Abstract
Shrimp is seafood that can commonly trigger allergic reactions. In this study, the ultrafast real-time PCR assay with portable device was developed to detect a shrimp-derived major allergen, tropomyosin, without complicated DNA extraction. For shrimp allergen detection, a specific primer pair was designed based on the shrimp tropomyosin gene and 18S ribosomal RNA gene as internal control. Primer specificity was assessed using 8 common seafood species. Serially diluted shrimp DNA was used to determine the limit of detection of the ultrafast PCR system, which was approximately 3.2 pg. Twenty-three food samples containing shrimp were evaluated to verify the applicability of a direct ultrafast PCR method for detecting shrimp allergens without DNA isolation. It took less than 30 min from sample preparation-to-result analysis to detect shrimp DNA in raw and processed samples. Therefore, this PCR system can be effectively and conveniently utilized in the field to detect shrimp in various food products.
Keywords: Shrimp, Tropomyosin, Allergen, On-site detection, Ultrafast PCR, Food products
Introduction
Fishery products are an important component of the human diet. Currently, fishery products are one of the major causes of food allergies, especially in coastal areas and in fish processing communities (Van Do et al., 2005). Food allergies have been identified as a major health issue with an estimated 4% of the total population affected by food allergies (Sicherer et al., 2004).
Shrimp is one of the most popular seafood in Korea. It has been consumed as raw materials, and also used in various processed products, such as shrimp powder, seasoning paste, retort, and fried snack foods. However, there is concern for an allergy-like reaction when shellfish is eaten. The major heat-stable allergen of shrimp has been identified as tropomyosin (Shanti et al., 1993). Tropomyosin is a major, well-known allergen in crustaceans, such as shrimp and crabs, and in mollusks, such as clams and oysters (Herrero et al., 2012; Lee et al., 2012). The amount of tropomyosin to cause an allergenic reaction depends on the sensitivity of individual (Herrero et al., 2014) and there is no clinical report yet.
For detecting food allergens, protein- and DNA-based detection methods were commonly used (Herrero et al., 2012). Protein-based method is advantageous in that is easy to use, is detectable in a short period of time, and directly reacts allergen-inducing antigens (Park et al., 2013). However, there has drawback of low sensitivity of the processed food processed by heat or pressure due to protein denaturation (Reisch et al., 2015), and low specificity among similar species (Eller and Jensen, 2013). In the case of DNA-based detection, DNA-based method is more heat-stable than protein, so it can be detected highly sensitively in processed foods, and can be high specific detection by comparing sequences even similar species. (Herrero et al., 2014; Pascoal et al., 2011). However, there has time-consuming compared with the protein-based method, and has limitation in that indirectly detect an allergen-inducing antigen (Pafundo et al., 2010). PCR is a powerful technique for detection of pathogens and GMOs and the identification of meat species (Kim et al., 2017; Kim and Kim, 2017; Song et al., 2017). PCR requires a thermal cycler to amplify the target DNA, and the use of a Peltier heater in conventional PCR systems typically takes approximately 1–2 h to perform 30 PCR cycles (Furutani et al., 2016; Son et al., 2015). To reduce the reaction time of thermal cycling in PCR, ultrafast PCR systems using microfluidic chips have been recently developed and applied within various fields (Aboud et al., 2013; Furutani et al., 2017; Houssin et al., 2016; Wheeler et al., 2011). Furthermore, for point-of-care (POC) molecular diagnostics, ultrafast PCR systems have been designed to be portable, simple, and easy to use.
