical PM displacement was observed during either IMR or FMR. The APM displaced laterally during FMR. Postero-lateral PPM displacement was observed during both, IMR and FMR.
Conclusions:
Experimental in-vivo ovine models suggest postero-lateral PPM displacement as predominant pathomechanism leading to apical leaflet tethering during IMR/FMR.
Graphical Abstract
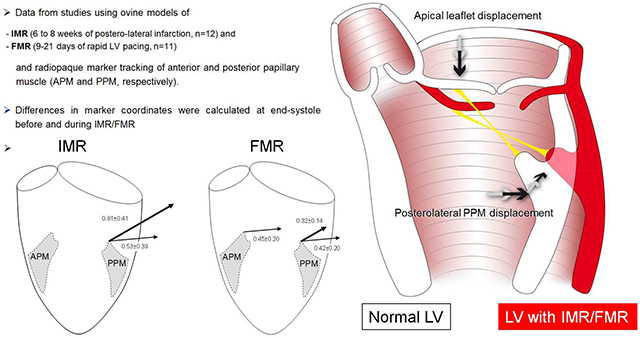
Graphical Abstract: Data from studies using ovine models of ischemic/functional mitral regurgitation (IMR/FMR) and radiopaque marker tracking of anterior and posterior papillary muscle tips (APM and PPM, respectively) suggest postero-lateral PPM displacement as predominant pathomechanism leading to apical leaflet tethering during IMR/FMR. LV=left ventricle
Central Picture: Data from experimental ovine studies suggest that apical anterior mitral leaflet (AML) tethering is associated with postero-lateral papillary muscle (PPM) dislocation in ischemic/functional mitral regurgitation (IMR/FMR).
Central Message
Consistent postero-lateral, but not apical dislocation of the posterior papillary muscle was observed with experimental IMR/FMR.
Introduction
In patients with ischemic/functional mitral regurgitation (IMR/FMR) mitral valve replacement is increasingly advocated as surgical treatment standard1 due to suboptimal results with reductive ring annuloplasty2,3. Poor repair outcomes are thought to be related to ongoing LV remodeling and papillary muscle displacement leading to recurrent leaflet tethering4. Nevertheless, lasting mitral repair may be superior to valve replacement5, and surgical strategies that specifically aim to address subvalvular distortions of PM geometry have been introduced clinically to improve repair durability6–13. However, due to incomplete understanding of the alterations of subvalvular geometry associated with IMR/FMR, no current consensus exists regarding optimal subvalvular repair. Mitral leaflet tethering has been shown to be associated with clinical and experimental IMR/FMR14–15, and apical papillary muscle displacement has been intuitively assumed to be the putative mechanism. The precise PM displacement in patients with IMR/FMR is, however, difficult to determine as clinical imaging modalities are unable to accurately track distinct anatomic landmarks over time. Experimental studies using radiopaque markers or sonomicrometry crystals have failed to demonstrate apical PPM displacement16–19 during IMR. To characterize 3-dimensional perturbations of papillary muscle geometry associated with IMR/FMR, we analyzed data from in vivo ovine studies using radiopaque marker tracking of the APM and PPM during experimental IMR and FMR.
Methods
Data from two experimental ovine studies performed by our research group were analyzed FMR to investigate 3-D vectors of APM and PPM displacement. These data have been partially published previously20–22. All animal protocols were approved by the Stanford Medical Center Laboratory Research Animal Review committee and conducted according to Stanford University policy. All studies included radiopaque marker placement on APM and PPM tips and around the mitral annulus using cardiopulmonary bypass and cardioplegic arrest. In the study investigating the effects of IMR, baseline data from 12 sheep were acquired 6 to 8 days after surgical placement of radiopaque markers (Control). Experimental IMR data were acquired 6 to 8 weeks after induction of a postero-lateral infarct (snare occlusion of obtuse marginal branches, see Tibayan et al., Ref.21,for details). In the model of experimental FMR, baseline data from eleven sheep were acquired 5 to 8 days after surgical placement of radiopaque markers (Control). A rapid-pacing pulse generator (Prodigy S 8164, Medtronic Medical) was inserted into a subcutaneous pocket and connected to the previously externalized LV electrode, and the animal was recovered. Rapid pacing was initiated 24 hours later. FMR data were obtained 9 to 21 days after rapid left ventricular pacing (180 to 230beats per minute, see Timek et al., Ref.22, for details). All data acquisitions were performed using biplane videofluoroscopy (60Hz) in standardized fashion, and marker positions from both views were digitized, merged, and analyzed using customized computer software23.
