Abstract
Arabinoxylans (AX) gels at 4% (w/v) were prepared using laccase (LAX gels) or peroxidase (PAX gels), and their cross-linking, rheological, structural, and spectroscopic characteristics were investigated. LAX gels presented lower amount of 5,5′-diferulic acid (11%), smaller mesh size (128 nm), and higher hardness (37 N) and elasticity (430 Pa) than the PAX gels (28%, 197 nm, 7 N, and 120 Pa, respectively). Microscopy of the LAX gels showed linked strands, while the system was less connected in the PAX gels. The Raman band at 2895 cm−1 of the LAX and PAX gels was less intense, indicating enhanced hydrogen bonding compared to that of AX. This decrease was less dramatic for the PAX gels. The greater content of 5,5′-diferulic acid in PAX gels could favor intrachain bonds, affecting their rheological, structural, and spectroscopic characteristics. Laccase may be a better option than peroxidase for AX gelation intended for food and biotechnological applications.
Keywords: Polysaccharides, Enzymes, Gelation, Ferulic acid, Swelling
Introduction
Polysaccharide-based gels are of considerable interest in the fields of food science, biotechnology, biomedical engineering, and pharmaceuticals (Chaturvedi et al., 2013). For these applications, different mild cross-linking methods have been studied to form covalent gels that can be used to encapsulate different compounds. Some enzymes can catalyze polysaccharide cross-linking under physiological conditions (Teixeira et al., 2012). Arabinoxylans (AX) are nonstarch polysaccharides found mainly in the cell walls of cereals. AX consists of a linear backbone of β-(1 → 4)-linked d-xylose residues to which α-l-arabinose substituents are attached through O-2 and/or O-3 (Izydorczyk and Biliaderis, 1995). Some of the arabinose moieties are ester-linked at (O)-5 to ferulic acid (FA) (Smith and Hartley, 1983). AX present interesting properties, such as emulsifying, stabilizing, antioxidant, prebiotic, and gel formation properties (Niño-Medina et al., 2010). AX can form an aqueous three-dimensional network through covalent cross-linking of FA upon oxidation by enzymatic free radical-generating agents. This oxidation favors the formation of diferulic (di-FA) and triferulic acid (tri-FA) bridges between adjacent AX chains (Carvajal-Millan et al., 2005a; Izydorczyk and Biliaderis, 1995). The AX gelation process is controlled by the establishment of both covalent bonds (di-FA and tri-AF) and physical interactions (hydrogen bonding and van der Waals interactions) between the polysaccharide chains (Carvajal-Millan et al., 2005a; Vansteenkiste et al., 2004). Five isomeric forms of di-FA (5,5′-, 8,5′-benzo, 8-O-4′, 8,5′- and 8,8′-) and a trimer (4-O-8′,5,5′) have been reported in AX gels (Carvajal-Millan et al., 2005b). AX gels have a neutral taste and odor, which are desirable properties for food applications (Izydorczyk and Biliaderis, 1995). In addition, AX cross-linking promotes the selective fermentation of this polysaccharide in the colonic region, limiting the growth of bacteria that are not considered beneficial (Bacteroides) and favoring the growth of probiotics such as Bifidobacteria (Hopkins et al., 2003). In this regard, the use of an enzymatic cross-linking agent capable of forming AX gels with effective interchain di-FA structures would improve the functionality of these gels and their potential use in the food industry. The ability of laccase or peroxidase to catalyze AX cross-linking has been described in previous studies (Martínez-López et al., 2011; Skendi et al., 2011), and it has been reported that AX gel characteristics are influenced by the polysaccharide structure and by factors such as the polymer concentration, AX purity, and free radical generating system. However, possible differences in the di-FA isomers as well as in the rheological, structural, and spectroscopic characteristics of AX gels formed by laccase or peroxidase catalysis have not been previously investigated. Laccase and peroxidase catalyze the AX cross-linking reaction by different oxidative mechanisms. Laccase oxidizes the substrate via direct interaction of the oxygen with the copper cluster in the enzyme, while peroxidase requires hydrogen peroxide as an oxidizing agent (Carunchio et al., 2001). These differences in the oxidation mechanisms of laccase and peroxidase could influence the AX gelation process and, consequently, the characteristics of the gels formed. The aim of this study was to cross-link AX using laccase (LAX gels) and peroxidase (PAX gels) to investigate the di-FA isomeric forms, rheology, microstructure, and spectroscopic characteristics of the gels formed.
Materials and methods
Materials
Ferulated arabinoxylans (AX) from maize bran were obtained and characterized as previously reported by Martínez-López et al. (2011). AX presented an arabinose to xylose ratio of 0.72 and 85% dry basis (d.b.) of pure AX (sum of arabinose and xylose). Horseradish peroxidase (P, donor: hydrogen peroxide oxidoreductase, EC 1.11.1.7, type I, 250 U/mg), laccase (benzenediol: oxygen oxidoreductase, E.C. 1.10.3.2) from Trametes versicolor (98 U/mg), and all other chemical products were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Gelation
AX solutions at 4% (w/v) were prepared in 0.05 M citrate phosphate buffer at pH 5. The buffer was filtered through a 0.2 μm membrane filter to prevent microbial contamination. Laccase and peroxidase were used as the cross-linking agents following the conditions described by Martínez-López et al. (2011; 2013), respectively. The H2O2 stock solution was prepared fresh every day. Gels were allowed to develop for 5 h at 25 °C.