Due to an expansion in food allergen labeling and revisions to allergen labeling methods in Korea, we developed a detection method for shrimp tropomyosin using an ultrafast PCR system that can complete 40 cycles within 20 min. A homogenization process in lysis buffer without the routine procedures for DNA extraction was combined with this system, allowing on-site detection of traces of shrimp allergen. In a previous study, Song et al. (2012) had developed a LabChip real-time PCR system as a POC tool to rapidly detect influenza A, which could achieve 30 PCR cycles in 15 min. Furutani et al. (2016) had reported a reciprocal-flow PCR system that was based on the TaqMan probe technology, which was able to detect E. coli with 50 cycles in 7 min. An ultrafast photonic PCR method developed by Son et al. (2015) combined an AU film as the light-to-heat converter and light-emitting diodes (LEDs) as the heat source, which could complete 30 cycles within 5 min. However, our method reported here has advantages over previously reported methods: our method has minimal time compared to previous report by Song et al. (2012), and does not require individual probe design, and visualization by electrophoresis on the gel after PCR amplification (Furutani et al., 2016; Son et al., 2015). In addition, PCR products amplified by the target-specific primer pair can be monitored and analyzed in real-time and confirmed through post-PCR melt curve analysis.
Therefore, to develop a rapid on-site detection method for shrimp allergens, a direct real-time ultrafast PCR assay was applied to 23 shrimp-containing food products. The assay allowed sample preparation-to-result analysis of various food products in less than 30 min. This PCR system can be effectively and conveniently utilized to detect the shrimp tropomyosin gene in raw and processed food products.
Materials and methods
Samples
Five shrimp species [white leg shrimp (Litopenaeus vannamei), black tiger shrimp (Penaeus monodon), Argentina red shrimp (Pleoticus muelleri), kuruma shrimp (Marsupenaeus japonicus), and Chinese white shrimp (Fenneropenaeus chinensis)] and 7 different species of seafood [crab (Portunus trituberculatus), squid (Todarodes pacificus), clam (Ruditapes philippinarum), mussel (Mytilus edulis), abalone (Haliotis discus hannai), oyster (Crassostrea gigas), and mackerel (Scomber japonicas)] were purchased at local Korean seafood markets. To evaluate the applicability of this method in processed food products, 23 commercial samples containing shrimp (5 seasoning, 4 fried rice, 3 snack, 3 dried shrimp, 2 porridge, 3 dumpling, 1 fried shrimp, 1 pizza, and 1 shrimp sushi) were purchased at markets in Korea. All samples were ground in liquid nitrogen using a mortar and pestle, and immediately stored at − 20 °C until use.
DNA extraction
Each DNA sample was isolated from the raw shrimp and food products using a DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The DNA concentration and purity were measured with a UV spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan). The final concentration of isolated DNA was adjusted to 10 ng/μL for use in ultrafast PCR. DNA with a 260/280 nm wavelength ratio between 1.8 and 2.0 was used as the template for PCR.
Primer design
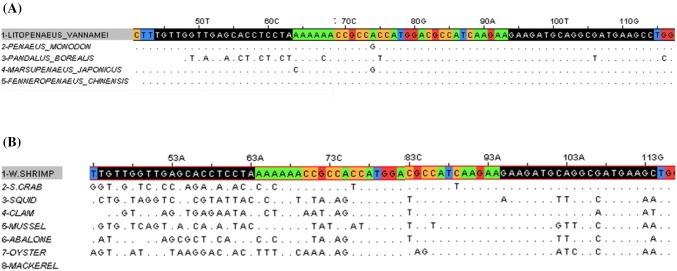
The shrimp tropomyosin gene sequence was obtained from GenBank (Accession No. EU410072) and aligned with the Clustal Omega program (Fig. 1). In addition, the primer pair M18S-F/R was used to amplify the 18S rRNA gene as an internal control. All primers were designed using the Primer Designer program version 3.0 (Scientific and Education Software, Durham, NC, USA) and synthesized by Bionics (Seoul, Korea). Table 1 shows primer pair information.
Fig. 1.
Sequence alignment of shrimp-specific tropomyosin primers between five shrimp species (A) and seven marine species (B)
Table 1.
