Abstract
Probiotic properties including antioxidant and immune-enhancing effects of Lactobacillus plantarum 200655 isolated from kimchi were evaluated. The tolerance of three strains (L. plantarum 200655, L. plantarum KCTC 3108, and L. rhamnosus GG to bile salts (0.3% oxgall, 24 h) was similar, and L. plantarum 200655 showed the highest tolerance to gastric juice (0.3% pepsin, 3 h). All strains presented similar autoaggregation ability. L. plantarum 200655 showed higher cell surface hydrophobicity and adhesion ability on HT-29 cells. L. plantarum 200655 did not produce β-glucuronidase and was sensitive to ampicillin, tetracycline, chloramphenicol, and doxycycline. Additionally, L. plantarum 200655 showed the highest antioxidant effects in DPPH and ABTS radical scavenging, and β-carotene bleaching assays. RAW 264.7 cells treated with L. plantarum 200655 produced more nitric oxide, induced nitric oxide synthase, and cytokine related to immune-enhancing effects such as interleukin-1β and interleukin-6. Therefore, L. plantarum 200655 could be useful as a probiotic strain for older people.
Keywords: Probiotics, Lactobacillus plantarum, Kimchi, Antioxidant activity, Immune-enhancing effect
Introduction
The world population of individuals over 60 years of age is increasing dramatically. Compared to 1980 when there were 382 million older people, studies have shown that the elderly population had doubled by 2017, with an elderly population of 962 million people worldwide (Population Division, 2017). Older persons show oxidative stress and weakened immune system, which eventually leads to various diseases such as infection and cancer (Castelo-Branco and Soveral, 2014).
An imbalance in the large amount of reactive oxygen species (ROS) and low antioxidant capacity is known as oxidative stress. ROS include superoxide radical, hydrogen peroxide, hydroxyl radical, and hypochlorite ion, which destabilize proteins, DNA, and cell structures by reacting strongly and oxidizing with other substances (Ji et al., 2015; Tang et al., 2017). Excessive ROS levels are related to aging, cancer, Alzheimer’s disease, and cardiovascular disease but can be controlled by consumption of antioxidant supplements (Mishra et al., 2015). Therefore, it is necessary to development of nutritional supplement for healthy life of older people.
In recent years, probiotics were shown to reduce oxidative stress and exhibit antioxidant effects by modifying antioxidative enzymes, and immune-enhancing effects (Chang et al., 2015; Mishra et al., 2015). Probiotics are defined as live microorganism which, when administered in proper amounts, confer a beneficial effect on the host (FAO/WHO, 2002). To be used as probiotics, microorganisms should have the capacity to withstand the physical and chemical conditions in the human body and colonize and adhere to intestinal epithelial cells (Han et al., 2017). They must also be non-pathogenic and sensitive to antibiotics (Shao et al., 2015). Probiotics are found in natural environment, feces of infants, and various fermented foods (Han et al., 2017).
Kimchi is a famous traditional vegetable food in Korea that is fermented by lactic acid bacteria (LAB) at low temperature (Jeon et al., 2017). During the storage period, LAB produce the unique flavor of kimchi and compounds beneficial for health by changing the composition of kimchi through fermentation (Lee et al., 2016). Particularly, Lactobacillus plantarum is the most important and dominant microorganism during the middle and later steps of kimchi fermentation (Lee et al., 2015a). L. plantarum is considered as a generally recognized as safe (GRAS) microorganism and has been used as probiotic strain and to ferment a variety of foods, including dairy products, meat, coffee, and many plants (Jeon et al., 2016). Additionally, many studies have been reported that L. plantarum has inflammatory attenuation, immune stimulation, and antioxidant activities (Jiang et al., 2016). However, there are few LAB having both antioxidant and immune-enhancing activities for older people.
The aim of this study was to identify L. plantarum 200655 isolated from kimchi and investigate its probiotic potentiality, including tolerance to gastric acid and bile salt conditions, enzyme production, adhesion ability to the intestine, and antibiotic susceptibility characteristics. Additionally, the antioxidant and immune-enhancing effects were evaluated in vitro as a beneficial LAB for older people.
