Abstract
In order to improve the slow ethanol fermentation during acetic acid fermentation process of black raspberry vinegar (BRV), the microbiological and physicochemical aspects of the effects of indigenous Saccharomyces cerevisiae JBCC-21A were examined. The selected S. cerevisiae JBCC-21A showed better growth and ethanol production rates than the commercial yeast strains. The ethanol production rate was 3-times faster than the traditional method. Acetic acid fermentation by S. cerevisiae JBCC-21A began 10 days earlier than the traditional method and reached up to 60 g/L acetic acid. Bacterial counts revealed Acetobacter pasteurianus was the only dominant species throughout the inoculated acetic acid fermentation. The physicochemical and functional properties of the fermented vinegar using indigenous S. cerevisiae JBCC-21A maintained a high quality similar to the traditional method, while being the faster fermentation process. Thus, it is suggested that inoculation of the indigenous S. cerevisiae strain in order to shorten the fermentation time without affecting the quality of traditional BRV.
Keywords: Black raspberry vinegar, Indigenous yeast, Saccharomyces cerevisiae, Ethanol fermentation, Acetic acid fermentation
Introduction
Technically, there are two types of methods used to produce vinegar: slow traditional processes and quick submerged (industrial purpose) methods. Unlike submerged methods, the vinegars produced by traditional static acetic acid fermentation are generally considered to be high quality due to diverse organic acids and amino acids being balanced during long-term surface culture fermentation (Lee et al., 2012). However, the traditional method, which is a lengthy process without microbial control delaying the alcoholic fermentation (AF) and acetic acid fermentation (AAF), may cause contamination and low acidity. Therefore, a technical strategy to achieve faster and higher acidity yield fermentation that maintains a high quality similar to the traditional method is needed.
Control of the ethanol production step by yeast inoculation is a priority in traditional vinegar fermentation, as it shortens the fermentation time and increases the safety of the product, thus ensuring the quality and reproducibility of the final product (Hidalgo et al., 2010). In yeast inoculated persimmon, balsamic, and strawberry vinegar fermentation, alcohol fermentation occurred faster in yeast-seeded vinegar due to the shorter lag phase without affecting the chemical composition or acetic acid bacteria (AAB) diversity (Hidalgo et al., 2010; 2012).
Many reports have established that the use of indigenous S. cerevisiae strains is preferred by many winemakers since active commercial dried yeasts reduce both the biodiversity of the strains and the wine complexity (Frezier and Dubourdieu, 1992). They also possess high fermentation capability, as native yeast populations are strongly adapted to specific environments, thus having important implications for the use of autochthonous wild yeast strains as starters (Nadal et al., 1996). The use of starter cultures has improved the reproducibility and predictability wine quality (Di Maro et al., 2007).
Traditional Muju black raspberry vinegar (TMBV) in Korea has been manufactured empirically with black raspberry extract (BRE) to facilitate the growth of desirable microorganisms in a crock using the back-slopping method with seed vinegar. Due to the health-related effects of black raspberry and black raspberry vinegar (BRV) containing nutritious components and various physiological activities, BRV has received great attention for its anti-oxidative, anti-tumor activity, anti-inflammatory, anti-high lipid, anti-high sugar, and antimicrobial activity (Hong et al., 2012; Lim et al., 2012). However, slow spontaneous alcohol fermentation affected slow starting acetification, which resulted in a low final total acidity even with a long fermentation time. To establish an effective BRV fermentation process, a reduction in the length of the AF and the control of the process are needed (Hidalgo et al., 2012).
In this study, we isolated the indigenous starter yeast from traditionally prepared TMBV and examined their biochemical and fermentation properties to improve the BRV fermentation process. Moreover, we also applied the selected indigenous starter yeast to the pilot-scale BRV fermentation process.