To assess the presence of apical PM displacement, the orthogonal distance from each papillary muscle marker to the least-squares mitral annular plane at end-systole was calculated before and during IMR or FMR.
In order to specify precise vectors of APM/PPM displacement, 3-D marker positions were determined at end-systole before and after induction of experimental IMR and FMR. A left-handed coordinate system was used with the lateral (y-) axis pointing towards the lateral mitral annular marker, the apical (x-) axis orthogonal to the y-axis in a plane with the LV apex marker. The z-axis was orthogonal to the x-y plane pointing posteriorly (Figure 1, small schematic). Displacement vectors were calculated as differences of x, y and z coordinates between baseline and respective experimental state. Data were compared using Student’s t-test for paired samples. The Bonferroni adjustment was applied to account for the different measurement conditions (three coordinate axes, distance to mitral annular plane) within the two different papillary muscles (overall significance level set to p <=0.05, Bonferroni corrected level of significance was set to p < 0.006). All results are presented with mean and standard deviation (SD). Statistical analysis was performed using SPSS 21 for Windows.
Figure 1:
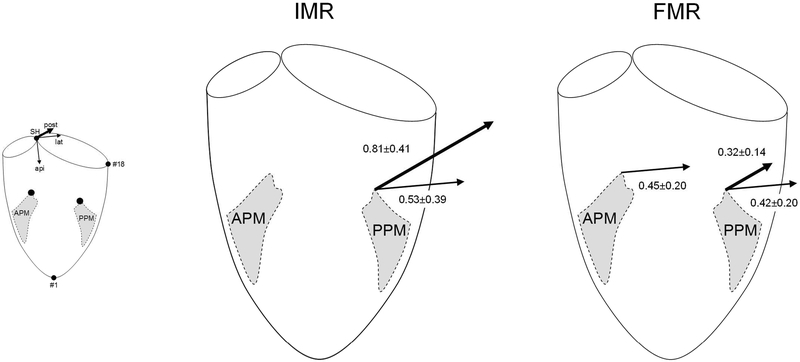
Schematic illustrations depicting three-dimensional anterior and posterior papillary muscle (APM and PPM, respectively) displacement vectors in experimental ovine models of ischemic and functional mitral regurgitation (IMR and FMR, respectively). Arrows indicate vectors that reached statistical significance according to Table 2. Arrow lengths are proportionate to the average of the differences between Control and the respective IMR/FMR values. The small schematic illustrates the coordinate system used (see Methods). SH=saddle horn, api=apical, lat=lateral, post=posterior, #18=mid-lateral mitral annular marker.
Results
Both animal subgroups (experimental IMR and FMR) developed significant MR and signs of LV failure as indicated by an increase in left-ventricular end-diastolic volume (Table 1). Table 2 displays the distances of APM and PPM to the mitral annular plane before and during IMR and FMR. During both experimental conditions (IMR and FMR), none of the changes in distances of either APM or PPM to the mitral annular plane was statistically significant, but a trend towards a decrease of the PPM distance to the mitral annular plane during both, IMR and FMR was observed (3.82±0.53 vs. 3.16±0.41, p=.014 and 3.01±0.80 vs. 2.69±0.67, p=.026, respectively). Table 3 demonstrates the APM and PPM positions before and during IMR and FMR. The APM did not displace significantly during experimental IMR and laterally during FMR (lateral: 0.97±0.47 vs. 1.43±0.43, p<.001). The PPM displaced postero-laterally during both, IMR (posterior: 0.58±0.40 vs. 1.39±0.46, p<.001, lateral: 0.66±0.55 vs. 1.19±0.70, p=.001) and FMR (posterior: 0.10±0.46 vs. 0.22±0.48, p<.001, lateral: 0.74±0.48 vs.1.16±0.45, p<.001). Figure 1 illustrates APM and PPM displacement vectors that reached statistical significance.