Materials and methods
Bacterial strains and sample preparation
L. plantarum 200655 was isolated from cabbage kimchi. Kimchi sample (1 g) was serially diluted and inoculated on de Man, Rogosa, and Sharpe (MRS; BD Biosciences, Franklin Lakes, NJ, USA) agar at 37 °C for 24 h. A colony was inoculated and incubated in MRS broth at 37 °C for 24 h. As a commercial probiotic strain, L. plantarum KCTC 3108 and L. rhamnosus GG were obtained from the Korean Collection for Type Cultures (KCTC, Daejeon, Korea) and were used as reference probiotic strains. LAB samples were prepared as follows: LAB strains were incubated in MRS broth at 37 °C for 18 h before use. To harvest the intact cells, bacterial cultures were centrifuged at 14,240×g at 4 °C for 10 min. The bacterial cells were washed three times and resuspended in phosphate-buffered saline (PBS; Gibco, Grand Island, NY, USA).
Identification of LAB strain
Lactobacillus plantarum 200655 was identified by 16S rRNA sequencing conducted by Bionics Inc. (Seoul, Korea). The sequencing results were analyzed by comparison with the GENBANK database using the Basic Local Alignment Search Tool (BLAST) website (http://blast.ncbi.nlm.nih.gov).
Tolerance to artificial gastric juice and bile salts
To measure the tolerance of Lactobacillus strains, artificial gastric juice and bile salts were prepared as described by Thirabunyanon and Thongwittaya (2012). The strains were incubated in MRS broth at 37 °C for 18 h. Artificial gastric juice [0.3% pepsin (Sigma-Aldrich, St. Louis, MO, USA), pH 2.5] and bile salts [0.3% oxgall (BD Biosciences, Franklin Lakes, NJ, USA)] were inoculated with the overnight culture of the LAB strains at a final concentration of 1 × 107 CFU/mL and incubated at 37 °C for 3 and 24 h, respectively. The percentage of surviving bacteria was determined by counting viable cells on the MRS plates.
Enzyme activity
Enzyme activity was assessed by using the API ZYM kit (BioMerieux, Lyon, France). Bacterial samples (65 μL) were inoculated in each cupule and incubated at 37 °C for 4 h. A drop of ZYM A and ZYM B reagents was added to each cupule. The level of enzyme activity was scored as 0 (no activity) to 5 (≥ 40 nM) based on the color change.
Antibiotic susceptibility
The antibiotic susceptibility of L. plantarum 200655 was determined by the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) standards. One hundred microliters of each Lactobacillus strain (1 × 107 CFU/mL) was spread onto MRS agar and paper disks were plated after a few minutes. Paper disks contained eight antibiotics: ampicillin (0.2 mg/mL), gentamicin (0.2 mg/mL), kanamycin (0.6 mg/mL), streptomycin (0.2 mg/mL), tetracycline (0.6 mg/mL), ciprofloxacin (0.1 mg/mL), chloramphenicol (0.6 mg/mL), and doxycycline (0.6 mg/mL). After incubation at 37 °C for 24 h, the inhibition zone (mm) was investigated.
Cell surface hydrophobicity
Cell surface hydrophobicity was measured as described by Iyer et al. (2010). Bacterial samples were adjusted to absorbance value of 0.5 ± 0.02 at 600 nm with PBS. Next, 3 mL of bacterial suspension and 1 mL xylene was mixed and incubated at 37 °C for 20 min. After incubation, the aqueous supernatant was harvested and absorbance was determined at 600 nm. Cell surface hydrophobicity (%) was calculated as follows:
where A0 and At are absorbance at 0 h and after incubation with xylene, respectively.
Adhesion ability to HT-29 cells
The adhesion ability of Lactobacillus strains was examined using a human colon adenocarcinoma cell line (HT-29, KCLB 30038). First, 1 × 105 cells/mL of HT-29 cells was inoculated into a 24-well cell culture plate and incubated at 37 °C. After 24 h, Lactobacillus strains (1 × 107 CFU/mL) were inoculated into HT-29 cells and incubated at 37 °C for 2 h. Non-adherent bacterial cells were eliminated by washing three times with PBS. Next, 1% Triton X-100 (Sigma-Aldrich) solution was used to detach the adherent bacterial cells. The number of adherent bacterial cells was determined by counting viable cells on the MRS plates.