Rheology
AX gelation using laccase (LAX gels) or peroxidase (PAX gels) was monitored by small-amplitude oscillatory shear by using a strain-controlled rheometer (Discovery HR-2 rheometer; TA Instruments, New Castle, DE, USA) as reported by Vansteenkiste et al. (2004). AX gelation was monitored for 5 h at 25 °C. All measurements were carried out at a frequency of 0.25 Hz and 5% strain (linearity range of the viscoelastic behavior). The mechanical spectra of gels were obtained by frequency sweep from 0.01 to 100 Hz at 25 °C. The hardness of the 4% (w/v) LAX and PAX gels, freshly made (5 h) in 6-mL glass flasks 30 mm tall and with an internal diameter of 25 mm, was analyzed with a TA.XT2i Texture Analyzer (RHEO Stable Micro Systems, Godalming, England) equipped with XTRAD software version 3.7. The gels were deformed by compression at a constant speed of 1.0 mm/s to a distance of 4 mm from the gel surface using a cylindrical plunger (diameter 25.4 mm). The peak height at 4 mm of compression was considered as the gel hardness (Carvajal-Millan et al., 2005b; Takahashi and Seib, 1988).
Covalent cross-linking
The quantification of FA, di-FA, and tri-FA in the LAX and PAX gels was performed by RP-HPLC after a de-esterification step as described by Vansteenkiste et al. (2004). An Alltima (Alltech, Deerfield, IL, USA) C18 column (250 × 4.6 mm) and a photodiode array detector Waters 996 (Millipore Co., Milford, MA, USA) were used. Detection was performed by measuring UV absorbance at 320 nm.
Structure
The LAX and PAX gels were allowed to swell as described by Carvajal-Millan et al. (2005a). The equilibrium swelling was reached when the weight of the samples changed by no more than 3% (0.06 g). The swelling ratio (q) was calculated as follows:
where Ws is the weight of the swollen gel and WAX is the weight of AX in the gel. From the swelling measurements, the structural parameters of the gels were calculated as reported by Carvajal-Millan et al. (2005a) using the model of Flory and Rehner (1943) modified by Peppas and Merrill (1976) for gels in which the cross-links are introduced in solution.
Atomic force microscopy (AFM)
The LAX and PAX gels were deposited onto freshly cleaved mica and allowed to dry in air according to Morales-Burgos et al. (2017). AFM imaging was performed at room temperature using a Nanoscope IIIa A and NanoScope Image software (Digital Instruments, Santa Barbara, CA, USA) in tapping mode. Antimony (n)-doped Si cantilevers with a spring constant of 42 N/m and a tip radius of 8 nm were used (Bruker Corporation, Camarillo, CA, USA).
Raman spectroscopy
Raman spectra were recorded between 500 and 3500 cm−1 Raman shift using a Micro-Raman IHR320 (HORIBA Jobin, Yvon S.A.S., Longjumeau, France) with an excitation wavelength of 532 nm and an objective 50 ×.
Statistical analysis
The results are expressed as the mean ± standard deviation (SD) from three replicates. The data were subjected to analysis of variance (ANOVA) (OriginPro software, version 8.6. Originlab Corporation, Northampton, MA, USA). These data were compared by Tukey’s test. A 95% confidence level was used.
Results and discussion
Rheology and covalent cross-linking
Figure 1 shows the evolution of the storage (G′) and loss (G″) moduli of 4% (w/v) AX solution as a function of oxidative gelation catalyzed by laccase or peroxidase at the same enzyme level (1.675 nkat/mg AX). The gelation profiles versus time of the gels exhibited similar trends with an initial increase in the G′ modulus followed by a plateau region. The increase in G′ indicated that both enzymes catalyzed the formation of a gel. However, the rheological gelation kinetics of the AX treated with laccase (LAX gels) or peroxidase (PAX gels) exhibited some differences. The crossover points of G′ and G″ (G′ > G″) were at 3 and 8 min for LAX and PAX treatments, respectively, indicating LAX had a faster initial rate. Figueroa-Espinoza and Rouau (1998) reported a more rapid increase in the viscosity of the AX solution treated with laccase relative to that of the peroxidase treatment. The G′ values of the AX gel in the plateau region (300 min) were 430 and 120 Pa for LAX and PAX, respectively, which could be related to the more rapid action of laccase by the direct interaction with oxygen. The values of tan δ (G″/G′) were 0.01 and 0.03 for the LAX and PAX gels, respectively, confirming the formation of an elastic covalent network. The slight increase in the tan δ values in the PAX gel indicated a higher viscous contribution to