Abstract
In this study, 19 indigenous mezcal Saccharomyces cerevisiae strains were screened for their tolerance to grow under different stress conditions and their potential use in fermentation. All strains were able to tolerate pH value of 3, significant levels of glucose (30%), ethanol (12% v/v), and temperature of 37 °C. Eleven of them were able to grow in presence of 15% of ethanol, but only CH7 and PA18 strains grew at 42 °C. Both were selected for evaluation of their fermentative abilities in maguey juice and in a synthetic medium incubated at 30 and 40 °C. Temperature of 40 °C had a positive effect on the ethanol production, increasing the productivity and efficiency in maguey fermentation. Ethyl acetate, isobutanol and isoamyl alcohols production was favored at 30 °C. Both evaluated strains presented a good fermentative capacity and production of volatile compounds, suggesting their potential use as starter cultures in mezcal fermentation.
Keywords: Saccharomyces cerevisiae, Stress tolerance, Fermentative capacity, Mezcal fermentation, Volatile compounds
Introduction
Mezcal is a Mexican alcoholic beverage produced by the distillation of fermented maguey (Agave genera) juice. This spirit is exclusively produced in regions within the appellation of origin. According to the Official Mexican Standards, mezcal must have an alcohol content between 35 and 55% (v/v) of ethanol at 20 °C (NOM-070-SCFI-2016). According to the specific process used for maguey cooking, milling, fermentation, and distillation, mezcal is classified as ancestral mezcal, artisanal mezcal, and mezcal (NOM-070-SCFI-2016). The elaboration processes of the first two categories, generally involve natural fermentation in which yeasts play an essential function in ethanol and volatile compounds production (Kirchmayr et al. 2017; Páez-Lerma et al. 2013; Verdugo-Valdez et al. 2011). The main yeast species involved in this process comprise Saccharomyces cerevisiae, Saccharomyces exiguous, Kluyveromyces marxianus, Torulaspora delbrueckii, Pichia membranefaciens, Pichia kluyvery, Pichia fermentans, Zygosaccharomyces bailii, Zygosaccharomyces rouxii, Zygosaccharomyces bisporus, Clavispora lusitaniae, Candida ethanolica, Candida diversa (Kirchmayr et al. 2017; Páez-Lerma et al. 2013; Verdugo-Valdez et al. 2011). Saccharomyces cerevisiae has been found as the predominant mezcal yeast (Kirchmayr et al. 2017; Páez-Lerma et al. 2013; Verdugo-Valdez et al. 2011) and is associated with their ability to grow under environmental conditions prevailing in the fermentation vats, such as the presence of maguey fibers, sugar content (120–300 g/L; which depends of the harvest season, in spring the must prepared can reach up to 300 g/L of sugar, due to fructans concentration in the maguey plant is higher than the one reached in fall season; Vera-Guzmán et al. 2012), pH value about 4, high temperature inside of the fermentation vats (35–40 °C; frequently, warm water is added to the bagasse contained in the wooden vats, so the temperature could be higher than 40 °C), and ethanol content about of 40 and 60 g/L (Kirchmayr et al. 2017). Natural fermentation of mezcal sometimes is sluggish or stuck, leaving a high amount of residual sugar, low ethanol yields, and an increase in total acidity due to the presence of the acetic acid bacteria, which are abundant in prolonged fermentation (Kirchmayr et al. 2017; Nuñez-Guerrero et al. 2016). One of the recommended alternatives to solve these problems is to use starter culture of selected local yeasts (Liu et al. 2016; Rodríguez et al. 2010). These microorganisms are better adapted to the local conditions, and they dominate the fermentation (Rodríguez et al. 2010). Yeast selection criteria for indigenous inoculum are based on the properties of the strains that are relevant to fermentation (Rodríguez et al. 2010). Some of the most important factors are tolerance to high ethanol and sugar concentration, resistance to low pH, potential of sugar exhaustion, high fermentation activity (Aparecida-Oliveira et al. 2008; Liu et al. 2016; Regodón et al. 1997), etc. These conditions are similar to the ones found in the final stage of the mezcal fermentation. In this phase, S. cerevisiae has been reported as the dominant species (Kirchmayr et al. 2017; Páez-Lerma et al. 2013; Verdugo-Valdez et al. 2011). This yeast, in addition to ethanol, also produces higher alcohols (amyl and isoamyl alcohols, n-propanol, 2-phenyl ethanol and isobutanol) and esters (isoamyl acetate and ethyl hexanoate) (Díaz-Montaño et al. 2008). Mezcal flavor is associated with a wide variety of volatile compounds, which are originated from the maguey plant, cooking, and fermentation, and they are concentrated during distillation step (Prado-Jaramillo et al. 2015). Volatile compounds present in relatively low concentrations are important for quality and authenticity of the spirit (Prado-Jaramillo et al. 2015).
In this context, the aim of this work was the screening and selection of wild S. cerevisiae yeast strains able to tolerate diverse stress conditions. Following this initial screening process, two selected yeast strains were evaluated in fermentations of maguey juice and compared with a synthetic medium. In order to know the effect of temperature on the ethanol and volatile compounds production, these experiments were carried out at 30 and 40 °C, considering that the prevailing temperature in natural fermentation has been reported around 30 °C, while in some regions the fermentation reaches up to 40 °C (Kirchmayr et al. 2017).