Antioxidant activity of LAB strains
The 2,2,-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was measured as described by Tang et al. (2017) with some modification. The DPPH solution (0.4 mM) was prepared in methanol, and 2 mL of bacterial samples or distilled water (control) were mixed with 2 mL of this solution. The mixtures were incubated at room temperature for 30 min in the dark. The absorbance of the mixtures was measured at 517 nm and calculated as follows:
The 2,2′-azinobis (2-ethyl benzothiazoline-6-sulfonate) (ABTS) radical scavenging activity was measured as described by Han et al. (2017) with some modifications. The ABTS solution was composed of 7 mM ABTS and 5 mM potassium persulfate diluted with 20 M sodium phosphate buffer (pH 7.4) to a final absorbance of 0.7 ± 0.02 at 734 nm. Next, 150 μL of bacterial samples and distilled water (control) were mixed with 150 μL of ABTS solution and incubated at 37 °C for 10 min. The absorbance of the mixtures was measured at 734 nm and calculated as follows:
β-Carotene bleaching assay was measured as described by Kachouri et al. (2015) with some modifications. Linoleic acid (66 μL), 3 mg of β-carotene, and 300 μL of Tween 80 were mixed with 10 mL of chloroform. The mixtures were evaporated to remove the chloroform at 40 °C under vacuum using a rotary evaporator and diluted with 75 mL of distilled water. Bacterial samples (200 μL) and the control (distilled water, 200 μL) were mixed with 4 mL of the emulsion and incubated in a water bath at 50 °C for 2 h. Absorbance was measured at 470 nm for 0 and 2 h. Inhibition of β-carotene and linoleic acid oxidation was calculated according to the following equation:
Nitro oxide (NO) production on RAW 264.7 cells
A murine macrophage cell line, RAW 264.7 cells, was obtained from the Korean Cell Line Bank (KCLB 40071; Korea). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Hyclone, Logan, UT, USA) with 10% (v/v) fetal bovine serum and 1% (v/v) streptomycin/penicillin solution (Hyclone, Logan, UT, USA). The amount of nitro oxide in the cell culture medium was evaluated according to Lee et al. (2015b). RAW 264.7 cells (2 × 105 cells/well) were plated into 96-well cell culture plates and incubated with lipopolysaccharide (LPS, 10 ng/mL) and LAB strains (105 CFU/mL) for 24 h. After incubation, 100 µL of the cell culture medium was added to the same amount of Griess reagent. The absorbance of the mixtures was measured at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). NO production was calculated by comparison with sodium nitrate as a standard.
Immune-enhancing effect of LAB strains
The immune-enhancing effect of LAB strains was determined as described by Chang et al. (2015) with some modifications. RAW 264.7 cells were incubated into a 6-well plate (1 × 106 cells/mL) for 24 h and incubated for 24 h again with LPS (10 ng/mL) or LAB strains (1 × 105 CFU/mL). Total RNA was isolated from RAW 264.7 cells using the RNeasy® Mini Kit (QIAGEN, Hilden, Limburg, Germany) and cDNA was synthesized using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). The expression levels of induced nitric oxide synthase and cytokine related to immune-enhancing effects were determined by using the SYBR Green PCR Master mix with semi-quantitative real-time PCR (PikoReal 96, Scientific Pierce, Waltham, MA, USA). The primers were listed in Table 1. Semi-quantitative real-time PCR was performed as follows: 95 °C for 2 min for polymerase activation, followed by 40 cycles of 95 °C for 5 s for denaturation, and 60 °C for 15 s for annealing/extension. The results were analyzed by using the delta–delta Cq method. The melting curve was used to analyze the measurement of reaction specificity.
Table 1.
Primer sequences related immune-enhancing effect used in semi-quantitative real-time PCR
| Primera | Sequence (5′–3′) | |
|---|---|---|
| iNOS | (Sense) | CCCTTCCGAAGTTTCTGGCAGC |
| (Antisense) | GGCTGTCAGAGCCTCGTGGCTTTGG | |
| IL-1β | (Sense) | CAGGATGAGGACATGAGCACC |
| (Antisense) | CTCTGCAGACTCAAACTCCAC | |
| IL-6 | (Sense) | GTACTCCAGAAGACCAGAGG |
| (Antisense) | TGCTGGTGACAACCACGGCC | |
| TNF-α | (Sense) | TTGACCTCAGCGCTGAGTTG |
| (Antisense) | CCTGTAGCCCACGTCGTAGC | |
| β-Actin | (Sense) | GTGGGCCGCCCTAGGCACCAG |
| (Antisense) | GGAGGAAGAGGATGCGGCAGT |
aiNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α
Statistical analysis
All experiments were repeated in at least triplicate, and one-way analysis of variance (ANOVA) and Duncan’s multiple rang tests were conducted using SPSS software (Version 24; SPSS, Inc., Chicago, IL, USA) to detect significant differences.