Materials and methods
Yeast strains
The yeast strains were isolated from traditionally prepared black raspberry wine and TMBV by using YM agar plates (Difco Co., Detroit, MI, USA) containing 25 U/mL penicillin–streptomycin (Sigma) to inhibit bacterial growth were used. After incubating for 2–3 days at 29 °C, yeast-like colonies were randomly picked and cultivated in YM broth for 48 h. Isolates were kept at − 80 °C in a deep freezer. S. cerevisiae KACC30008 and commercial dried yeast S. cerevisiae no. 7013 (DSM Food Specialties, INRA NARBONNE, Servian, France) were used as the standard control yeast strains.
Glucose, metabisulfite and ethanol tolerance
To determine the environmental tolerance capability of the strains, the yeast cells were grown in YM broth with different concentrations of glucose (200 and 300 g/L), metabisulfite (200 and 500 mg/L), and ethanol (0, 5, 10, and 15%, v/v). Yeast strains were cultivated at 29 °C, 180 r/min for 2 days. The absorbance was monitored at 12, 24 and 48 h at 600 nm using a spectrophotometer (UV–VIS Spectrophotometer, Beckman Coulter, USA).
Biogenic amine (BA) analysis
The BA standards, including tryptamine, 2-phenylethylamine, putescine, cadaverine, histamine, serotonin, trymine, spermidine, spermine, L-noradrenaline, and dopamine, were obtained from Sigma-Aldrich (St. Louis, MO, USA). An analysis of the concentration of BA was carried out using the dansyl chloride derivatization reaction. After derivatization of the samples, an aliquot of samples was submitted to high-pressure liquid chromatography (Agilent Technologies, Santa Clara, CA, USA) with a UV detector and CapcellPak C18 Column (4.6 × 250 mm, 5 μm, Shiseido Co., Tokyo, Japan), as described previously (Moon et al., 2010).
Ethanol analysis
Ethanol production by yeast strains was analyzed using gas chromatography (HP 6890, Hewlett Packard, CA, USA) with a flame ionization detector (FID) and a DB-5 capillary column (30 m × 0.25 mm, 0.25 μm; J&W Scientific, CA, USA), following the methods of Song et al. (2016b). The temperature was programmed to rise from 70 to 150 °C at a rate of 20 °C/min, followed by an increase to 220 °C, which was held for 1 min.
Yeast identification
Restriction fragment length polymorphism (RFLP), internal transcribed spacer (ITS), and 26S rDNA sequencing of yeasts were performed as described previously (Solieri et al., 2006; Song et al., 2016b).
Black raspberry wine and vinegar fermentation process
The BRE and seed vinegar obtained as described previously (Song et al., 2016a; 2016b). The BRE was heated at 85 °C for 15–20 min, and then used for black raspberry wine (BRW) fermentation. Wine fermentation was performed using a two-step fermentation process. First, the log 8–9 CFU/mL of starter yeast inoculated in 20°Brix of BRE and incubated it at 25 °C, 160 rpm for 48 h. Then, the AF was performed in the stationary phase for 27 days. The obtained BRW (700 L) was mixed with 100 mL/L of seed vinegar. AAF was performed in a bioreactor crock at 23 °C without agitation. Every week, 50 mL samples were collected and immediately used for chemical analysis, traditional microbial counts and culture-independent microbiological analysis. The total soluble solids (°Brix), pH and acidity were analyzed as previously described by Song et al. (2016b).
Yeast and acetic acid bacteria counts
Samples obtained from AAF were serially diluted with sterilized water and spread onto agar plates to isolate microorganisms. YM agar plates containing 25 U/mL penicillin–streptomycin (Sigma) as described above were used for yeast counting. For AAB isolation, two different media, GYC agar (5% glucose, 1% yeast extract, 3% ethanol, 0.8% agar) and GYEC agar plates (5% glucose, 1% yeast extract, 3% ethanol, 1.5% calcium carbonate, 0.8% agar), were used for bacterial counting. Counting was conducted after incubating for 2–3 days at 29 °C.