Table 1:
Hemodynamics
| Control | IMR | p | Control | FMR | p | |
|---|---|---|---|---|---|---|
| MR (0–4+) | 0.6±0.5 | 2.5±0.6 | <.001* | 0.2±0.3 | 2.2±0.9 | <.001* |
| LV dP/dtmax (mm Hg/s) | 1979±785 | 1256±506 | .002* | 1350±219 | 1162±374 | .290 |
| EDV (ml/m2) | 128±36 | 180±40 | <.001* | 160±57 | 201±67 | .001* |
Values are mean±1 SD, MR = mitral regurgitation; LV dP/dtmax, maximum of first derivative of pressure vs. time; EDV, end-diastolic volume,
= statistically significant after Holm-Bonferroni adjustment.
Table 2:
Distances of APM and PPM to the mitral annular (MA) plane before and during IMR and FMR
| Control | IMR | p | Control | FMR | p | |
|---|---|---|---|---|---|---|
| Distance to MA plane (cm) | ||||||
| -APM | 3.81±0.70 | 3.70±0.39 | .627 | 2.50±0.85 | 2.52±0.79 | .867 |
| -PPM | 3.82±0.53 | 3.16±0.41 | .014 | 3.01±0.80 | 2.69±0.67 | .026 |
Values are mean±1SD at end-systole, APM/PPM=anterior/posterior papillary muscle, IMR/FMR=ischemic/functional mitral regurgitation.
Table 3:
Anterior and posterior PM positions at end-systole before and during IMR and FMR
| Control | IMR | p | Control | FMR | p | |
|---|---|---|---|---|---|---|
| APM position | ||||||
| -lateral | 1.30±0.85 | 1.69±0.98 | .498 | 0.97±0.47 | 1.43±0.43 | <.001* |
| -posterior | −2.05±0.72 | −2.18±0.54 | .488 | −2.15±0.28 | −2.62±0.54 | .009 |
| -apical | 5.06±1.02 | 4.79±0.86 | .029 | 3.05±0.55 | 3.01±0.62 | .743 |
| PPM position | ||||||
| -lateral | 0.66±0.55 | 1.19±0.70 | .001* | 0.74±0.48 | 1.16±0.45 | <.001* |
| -posterior | 0.58±0.40 | 1.39±0.46 | <.001* | −0.10±0.46 | 0.22±0.48 | <.001* |
| -apical | 5.40±1.04 | 4.94±0.59 | .282 | 4.11±0.67 | 4.46±1.01 | .153 |
mean±1SD and describe papillary muscle (PM) positions at end-systole (see Methods for coordinate system used). Negative values represent a PM position in the opposite direction as indicated (i.e. septal, anterior and towards the LV base as opposed to lateral, posterior and apical, respectively).
= statistically significant after Holm-Bonferroni adjustment. APM/PPM=anterior/posterior papillary muscle, IMR/FMR=ischemic/functional mitral regurgitation.
Discussion
The main findings of this experimental work are displayed in the graphical abstract. During both, experimental IMR and FMR: 1) no apical PM displacement is observed; 2) the displacement vector of the PPM is directed postero-laterally. These findings suggest posterolateral PPM displacement as predominant pathomechanism leading to apical leaflet tethering during IMR/FMR.
Surgical mitral valve repair in patients with IMR/FMR using a ring annuloplasty alone is associated with high rates of residual/recurrent mitral regurgitation2 as prosthetic ring implantation may enhance leaflet tethering while ongoing left ventricular dilatation leads to further PM displacement and recurrent MR24. As a consequence, several authors have introduced adjunctive subvalvular techniques (in addition to annuloplasty) to counteract PM displacement6–9,25.