Results and discussion
Identification of isolated LAB strain
Ageing changes the composition of the intestinal microorganisms, especially the number of Bifidobacterium and Lactobacillus strains decreased (Tiihonen et al., 2010). In addition, these changes in intestinal tract were related to disease outbreaks (Hopkins et al., 2001). Therefore, reasonable intake of Lactobacillus probiotic strain could be helpful to older people. Isolated Lactobacillus strain was isolated from kimchi and identified by 16S rRNA sequencing analysis. By comparing the rRNA gene sequence of the isolated LAB strain and GenBank data, the isolated LAB strain was identified as L. plantarum, with 99% similarity to L. plantarum a66, GenBank number KX057547.1 (data not shown).
Tolerance to artificial gastric juice and bile salts
Ingested substances pass through the stomach where the pH is 2.5–3.5 to prevent the entry of pathogens and through the intestinal tract which contains 0.3% (w/v) bile salts (Han et al., 2017; Jeon et al., 2016). Therefore, probiotic LAB strains must be tolerant to acid and bile salts. The tolerance of Lactobacillus strains to artificial gastric juice and bile salts was presented in Table 2. There was significant difference (p < 0.01) in acid conditions among the LAB strains. While L. plantarum 200655 was increased by 0.18 Log CFU/mL in an acidic environment, viable cells of L. rhamnosus GG and L. plantarum KCTC 3108 were decreased by 0.34 and 0.16 Log CFU/mL, respectively. Under basic conditions for 24 h, viable cells of Lactobacillus strains increased to more than the initial cell number. Specifically, the number of L. plantarum 200655 showed the greatest increase in bile salts compared to other commercial strains. However, there was no significant difference (p > 0.05) in bile salts conditions among the LAB strains. L. casei Zhang exhibited similar tolerance to basic conditions, while its tolerance to acidic conditions (pH 2.5, 0.3% pepsin) was less than 70% (Guo et al., 2009). In another study, the survival rate of L. plantarum KLDS1.0391 was 92.67% and 97.62% in the presence of 0.3% pepsin and 0.3% bile salt, respectively (Han et al., 2017). Because L. plantarum 200655 can withstand the artificial acidic and basic conditions, this strain could be expected to survive in the human gastrointestinal tract.
Table 2.
Tolerance of LAB strains to artificial gastric juice and bile salts
| Treatment | Viable cell number (Log CFU/mL) | ||
|---|---|---|---|
| L. rhamnosus GG | L. plantarum 200655 | L. plantarum KCTC 3108 | |
| Tolerance to artificial gastric juice | |||
| Initial cell number | 8.89 ± 0.07 | 8.30 ± 0.26 | 8.36 ± 0.04 |
| 0.3% pepsin, pH 2.5, 3 h | 8.55 ± 0.09 | 8.48 ± 0.04 | 8.20 ± 0.02 |
| Survival rate (%) | 96.69 ± 1.04b1 | 100.03 ± 0.43a | 98.09 ± 0.58b |
| Tolerance to artificial bile salts | |||
| Initial cell number | 8.89 ± 0.07 | 8.30 ± 0.26 | 8.36 ± 0.04 |
| 0.3% oxgall, 24 h | 9.00 ± 0.08 | 8.69 ± 0.03 | 8.42 ± 0.09 |
| Survival rate (%) | 101.83 ± 0.87 | 102.48 ± 0.34 | 100.76 ± 1.42 |
1a–bDifferent superscript letters in the same row indicate significant differences in each characteristic (p < 0.01). All values are mean ± standard deviation of triplicate experiments
Enzyme production of L. plantarum 200655
Probiotics must be evaluated appropriate production of enzymes to prevent from producing of possible toxic substances (Lee et al., 2015b). According to the results obtained using the API ZYM kit, the degree to 19 enzymes production was confirmed (data not shown). L. plantarum 200655 did not produce β-glucuronidase, alkaline phosphate, esterase, esterase lipase, lipase, cystine arylamidase, trypsin, α-chymotrypsin, acid phosphatase, α-mannosidase, and α-fucosidase. Because β-glucuronidase is a bacterial carcinogenic enzyme that exerts negative effects in the liver, LAB should not produce β-glucuronidase (Jeon et al., 2017). The potential probiotic L. brevis FI10700 and L. perolens FI10842 did not produce β-glucuronidase (Son et al., 2017). In contrast, leucin arylamidase, valine arylamidase, naphthol-AS-BI-phosphohydrolase, α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, and N-acetyl-β-glucosaminidase were produced by L. plantarum 200655 (data not shown). β-Galactosidase hydrolyzes lactose into glucose and galactose, and is used to diminishing the lactose intolerance problem (Vasiljevic and Jelen, 2001). β-Glucosidase plays major role in bioconversion of gensenoside, isoflavone, and phenolic compound by breaking down of glycosidic bonds (Son et al., 2017). Therefore, L. plantarum 200655 could be safe against β-glucuronidase and produce useful enzymes.