Materials and methods
Yeast strains
Nineteen Saccharomyces cerevisiae strains from the yeast collection of the Department of Food and Biotechnology from the Universidad Nacional Autónoma de México, Mexico were evaluated (Table 1). All strains were previously isolated from different mezcal distilleries in Oaxaca State during the step of maguey fermentation. The strains were stored at − 80 °C in glycerol (30%), reactivated by streaking onto YPD agar (yeast extract 10 g/L, peptone 20 g/L, dextrose 20 g/L, and agar 20 g/L) and incubated at 30 °C for 48 h.
Table 1.
Analysis of yeast tolerance to different stress conditions
| Strain | pH | Temperature (°C) | Glucose % (w/v) | Ethanol % (v/v) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 37 | 42 | 45 | 20 | 25 | 30 | 8 | 10 | 12 | 15 | ||
| CH1 | 5 | 4 | nd | nd | 5 | 5 | 4 | 4a | 4a | 4b | 2b | |
| CH2 | 4 | 4 | nd | nd | 4 | 4 | 4 | 5a | 4a | 4a | nd | |
| CH3 | 4 | 4 | nd | nd | 4 | 4 | 4 | 4a | 4a | 4b | 3b | |
| CH7 | 4 | 5 | 4 | nd | 4 | 4 | 4 | 4* | 4* | 4a | 1b | |
| DI1 | 5 | 4 | nd | nd | 4 | 4 | 3 | 5a | 4a | 4b | 3b | |
| DI14 | 4 | 4 | nd | nd | 4 | 4 | 4 | 4a | 4a | 4b | nd | |
| DH1 | 4 | 5 | nd | nd | 4 | 4 | 4 | 4a | 4a | 4b | nd | |
| DH2 | 4 | 4 | nd | nd | 4 | 4 | 4 | 5a | 1a | 1b | nd | |
| DI3 | 5 | 4 | nd | nd | 4 | 3 | 3 | 4a | 4a | 4b | 3b | |
| DI10 | 4 | 4 | nd | nd | 4 | 4 | 4 | 4a | 4a | 4b | 4b | |
| DI11 | 5 | 4 | nd | nd | 4 | 4 | 4 | 4a | 4a | 4b | 4b | |
| DI12 | 5 | 4 | nd | nd | 4 | 4 | 4 | 5a | 5a | 5b | nd | |
| FF7 | 5 | 5 | nd | nd | 5 | 4 | 4 | 5a | 4a | 4a | 4a | |
| FV10 | 4 | 5 | nd | nd | 4 | 4 | 4 | 4a | 4a | 4b | 4b | |
| FV11 | 5 | 5 | nd | nd | 4 | 4 | 4 | 5a | 5a | 5b | 4b | |
| LC12 | 4 | 4 | nd | nd | 4 | 4 | 4 | 4* | 4* | 4a | nd | |
| LC14 | 4 | 5 | nd | nd | 4 | 4 | 4 | 5a | 4a | 4b | nd | |
| PA18 | 4 | 4 | 4 | nd | 4 | 4 | 4 | 5* | 5* | 4a | 4b | |
| ZA6 | 5 | 4 | nd | nd | 4 | 4 | 3 | 4a | 4a | 4b | nd | |
Tolerance to the different fermentation stress factors is indicated by numbers from 0 (absence to growth) to 6 (colony development at the sixth dilution); all plates were incubated during 24–120 h. Colony growth at pH value of 3 was observed at 24 h of incubation at 30 °C; growth on plates at 20, 25 and 30% of glucose was observed at 24 h of incubation at 30 °C; colony growth on plates incubated at 37 and 42 °C was observed at 24 h of incubation
*Colony growth was observed at 24 h of incubation at 30 °C
aColony growth was observed at 72 h of incubation at 30 °C
bGrowth was observed at 120 h of incubation at 30 °C; nd: not detected
Strain typing of S. cerevisiae isolates
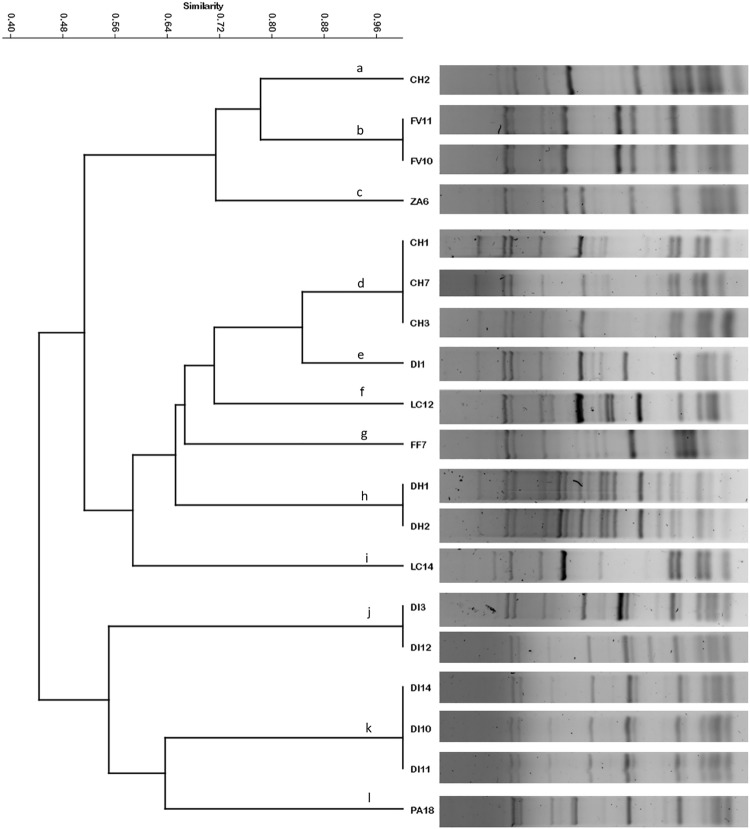
In order to distinguish different S. cerevisiae strains, interdelta sequence amplifications of all S. cerevisiae strains were carried out using delta 12 and delta 21 primers according to Legras and Karst (2003). The PCR products were separated by electrophoresis on 1.0% (w/v) agarose gels, which were applied with 90 V for 90 min in 0.5 X TBE buffer (44.5 mM Tris (Sigma-Aldrich, St. Louis, MO, USA), 44.5 mM boric acid (Sigma-Aldrich), 1 mM Na2-EDTA (Sigma-Aldrich)) and photographed under UV light trans-illumination. The approximate sizes of the amplicons were determined using a standard molecular weight marker (1 kb plus DNA ladder; Invitrogen). The digital images were obtained using the ChemiDoctm and Image Lab 4.1 software (Bio-Rad, Hercules, CA, USA), and analyzed using the PyElph 1.4 software (Pavel and Vasile 2012). The profiles were grouped using unweighted pair group average (UPGMA) cluster analysis based on the Jaccard similarity index using the PAST software version 2.17c.
Analysis of S. cerevisiae strains to several stress conditions