In the clinical literature, papillary muscle displacements have frequently been associated with an apically directed vector26–30. Based on increases in echocardiographically measured tethering lengths of both anterior and posterior PM in patient with FMR, Yiu and colleagues concluded that apical displacement of papillary muscles is a major determinant of valvular tenting29. Numerous other clinical studies have used tethering lengths to characterize three dimensional changes of the subvalvular apparatus in patients with FMR/IMR24,29,31–35. Tethering length, however, is a two-dimensional parameter and does not allow drawing conclusions about the 3-D vectors of PM displacement.
Several experimental studies exist that report three-dimensional alterations of the geometry of the PMs during IMR. Gorman and colleagues demonstrated a decrease in the distance between the PPM and the posterior commissure in an ovine model of IMR17 which suggests a lack of apical PPM displacement and is in accordance with our findings. Similar geometric perturbation of posterior papillary muscle geometry were reported by Lai during acute ovine IMR36. Myocardial marker tagging of multi-headed papillary tips also failed to demonstrate apical displacement of any papillary muscle heads during acute ovine IMR19. The data from these acute studies demonstrate similar trends compared to our model of longer term IMR/FMR. Interestingly, in our IMR/FMR models the PPM distance to the mitral annular plane during both, IMR and FMR tended to decrease (3.82±0.53 vs. 3.16±0.41, p=.014 and 3.01±0.80 vs. 2.69±0.67, p=.026, respectively). This trend may have become significant with a bigger sample size and suggests that apical leaflet tethering may occur even if the papillary muscles move closer to the annular plane.
Our finding of postero-lateral, but not apical displacement of the PPM during IMR/FMR is also indirectly supported by other reports. Balloon repositioning of the displaced PPM in ovine IMR purely in the antero-septal direction decreased leaflet tethering and reduced mitral insufficiency25,37. The Coapsys device which primarily repositions the PPM towards the LV septum has demonstrated a similar effect clinically38. On the contrary, pulling the posterior PM towards the posterior commissure (which includes a vector mainly directed towards the LV base) during acute ovine IMR did not ameliorate mitral regurgitation39 whereas PPM relocation towards the right fibrous trigone (which includes a septal and anterior vector) in addition to reductive annuloplasty decreased leaflet tethering in a porcine model of IMR/FMR40.
A recent prospective, randomized trial in patients with severe IMR demonstrated improved long-term cardiac outcomes in patients undergoing restrictive mitral annuloplasty and a PM sling as compared to patients receiving a restrictive annuloplasty alone11. In this novel subvalvular repair, PMs are approximated using a PTFE tube with the relocation vector of the PPM primarily directed anteriorly and towards the septum, but not towards the LV base.
Clinical Inference
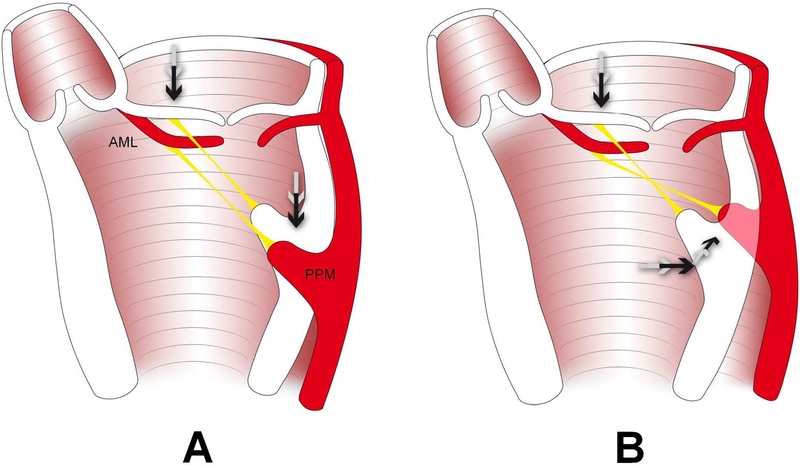
For a meaningful discussion of clinical inferences the limitations of our data (see paragraph below) including potential differences to patients with long lasting IMR/FMR must be considered. An important goal of this work is therefore to stimulate further clinical research investigating three-dimensional papillary muscle displacement vectors during IMR/FMR. As mitral leaflets are often observed to be tethered apically in IMR/FMR, it is frequently assumed that apical leaflet tethering results from apical PPM displacement (Figure 2A). Our analysis of two different in vivo animal data sets from our laboratory, however, revealed that mitral leaflet tethering in experimental ovine in vivo models does not result from apical, but from postero-lateral PPM dislocation (Figure 1 B). In case these findings translate to patients with IMR/FMR these data suggest that subvalvular surgical or interventional approaches to IMR/FMR should focus on relocating the PPM anteriorly and towards the septum.