General and functional component analysis
Mineral contents, free sugars, and organic acids were analyzed following the methods of Song et al., (2016b). The total polyphenol contents of the samples were determined using the Folin–Ciocalteu method with gallic acid as a standard phenolic compound (Folin and Denis, 1912). The total flavonoid content of the BRV samples was determined using a colorimetric method described in the literature (Davis, 1947) and catechin used as a standard. The DPPH radical scavenging activity was measured as described by Meda et al. (2005).
DNA extraction and PCR-DGGE analysis
Total DNA for DGGE analysis was extracted directly from the vinegar samples using the i-genomic soil DNA extraction mini kit (Intron biotechnology, INC., Seoul, Korea) following the manufacturer’s instructions. DNA from each single band was extracted and purified using the Genomic DNA Purification kit from Promega (Madison, WI, USA). For yeast identification, DNA extracted from the DGGE gels was amplified by PCR with NL1/NL4 and NL1/LS2 primers, respectively, using the previously reported protocol (Mills et al., 2002). The DNA sequences of bacteria were amplified with the primer pair 357f-GC clamp and 517r following a reported protocol (Muyzer et al., 1993). The DGGE analysis of PCR amplicons was carried out following the method of De Vero et al. (2006).
Statistics
The data were analyzed using the Student’s t test with the SPSS version 20.0 software. The results of the analyses, which were performed in triplicate, were expressed as the mean ± standard deviation (SD). The differences among groups were assessed using Duncan’s multiple range tests.
Results and discussion
Selection, characterization and identification of the indigenous strain
To select starter yeast for rapid and controlled AF, approximately 200 yeast strains were isolated from traditionally fermented black raspberry wine or TMBV in this study. The JBCC-21A strain was finally selected due to its high growth properties with glucose or metabisulfites, ethanol productivity (13%, v/v), and BA degradation activity (Table 1). In particular, the selected JBCC-21A strain was able to remove 100% cadaverine, spermidine, and 15% histamine, which was 3–8 times lower than the normal toxic dose (Soufleros et al., 1998). The strain was identified via ITS-RFLP, ITS, and 26S rDNA sequencing, showing 99% homology with S. cerevisiae.
Table 1.
Characterization of indigenous Saccharomyces cerevisiae JBCC-21A and standard control yeasts
| Tolerance | JBCC-21A | S. cerevisiae no. 7013 | S. cerevisiae KACC30008 |
|---|---|---|---|
| Ethanol production | Over 10% (v/v) | 15.8–16.4 (%, v/v) (Lee and Ahn, 2009) | 16 (%, v/v) (Jang et al., 2011) |
| 11 ± 0.1 (%, v/v) from 21°Brix of the must) (Lee et al., 2006) | |||
| Glucose tolerance (g/L) | – | – | |