the network structure (Doubler and Cuvelier, 1996). This behavior has also been observed in ferulated sugar beet pectin gels, and in these cases, a stronger gel was developed via laccase catalysis than via peroxidase catalysis (Norsker et al., 2000). The mechanical spectra of the cured gels (5 h) are shown in Fig. 2. The LAX and PAX gels exhibited solid-like behavior with G′ > G″, and the G′ values were independent of the frequency from 0.01 to 10 Hz, whereas G″ was highly dependent on frequency. The dependence of G″ on frequency has been related to the presence of physical entanglements in the gel (Martínez-López et al., 2016). Similar mechanical spectra have been previously reported for covalent cross-linked pectin gels catalyzed by laccase and peroxidase (Zaidel and Meyer, 2012). Hardness is a useful parameter for characterizing the stiffness of the polymer network. In the present study, this textural property was significantly affected by the type of enzyme used. LAX gels presented an average hardness of 37 N, fivefold higher than the value measured for PAX gels (7 N). Both gels presented higher hardness values than those previously reported for laccase-induced AX gels (0.28 N) (Carvajal-Millan et al., 2005b). This difference could be related to the AX concentration in the gel; in the present study, the gels were formed at 4% (w/v), while those studied by Carvajal-Millan et al. (2005b) were prepared at 1% (w/v). The differences in the mechanical spectra and hardness values of the LAX and PAX gels could be related to the covalent cross-linking content and/or the proportions of the di-FA isomers. The FA and di-FA contents in the LAX and PAX gels were measured during the cross-linking process (Fig. 3). Only traces of tri-FA were detected in the AX gels. In both the LAX and PAX gels, a substantial portion of the FA was oxidized (47% of initial FA content) during the first 10 min of enzyme exposure; however, a slower decrease in the FA content occurred with laccase than with peroxidase. Laccase-mediated gelation produced an increase in di-FA during the first 2.5 min followed by a decrease in these compounds from 2.5 to 10 min of the reaction; peroxidase catalysis caused a decrease in the di-FA content during the first 5 min of reaction. These differences in AX cross-linking could be explained by the enzyme reaction mechanisms. Laccase catalyzes the oxidation of FA esterified on the AX by the interaction between O2 and the copper cluster in the enzyme, resulting in a delayed reaction. In peroxidase catalysis, H2O2 can rapidly oxidize the FA and di-FA present in the AX, allowing a faster reaction. In addition, during peroxidase catalysis, excess H2O2 might affect the reaction by further oxidizing the di-FA to generate other FA structures, explaining the decrease in di-FA after the first few minutes of the reaction (Zaidel and Meyer, 2012). The di-FA levels remained almost constant for laccase and peroxidase treatment after 10 and 7.5 min of reaction, respectively. At the end of the laccase and peroxidase cross-linking reactions, the initial FA content in the AX decreased by 95%, while the amount of di-FA produced never accounted for all of the FA monomers that had been consumed. This behavior in AX gels, suggesting the formation of other ferulated cross-linking structures and/or the participation of lignin residues coupled with FA during the gelation process, has been previously reported by several authors (Carvajal-Millan et al., 2007; Lapierre et al., 2001). Regarding di-FA isomers, both gels contained mainly the 8,5′ form (80 and 67% for LAX and PAX, respectively), followed by 5,5′- (11 and 28% for LAX and PAX, respectively) and 8-O-4′ forms (9 and 5% for LAX and PAX, respectively). The predominance of 8,5′-di-FA has been reported for AX gels previously (Carvajal-Millan et al., 2006; Martínez-López et al., 2011). The higher percentage of 5,5′-di-FA found in PAX gel (28%) than in LAX gel (11%) might favor the intrapolysaccharide chain bonds (Hatfield and Ralph, 1999). This result suggests that the slower initial formation of LAX gels could favor the development of a more effective polymeric network structure containing more interchain bonds than those formed in PAX gels.
Fig. 1.
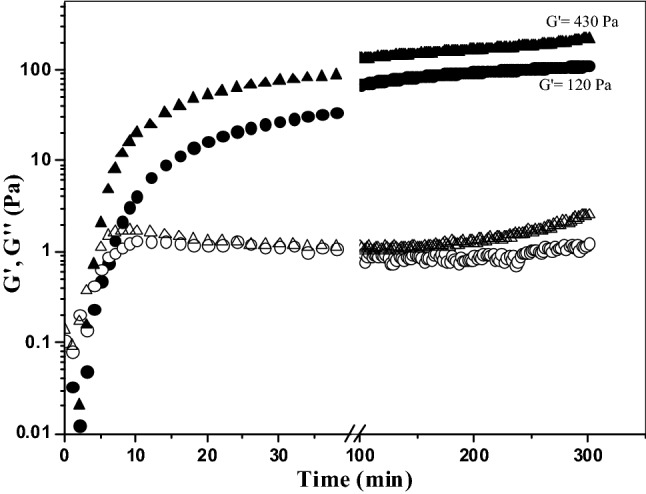
Monitoring the storage (G′) and loss (G″) moduli of an AX solution at 4% (w/v) during gelation by laccase (G′ filled triangle, G″ open triangle) or peroxidase (G′ filled circle, G″ open circle) at 0.25 Hz, 5% strain and 25 °C
Fig. 2.