Figure 2:
Schematic illustrating the hypothesized predominant mechanism leading to leaflet tethering during IMR/FMR: Apical leaflet displacement is not – as frequently hypothesized – associated with apical (A), but with a postero-lateral displacement of the posteromedial papillary muscle (PPM) (B). AML=anterior mitral leaflet.
Study Limitations
The results of the presented data must be viewed in the context of several limitations. First, left ventricular alterations in these experimental models of IMR/FMR significantly differ from patients with long term IMR/FMR. Apical PM displacement could occur in IMR/FMR patients even secondary to postero-lateral PPM displacement. Clinical studies assessing 3-dimensional changes of the PM geometry in IMR/FMR patients over a longer time period are needed to resolve this issue. Second, our analyses focus solely on 3-D changes in the PM geometry and no additional hemodynamic data or data from alterations in LV or leaflet geometry were calculated. These data have, however, been published earlier and have been shown to be consistent with typical alterations observed during experimental IMR or FMR20,22,41. Third, species differences in papillary muscle blood supply may limit the extrapolation of these results to patients42 and mitral valve anatomy may be heterogeneous between patients43. Fourth, we investigated only one time point in the cardiac cycle (end-systole). Our analyses therefore do not provide insight into cases where FMR/IMR arises due to dynamic papillary muscle dysfunction44. Lastly, recently published algorithms for the surgical treatment of IMR/FMR suggest the addition of subvalvular repair based on LV wall perfusion or motion abnormalities45. In our study, no analysis of LV motion was performed, and it is possible that PM displacement vectors differ in individual patient. However, it is reasonable to assume that the observed alterations of PM geometry in our study apply to the majority of patients with IMR/FMR.
In conclusion, experimental in-vivo ovine models suggest postero-lateral PPM displacement as predominant pathomechanism leading to apical leaflet tethering during IMR/FMR.
Supplementary Material
Video: Presentation illustrating the key findings of this study.
Perspective Statement.
Papillary muscle (PM) displacement contributes to apical leaflet tethering during IMR/FMR. In these experimental ovine IMR/FMR studies consistent postero-lateral, but not apical displacement of the posterior PM was observed.
Acknowledgments
We acknowledge the superb technical assistance provided by Carol W. Mead, BA, and Maggie Brophy, RVT.
Funding Sources/Disclosures: D. Craig Miller received R01 research grants, NHLBI, National Institutes of Health HL29589 (1982–2008) and HL67025 (2001–2010) and is a Consultant for Medtronic CardioVascular Division. There is no conflict of interest.