Primer pairs for ultrafast PCR
| Primer name | Sequences (5′→3′) | Target genes | Amplicon size (bp) | Access no. |
|---|---|---|---|---|
| M18S-F | CAGGTCTGTGATGCCCTTAG | 18S rRNA | 160 | – |
| M18S-R | ACTGGGAATTCCTCGTTC | |||
| Strop-F | TGTTGGTTGAGCACCTCCTA | Shrimp tropomyosin (Lit v 1) |
71 | EU410072 |
| Strop-R | GCTTCATCGCCTGCATCTTC |
Ultrafast PCR reaction
For the rapid and on-site detection of shrimp allergen, an ultrafast real-time PCR system (GENECHECKER®) based on microfluidic technology was developed. The PCR system is coupled with a microfluidic PCR chip (Rapi:chip™) that is made of a thin polymer film with a well volume of only 10 μL, which included 5 μL of SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories, CA, USA), 1 μL of each primer pair (400 nM), and 1 μL of DNA template (10 ng/μL). The following PCR conditions were used: pre-denaturation for 1 min at 95 °C, followed by 40 cycles of denaturation at 94 °C for 5 s, annealing at 58 °C for 5 s, extension at 72 °C for 5 s. PCR was followed by melting curve analysis (60–90 °C) to confirm the melting temperature of each amplified sample using the portable fluorometer. Non-template samples were used as negative controls in all reaction.
Direct ultrafast PCR reaction
20 mg of ground sample was washed three times with 1 mL of distilled water (D.W.) and then added to 1.5 mL of Direct PCR Lysis Buffer (Genesystem, Daejeon, Korea). The sample was mixed vigorously using a vortex mixer for 1 min, incubated for 5 min at room temperature, and then centrifuged. The supernatant was transferred to a new tube and diluted by one-fourth with D.W. Diluted supernatant (2 μL) was used as a template for the ultrafast PCR reaction and was carried out under the same conditions listed previously.
Intra-laboratory validation of direct ultrafast PCR
The developed ultrafast PCR assay was validated using each different ultrafast PCR instrument by two users. To verify the reliability of this assay, each user performed sample preparation of 23 food products and analyzed the presence of shrimp by using ultrafast PCR, as described above.
Results and discussion
Development and specificity of portable ultrafast PCR system
The portable ultrafast PCR system was developed using a microfluidic polymer chip for rapid molecular diagnostics and research applications, which can perform 40 PCR cycles in less than 20 min. This system could be easily applied to molecular diagnostics in various fields with no need for individual probe design due to the use of a fluorescent dye. Furthermore, because the device was compact and powered by a battery, the portable ultrafast PCR system may be suitable for on-site detection.
In this study, five shrimp species [white leg shrimp (Litopenaeus vannamei), black tiger shrimp (Penaeus monodon), Argentina red shrimp (Pleoticus muelleri), kuruma shrimp (Marsupenaeus japonicus), and Chinese white shrimp (Fenneropenaeus chinensis)] were tested for allergen with the ultrafast PCR system. Among them, white leg shrimp and black tiger shrimp are produced more than 80% of the farmed shrimp worldwide (Pascoal et al., 2011), and are eaten the most commonly in Asia (Lee et al., 2012).
The tropomyosin gene is shown high sequence homology across crustacean species (Leung and Chu, 1998). A shrimp-specific primer pair, Strop-F/R, was designed by comparing tropomyosin sequences between several crustaceans (Fig. 1). Using the primer Strop-F/R, PCR products were amplified from these five shrimp species (Table 2). The ultrafast PCR system included a post-PCR DNA melt curve analysis using the EvaGreen dye, which exhibits lower PCR inhibition than SYBR Green I (Ali et al., 2014; Mao et al., 2007) and showed threshold cycle number (Ct) and the melting temperature (Tm) amplification for each target. Therefore, the results of the PCR test were confirmed as positive or negative by comparing their melting curves and peaks.
Table 2.