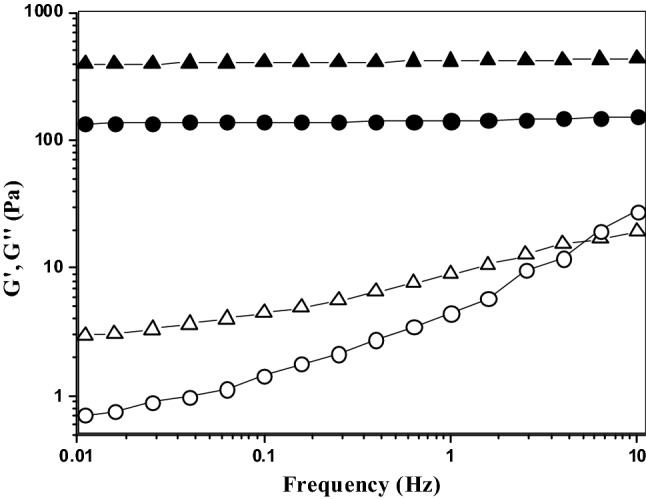
Mechanical spectrum of AX gels formed by laccase (LAX gels, G′ filled triangle, G″ open triangle) or peroxidase (PAX gels, G′ filled circle, G″ open circle) as catalysts. Rheological measurements were carried out at 25 °C and 5% strain
Fig. 3.
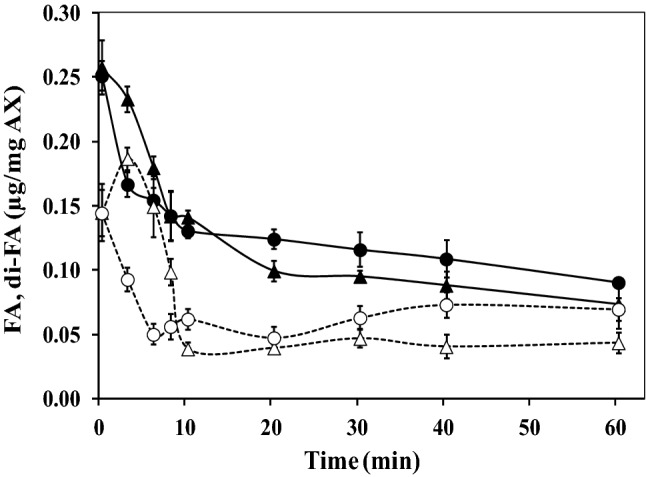
Decrease in ferulic acid (FA) content and the formation of diferulic acid (di-FA) during oxidative cross-linking of AX at 4% (w/v) catalyzed by laccase (FA filled triangle, di-FA open triangle) or peroxidase (FA filled circle, di-FA open circle)
Structure
The swelling ratio (q) is an important parameter for determining the physical properties of gels. The swelling behavior of the LAX and PAX gels at 4% (w/v) submerged in water at 25 °C was monitored for 18 h, and the gels reached equilibrium at 8 and 15 h, respectively. The swelling ratios (g water/g of AX) of the LAX gels was smaller than in PAX gel (Table 1), which could be attributed to the higher percentage of 5,5′-di-FA in the PAX gels (less effective elastic intrachain bonds). Intrachain bonds gave rise to longer uncross-linked polymer chain sections in the gel, which can expand quickly, resulting in a high water absorption capacity (Meyvis et al., 2000). The q values found for both gels were similar to those previously reported for AX gels catalyzed by peroxidase (4% w/v, q value of 42) or laccase (3.5% w/v, q value of 20) (Berlanga-Reyes et al., 2014; Martínez-López et al., 2011). The structural parameters were calculated from the equilibrium swelling tests. The mesh size (ξ), the cross-linking density (ρc) and the molar mass between two cross-links (Mc) values for LAX and PAX gels are presented in Table 1. The gels showed no significant (p > 0.05) differences in their structural parameters, except for ξ (128 and 197 nm, for the LAX gels and PAX gels, respectively). The differences in ξ could be associated with the higher percentage of 8,5′-di-FA and lower proportion of 5,5′-di-FA present in the LAX gels compared with the PAX gels. This macroporous structure has been observed in several previous studies of AX gels (Carvajal-Millan et al., 2005a; Niño-Medina et al., 2010).
Table 1.