| 200 | +, OD600 < 15 | ||
| 300–500 | ++, 20 < OD600 | ||
| Na 2 S 2 0 5 tolerance (mg/L) | – | ||
| 200 | ++, 7 < OD600 | ≤ 4 mM SO2 (active growth) (Barbosa et al., 2014) | |
| 500 | ++, 7 < OD600 | ||
| Ethanol tolerance (%, v/v) | – | ||
| 5 | +++, 6 < OD600 | ≤ 10 (%, v/v) (medium growth) | |
| 10 | +, 1 < OD600 | ||
| 15 | −, OD600 < 1 | > 12.5 (%, v/v) (no growth) (Barbosa et al., 2014) | |
| Biogenic amine degradation | – | – | |
| Cadaverine | 100% | ||
| Histamine | 15.07% | ||
| Spermidine | 100% | ||
| Identification | |||
| ITS-RFLP (5.8S rDNA) | S. cerevisiae (100%, JN083825) | ||
| ITS (ITS1, ITS4) | S. cerevisiae 99% | ||
| 26S rDNA | S. cerevisiae 100% |
Ethanol fermentation properties of the selected indigenous S. cerevisiae JBCC-21A strain on black raspberry extract
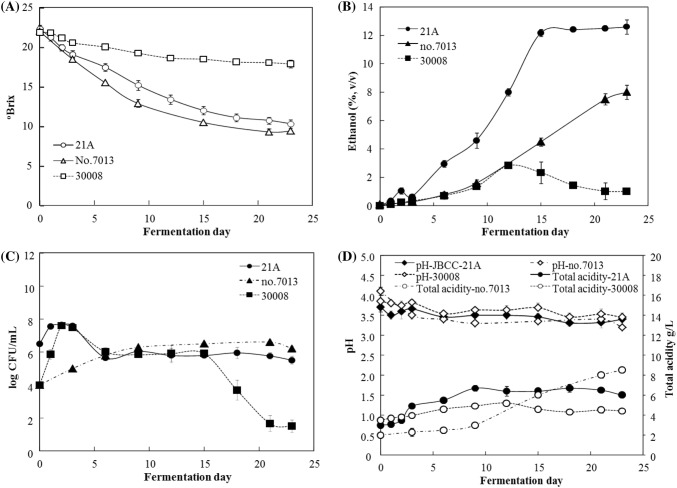
During AF, S. cerevisiae JBCC-21A showed similar growth properties than S. cerevisiae KACC30008 (Fig. 1C), which initially showed identical growth. However, S. cerevisiae KACC30008 eventually began decreasing dramatically starting from day 14 and S. cerevisiae no. 7013 showed slow growth. S. cerevisiae JBCC-21A and S. cerevisiae no. 7013 showed similar sugar consumption, but that of S. cerevisiae KACC30008 was significantly low. These results clearly show that indigenous S. cerevisiae JBCC-21A has better adaptation and fermentation capacity in the black raspberry environment than do standard control yeast strains.
Fig. 1.
Physicochemical changes in sugar consumption (A), ethanol production (B), yeast population (C), total acidity and pH (D) during alcohol fermentation by indigenous strain and standard yeasts. 21A, Saccharomyces cerevisiae JBCC-21A; no. 7013, commercial S. cerevisiae no. 7013; 30008, type strain S. cerevisiae KACC30008
As shown in Fig. 1B, the ethanol concentration increased up to 12.2% (v/v) when S. cerevisiae JBCC-21A was used as a starter until day 15, also exhibiting a decrease in total sugar (Fig. 1A). Commercial yeast S. cerevisiae no. 7013, which is broadly used in industrial fermentation, only produced 8% (v/v) ethanol in BRE (Fig. 1B); this value is twice as low as the ethanol production rate for the selected S. cerevisiae JBCC-21A. The BRW prepared using S. cerevisiae JBCC-21A (BRW-21A) showed a slightly lower pH than that of S. cerevisiae KACC30008 (BRW-K30008) and S. cerevisiae no. 7013 (BRW-7013) (Fig. 1D). As shown in Fig. 1D, the total acidity of BRW-21A increased up to 6.0 g/L on day 18, which was almost identical to that for grape wine at 5.6–9.6 g/L (Kim et al., 2010). BRW-K30008 showed a slightly lower total acidity (4.4 g/L), whereas BRW-7013 increased up to 8.5 g/L through day 23. Hence, the isolated indigenous strain S. cerevisiae JBCC-21A was demonstrated to have efficient ethanol fermentation properties upon BRE.