Antibiotic susceptibility of LAB strains
Probiotic strains should be tested for antibiotic susceptibility to confirm their safety. Transmission of antibiotic resistance genes in LAB may result in novel antibiotic-resistant pathogens (Teuber et al., 1999). L. rhamnosus GG, L. plantarum 200655, and L. plantarum KCTC 3108 were sensitive to ampicillin, tetracycline, chloramphenicol, and doxycycline. However, these LAB strains were resistant to gentamycin, kanamycin, streptomycin, and ciprofloxacin (data not shown). Generally, L. plantarum are resistant to gentamycin, kanamycin, streptomycin, and ciprofloxacin. There were the results that potential probiotic L. plantarum Lb41 and L. plantarum IMAU20697 were resistant to the same antibiotics and accepted CLIS guideline (Jeon et al., 2016; Shao et al., 2015). Therefore, ingestion of L. plantarum 200655 could be safe against antibiotic resistance problems.
Adhesion ability of LAB strains to intestinal epithelial cells
The adhesion ability of LAB to intestinal cells is related the bacteria’s effects, colonization in the human body, and prevention of pathogen infections (García-Cayuela et al., 2014). Due to reduced adhesion to the intestinal mucus of older people, adhesion ability is needed as one of the probiotics properties (Tiihonen et al., 2010). To measure the adhesion and colonization of LAB to human intestinal epithelial cells, cell surface hydrophobicity, and adhesion ability to HT-29 cells were analyzed (Table 3). There was significant difference (p < 0.001) in cell surface hydrophobicity, and adhesion ability to HT-29. Cell surface hydrophobicity of the isolated strains was highly variable (< 13–79%) depending on the LAB cell. L. rhamnosus GG and L. plantarum KCTC 3108 showed hydrophobicity values of 64.02% and 13.62%, respectively. L. plantarum 200655 exhibited a higher hydrophobicity of 78.45% compared to the other LAB tested.
Table 3.
Cell surface hydrophobicity, and adhesion abilities of LAB strains
| Subjects | LAB strains | ||
|---|---|---|---|
| L. rhamnosus GG | L. plantarum 200655 | L. plantarum KCTC 3108 | |
| Cell surface hydrophobicity (%) | 64.02 ± 4.06b | 78.45 ± 2.36a | 13.62 ± 2.55c |
| Adhesion ability (%) | 6.30 ± 0.51b | 14.03 ± 3.37a | 1.38 ± 0.08c |
1a–cDifferent superscript letters in the same row indicate significant differences in each characteristic (p < 0.001). All values are mean ± standard deviation of triplicate experiments
L. plantarum 200655 showed the best adhesion ability (14.03%) compared to L. rhamnosus GG (6.30%) and L. plantarum KCTC 3108 (1.38%). The results of the analysis of adhesion ability to HT-29 cells showed a similar trend as cell surface hydrophobicity in this study and previous research. García-Cayuela et al. (2014) and Son et al. (2017) reported that bacteria with high cell surface hydrophobicity showed higher adhesion ability to intestinal epithelium cells and helps to maintain bacterial cell adhesion. Han et al. (2017) was reported that charge, hydrophobicity, and adhesion to hydrocarbon of bacterial cell were related to adhesion capacity to intestinal epithelial cells. Compared with commercial strains, these results showed that L. plantarum 200655 could attach and colonize intestinal epithelial cells.
Antioxidant effects of LAB strains
Christen (2000) reported that as people get older, the accumulation of ROS damage to cells structure and caused the neurodegenerative disease and cancer. To prevent generating ROS, antioxidant activity of LAB was evaluated by using radical scavenging and lipid peroxidation inhibition activity. The results of analysis of the in vitro antioxidant effect of LAB strains are shown in Table 4 and showed significant different in antioxidant activities with three different assays (p < 0.001). The DPPH radical scavenging assay is widely used to evaluate the hydrogen donation of antioxidants (Das and Goyal, 2015). L. plantarum 200655 showed a maximum DPPH scavenging activity of 30.51% at 108 CFU/mL, which was 2.58% and 10.31% higher than those of L. rhamnosus GG (27.93%) and L. plantarum KCTC 3108 (20.20%), respectively (p < 0.001).
Table 4.