Swelling and structural characteristics of AX gels catalyzed by laccase (LAX gel) or peroxidase (PAX gel) at 4% (w/v)
| q (g water/g AX) | Mc × 103 (g/mol) | ρc × 10−5 (mol/cm3) | ξ (nm) | |
|---|---|---|---|---|
| LAX gel | 18 ± 1 | 48 ± 0.4 | 29 ± 0.1 | 128 ± 3 |
| PAX gel | 43 ± 2 | 49 ± 0.2 | 30 ± 0.1 | 197 ± 1 |
q, equilibrium swelling ratio; Mc, molar mass between two cross-links; ρc, cross-linking density obtained from Mc values; ξ, mesh size
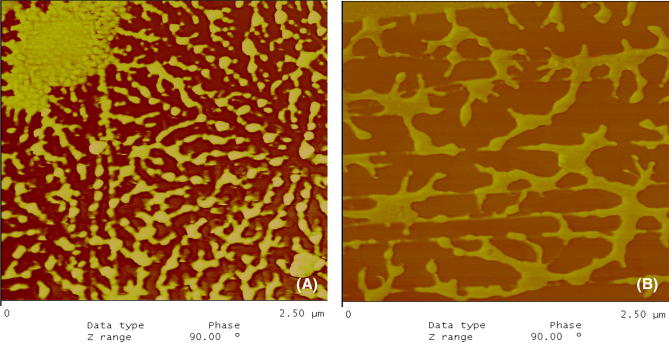
Microstructure
A representative atomic force microscopy (AFM) image of a LAX gel and a PAX gel is presented in Fig. 4(A), (B). To the best of our knowledge, no AFM analysis of PAX gels has been reported to date. The LAX gels [Fig. 4(A)] exhibit patterns of connected strands resembling a sponge-like microstructure, similar to that recently reported by Marquez-Escalante et al. (2018) and Morales-Burgos et al. (2017). The PAX gel [Fig. 4(B)] shows a less connected system, suggesting that peroxidase may limit the establishment of the AX network (probably due to the high proportion of 5,5′-di-FA formed). It has been previously suggested that the flexibility and the threefold helical conformation of the AX chains could favor different spatial arrangements of FA during AX cross-linking, allowing a superior molecular arrangement (Almond and Sheehan, 2003; Nieduszynski and Marchessault, 1972; Yui et al., 1995). In the AFM images of the PAX gels, non-connected strands were visible. Similar AFM images of un-crosslinked AX have been previously described (Adams et al., 2003; Adams et al., 2004; Gunning et al., 2000). These results suggest that the majority of the AX chains absorbed on the mica were more aggregated than crosslinked with each other. For the LAX gels, the AFM images showed a rough surface, which may be caused by the superposition of chains on top of each other, where the sizes of cavities were heterogeneous, resulting in a sponge-like microstructure similar to that reported for other laccase-mediated AX gels (Marquez-Escalante et al., 2018; Morales-Burgos et al., 2017). The particular AFM characteristics of the LAX and PAX gels could be explained by the different proportions of the di-FA isomers (especially the higher percentage of 5,5′-di-FA in the PAX gels) and/or weak linkages between the polysaccharide chains.
Fig. 4.
Representative atomic force microscopy image of a LAX gel (A) and a PAX gel (B) (2.5 µm × 2.5 µm)
Raman spectroscopy
Vibrational spectral signatures of the LAX gels and PAX gels were collected by Raman spectroscopy to investigate the specific patterns in these samples. To date, neither the Raman spectrum of AX gel nor the effect of the type of cross-linking enzyme on the Raman spectrum of the formed gel has been reported in the literature. Figure 5(A) corresponds to the Raman spectrum of un-crosslinked AX, which contained characteristic bands in the polysaccharide fingerprint region (899, 1094–1126, and 1226–1462 cm−1) that have been reported to be related to this polysaccharide (Piot et al., 2001). The bands in the 1400–1300 cm−1 spectral region have been assigned to CH and COH bending, while the COC, CO, and CC stretching vibrations can be observed in the 1000–1200 cm−1 range. The Raman band at 899 cm−1 revealed the presence of β(1→4) glycosidic linkages (Kacurakova et al., 1999). Raman vibrations at 1600–1632 cm−1 associated with the stretching vibrations of the phenolic ring confirmed the presence of phenolic structures, such as ferulic acid, which is the most abundant hydroxycinnamic acid in cereal cell wall components (Parker et al., 2005). In Fig. 5(A), the major Raman band was observed at 2895 cm−1, and this signal has been previously attributed to the strong C–H stretching in hemicellulose (Adapa et al., 2009). In general, the AX Raman spectrum in Fig. 5(A) is similar to that reported by Barron and Rouau (2008) for wheat pericarp tissue components that include AX.
Fig. 5.
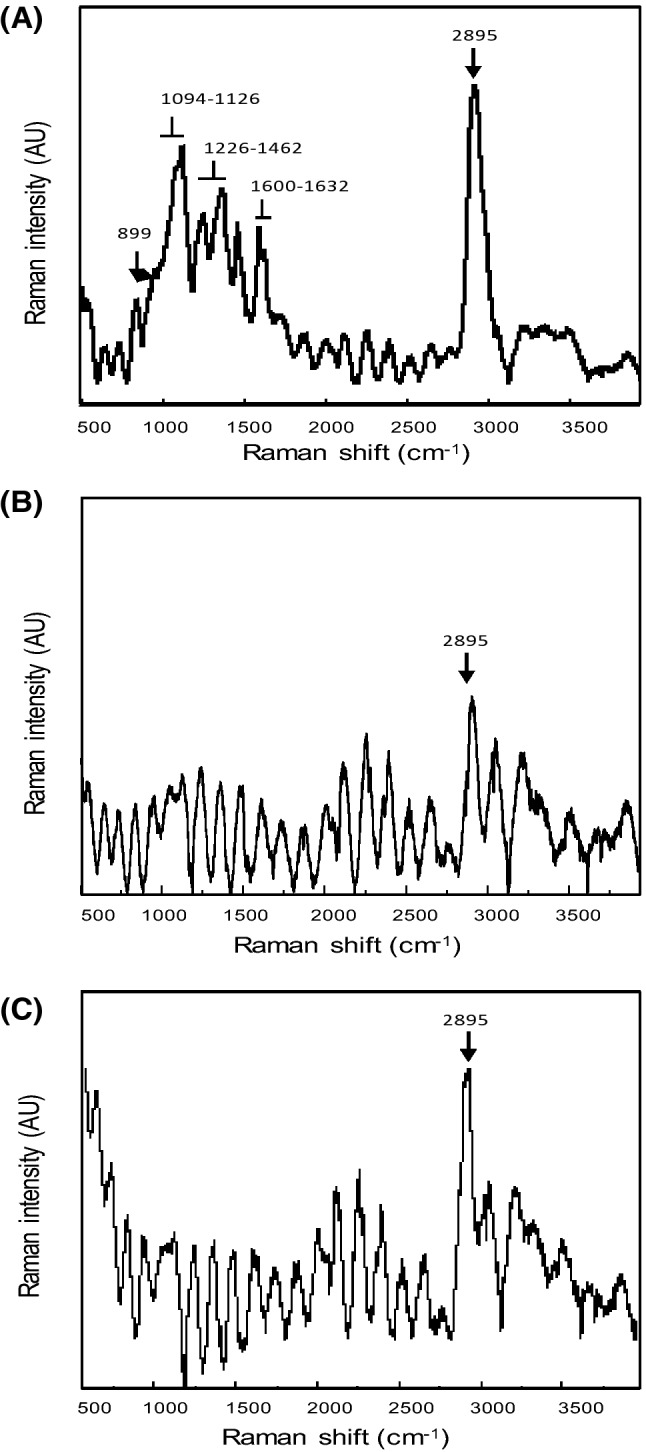