Physicochemical changes by S. cerevisiae JBCC-21A during BRV fermentation
The effects of S. cerevisiae JBCC-21A on physicochemical changes during BRV fermentation at a pilot scale (700 L, stainless barrel) are shown in Table 2. After inoculating activated S. cerevisiae JBCC-21A, the ethanol concentration increased up to 12.6% (v/v) until day 27, which was similar to that of the traditional fermentation method (Song et al., 2016b). However, the ethanol concentration by the traditional method reached its maximum concentration very slowly until day 59, which was almost three times longer than S. cerevisiae JBCC-21A. The levels of soluble solids (°Brix) decreased up to 9.5°Brix and reached a maximum of 12.6% (v/v) ethanol until the final stage of AF (day 27). After the ethanol production step was almost finished at 35 days, the seed vinegar (18.8°Brix) was inoculated to initiate the second step of AAF. Acetic acid production was activated after the acidity reached 2%, which is the appropriate initial acidity for acetic acid fermentation (Hong et al., 2012), and the total acidity increased from 20 g/L (day 35) to 60 g/L (day 144). Consequently, these results suggest that a larger acidity can be achieved quicker and more efficiently via the BRV fermentation process by adopting the isolated S. cerevisiae JBCC-21A compared to TMBV (Song et al., 2016b).
Table 2.
Changes of microbial enumeration and physicochemical components during black raspberry vinegar fermentation process using Saccharomyces cerevisiae JBCC-21A
| Fermentation method | Stage | Sampling | Day | Yeast (log CFU/mL) | Ethanol (%) | Soluble solids (oBrix) | AAB (log CFU/mL) | Total titratable acidity (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|
| Inoculated alcohol fermentation | Initial | 0 | 0 | 5.48 ± 0.32 | 0.1 ± 0.00 | 22.3 ± 0.01 | < log 3.0 | 2.0 ± 0.5 | 4.1 ± 0.02 |
| 1 | 10 | 7.59 ± 0.21 | 4.6 ± 0.12 | 15.2 ± 0.02 | < log 3.0 | 3.0 ± 0.1 | 3.3 ± 0.01 | ||
| Middle | 2 | 20 | 5.78 ± 0.30 | 12.5 ± 0.23 | 12 ± 0.01 | < log 3.0 | 8.2 ± 0.2 | 3.36 ± 0.01 | |
| Final | 3 | 27 | 5.5 ± 0.22 | 12.6 ± 1.21 | 10.3 ± 0.02 | < log 3.0 | 13.1 ± 2.0 | 3.2 ± 0.03 | |
| Seed vinegar inoculated acetic acid fermentation | Initial | 4 | 35 | 5.47 ± 0.26 | 7.16 ± 1.30 | 10.3 ± 0.02 | 3.5 ± 0.22 | 19.5 ± 2.1 | 2.97 ± 0.01 |
| 5 | 45 | 5.38 ± 0.25 | 7.17 ± 2.10 | 10.6 ± 0.03 | 3.5 ± 0.32 | 24.3 ± 2.2 | 3.13 ± 0.01 | ||
| 6 | 52 | 5.47 ± 0.21 | 7.44 ± 1.54 | 11.3 ± 0.02 | 4.5 ± 0.05 | 25.8 ± 3.3 | 3.09 ± 0.02 | ||
| 7 | 59 | 5.65 ± 0.31 | 7.0 ± 2.41 | 10.9 ± 0.04 | 4.85 ± 0.21 | 27.4 ± 3.5 | 3.06 ± 0.03 | ||