Glossary of Abbreviations
- IMR/FMR
ischemic/functional mitral regurgitation
- LV
left ventricle
- PM
papillary muscle
- APM/PPM
anterior/posterior papillary muscle
- LCx
left circumflex artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salmasi MY, Acharya M, Humayun N, Baskaran D, Hubbard S, Vohra H. Is valve repair preferable to valve replacement in ischaemic mitral regurgitation? A systematic review and meta-analysis. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2016;50(1):17–28. doi: 10.1093/ejcts/ezw053 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med. 2016;374(4):344–353. doi: 10.1056/NEJMoa1512913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med. 2016;374(20):1932–1941. doi: 10.1056/NEJMoa1602003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kainuma S, Funatsu T, Kondoh H, et al. Beneficial effects of restrictive annuloplasty on subvalvular geometry in patients with functional mitral regurgitation and advanced cardiomyopathy. J Thorac Cardiovasc Surg. December 2017. doi: 10.1016/j.jtcvs.2017.11.090 [DOI] [PubMed] [Google Scholar]
- 5.Bolling SF. How Do We Ensure a “Good” Repair in Ischemic Mitral Regurgitation? J Am Coll Cardiol. 2016;67(20):2347–2348. doi: 10.1016/j.jacc.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 6.Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thomc Surg. 2002;74(2):600–601. [DOI] [PubMed] [Google Scholar]
- 7.Bothe W, Doenst T. Ring-Noose-String Technique Allows Adjustable Papillary Muscle Repositioning During Minimally Invasive Mitral Valve Repair in Patients with Functional/Ischemic Mitral Regurgitation. Thorac Cardiovasc Surg. April 2015. doi: 10.1055/s-0035-1549264 [DOI] [PubMed] [Google Scholar]
- 8.Langer F, Groesdonk H-V, Kunihara T, Schäfers H-J. Dynamic RING + STRING for ischemic mitral regurgitation: papillary muscle repositioning and modification of the septal-lateral diameter in the loaded beating heart under echocardiographic guidance. J Thorac Cardiovasc Surg. 2011;141(5):1315–1316. doi: 10.1016/j.jtcvs.2010.07.078 [DOI] [PubMed] [Google Scholar]
- 9.Ueno T, Sakata R, Iguro Y, Nagata T, Otsuji Y, Tei C. New surgical approach to reduce tethering in ischemic mitral regurgitation by relocation of separate heads of the posterior papillary muscle. Ann Thorac Surg. 2006;81(6):2324–2325. doi: 10.1016/j.athoracsur.2005.03.059 [DOI] [PubMed] [Google Scholar]
- 10.Mihos CG, Larrauri-Reyes M, Santana O. A Meta-Analysis of Ring Annuloplasty Versus Combined Ring Annuloplasty and Subvalvular Repair for Moderate-to-Severe Functional Mitral Regurgitation. J Card Surg. 2016;31(1):31–37. doi: 10.1111/jocs.12662 [DOI] [PubMed] [Google Scholar]
- 11.Nappi F, Lusini M, Spadaccio C, et al. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. J Am Coll Cardiol. 2016;67(20):2334–2346. doi: 10.1016/j.jacc.2016.03.478 [DOI] [PubMed] [Google Scholar]
- 12.Mihos CG, Yucel E, Santana O. The role of papillary muscle approximation in mitral valve repair for the treatment of secondary mitral regurgitation. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. December 2016. doi: 10.1093/ejcts/ezw384 [DOI] [PubMed] [Google Scholar]
- 13.Nappi F, Spadaccio C, Nenna A, et al. Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? Subanalysis of the Papillary Muscle Approximation trial. J Thorac Cardiovasc Surg. 2017;153(2):286–295.e2. doi: 10.1016/j.jtcvs.2016.09.050 [DOI] [PubMed] [Google Scholar]
- 14.Bothe W, Kvitting J-PE, Stephens EH, et al. Effects of different annuloplasty ring types on mitral leaflet tenting area during acute myocardial ischemia. J Thorac Cardiovasc Surg. 2011;141(2):345–353. doi: 10.1016/j.jtcvs.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golba K, Mokrzycki K, Drozdz J, et al. Mechanisms of functional mitral regurgitation in ischemic cardiomyopathy determined by transesophageal echocardiography (from the Surgical Treatment for Ischemic Heart Failure Trial). Am J Cardiol. 2013;112(11):1812–1818. doi: 10.1016/j.amjcard.2013.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komeda M, Glasson JR, Bolger AF, et al. Geometric determinants of ischemic mitral regurgitation. Circulation. 1997;96(9 Suppl):II–128- 133. [PubMed] [Google Scholar]