Antioxidant activity of LAB strains
| Subjects | LAB strains | ||
|---|---|---|---|
| L. rhamnosus GG | L. plantarum 200655 | L. plantarum KCTC 3108 | |
| Inhibition rate of β-carotene and linoleic acid oxidation (%) | 43.34 ± 0.58b1 | 48.95 ± 1.41a | 11.04 ± 1.17c |
| DPPH radical scavenging activity (%) | 27.93 ± 1.97a | 30.51 ± 1.60a | 20.20 ± 1.22b |
| ABTS radical scavenging activity (%) | 24.76 ± 1.58c | 38.13 ± 0.10a | 35.03 ± 0.66b |
1a–cDifferent superscript letters in the same row indicate significant differences in each characteristic (p < 0.001). All values are mean ± standard deviation of triplicate experiments
ABTS is oxidized by potassium persulfate to its radical form, ABTS+∙, which is a dark color, and antioxidant ability can be evaluated as the decolorization of the ABTS radical (Mishra et al., 2015). The results of ABTS radical scavenging activity (%) showed that LAB strains exhibited various scavenging activities in the following order: L. plantarum 200655 (38.13%), L. plantarum KCTC 3108 (35.03%), and L. rhamnosus GG (24.76%).
As indicated in Table 4, LAB strains could prevent lipid peroxidation. Particularly, L. plantarum 200655 showed the highest inhibition rate of β-carotene and linoleic acid oxidation of 48.95%. The inhibition rates of L. rhamnosus GG and L. plantarum KCTC 3108 were 43.34% and 11.04%, respectively (p < 0.001).
The antioxidant effects of LAB were determined by evaluating radical scavenging activities, antioxidant enzymes in bacterial cells, and antioxidant-related genes. A previous study by Tang et al. (2017) found the highest radical scavenging capacity of the intact cells of L. plantarum MA2 compared to the culture supernatant and cell-free extract. This effect was related to cell surface active substances such as protein, polysaccharides, and lipoteichoic acid. Additionally, antioxidant enzymes, Mn2+ ion, mercapto compounds, bioactive peptide, and exopolysaccharides present in LAB cells were reported to influence antioxidant effects (Kullisaar et al., 2002; Li et al., 2014). Based on the radical scavenging activities and potential active substances, L. plantarum 200655 has superior antioxidant effects.
Immune-enhancing effect of LAB strains
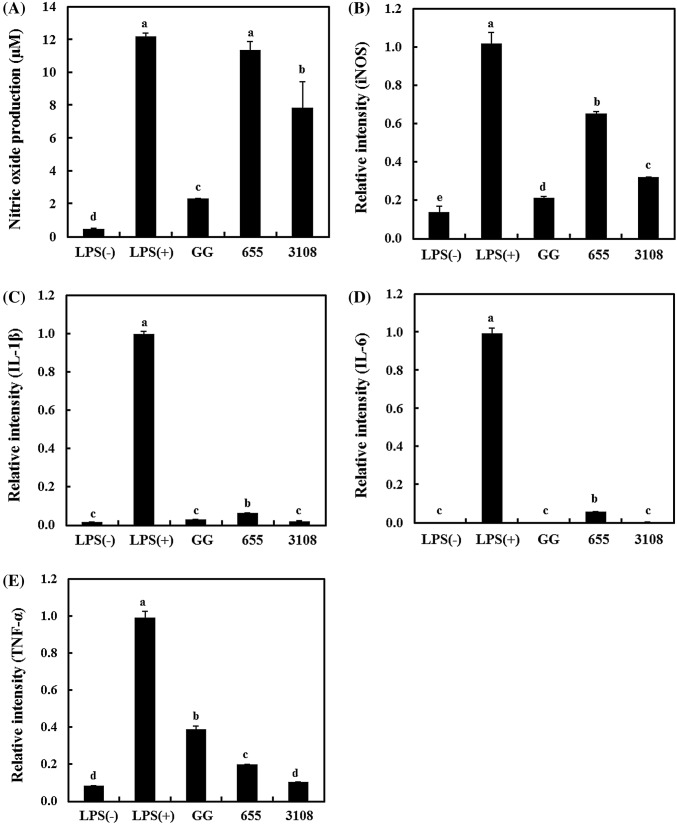
To confirm the immune-enhancing effect of LAB, NO production was measured in RAW 264.7 cells (Fig. 1A) and the treated LPS (10 ng/mL) cells was used as a positive control to stimulate RAW 264.7 cells. All treated LAB samples produced more NO compared to cells not treated with LPS(−) (0.47 μM). The treated LPS and L. plantarum 200655 cells produced higher NO concentration of 12.18 μM and 11.38 μM, respectively compared to cells cultured with L. plantarum KCTC 3108 (7.83 μM), and L. rhamnosus GG (2.31 μM). Based on the fact that NO is generated by RAW 264.7 cells, induced nitric oxide synthase (iNOS) and immune-related cytokine expression was examined (Fig. 1B–D). Because NO is induced via oxidation of l-arginine by iNOS, iNOS production by LAB strains showed results similar to those of NO production (Lee et al., 2007).