| Middle | 8 | 72 | 5.15 ± 0.33 | 6.0 ± 2.30 | 10.9 ± 0.03 | 5.01 ± 0.30 | 30.4 ± 2.4 | 3.05 ± 0.02 | |
| 9 | 77 | 4.88 ± 0.26 | 4.79 ± 1.10 | 10.8 ± 0.05 | 5.2 ± 0.22 | 30.4 ± 5.6 | 3.05 ± 0.00 | ||
| 10 | 87 | 4.21 ± 0.24 | 3.62 ± 1.01 | 10.2 ± 0.01 | 5.1 ± 0.25 | 27.4 ± 3.1 | 3.02 ± 0.01 | ||
| 11 | 95 | 3.8 ± 0.28 | 3.5 ± 2.21 | 10.0 ± 0.02 | 5.72 ± 0.34 | 31.2 ± 1.2 | 2.96 ± 0.00 | ||
| 12 | 104 | 3.52 ± 0.35 | 3.15 ± 1.41 | 10.0 ± 0.03 | 5.48 ± 0.24 | 35.0 ± 2.1 | 3.04 ± 0.01 | ||
| 13 | 119 | 2.83 ± 0.24 | 2.8 ± 0.23 | 10.1 ± 0.02 | 5.71 ± 0.22 | 41.0 ± 0.8 | 2.96 ± 0.00 | ||
| Final | 14 | 144 | 2.71 ± 0.22 | 0.93 ± 0.02 | 9.5 ± 0.03 | 5.8 ± 0.30 | 62.3 ± 1.2 | 2.94 ± 0.02 | |
| Traditional alcohol fermentationa | 0 | 0 | 5.0 ± 0.05 | 0.2 ± 0.05 | 19.5 ± 0.01 | 0.5 ± 0.33 | 0.4 ± 0.05 | 4.0 ± 0.05 | |
| 1 | 10 | 7.29 ± 0.2 | 4.5 ± 0.40 | 14.7 ± 0.03 | 1.5 ± 0.41 | 1.06 ± 0.1 | 3.16 ± 0.1 | ||
| 2 | 20 | 7.37 ± 0.3 | 4.93 ± 0.06 | 7.8 ± 0.04 | 1.86 ± 0.22 | 1.12 ± 0.2 | 3.26 ± 0.2 | ||
| 3 | 27 | 7.29 ± 0.1 | 5.25 ± 0.02 | 6.0 ± 0.03 | 2.0 ± 0.12 | 1.37 ± 0.2 | 3.22 ± 0.2 | ||
| Traditional acetic acid fermentationa | 4 | 35 | 7.58 ± 0.3 | 11.81 ± 0.42 | 5.9 ± 0.04 | 2.65 ± 0.34 | 1.55 ± 0.1 | 3.13 ± 0.05 | |
| 5 | 45 | 6.79 ± 0.05 | 11.56 ± 0.31 | 5.6 ± 0.02 | 3.0 ± 0.50 | 1.98 ± 0.3 | 3.21 ± 0.05 | ||
| 6 | 52 | 6.63 ± 0.2 | 11.71 ± 0.33 | 5.9 ± 0.03 | 3.34 ± 0.31 | 1.98 ± 0.2 | 3.16 ± 0.1 | ||
| 7 | 59 | 6.82 ± 0.4 | 12.89 ± 0.52 | 5.7 ± 0.01 | 3.50 ± 0.24 | 2.13 ± 0.05 | 3.09 ± 0.2 | ||
| 8 | 72 | 3.65 ± 0.05 | 6.14 ± 0.13 | 5.7 ± 0.03 | 3.78 ± 0.12 | 2.28 ± 0.1 | 3.09 ± 0.1 | ||
| 9 | 77 | 3.5 ± 0.05 | 5.82 ± 0.00 | 5.7 ± 0.02 | 4.85 ± 0.32 | 2.43 ± 0.1 | 3.09 ± 0.05 | ||
| 10 | 87 | 3.5 ± 0.2 | 5.49 ± 0.05 | 5.4 ± 0.05 | 5.51 ± 0.21 | 2.13 ± 0.2 | 3.04 ± 0.2 | ||
| 11 | 95 | 3.3 ± 0.3 | 5.16 ± 0.21 | 5.1 ± 0.03 | 5.54 ± 0.16 | 2.66 ± 0.3 | 2.96 ± 0.2 | ||
| 12 | 104 | 3.0 ± 0.3 | 3.72 ± 0.21 | 5.1 ± 0.03 | 5.82 ± 0.23 | 2.89 ± 0.05 | 3.05 ± 0.1 | ||
| 13 | 119 | 2.6 ± 0.1 | 3.50 ± 0.10 | 5.3 ± 0.01 | 5.62 ± 0.22 | 3.19 ± 0.1 | 3.0 ± 0.05 | ||
| 14 | 144 | 2.3 ± 0.4 | 1.97 ± 0.11 | 5.4 ± 0.04 | 5.16 ± 0.30 | 4.41 ± 0.2 | 3.02 ± 0.1 |
aTraditional fermentation method (Song et al., 2016b)
Analysis of BRV fermented using indigenous S. cerevisiae JBCC-21A