Primer pair specificity results used in this study
| Common name (Scientific name) | Shrimp-specific ultrafast PCR system (Tm, °C) | Universal ultrafast PCR system (Tm, °C) | |
|---|---|---|---|
| 1 | White leg Shrimp (Litopenaeus vannamei) | + (78.80) | + (84.63) |
| 2 | Black tiger shrimp (Penaeus monodon) | + (78.80) | + (84.31) |
| 3 | Red shrimp (Pleoticus muelleri) | + (78.47) | + (83.66) |
| 4 | Kuruma Shrimp (Marsupenaeus japonicas) | + (79.12) | + (83.98) |
| 5 | Chinese white shrimp (Fenneropenaeus chinensis) | + (78.47) | + (84.63) |
| 6 | Crab (Portunus trituberculatus) | Nd | + (81.71) |
| 7 | Squid (Todarodes pacificus) | Nd | + (84.31) |
| 8 | Clam (Ruditapes philippinarum) | Nd | + (84.31) |
| 9 | Mussel (Mytilus edulis) | Nd | + (83.66) |
| 10 | Abalone (Haliotis discus hannai) | Nd | + (83.01) |
| 11 | Oyster (Crassostrea gigas) | Nd | + (83.98) |
| 12 | Mackerel (Scomber japonicas) | Nd | + (84.31) |
Nd not detected
As shown in Table 2, the specificity of the primer Strop-F/R for the shrimp tropomyosin gene was confirmed to not cross-amplify using different species of seafood, such as crab (Portunus trituberculatus), squid (Todarodes pacificus), clam (Ruditapes philippinarum), mussel (Mytilus edulis), abalone (Haliotis discus hannai), oyster (Crassostrea gigas), and mackerel (Scomber japonicas). In addition, the melting temperatures (Tm) of amplified products from five shrimp species ranged from 78.47 to 79.12 °C.
A universal primer pair targeting the 18S rRNA was also used as an internal positive control and was amplified to confirm the presence of DNA in all samples used for specificity test (Martin et al., 2009; Safdar and Jenejo, 2015). As shown in Table 2, positive signals in all seafood samples, including the five shrimp species, were obtained by the eukaryotic primer pair. Thus, these results showed that shrimp-specific primer pair designed in this study specifically amplified target species except different species.
Sensitivity of the ultrafast PCR system
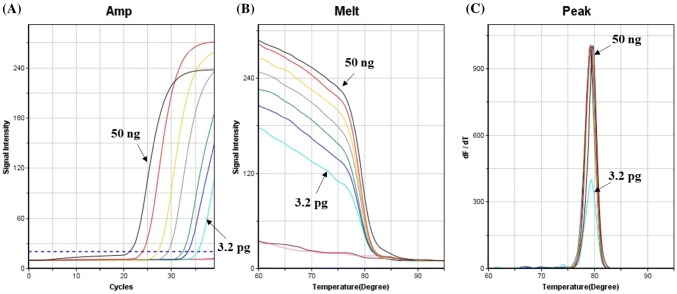
The detection of allergens in food products can be very difficult since allergens are frequently present only in trace quantities (Poms et al., 2004). Therefore, the development of precise and highly sensitive detection methods is significant for the food industry. To determine the limit of detection by the ultrafast PCR assay, DNA extracted from the shrimp was 5-fold serially diluted from 50 ng to 0.64 pg, and then the Ct values and melting temperatures (Tm) were analyzed. The Ct values of the shrimp DNA samples ranged from 23.58 to 34.61, and the Tm’s of all samples were 79.35 ± 0.24 °C. The detection limit of this assay was 3.2 pg (Fig. 2). This value was lower than the 5 pg of DNA reported by Taguchi et al. (2011). Moreover, since the ultrafast PCR technique for detecting the presence of shrimp is completed in 20 min and analyzed using minimal and battery-powered equipment, our method is much faster and is applicable to on-site detection compared to other PCR-based methods such as real-time PCR and RFLP-PCR.
Fig. 2.