Raman spectra in the 500–4000 cm−1 region of un-crosslinked AX (A), LAX gel (B), and PAX gel (C) studied in the present work
Figure 5(B), (C) present the Raman spectra of the LAX gels and PAX gels, respectively, where the Raman band at 2895 cm−1 related to hemicellulose C–H vibrations is more intense than the bands registered prior to gelation [Fig. 5(A)]. This change in the Raman spectrum after AX gelation could be related to the appearance of several shoulders (1065 cm−1 attributed to the stretching of the C–O and C–C bonds of the carbohydrate ring, 1349 cm−1 related to the angular deformation of the C–OH bond, and 1396 cm−1 attributed to the angular bending of the CH2 radical), as reported by Kizil and Irudayaraj (2007). The Raman band at 2895 cm−1 was less intense in the LAX gels and PAX gels than in AX. The weakening of the 2895 cm−1 Raman band (C–H stretching) in spectrum of the LAX gels and PAX gels suggest enhanced hydrogen bonding in comparison to un-crosslinked AX. However, this decrease in the 2895 cm−1 band was less noticeable for PAX gels, suggesting higher molecular motion, probably due to the development of a less effective polymeric network structure (more 5,5′-di-FA may form intra-AX chain bonds).
The results discussed above indicate that the enzyme used to cross-link the AX has an important effect on the proportion of di-FA isomers and the rheological, structural, microstructural, and spectroscopic characteristics of the formed gel. The LAX gels were consistently stronger than the PAX gels, as shown by the higher G′ and the greater hardness values. In addition, the LAX gels showed a more compact structure with a smaller mesh size than the PAX gels. These differences could be due to the higher 5,5′-di-FA content in PAX gels. Peroxidase catalysis may favor intrachain interactions, reducing the connectivity of the AX network, as suggested by the AFM and RAMAN spectroscopy analyses. The results of this study show that the use of laccase as an agent for AX cross-linking may be a better choice than peroxidase for potential food and biotechnological applications of AX gels.
Acknowledgements
This research was supported by “Fondo Institucional CONACyT - Problemas Nacionales 2015”, Mexico (Grant 2015-01-568 to E. Carvajal-Millan).
References
- Adams EL, Kroon PA, Williamson G, Gilbert HJ, Morris VJ. Inactivated enzymes as probes of the structure of arabinoxylans as observed by atomic force microscopy. Carbohydr. Res. 2004;339:579–590. doi: 10.1016/j.carres.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Adams EL, Kroon PA, Williamson G, Morris VJ. Characterization of heterogeneous arabinoxylans by direct imaging of individual molecules by atomic force microscopy. Carbohydr. Res. 2003;338:771–780. doi: 10.1016/S0008-6215(03)00017-X. [DOI] [PubMed] [Google Scholar]
- Adapa P, Karunakaran C, Tabil L, Schoenau G. Potential applications of infrared and Raman spectromicroscopy for agricultural biomass. Agricultural Engineering International: the CIGR. Ejournal. XI (2009)
- Almond A, Sheehan JK. Predicting the molecular shape of polysaccharides from dynamic interactions with water. Glycobiology. 2003;13:255–264. doi: 10.1093/glycob/cwg031. [DOI] [PubMed] [Google Scholar]
- Barron C, Rouau X. FTIR and Raman signatures of wheat grain peripheral tissues. Cereal Chem. 2008;85:619–625. doi: 10.1094/CCHEM-85-5-0619. [DOI] [Google Scholar]
- Berlanga-Reyes CM, Carvajal-Millan E, Hicks KB, Yadav M, Rascón-Chu A, Lizardi-Mendoza J, Toledo-Guillén AR, Islas-Rubio AR. Protein/arabinoxylans gels: effect of mass ratio on the rheological, microstructural and diffusional characteristics. Int. J. Molecular Sci. 2014;15:19106–19118. doi: 10.3390/ijms151019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carunchio F, Crescenzi C, Girelli AM, Messina A, Tarola AM. Oxidation of ferulic acid by laccase-induced: identification of the products and inhibitory effects of some dipeptides. Talanta. 2001;55:189–200. doi: 10.1016/S0039-9140(01)00417-9. [DOI] [PubMed] [Google Scholar]