- 17.Gorman JH, Gorman RC, Jackson BM, Enomoto Y, St John-Sutton MG, Edmunds LH. Annuloplasty ring selection for chronic ischemic mitral regurgitation: lessons from the ovine model. Ann Thorac Surg. 2003;76(5):1556–1563. [DOI] [PubMed] [Google Scholar]
- 18.Lai DT, Timek TA, Dagum P, et al. The effects of ring annuloplasty on mitral leaflet geometry during acute left ventricular ischemia. J Thorac Cardiovasc Surg. 2000;120(5):966–975. doi: 10.1067/mtc.2000.110186 [DOI] [PubMed] [Google Scholar]
- 19.Timek TA, Lai DT, Bothe W, et al. Geometric perturbations in multiheaded papillary tip positions associated with acute ovine ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;150(1):232–237. doi: 10.1016/j.jtcvs.2015.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timek TA, Lai DT, Tibayan F, et al. Ischemia in three left ventricular regions: Insights into the pathogenesis of acute ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2003;125(3):559–569. doi: 10.1067/mtc.2003.43 [DOI] [PubMed] [Google Scholar]
- 21.Tibayan FA, Rodriguez F, Zasio MK, et al. Geometric distortions of the mitral valvular-ventricular complex in chronic ischemic mitral regurgitation. Circulation. 2003;108 Suppl 1:11116–121. doi: 10.1161/01.cir.0000087940.17524.8a [DOI] [PubMed] [Google Scholar]
- 22.Timek TA, Dagum P, Lai DT, et al. Pathogenesis of mitral regurgitation in tachycardia-induced cardiomyopathy. Circulation. 2001;104(12 Suppl 1):I47–53. [DOI] [PubMed] [Google Scholar]
- 23.Niczyporuk MA, Miller DC. Automatic tracking and digitization of multiple radiopaque myocardial markers. Comput Biomed Res Int J. 1991. ;24(2):129–142. [DOI] [PubMed] [Google Scholar]
- 24.Yu H-Y, Su M-Y, Chen Y-S, Lin F-Y, Tseng W-YI. Mitral tetrahedron as a geometrical surrogate for chronic ischemic mitral regurgitation. Am J Physiol Heart Circ Physiol. 2005;289(3):H1218–1225. doi: 10.1152/ajpheart.00169.2005 [DOI] [PubMed] [Google Scholar]
- 25.Hung J, Solis J, Guerrero JL, et al. A novel approach for reducing ischemic mitral regurgitation by injection of a polymer to reverse remodel and reposition displaced papillary muscles. Circulation. 2008;118(14 Suppl):S263–269. doi: 10.1161/CIRCULATIONAHA.107.756502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee AP-W, Acker M, Kubo SH, et al. Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy: importance of distal anterior leaflet tethering. Circulation. 2009;119(19):2606–2614. doi: 10.1161/CIRCULATIONAHA.108.796151 [DOI] [PubMed] [Google Scholar]
- 27.Dai-Bianco JP, Aikawa E, Bischoff J, et al. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 2009;120(4):334–342. doi: 10.1161/CIRCULATIONAHA.108.846782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agricola E, Oppizzi M, Pisani M, Meris A, Maisano F, Margonato A. Ischemic mitral regurgitation: mechanisms and echocardiographic classification. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol. 2008;9(2):207–221. doi: 10.1016/j.euje.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 29.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000;102(12):1400–1406. [DOI] [PubMed] [Google Scholar]
- 30.Levine RA, Hagége AA, Judge DP, et al. Mitral valve disease--morphology and mechanisms. Nat Rev Cardiol. 2015;12(12):689–710. doi: 10.1038/nrcardio.2015.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uemura T, Otsuji Y, Nakashiki K, et al. Papillary muscle dysfunction attenuates ischemic mitral regurgitation in patients with localized basal inferior left ventricular remodeling: insights from tissue Doppler strain imaging. J Am Coll Cardiol. 2005;46(1):113–119. doi: 10.1016/j.jacc.2005.03.049 [DOI] [PubMed] [Google Scholar]