One colony was used to inoculate 5 mL of YPD broth and incubated overnight at 30 °C on a shaker at 250 rpm. After that, the inoculum preparation was performed as described by Belloch et al. (2008). Four stress factors were assessed: Ethanol (8, 10, 12 and 15% v/v), temperature (37 °C, 42 °C, and 45 °C), glucose (20, 25, and 30% w/v), and pH of 3. Stress media were prepared using YPD agar supplemented with adequate amounts of each stress factor. Temperature stress was performed on agar YPD medium incubated at the respective temperature. pH stress was evaluated on agar YPD adjusted to value of 3. All plates were incubated during 1–5 days at 30 °C, except for those testing temperature stress.
Selected yeast strains and culture media
Strains CH7 and PA18, were selected based on tolerated stress conditions. Fermentative capacity was evaluated using two fermentation media: A synthetic medium designed by Segura-García et al. (2015), which consisted of fructose 100 g/L, (NH4)2SO4 1 g/L, K2HPO4 2.2 g/L, yeast extract 1 g/L, MgSO4·7H2O 3.27 g/L, and Ca(NO3)2·4H2O 0.93 g/L; and a natural medium which consisted of maguey juice from Agave angustifolia. The latter was prepared as follows: Maguey pines were cut into small pieces, placed in a container and cooked at 105 °C for 3 h using an autoclave. The juice of the cooked maguey was extracted using a kitchen juice extractor. The concentrated juice was filtered, diluted with distilled water up to 10 ºBx, and supplemented with ammonium sulphate (1 g/L; this nitrogen source was added to mimic the must conditions, such as is practiced in some mezcal producing regions). The diluted juice was sterilized at 121 °C for 15 min.
Fermentation conditions
Fermentation tests were carried out in 450 mL jacketed tubes with recirculation containing 250 mL of medium. The culture medium was inoculated with the corresponding strains to reach an initial concentration of 1 × 106 cells/mL, using overnight pre-cultures grown in YEPD broth. Fermentation was performed at 30 and 40 °C without agitation during 60 h. Every 12 h, samples were collected during the course of fermentation.
Biomass quantification
Cell concentration in both maguey juice and synthetic medium was determined by optical density (measurement of absorbance at 600 nm using a spectrophotometer; GBC cintral 101, Hampshire, IL, USA) and a standard curve of cell count in a Neubauer chamber.
Sugar and acetic acid concentration
Sugar (glucose and fructose), ethanol, and acetic acid concentrations were determined using a liquid chromatography system (Waters Corporation, Milford, MA, USA), an ion-exclusion aminex HPX-87H column (300 × 7.8 mm; BIO-RAD) and a refraction index detector (Waters 2414, Milford, MA, USA), following the methodology described by Santiago-Urbina et al. (2015).
Volatile compound determination
Higher alcohols (1-propanol, isobutyl alcohol, amyl alcohol, and isoamyl alcohol) and ethyl acetate concentrations were determined using a head space solid phase microextraction (HS-SPME) followed by gas chromatography. The volatile compounds were extracted, in duplicate, by headspace using a Poly dimethylsiloxane/Divinylbenzene (PDMS/DVB) fiber (Supelco, PA, USA). 1.4 mL of sample was placed in a head space vial, 40% of NaCl was added. Fiber was inserted into the headspace and the solution was swirled in a magnetic stirrer at 14 000 rpm and 60 °C for 15 min, after that the fiber was transferred to the injector for desorption at 250 °C for 15 min. Analysis was performed on an Agilent 6890 N gas chromatograph equipped with a flame ionization detector (FID) and an Optima Wax column of 60 m × 0.25 mm. The temperature program was 40 °C (5 min) to 140 °C at 5 °C/min, and then to 240 °C at 10 °C/min. Injector and detector temperatures were set at 250 °C. Nitrogen was used as the carrier gas at 28.3 mL/min. Air flow of 400 mL/min and hydrogen flow at 40 mL/min were used. The identification of the volatile compound was achieved by comparing the retention time with those of standards. And the quantification was carried out following the internal standard method. Standard used were purchased in Sigma-Aldrich.