Fig. 1.
Immune-enhancing effects of LAB strains on RAW.264.7 cells. (A) Nitric oxide expression (μM); relative intensity of (B) iNOS, (C) IL-1β, (D) IL-6, and (E) TNF-α expression. LPS(−), non-treated; LPS(+), 10 ng/mL of lipopolysaccharide-treated; GG, L. rhamnosus GG-treated; 655, L. plantarum 200655-treated; 3108, L. plantarum KCTC 3108-treated. Different letters on each bars indicate significant difference between values (p < 0.001)
Cytokine produced by cells in response to external stimulation are small proteins with approximately 25-kDa molecular weights and affect other cells by binding to specific receptors on cell surfaces. The major cytokine synthesized by activated macrophages are interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF-α) (Parham, 2015). Furthermore, the group cultured with L. plantarum 200655 showed higher cytokine production of IL-1β and IL-6. Among the cells treated with LAB strains, TNF-α production was not the highest, but higher than that in untreated cells. These results indicate that L. plantarum 200655 exerts immune-enhancing effects by stimulating macrophages.
Jeong et al. (2015) reported that NO production and cytokine induction were increased depending on the dose and treatment time of lipoteichoic acid (LTA) isolated from four LAB strains including L. plantarum, L. sakei, L. rhamnosus, and L. delbrueckii. TNF-α production was induced by LTAs isolated from L. sakei K101 and LTAs isolate from L. plantarum and L. rhamnosus increased IL-10 concentration. The high immune-enhancing effect of L. plantarum 200655 may be related to the interaction of LTA on RAW 264.7 cells.
In conclusion, L. plantarum 200655 isolated from kimchi showed higher tolerance to gastric acid and bile salt, cell surface hydrophobicity, and adhesion ability to HT-29 cells. In addition, L. plantarum 200655 was safe against production of toxic substances and antibiotic resistance. The results of three different antioxidant activities in vitro showed that L. plantarum 200655 exhibited radical scavenging activity and lipid peroxidation inhibition activity. L. plantarum 200655 enhanced immunity on RAW 264.7 cells according to the results of NO production and mRNA expression of iNOS and cytokines. Therefore, L. plantarum 200655 could be used as potential probiotics and help to promote health for older people.
Acknowledgements
This research was supported by the “Leaders in INdustry-university Cooperation +” Project of the Ministry of Education and National Research Foundation of Korea (Grant Number G2017A0030055) and the High Value-added Food Technology Development Program of the Ministry of Agriculture, Food, and Rural Affairs (Grant Number 314073-03).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Castelo-Branco C, Soveral I. The immune system and aging: A review. Gynecol. Endocrinol. 2014;30:16–22. doi: 10.3109/09513590.2013.852531. [DOI] [PubMed] [Google Scholar]
- Chang CK, Wang SC, Chiu CK, Chen SY, Chen ZT, Duh PD. Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac. J. Trop. Biomed. 2015;5:281–286. doi: 10.1016/S2221-1691(15)30346-4. [DOI] [Google Scholar]
- Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- Das D, Goyal A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-Food Sci. Technol. 2015;61:263–268. doi: 10.1016/j.lwt.2014.11.013. [DOI] [Google Scholar]
- FAO/WHO. Guideline for the Evaluation of Probiotics in Food. London, Ontario, Canada (2002)
- García-Cayuela T, Korany AM, Bustos I, Gómez de Cadiñanos LP, Requena T, Peláez C, Martínez-Cuesta MC. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014;57:44–50. doi: 10.1016/j.foodres.2014.01.010. [DOI] [Google Scholar]
- Guo Z, Wang J, Yan L, Chen W, Liu XM, Zhang HP. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT-Food Sci. Technol. 2009;42:1640–1646. doi: 10.1016/j.lwt.2009.05.025. [DOI] [Google Scholar]
- Han Q, Kong B, Chen Q, Sun F, Zhang H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Food. 2017;32:391–400. doi: 10.1016/j.jff.2017.03.020. [DOI] [Google Scholar]
- Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture. 16S rRNA abundance and community cellular fatty acid profiles. Gut. 2001;48:198–205. doi: 10.1136/gut.48.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Tomar SK, Kapila S, Mani J, Singh R. Probiotic properties of folate producing Streptococcus thermophilus strains. Food Res. Int. 2010;43:103–110. doi: 10.1016/j.foodres.2009.09.011. [DOI] [Google Scholar]