Sensitivity results of the ultrafast PCR using fivefold serially diluted shrimp DNAs (50 ng–0.64 pg). Fluorescence of amplification for shrimp was detected in real-time (A), and then melting curve analysis was performed after PCR reaction (B)
Analysis of commercial food products
To confirm the applicability of the developed ultrafast PCR system in commercial food samples, 23 food products were analyzed using extracted DNA and samples without DNA extraction (Table 3). All reactions were carried out in duplicate to verify the repeatability of the assay. The 23 shrimp-containing foods included seasoning, fried rice, snacks, dried shrimp, porridge, dumplings, fried shrimp, pizza, and sushi. The shrimp allergen tropomyosin was successfully amplified by primers within 20 min in a variety of food products (Table 3). The most of Ct values measured by direct ultrafast PCR assay without the routine procedures for DNA extraction were delayed compared to the Ct values measured by ultrafast PCR assay using DNAs extracted from food products, because some components in food matrix could inhibit PCR reaction (Kim and Kim, 2017; Kitpipit et al., 2014). However, melting peaks performed after the amplification reaction were between 78.47 and 80.74 °C for both extracted DNA and samples without DNA extraction (Table 3). These results showed that the same PCR products were amplified by the shrimp-specific primers in both methods and applicability of this developed system in various foods including highly processed samples. PCR-based methods for food allergen detection can be successfully applied to highly processed samples due to more stable property of genomic material than protein (Herrero et al., 2014). The shrimp tropomyosin gene was detected without a DNA isolation procedure in all food samples in less than 30 min from sampling to time of analysis. In addition, since the procedure is easy and only requires buffers at room temperature, this direct ultrafast PCR system could be utilized for the detection of shrimp in the field.
Table 3.
Food product results using the shrimp-specific ultrafast PCR system
| No. | Food products | Ultrafast PCR system using extracted DNA (Tm, °C) | Direct ultrafast PCR system without DNA extraction (Tm, °C) | |
|---|---|---|---|---|
| User 1 | User 2 | |||
| 1 | Seasoning A | + (80.09) | + (80.42) | + (79.77) |
| 2 | Seasoning B | + (80.09) | + (80.74) | + (79.44) |
| 3 | Seasoning C | + (79.77) | + (80.42) | + (79.77) |
| 4 | Seasoning D | + (79.77) | + (81.06) | + (79.12) |
| 5 | Seasoning E | + (79.77) | + (80.74) | + (79.12) |
| 6 | Fried rice A | + (80.42) | + (80.09) | + (78.80) |
| 7 | Fried rice B | + (80.09) | + (79.77) | + (78.80) |
| 8 | Fried rice C | + (80.74) | + (80.09) | + (78.80) |
| 9 | Fried rice D | + (80.74) | + (80.09) | + (78.80) |
| 10 | Snack A | + (80.42) | + (80.42) | + (79.44) |
| 11 | Snack B | + (79.77) | + (80.09) | + (79.44) |
| 12 | Snack C | + (80.42) | + (79.77) | + (80.09) |
| 13 | Dried shrimp A | + (79.44) | + (80.74) | + (79.12) |
| 14 | Dried shrimp B | + (80.09) | + (79.44) | + (78.80) |
| 15 | Dried shrimp C | + (79.77) | + (79.77) | + (80.09) |
| 16 | Porridge A | + (79.12) | + (79.12) | + (78.47) |
| 17 | Porridge B | + (79.44) | + (79.12) | + (79.12) |
| 18 | Dumpling A | + (79.77) | + (79.44) | + (78.80) |
| 19 | Dumpling B | + (79.44) | + (79.44) | + (79.12) |
| 20 | Dumpling C | + (79.44) | + (79.12) | + (78.80) |
| 21 | Fried shrimp | + (78.80) | + (79.44) | + (78.80) |
| 22 | Pizza | + (78.47) | + (78.47) | + (78.80) |
| 23 | Shrimp sushi | + (80.09) | + (78.47) | + (78.47) |
Each reaction was repeated two times. The mean Tm are tabulated
Intra-laboratory validation study of the direct ultrafast PCR system
An intra-validation study was performed to assess the reliability and applicability of the direct ultrafast PCR system. Each of two users prepared 23 of food samples using the simplified extraction method and carried out each different ultrafast PCR to detect shrimp in commercial products. Results analyzed by two users showed all samples containing shrimp were observed as positive signals and target species was confirmed through Tm values analysis (Table 3). Thus, this developed assay can provide as a reliable and robust method to identify shrimp in processed products and is available for food allergen control in process chain.