Statistical analysis was performed by one-way ANOVA using Statgraphics Centurion XVII.II.
Results and discussion
Typing of S. cerevisiae strains
The identification and differentiation of indigenous yeast is an important step to starter culture selection (Sun et al. 2014). In order to identify S. cerevisiae at strain level, an interdelta PCR typing was performed, 19 Saccharomyces cerevisiae strains were typed in 12 different patterns of amplification (Fig. 1), revealing a high discrimination among autochthonous yeast strains. Several studies on strain typing of S. cerevisiae isolates have demonstrated the high discriminatory power of the interdelta analysis (Settani et al. 2012; Sun et al. 2014). The differences observed in the interdelta profile (Fig. 1) were mainly due to the gain or loss of some interdelta fragments. Strains FV10 and FV11 isolated from FV distillery were grouped in a single cluster. Distillery CH presented two different S. cerevisiae strains, which were included in “a” and “d” clusters (Fig. 1). While, DI distillery revealed the presence of 3 different strains, which were grouped in “e”, “j”, and “k” clusters. The findings showed that the population of S. cerevisiae in a single distillery included more than one strain, and each distillery showed interdelta profile that are not detected in other place. It suggests that the use of these yeasts could contribute to the production of mezcal with typical sensory characteristic of each region, such as suggested by Sun et al. (2014). These results are similar to the ones found in the study of Badotti et al. (2010), who investigated the differentiation strains of S. cerevisiae from six cachaça distilleries located in different regions in Brazil. The prevalence of the most adapted S. cerevisiae strains is influenced by different stress conditions such as changes in pH, ethanol content and sugar concentration (Badotti et al., 2010). With this test, we assure that S. cerevisiae strains selected and used in subsequent experiment as starter in maguey juice fermentation, did not correspond to the same strain.
Fig. 1.
Dendrogram illustrating the similarity of 19 yeast strains based on interdelta PCR patterns. The letters represent the groups with 100% similarity
Yeast grown under stress conditions
All autochthonous mezcal S. cerevisiae strains, were investigated for characteristics of mezcal production interest, such as resistance to ethanol, osmotic stress, temperature, and pH stress, similar to the environmental conditions occurring in maguey juice fermentation vats (Vera-Guzmán et al. 2012). According to Nuñez-Guerrero et al. (2016) a good fermentative capacity (high sugar intake, high ethanol production, and low acetic acid production), good ethanol tolerance (up to 8%), and neutral killer phenotypes are required features of yeast for use in the industrial level. Table 1 compares the capacity of each strain to develop colonies in the dilutions at the stress environments tested in this study. Colony development observed at 72 and 120 h showed that strains experienced more stress. After 24 h of incubation, only CH7, LC12, and PA18 grew in culture media containing 8 and 10% (v/v) of ethanol. After 72 h, these strains like CH02 and FF07 were able to grow in YEPD with 12% (v/v) of ethanol. In addition, the FF07 strain also showed growth in a culture medium containing 15% (v/v) of ethanol. At the end of the experiment, 11 strains (CH1, CH3, CH7, DI1, DI3, DI10, DI11, FF07, FV10, FV11, and PA18) had been already adapted to the medium with 15% (v/v) of ethanol. These results agree with the investigation of ethanol tolerance in cachaça yeast, which reported that the PE5 and SC6 strains of S. cerevisiae were able to grow at 15% ethanol (Badotti et al., 2010). Ethanol concentration in fermentation media increases over time, this metabolite changes the membrane organization and increases their permeability and consequently inhibition of glucose and amino acid uptake (Stanley et al. 2010). It has been reported that ethanol tolerance is a good factor to evaluate the adaptive laboratory evolution to improve the technological properties of industrial wine yeast strains (Novo et al. 2014).
The maguey juice at the beginning of the fermentation can contain up to 300 g/L of sugar, which could result in inhibition of yeast growth. It has been reported that osmotic stress caused by high sugar concentration during alcoholic fermentation affect S. cerevisiae cells during their growth (Jiménez-Martí et al. 2011). So, the use of osmotolerant strains could be a suitable alternative in the mezcal production. In this study all mezcal S. cerevisiae strains were able to grow in presence of significant concentrations of glucose (20, 25, and 30% w/v). Growth at 37 °C was positive for all strains, whereas only CH7 and PA18 strains grew at 42 °C (Table 1) and no strains grew at 45 °C. These strains showed better temperature tolerance (42 °C) than that resisted (37 °C) by cachaça yeast (Badotti et al. 2010). In this way, temperature was a discriminating factor between strains and helped us select them. This factor plays an important role in fermentation, because the temperature during this process in the vats is about 40 °C, depending on the environmental conditions. Natural fermentation of maguey juice is given at pH value around 4; therefore, tolerance to low pH is another important factor for yeast selection. Findings showed that ability to growth at low pH (pH value of 3) was common for all strains evaluated; Serra et al. (2005) and Belloch et al. (2008) reported that pH does not have a significant influence on Saccharomyces growth.