The biochemical and functional properties of BRV fermented using indigenous S. cerevisiae JBCC-21A were analyzed to understand the effects of indigenous S. cerevisiae JBCC-21A on the BRV fermentation process. The remaining carbon source of BRV was fructose (2.07 g/L), whereas glucose was not detected (Table 3). The total acidity and final ethanol concentration of TMBV were 44 g/L and 1.9% (v/v), respectively (Song et al., 2016b), while those for BRV were 60 g/L and 0.93% (v/v), respectively. As shown in Table 3, acetic acid (2.82 g/L) and oxalic acid (3.38 g/L) were the two major organic acids in BRW. Acetic acid (59.11 g/L), oxalic acid (7.7 g/L) and citric acid (1.18 g/L) were mainly generated after AAF, but both malic acid and lactic acid were reduced. The different organic acid profiles obtained in this study, which were comparable to those for the traditional method (Song et al., 2016b), could be explained by the shortened AF period and ethanol oxidation. In particular, the faster oxidation of some organic acids by AABs may have been due to the shortened AAB proliferation and ethanol consumption period caused by large and fast ethanol production (Giudici et al., 2009). Similarly, different organic acid and volatile compounds between traditional balsamic vinegar (TBV) and commercial balsamic vinegar (BV), which were manufactured using different methods were also observed (Li et al., 2015). The large amount of acetic acid may be due to the rapid consumption of ethanol obtained from the inoculated AF. Further, some of the changes in the organic acids are a result of the acetate overoxidation during diauxic growth of the Acetobacter species (Li et al., 2015).
Table 3.
Biochemical analysis of black raspberry wine and vinegar using inoculated method
| Samples | Residual sugars (g/L) | Organic acid (g/L) | Mineral (ppb) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Acetic acid | Oxalic acid | Citric acid | Lactic acid | Malic acid | Mg | Fe | |
| BRW | nd | 2.49 ± 0.6a | nd | 2.82 ± 1.36b | 3.38 ± 1.77a | 0.06 ± 0.01b | 0.77 ± 0.08 | 0.5 ± 0.1 | 33,308b | 2511b |
| BRV | nd | 2.07 ± 0.0a | nd | 59.11 ± 8.48a | 7.7 ± 4.15a | 1.18 ± 0.29a | nd | nd | 83,270a | 4813a |
| TMBVc | nd | 10.01 ± 1.04 | nd | 72.12 ± 19.58 | nd | nd | 1.81 ± 1.38 | 1.07 ± 0.03 | ||
Data is expressed as mean ± standard deviation (n = 3)
BRW black raspberry wine fermented by indigenous yeast, S. cerevisiae JBCC-21A, BRV black raspberry vinegar fermented by 100 mL/L seed vinegar inoculation into BRW
a,bSmall letters in the same column indicate significant differences of values between two samples by student’s t-test (p < 0.05)
cTMBV, traditional Muju black raspberry vinegar (Song et al. 2016b)
The minerals, Mg reacted with citric acid presented in BRV may stabilize the vinegar forming soluble complexes (Casale et al., 2006) and higher Fe concentration plays a fundamental role as redox catalysts on the antioxidant properties of BRV.