Acknowledgments
This research was supported by a Grant 16163MFDS003 from the Ministry of Food and Drug Safety in Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Aboud M, Oh HH, McCord B. Rapid direct PCR for forensic genotyping in under 25 min. Electrophoresis. 2013;34:1539–1547. doi: 10.1002/elps.201200570. [DOI] [PubMed] [Google Scholar]
- Ali ME, Razzak MA, Hamid SBA. Multiplex PCR in species authentication: probability and prospects—a review. Food Anal. Method. 2014;7:1933–1949. doi: 10.1007/s12161-014-9844-4. [DOI] [Google Scholar]
- Eller E, Jensen BC. Clinical value of component-resolved diagnostics in peanut-allergic patients. Allergy. 2013;68:190–194. doi: 10.1111/all.12075. [DOI] [PubMed] [Google Scholar]
- Furutani S, Naruishi N, Hagihara Y, Nagai H. Development of an on-site rapid real-time polymerase chain reaction system and the characterization of suitable DNA polymerases for TaqMan probe technology. Anal. Bioanal. Chem. 2016;408:5641–5649. doi: 10.1007/s00216-016-9668-8. [DOI] [PubMed] [Google Scholar]
- Furutani S, Hagihara Y, Nagai H. On-site identification of meat species in processed foods by a rapid real-time polymerase chain reaction system. Meat Sci. 2017;131:56–59. doi: 10.1016/j.meatsci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Herrero B, Vieites JM, Espiñeira M. Fast real-time PCR for the detection of crustacean allergen in foods. J. Agric. Food Chem. 2012;60:1893–1897. doi: 10.1021/jf2043532. [DOI] [PubMed] [Google Scholar]
- Herrero B, Vieites JM, Espiñeira M. Development of an in-house fast real-time PCR method for detection of fish allergen in foods and comparison with a commercial kit. Food Chem. 2014;151:415–420. doi: 10.1016/j.foodchem.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Houssin T, Cramer J, Grojsman R, Bellahsene L, Clas G, Moulet H, Minnella W, Pannetier C, Leberre M, Plecis A, Chen Y. Ultrafast, sensitive and large-volume on-chip real-time PCR for the molecular diagnosis of bacterial and viral infections. Lab Chip. 2016;16:1401–1411. doi: 10.1039/C5LC01459J. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ryu JO, Song JY, Kim HY. Multiplex PCR for identification of Shigellae and Shigella species using novel genetic markers screened using comparative genomics. Foodborne Pathog. Dis. 2017;14(7):400–406. doi: 10.1089/fpd.2016.2221. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim HY. Species identification of commercial jerky products in food and feed using direct pentaplex PCR assay. Food Control. 2017;78:1–6. doi: 10.1016/j.foodcont.2017.02.027. [DOI] [Google Scholar]
- Kitpipit T, Sittichan K, Thanakiatkrai P. Direct-multiplex PCR assay for meat species identification in food products. Food Chem. 2014;163:77–82. doi: 10.1016/j.foodchem.2014.04.062. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Gerez I, Shek LPC, Lee BW. Shellfish allergy—an Asia-Pacific perspective. Asian Pac. J. Allergy. 2012;30(1):3–10. [PubMed] [Google Scholar]
- Leung PS, Chu KH. Molecular and immunological characterization of shellfish allergens. Front Biosci. 1998;3:306–312. doi: 10.2741/A243. [DOI] [PubMed] [Google Scholar]
- Mao F, Leung WY, Xin X. Characterization of EvaGreen and the implication of its physicochemical properties for qPCR applications. BMC Biotechnol. 2007;7:76. doi: 10.1186/1472-6750-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Garcia T, Fajardo V, Rojas M, Pegels N, Hemandez PE, Gonzalez I, Martin R. SYBR-Green real-time PCR approach for the detection and quantification of pig DNA in feedstuffs. Meat Sci. 2009;82(2):252–259. doi: 10.1016/j.meatsci.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pafundo S, Gulli M, Marmiroli N. Multiplex real-time PCR using SYBR® GreenER™ for the detection of DNA allergens in food. Anal. Bioanal Chem. 2010;396:1831–1839. doi: 10.1007/s00216-009-3419-z. [DOI] [PubMed] [Google Scholar]