Fermentation of maguey juice
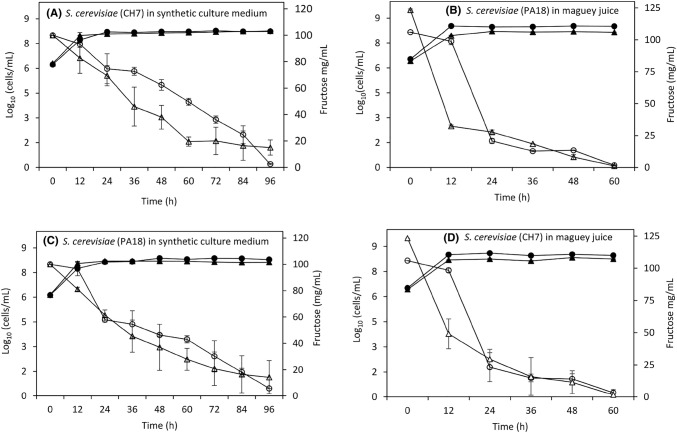
Based on the selection criteria previously described, especially on their ethanol and temperature tolerance, strains CH7 and PA18 were selected and tested as starters in maguey juice and synthetic medium fermentation at laboratory scale at 30 and 40 °C. Results of an interdelta analysis revealed that the two selected yeasts belong to different strains. In this way it was ensured that the composition of culture medium, strain, and temperature of fermentation had different effects on the metabolite production. Figure 2 shows the growth of S. cerevisiae strains and sugar consumption in both maguey juice [Fig. 2(B) and (D)] and synthetic medium [Fig. 2(A) and (C)]. All strains presented similar growth behavior: Initial cell concentration in all fermentations was ~ 6 log cells/mL, it concentration increased to over 8 log cells/mL in the first 12 h, and after time biomass remained stable throughout the fermentation process. In maguey juice fermentation performed at 40 °C, both PA18 and CH7 strains consumed about 73% and 60% of the sugar in the first 12 h [Fig. 2(B) and (D)], curve with empty triangles), respectively. While, in fermentations carried out at 30 °C, strains only consumed less than 20% of the sugars (curves with empty circles). Results showed that the yeasts were better adapted to the maguey juice than to the synthetic medium. After 24 h of fermentation, the maguey juice contained 25 mg/mL of residual fructose, while the synthetic medium contained about 60 and 70 mg/mL (Fig. 2). Maguey fermentation was completed in 60 h, and in this time more than 97% of the sugars were consumed. In the fermentation of the synthetic medium, sugar was not completely exhausted after 96 h. At the end of the fermentation, the ethanol concentration ranged between 18.77 and 44.28 g/L (Table 2). Findings reveal that the temperature and composition of the culture medium impacted on the amount of ethanol produced. Maguey fermentation performed at 40 °C reached a higher ethanol concentration than these obtained at 30 °C. Conversely, the ethanol production in synthetic medium was favored at 30 °C (Table 2). The highest ethanol yields (ethanol/sugar yield) and efficiencies (percentage of sugars that were converted to ethanol) were obtained using the synthetic medium (Table 2). The highest efficiency was found with CH7 strain at 30 and 40 °C, as well as with PA18 at 30 °C. The highest productivity was reached in fermentations with CH7 and PA18 in maguey juice at 40 °C. The high temperature had a positive effect on the ethanol production in maguey juice fermentation. This observation could be due to this strain being better adapted to the maguey juice and at high temperature (40 °C), as the maguey juice could have major essential nutrients for alcoholic fermentations. This explanation is supported by the fact that both yeasts (CH7 and PA18) consumed the sugar in 60 h, while fermentation in a synthetic medium was not completed even after 96 h. These results were shown with the high productivity reached at 40 °C in the juice. Environmental parameters such as the initial concentration of nitrogen and temperature have an effect on the synthesis of non-volatile and volatile compounds (Rollero et al. 2015). Under the conditions studied, we found that the ethanol production depended on the fermentation temperature and the culture medium composition and not on the strain.
Fig. 2.
Growth and fructose consumption in fermentations performed by CH7 (graphs A and D) and PA18 (graphs B and C) strains. Empty circle indicates fructose concentration in fermentation performed at 30 °C; filled circle indicates logarithm of cell concentration in fermentations performed at 30 °C; empty triangle indicates fructose concentration in fermentations performed at 40 °C; filled triangle indicates logarithm of cell concentration in fermentations performed at 40 °C
Table 2.