- Carvajal-Millan E, Landillon V, Morel MH, Rouau X, Doublier JL, Micard V. Arabinoxylan gels: impact of the feruloylation degree on their structure and properties. Biomacromolecules. 2005;6:309–317. doi: 10.1021/bm049629a. [DOI] [PubMed] [Google Scholar]
- Carvajal-Millan E, Guigliarelli B, Bell V, Rouau X, Micard V. Storage stability of arabinoxylan gels. Carbohydr. Polym. 2005;59:181–188. doi: 10.1016/j.carbpol.2004.09.008. [DOI] [Google Scholar]
- Carvajal-Millan E, Guilbert S, Doublier JL, Micard V. Arabinoxylan/protein gels: Structural, rheological and controlled release properties. Food Hydrocolloid. 2006;20:53–61. doi: 10.1016/j.foodhyd.2005.02.011. [DOI] [Google Scholar]
- Carvajal-Millan E, Rascón-Chu A, Márquez-Escalante J, Ponce-de-León N, Micard V. Gardea A (2007) Maize bran gum: extraction, characterization and functional properties. Carbohydr. Polym. 2007;69:280–285. doi: 10.1016/j.carbpol.2006.10.006. [DOI] [Google Scholar]
- Chaturvedi K, Ganguly K, Nadagouda MN, Aminabhavi TM. Polymeric hydrogels for oral insulin delivery. J. Control. Release. 2013;165:129–138. doi: 10.1016/j.jconrel.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Doubler JL, Cuvelier G. Gums and hydrocolloids: functional aspects. In: Eliasson AC, editor. Carbohydrates in food. New York: Marcel Dekker; 1996. pp. 283–318. [Google Scholar]
- Figueroa-Espinoza MC, Rouau X. Oxidative cross-linking of pentosans by a fungal laccase-induced and horseradish peroxidase: mechanism of linkage between feruloylated arabinoxylans. Cereal Chem. J. 1998;75:259–265. doi: 10.1094/CCHEM.1998.75.2.259. [DOI] [Google Scholar]
- Flory PJ, Rehner J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Physics. 1943;11:512. doi: 10.1063/1.1723791. [DOI] [Google Scholar]
- Gunning AP, Mackie AR, Kirby AR, Kroon P, Williamson G, Morris VJ. Motion of a cell wall polysaccharide observed by atomic force microscopy. Macromolecules. 2000;33:5680–5685. doi: 10.1021/ma000331w. [DOI] [Google Scholar]
- Hatfield RD, Ralph J. Modelling the feasibility of intramolecular dehydrodiferulate formation in grass walls. J. Sci. Food Agric. 1999;79:425–427. doi: 10.1002/(SICI)1097-0010(19990301)79:3<425::AID-JSFA282>3.0.CO;2-U. [DOI] [Google Scholar]
- Hopkins MJ, Englyst HN, Macfarlane S, Furrie E, Macfarlane GT, McBain AJ. Degradation of cross-linked and non-cross-linked arabinoxylans by the intestinal microbiota in children. App. Environ. Microbiol. 2003;69:6354–6360. doi: 10.1128/AEM.69.11.6354-6360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izydorczyk MS, Biliaderis CG. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr. Polym. 1995;28:33–48. doi: 10.1016/0144-8617(95)00077-1. [DOI] [Google Scholar]
- Kacurakova M, Wellner N, Ebringerova A, Hromadkova Z, Wilson RH, Belton PS. Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocolloid. 1999;13:35–41. doi: 10.1016/S0268-005X(98)00067-8. [DOI] [Google Scholar]
- Kizil R, Irudayaraj J. Rapid evaluation and discrimination of & #x03B3;-irradiated carbohydrates using FT-Raman spectroscopy and canonical discriminant analysis. J. Sci. Food Agric. 2007;87:1244–1251. doi: 10.1002/jsfa.2830. [DOI] [Google Scholar]
- Lapierre C, Pollet B, Ralet MC, Saulnier L. The phenolic fraction of maize bran: evidence for lignin-heteroxylan association. Phytochemistry. 2001;57:765–772. doi: 10.1016/S0031-9422(01)00104-2. [DOI] [PubMed] [Google Scholar]
- Marquez-Escalante J, Carvajal-Millan E, Yadav MP, Kale M, Rascón-Chu A, Gardea-Bejar A, Valenzuela E, López-Franco Y, Lizardi-Mendoza J, Faulds C. Rheology and microstructure of gels based on wheat arabinoxylans enzymatically modified in arabinose to xylose ratio. J. Sci. Food Agric. 2018;98:914–922. doi: 10.1002/jsfa.8537. [DOI] [PubMed] [Google Scholar]
- Martínez-López AL, Carvajal-Millan E, Lizardi-Mendoza J, López-Franco YL, Rascón-Chu A, Salas-Muñoz E, Barron C, Micard V. The peroxidase/H2O2 system as a free radical-generating agent for gelling maize bran arabinoxylans: rheological and structural properties. Molecules. 2011;16:8410–8418. doi: 10.3390/molecules16108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-López AL, Carvajal-Millan E, Rascón-Chu A, Márquez-Escalante J, Martínez-Robinson K. Gels of ferulated arabinoxylans extracted from nixtamalized and non-nixtamalized maize bran: rheological and structural characteristics. CyTA J. Food. 2013;11(S1):22–28. doi: 10.1080/19476337.2013.781679. [DOI] [PubMed] [Google Scholar]