- Park YC, Kim MR, Shin JH, Kim KH, Lee JH, Cho TY, Lee HJ, Lee SJ, Han SB. Development of PCR method for rapid detection of allergic materials in foods. J. Food Hyg. Saf. 2013;28(2):124–129. doi: 10.13103/JFHS.2013.28.2.124. [DOI] [Google Scholar]
- Pascoal A, Barros-Velazques J, Ortea I, Cepeda A, Gallardo JM, Calo-Mata P. Molecular identification of the black tiger shrimp (Penaeus monodon), the white leg shrimp (Litopenaeus vannamei) and the Indian white shrimp (Fenneropenaeus indicus) by PCR targeted to the 16S rRNA mtDNA. Food Chem. 2011;125:1457–1461. doi: 10.1016/j.foodchem.2010.10.053. [DOI] [Google Scholar]
- Poms RE, Klein CL, Anklam E. Methods for allergen analysis in food: a review. Food Addit. Contam. A. 2004;21:1–31. doi: 10.1080/02652030310001620423. [DOI] [PubMed] [Google Scholar]
- Reisch MP, Hochegger R, Stumr S, Korycanva K, Markl MC. Validation and comparison of two commercial ELISA kits and three in-house developed real-time PCR assays for the detection of potentially allergenic mustard in food. Food Chem. 2015;174:75–81. doi: 10.1016/j.foodchem.2014.10.132. [DOI] [PubMed] [Google Scholar]
- Safdar M, Jenejo Y. A multiplex-conventional PCR assay for bovine, ovine, caprine and fish species identification in feedstuffs: Highly sensitive and specific. Food Control. 2015;50:190–194. doi: 10.1016/j.foodcont.2014.08.048. [DOI] [Google Scholar]
- Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J. Immunol. 1993;151:5354–5363. [PubMed] [Google Scholar]
- Sicherer SH, Muňoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004;114:159–165. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Son JH, Cho BR, Hong SW, Lee SH, Hoxha O, Haack AJ, Lee LP. Ultrafast photonic PCR. Light Sci. Appl. 2015;4:e280. doi: 10.1038/lsa.2015.53. [DOI] [Google Scholar]
- Song HO, Kim JH, Ryu HS, Lee DH, Kim SJ, Kim DJ, Suh IB, Choi DY, In KH, Kim SW, Park H. Polymeric LabChip real-time PCR as a point-of-care-potential diagnostic tool for rapid detection of influenza A/H1N1 virus in human clinical specimens. PLoS One. 2012;7:e53325. doi: 10.1371/journal.pone.0053325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Kim JH, Kim HY. Detection of unapproved genetically modified potatoes in Korea using multiplex polymerase chain reaction. Food Control. 2017;80:19–22. doi: 10.1016/j.foodcont.2017.04.026. [DOI] [Google Scholar]
- Taguchi H, Watanabe S, Tsmmei Y, Hirao T, Akiyama H, Sakai S, Adachi R, Sakata K, Urisu A, Teshima R. Different detection of shrimp and crab for food labeling using polymerase chain reaction. J. Agric. Food Chem. 2011;59:3510–3519. doi: 10.1021/jf103878h. [DOI] [PubMed] [Google Scholar]
- Van Do T, Hordvik I, Endresen C, Elsayed S. Characterization of parvalbumin, the major allergen in Alaska pollack, and comparison with codfish Allergen M. Mol. Immunol. 2005;42:345–353. doi: 10.1016/j.molimm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Wheeler EK, Hara CA, Frank J, Deotte J, Hall SB, Benett W, Spadaccini C, Beer NR. Under-three minute PCR: Probing the limits of fast amplification. Analyst. 2011;136:3707–3712. doi: 10.1039/c1an15365j. [DOI] [PubMed] [Google Scholar]