Effect of the temperature and culture medium on the fermentative capacity of S. cerevisiae strains
| CH7, 30 °C MJ |
CH7, 40 °C MJ |
PA18, 30 °C MJ |
PA18, 40 °C MJ |
CH7, 30 °C SM | CH7, 40 °C SM |
PA18, 30 °C SM |
PA18, 40 °C SM | |
|---|---|---|---|---|---|---|---|---|
| Ethyl acetate (mg/L) | 55.21 ± 2.34e | 17.18 ± 1.71ab | 44.69 ± 0.87d | 19.11 ± 0.95b | 37.57 ± 0.63c | 12.89 ± 2.77a | 41.16 ± 2.54 cd | 14.4 ± 5ab |
| n-propanol (mg/L) | 11.26 ± 1.5b | 13.9 ± 1.04b | 12.67 ± 1.04b | 12.61 ± 0.61b | 3.9 ± 0.78a | 5.25 ± 1.30a | 3.16 ± 0.26a | 5.74 ± 2.69a |
| Isobutanol (mg/L) | 50.43 ± 6.33e | 12.23 ± 0.55a | 51.24 ± 4.23e | 12.6 ± 0.02a | 47.89 ± 4.33de | 31.54 ± 0b | 39.91 ± 2.02cd | 32.93 ± 4.76bc |
| Amyl alcohol (mg/L) | 5.85 ± 1.11b | 2.75 ± 0.11a | 5.5 ± 0.36b | 2.79 ± 0.14a | 8.98 ± 0.2c | 8.15 ± 0.40c | 7.09 ± 0.83bc | 8.32 ± 1.90c |
| Isoamylic alcohol (mg/L) | 47.54 ± 10.76bc | 15.34 ± 0.02a | 38.62 ± 7.89b | 15.44 ± 0.59a | 52.28 ± 0.77c | 42.03 ± 3.73bc | 38.91 ± 0.55b | 42.44 ± 9.06bc |
| Ethanol (g/L) | 18.77 ± 0.19a | 36.73 ± 0.37de | 23.34 ± 2.00ab | 31.05 ± 3.97bcd | 44.28 ± 3.11e | 33.89 ± 2.69 cd | 38.74 ± 7.73de | 28.50 ± 0.02bc |
| Ethanol yield (g/g) | 0.18 ± 0.00a | 0.30 ± 0.00bcd | 0.22 ± 0.02ab | 0.25 ± 0.03abc | 0.45 ± 0.03 | 0.40 ± 0.00def | 0.41 ± 0.08ef | 0.34 ± 0.07cde |
| Productivity (g/Lh) | 0.31 ± 0.00ab | 0.61 ± 0.01e | 0.39 ± 0.03abc | 0.52 ± 0.07de | 0.46 ± 0.03cd | 0.35 ± 0.03ab | 0.40 ± 0.08bc | 0.30 ± 0.00a |
| Efficient (%) | 36 ± 0.00a | 59 ± 1.00bcd | 44 ± 3.00ab | 50 ± 6.00abc | 89 ± 3.00f | 78 ± 3.00def | 81 ± 8.00ef | 66 ± 0.00cde |
Ethanol yield (Yp/s) was calculated as grams of ethanol produced per gram of utilized sugar. Ethanol productivity was calculated by ratio of ethanol production and fermentation time (60 h for maguey juice fermentation and 96 h for synthetic medium fermentation). The efficiency of sugar conversion was calculated by ratio of the experimental yield (Yp/s) and the theoretical yield (0.51 g/g) multiplied by 100
Each value is the mean of two repetitions ± standard deviation
Value with different superscript letters in the same row are significantly different (p ≤ 0.05)
MJ maguey juice; SM synthetic medium
Production of volatile compounds
Maguey juice fermented by S. cerevisiae strain CH7 presented 55 mg/L of ethyl acetate at 30 °C (Table 2). While this strain only produced 37 mg/L in fermentation of the synthetic medium under the same conditions. Fermentation performed at 40 °C using both CH7 and PA18 strains produced lower ethyl acetate concentrations than that reached at 30 °C (Table 2). Our results showed that ethyl acetate production depends on the culture medium composition and the fermentation temperature. It is produced in greater concentration at 30 °C (significant differences were observed) than that obtained at 40 °C. It has been reported that ethyl acetate is produced in higher concentration when fermentation is performed at low temperature 20 °C probably due to the differential expression of genes involved in ester formation (Beltran et al. 2006; Molina et al. 2007). Heat stress causes a repression of the genes encoding for alcohol acetyl transferase enzymes (ATF1), which is responsible for ester acetate formation (Verstrepen et al. 2003). This ester concentration was also higher in maguey juice fermentation than that reached in the synthetic medium. This finding could be attributed to the nutritional complexity of the juice, considering that naturally it contains amino acids and that ammonium sulphate was added. It has been published that a high nitrogen concentration in the fermentation medium increases the ethyl acetate concentration (Torrea et al. 2011).