- 32.Kumanohoso T, Otsuji Y, Yoshifuku S, et al. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg. 2003;125(1):135–143. doi: 10.1067/mva.2003.78 [DOI] [PubMed] [Google Scholar]
- 33.Yu H-Y, Su M-Y, Liao T-Y, Peng H-H, Lin F-Y, Tseng W-YI. Functional mitral regurgitation in chronic ischemic coronary artery disease: analysis of geometric alterations of mitral apparatus with magnetic resonance imaging. J Thomc Cardiovasc Surg. 2004;128(4):543–551. doi: 10.1016/j.jtcvs.2004.04.015 [DOI] [PubMed] [Google Scholar]
- 34.D’Ancona G, Biondo D, Mamone G, et al. Ischemic mitral valve regurgitation in patients with depressed ventricular function: cardiac geometrical and myocardial perfusion evaluation with magnetic resonance imaging. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2008;34(5):964–968. doi: 10.1016/j.ejcts.2008.07.056 [DOI] [PubMed] [Google Scholar]
- 35.Veronesi F, Corsi C, Sugeng L, et al. Quantification of mitral apparatus dynamics in functional and ischemic mitral regurgitation using real-time 3-dimensional echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2008;21(4):347–354. doi: 10.1016/j.echo.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 36.Lai DT, Timek TA, Tibayan FA, et al. The effects of mitral annuloplasty rings on mitral valve complex 3-D geometry during acute left ventricular ischemia. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2002;22(5):808–816. [DOI] [PubMed] [Google Scholar]
- 37.Hung J, Chaput M, Guerrero JL, et al. Persistent reduction of ischemic mitral regurgitation by papillary muscle repositioning: structural stabilization of the papillary muscle-ventricular wall complex. Circulation. 2007;116(11 Suppl):I259–263. doi: 10.1161/CIRCULATIONAHA.106.679951 [DOI] [PubMed] [Google Scholar]
- 38.Mishra YK, Mittal S, Jaguri P, Trehan N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation:1-year results. Am Thorac Surg. 2006;81(1):42–46. doi: 10.1016/j.athoracsur.2005.06.023 [DOI] [PubMed] [Google Scholar]
- 39.Langer F, Rodriguez F, Ortiz S, et al. Subvalvular repair: the key to repairing ischemic mitral regurgitation? Circulation. 2005;112(9 Suppl):I383–389. doi: 10.1161/CIRCULATIONAHA.104.523464 [DOI] [PubMed] [Google Scholar]
- 40.Jensen H, Jensen MO, Smerup MH, et al. Impact of papillary muscle relocation as adjunct procedure to mitral ring annuloplasty in functional ischemic mitral regurgitation. Circulation. 2009;120(11 Suppl):S92–98. doi: 10.1161/CIRCULATIONAHA.108.817833 [DOI] [PubMed] [Google Scholar]
- 41.Tibayan FA, Rodriguez F, Langer F, et al. Annular or subvalvular approach to chronic ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2005;129(6):1266–1275. doi: 10.1016/j.jtcvs.2005.01.021 [DOI] [PubMed] [Google Scholar]
- 42.Walmsley R Anatomy of human mitral valve in adult cadaver and comparative anatomy of the valve. Br Heart J. 1978;40(4):351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krawczyk-Ozog A, Hoida MK, Bolechaia F, et al. Anatomy of the mitral subvalvular apparatus. J Thorac Cardiovasc Surg. 2018;155(5):2002–2010. doi: 10.1016/j.jtcvs.2017.12.061 [DOI] [PubMed] [Google Scholar]
- 44.Messas E, Guerrero JL, Handschumacher MD, et al. Paradoxic decrease in ischemic mitral regurgitation with papillary muscle dysfunction: insights from three-dimensional and contrast echocardiography with strain rate measurement. Circulation. 2001;104(16):1952–1957. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez-Vaquero D, Díaz R, Álvarez-Cabo R, Vigil-Escalera C, Silva J. Mitral valve repair for moderate ischemic mitral regurgitation. J Thorac Dis. 2016;8(7):1410–1413. doi: 10.21037/jtd.2016.05.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video: Presentation illustrating the key findings of this study.