- Martínez-López AL, Carvajal-Millan E, Micard V, Rascón-Chu A, Brown-Bojorquez F, Sotelo-Cruz N, López-Franco YL, Lizardi-Mendoza J. In vitro degradation of covalently cross-linked arabinoxylan hydrogels by bifidobacteria. Carbohydr. Polym. 2016;144:76–82. doi: 10.1016/j.carbpol.2016.02.031. [DOI] [PubMed] [Google Scholar]
- Meyvis TKL, De Smedt SC, Demeester J, Hennink WE. Influence of the Degradation Mechanism of Hydrogels on Their Elastic and Swelling Properties during Degradation. Macromolecules. 2000;33:4717–4725. doi: 10.1021/ma992131u. [DOI] [Google Scholar]
- Morales-Burgos AM, Carvajal-Millan E, López-Franco YL, Rascón-Chu A, Lizardi-Mendoza J, Sotelo-Cruz N, Brown-Bojórquez F, Burgara-Estrella A, Pedroza-Montero M. Syneresis in gels of highly ferulated arabinoxylans: characterization of covalent cross-linking, rheology, and microstructure. Polymers. 2017;9:164. doi: 10.3390/polym9050164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieduszynski IA, Marchessault RH. Structure of β, D(1–> 4′)-xylan hydrate. Biopolymers. 1972;11:1335–1344. doi: 10.1002/bip.1972.360110703. [DOI] [Google Scholar]
- Niño-Medina G, Carvajal-Millan E, Rascon-Chu A, Márquez-Escalante JA, Guerrero V, Salas-Muñoz E. Feruloylated arabinoxylans and arabinoxylan gels: structure, sources and applications. Phytochem. Reviews. 2010;9:111–120. doi: 10.1007/s11101-009-9147-3. [DOI] [Google Scholar]
- Norsker M, Jensen M, Adler-Nissen J. Enzymatic gelation of sugar beet pectin in food products. Food Hydrocolloid. 2000;14:237–243. doi: 10.1016/S0268-005X(00)00016-3. [DOI] [Google Scholar]
- Parker ML, Ng A, Waldron KW. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L cv Avalon) grains. J. Sci. Food Agric. 2005;85:2539–2547. doi: 10.1002/jsfa.2304. [DOI] [Google Scholar]
- Peppas NA, Merrill EW. Poly(vinyl alcohol) hydrogels: Reinforcement of radiation-crosslinked networks by crystallization. J. Polym. Sci. 1976;14:441–457. [Google Scholar]
- Piot O, Autran JC, Manfait M. Investigation by confocal raman microspectroscopy of the molecular factors responsible for grain cohesion in the Triticum aestivum bread wheat Role of the cell walls in the starchy endosperm. J. Cereal Sci. 2001;34:191–205. doi: 10.1006/jcrs.2001.0391. [DOI] [Google Scholar]
- Skendi A, Biliaderis CG, Izydorczyk MS, Zervou M, Zoumpoulakis P. Structural variation and rheological properties of water-extractable arabinoxylans from six Greek wheat cultivars. Food Chem. 2011;126:526–536. doi: 10.1016/j.foodchem.2010.11.038. [DOI] [Google Scholar]
- Smith MM, Hartley RD. Occurrence and nature of ferulic acid substitution of cell-wall polysaccharides in graminaceous plants. Carbohydr. Res. 1983;118:65–80. doi: 10.1016/0008-6215(83)88036-7. [DOI] [Google Scholar]
- Takahashi S, Seib PA. Paste and gel properties of prime corn and wheat starches with and without native lipids. Cereal Chem. 1988;65:474–483. [Google Scholar]
- Teixeira LSM, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33:1281–1290. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste E, Babot C, Rouau X, Micard V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocolloid. 2004;18:557–564. doi: 10.1016/j.foodhyd.2003.09.004. [DOI] [Google Scholar]
- Yui T, Imada K, Shibuya N, Ogawa K. Conformation of an arabinoxylan isolated from the rice endosperm cell wall by X-ray diffraction a conformational analysis. Biosci. Biotechnol. Biochem. 1995;59:965–968. doi: 10.1271/bbb.59.965. [DOI] [PubMed] [Google Scholar]
- Zaidel DNA, Meyer AS. Biocatalytic cross-linking of pectic polysaccharides for designed food functionality: Structures, mechanisms, and reactions. Biocatal. Agric. Biotechnol. 2012;1:207–219. doi: 10.1016/j.bcab.2012.03.007. [DOI] [Google Scholar]