As shown in Table 2, the n-propanol concentration was higher in maguey juice fermentation that in synthetic medium. Both strains produced equal concentrations of isobutanol (50 mg/L) in maguey juice at 30 °C, while at 40 °C only 12 mg/L was produced. In a similar way, in synthetic medium at 30 °C this metabolite was higher than that obtained at 40 °C (Table 2). The concentration of amyl alcohol varied in a range of 2.75 to 8.98 mg/L, being the higher concentration (~ 8 mg/L) in synthetic medium. In maguey juice, isoamyl alcohol was found at a higher concentration in fermentation performed at 30 °C than that obtained at 40 °C (Table 2). In this study, under fermentation conditions evaluated, acetic acid was not detected. The production of n-propanol was higher in the maguey juice than in synthetic medium. This alcohol is positively affected when the initial nitrogen concentration in the medium is high (Rollero et al. 2015), and it is derived exclusively from nitrogen metabolism, mainly of threonine consumption (Mouret et al. 2004). The production of isobutanol and isoamyl alcohol was affected by the fermentation temperature. They reached higher concentrations at 30 °C than 40 °C. The synthesis of these metabolites is dependent both on amino-acid precursors and central carbon metabolism, where the temperature had different metabolic and genomic effects on the synthesis pathways (Mouret et al. 2004). Lower temperatures generally support the synthesis of higher alcohols (Molina et al. 2007). The results based on the fermentation temperature help to partially explain the variety in organoleptic characteristics between mezcals produced in regions where the temperature is different or those that are produced in distinct seasons of the year. Both temperature and culture medium were factors that influenced on the ethanol and volatile compounds production. In this study important physiological traits of the indigenous Saccharomyces cerevisiae isolated from maguey juice suggested their possible application as starter cultures in maguey fermentation.
Acknowledgements
This work was supported by Dirección General de Asuntos del Personal Académico de la Universidad Nacional Autónoma de México (DGAPA-UNAM), project IT-200812; and Programa de Apoyo a la Investigación y al posgrado (PAIP-UNAM), Project 50009100.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflicts of interest.
References
- Aparecida-Oliveira V, Araújo-Vicente M, Gomes-Fieeto L, de Miranda-Castro I, Coutrim MX, Schüller D, Alves H, Casal M, de Oliveira-Santos J, Dias-Araújo L, Alves da Silva PH, Lopes-Brandão R. Biochemical and molecular characterization of Saccharomyces cerevisiae strains obtained from sugar-cane juice fermentations and their impact in cachaça production. Appl. Environ. Microbiol. 74: 693–701 (2008) [DOI] [PMC free article] [PubMed]
- Badotti F, Belloch C, Rosa CA, Barrio E, Querol A. Physiological and molecular characterisation of Saccharomyces cerevisiae cachaça strains isolated from different geographic regions in Brazil. World J. Microbiol. Biotechnol. 2010;26:579–587. doi: 10.1007/s11274-009-0206-0. [DOI] [Google Scholar]
- Belloch C, Orlic S, Barrio E, Querol A. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int. J. Food Microbiol. 2008;122:188–195. doi: 10.1016/j.ijfoodmicro.2007.11.083. [DOI] [PubMed] [Google Scholar]
- Beltran G, Novo M, Leberre V, Sokol S, Labourdette D, Guillamon JM, Mas A, François J, Rozes N. Integration of transcription and metabolic analysis for understanding the global responses of low-temperature winemaking fermentations. FEMS Yeast Res. 2006;6:1167–1183. doi: 10.1111/j.1567-1364.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Montaño DM, Délia ML, Estarrón-Espinosa M, Strehaiano P. Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzyme Microb. Tech. 2008;42:608–616. doi: 10.1016/j.enzmictec.2007.12.007. [DOI] [Google Scholar]
- Jiménez-Martí E, Zuzuarregui A, Gomar-Alba M, Gutiérrez D, Gil C, del Olmo M. Molecular response of Saccharomyces cerevisiae wine and laboratory strains to high sugar stress conditions. Int. J. Food Microbiol. 2011;145:211–220. doi: 10.1016/j.ijfoodmicro.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Kirchmayr MR, Segura-García LE, Lappe-Oliveras P, Moreno-Terrazas R, de la Rosa M, Gschaedler-Mathis A. Impact of enviromental conditions and process modifications on microbial diversity, fermentation efficiency and chemical profile during the fermentation of mezcal in Oaxaca. LWT - Food Sci. Technol. 2017;79:160–169. doi: 10.1016/j.lwt.2016.12.052. [DOI] [Google Scholar]
- Legras JL, Karst F. Optimization of interdelta analysis for Saccharomyces cerevisiae strain characterization. FEMS Microbiol. lett. 2003;221:249–255. doi: 10.1016/S0378-1097(03)00205-2. [DOI] [PubMed] [Google Scholar]
- Liu PT, Lu L, Duan CQ, Yan GL. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT - Food Sci. Technol. 2016;71:356–363. doi: 10.1016/j.lwt.2016.04.031. [DOI] [Google Scholar]
- Molina AM, Swiegers JH, Valera C, Pretorius IS, Agosin E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007;77:675–687. doi: 10.1007/s00253-007-1194-3. [DOI] [PubMed] [Google Scholar]