- Jeon EB, Son SH, Jeewanthi RKC, Lee NK, Paik HD. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Sci. Biotechnol. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HL, Lee NK, Yang SJ, Kim WS, Paik HD. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017;26:1641–1648. doi: 10.1007/s10068-017-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Jang S, Jung BJ, Jang KS, Kim BG, Chung DK, Kim H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology. 2015;220:460–466. doi: 10.1016/j.imbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Ji K, Jang NY, Kim YT. Isolation of lactic acid bacteria showing antioxidative and probiotic activities from kimchi and infant feces. J. Microbiol. Biotechn. 2015;25:1568–1577. doi: 10.4014/jmb.1501.01077. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang F, Wan C, Xiong Y, Shah NP, Wei H, Tao X. Evaluation of probiotic properties of Lactobacillus plantarum WLPL04 isolated from human breast milk. J. Dairy Sci. 2016;99:1736–1746. doi: 10.3168/jds.2015-10434. [DOI] [PubMed] [Google Scholar]
- Kachouri F, Ksontini H, Kraiem M, Setti K, Mechmeche M, Hamdi M. Involvement of antioxidant activity of Lactobacillus plantarum on functional properties of olive phenolic compounds. J. Food Sci. Tech. Mys. 2015;52:7924–7933. doi: 10.1007/s13197-015-1912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002;72:215–224. doi: 10.1016/S0168-1605(01)00674-2. [DOI] [PubMed] [Google Scholar]
- Lee KW, Shim JM, Park SK, Heo HJ, Kim HJ, Ham KS, Kim JH. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT-Food Sci. Technol. 2016;71:130–137. doi: 10.1016/j.lwt.2016.03.029. [DOI] [Google Scholar]
- Lee ME, Jang JY, Lee JH, Park HW, Choi HJ, Kim TW. Starter cultures for kimchi fermentation. J. Microbiol. Biotechn. 2015;25:559–568. doi: 10.4014/jmb.1501.01019. [DOI] [PubMed] [Google Scholar]
- Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Sci. Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- Lee TH, Kwak HB, Kim HH, Lee ZH, Chung DK, Baek NI, Kim J. Molecules and methanol extracts of Stewartia koreana inhibit cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) gene expression by blocking NF-κB transactivation in LPS-activated. Mol. Cell. 2007;23:398–404. [PubMed] [Google Scholar]
- Li W, Ji J, Chen X, Jiang M, Rui X, Dong M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014;102:351–359. doi: 10.1016/j.carbpol.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015;63:3615–3626. doi: 10.1021/jf506326t. [DOI] [PubMed] [Google Scholar]
- Population Division. DESA. World population ageing 2017. United Nations. 1–124 (2017)
- Parham P. The immune system. 4rd ed. Garland Science. USA. pp. 62 (2015)
- Shao Y, Zhang W, Guo H, Pan L, Zhang H, Sun T. Comparative studies on antibiotic resistance in Lactobacillus casei and Lactobacillus plantarum. Food Control. 2015;50:250–258. doi: 10.1016/j.foodcont.2014.09.003. [DOI] [Google Scholar]
- Son SH, Jeon HL, Yang SJ, Lee NK, Paik HD. In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb. Pathog. 2017;112:135–141. doi: 10.1016/j.micpath.2017.09.053. [DOI] [PubMed] [Google Scholar]
- Tang W, Xing Z, Li C, Wang J, Wang Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017;221:1642–1649. doi: 10.1016/j.foodchem.2016.10.124. [DOI] [PubMed] [Google Scholar]
- Teuber M, Meile L, Schwarz F. Acquired antibiotic resistance in lactic acid bacteria from food. Antonie Leeuwenhoek. 1999;76:115–137. doi: 10.1023/A:1002035622988. [DOI] [PubMed] [Google Scholar]
- Thirabunyanon M, Thongwittaya N. Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella Enteritidis infection. Res. Vet. Sci. 2012;93:74–81. doi: 10.1016/j.rvsc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010;9:107–116. doi: 10.1016/j.arr.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Vasiljevic T, Jelen P. Production of β-galactosidase for lactose hydrolysis in milk and dairy products using thermophilic lactic acid bacteria. Innov. Food Sci. Emerg. Technol. 2001;2:75–85. doi: 10.1016/S1466-8564(01)00027-3. [DOI] [Google Scholar]