- Mouret JR, Camarasa C, Angenieux M. Aguera E, Perez M, Farines V, Sablayrolles JM. Kinetic analysis and gas-liquid balances of the production of fermentative aromas during winemaking fermentations: effect of assimilable nitrogen and temperature. Food Res. Int. 62: 1-10 (2004)
- Norma Oficial Mexicana NOM-070-SCFI-2016. Bebidas alcohólicas-mezcal-especificaciones. Secretaria de economía. Publicada en el Diario Oficial de la Federación el 23 de febrero de 2017
- Novo M, Gonzalez R, Bertran E, Martínez M, Yuste M, Morales P. Improvement fermenation kinetics by wine yeast strains evolved under ethanol stress. LWT-Food Sci. Technol. 2014;58:166–172. doi: 10.1016/j.lwt.2014.03.004. [DOI] [Google Scholar]
- Nuñez-Guerrero ME, Páez-Lerma JB, Rutiaga-Quiñones OM, González-Herrera SM, Soto-Cruz NO. Performance of mixtures of Saccharomyces and non-Saccharomyces native yeasts during alcoholic fermentation of Agave duranguensis juice. Food Microbiol. 2016;54:91–97. doi: 10.1016/j.fm.2015.10.011. [DOI] [Google Scholar]
- Páez-Lerma JB, Arias-García A, Rutiaga-Quiñones OM, Barrio E, Soto-Cruz NO. Yeasts isolation from the alcoholic fermentation of Agave duranguensis during mezcal production. Food Biotechnol. 2013;27:342–356. doi: 10.1080/08905436.2013.840788. [DOI] [Google Scholar]
- Pavel AB, Vasile CI. PyElph-a software tool for gel image analysis and phylogenetics. BMC Bioinformatics. 2012;13:9–14. doi: 10.1186/1471-2105-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Jaramillo N, Estarrón-Espinosa M, Escalona-Buendía H, Cosío-Ramírez R, Martín-del-Campo S. Volatile compound generation during different stages of the tequila production process. A preliminary study. LWT-Food Sci. Technol. 2015;61:471–483. doi: 10.1016/j.lwt.2014.11.042. [DOI] [Google Scholar]
- Regodón JA, Peréz F, Valdés ME, De Miguel C, Ramírez M. A simple and effective procedure for selection of wine yeast strains. Food Microbiol. 1997;14:247–254. doi: 10.1006/fmic.1996.0091. [DOI] [Google Scholar]
- Rodríguez ME, Infante JJ, Molina M, Domínguez M, Rebordinos L, Cantoral JM. Genomic characterization and selection of wine yeast to conduct industrial fermentations of a white wine produced in a SW Spain winery. J. Appl. Microbiol. 2010;108:1292–1302. doi: 10.1111/j.1365-2672.2009.04524.x. [DOI] [PubMed] [Google Scholar]
- Rollero S, Bloem A, Camarasa C, Sanchez I, Ortiz-Julien A, Sablayrolles JM, Dequin S, Mouret JR. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015;99:2291–2304. doi: 10.1007/s00253-014-6210-9. [DOI] [PubMed] [Google Scholar]
- Santiago-Urbina JA, Arias-García JA, Ruiz-Terán F. Yeast species associated with spontaneous fermentation of taberna, a traditional palm wine from the southeast of Mexico. Ann. Microbiol. 2015;65:287–296. doi: 10.1007/s13213-014-0861-8. [DOI] [Google Scholar]
- Segura-García LE, Taillandier P, Brandam C, Gschaedler A. Fermentative capacity of Saccharomyces and non-Saccharomyces in agave juice and semi-synthetic medium. LWT-Food Sci. Technol. 2015;60:284–291. doi: 10.1016/j.lwt.2014.08.005. [DOI] [Google Scholar]
- Serra A, Strehaiano P, Taillandier P. Influence of temperature and pH on Saccharomyces bayanus var. uvarum growth; impact of a wine yeast interspecific hybridization on these parameters. Int. J. Food Microbiol. 104: 257–265 (2005) [DOI] [PubMed]
- Settani L, Sannino C, Francesca N, Guarcello R, Moschetti G. Yeast ecology of vineyards within Marsala wine area (Western Sicily) in two consecutives vintages and selection of autochthonous Saccharomyces cerevisiae strains. J. Biosci. Bioeng. 2012;114:606–614. doi: 10.1016/j.jbiosc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Stanley D, Bandara A, Fraser S, Chamber PJ, Stanley GA. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010;109:13–24. doi: 10.1111/j.1365-2672.2009.04657.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Guo J, Liu F, Liu Y. Identification od indigenous yeast flora isolated from the five winegrape varieties harvested in Xiangning, China. Antonie Van Leeuwenhoek. 2014;105:533–540. doi: 10.1007/s10482-013-0105-0. [DOI] [PubMed] [Google Scholar]
- Torrea D, Valera C, Ugliano M, Ancin-Azpilicuenta C, Francis IL, Henschke PA. Comparison of inorganic and organic nitrogen supplementation of grape juice-effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011;127:1072–1083. doi: 10.1016/j.foodchem.2011.01.092. [DOI] [PubMed] [Google Scholar]
- Vera-Guzmán AM, López MG, Chávez-Servia JL. Chemical composition and volatile compounds in the artisanal fermentation of mezcal in Oaxaca. Mexico. Afr. J. Biotechnol. 2012;11:14344–14353. [Google Scholar]
- Verdugo-Valdez A, Segura García L, Kirchmayr M, Ramírez-Rodríguez P, González-Esquinca A, Coria R, Gschaedler-Mathis A. Yeast communities associated with artisanal mezcal fermentations from Agave salmiana. A. Van Leeuw. J. Microb. 2011;100:497–506. doi: 10.1007/s10482-011-9605-y. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Derdelinckx G, Dufour JP, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR. The Saccharomyces cerevisiae alcohol acetyl transferase gene ATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res. 2003;4:285–296. doi: 10.1016/S1567-1356(03)00166-1. [DOI] [PubMed] [Google Scholar]