As shown in Fig. 2, the BRV fermented using indigenous S. cerevisiae JBCC-21A contained significantly higher phenolic content than BRW (p < 0.001), and the total flavonoid contents of BRW and BRV were 1.68 and 2.56 g/L, respectively (p < 0.01). The total polyphenol content was higher than 25.19 mg/100 mL for the black raspberry vinegar from the Gochang area (Hong et al., 2012). The total polyphenol, total flavonoid, and DPPH radical scavenging activities of TMBV were 1.98 g/L, 1.91 g/L, and 1.63 g AEAC/L (g of ascorbic acid equivalent antioxidant content, data not shown), respectively (Song et al., 2016b); however, in this study, BRV fermented by indigenous S. cerevisiae JBCC-21A showed higher values of 2.61 g/L, 2.56 g/L, and 1.88 g AEAC/L, respectively. This result was in agreement with the higher total polyphenol values caused from inoculated wines compared to those of vinegars from spontaneous fermentation (Ubeda et al., 2011). This result may be due to the microbial changes accompanying the physicochemical components. Some of the non-Saccharomyces strains that resulted from environmental changes after adding seed vinegar also affected the values of polyphenol, anthocyanin and antioxidant activity (Li et al., 2015; Tu et al., 2005).
Fig. 2.
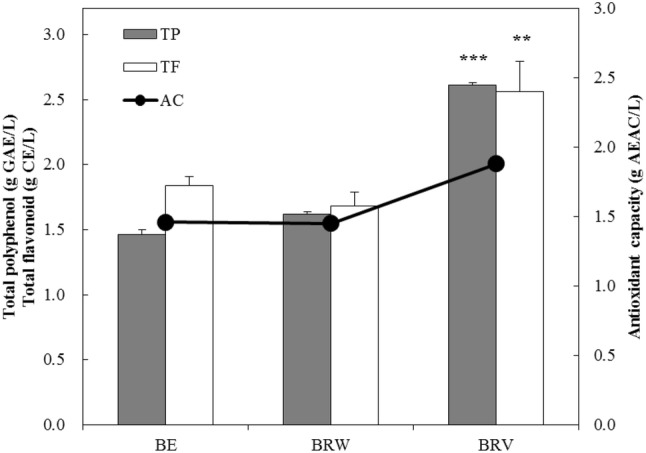
Functional component analysis of black raspberry wine and vinegar fermented by inoculated method. BRE, black raspberry extract; BRW, black raspberry wine fermented by indigenous yeast, Saccharomyces cerevisiae JBCC-21A; BRV, black raspberry vinegar fermented by 100 mL/L seed vinegar inoculation into BRW; TP, total polyphenol expressed by gallic acid equivalent (GAE); TF, total flavonoid expressed by catechin equivalent (CE); AC, ascorbic acid equivalent antioxidant content (AEAC) with DPPH radical scavenging activity. *, small asterisk in the same bar indicate significant differences of values between two samples by student’s t-test (**p < 0.01; ***p < 0.001)
Microbiological characterization during BRV fermentation by S. cerevisiae JBCC-21A
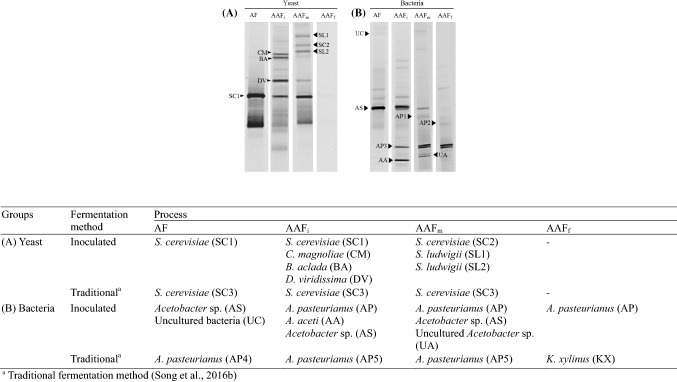
After inoculating S. cerevisiae JBCC-21A, their population rapidly proliferated until 10 days, and decreased to log 2.71 CFU/mL in the final BRV as shown in Table 2. Vinegar fermentation followed with rapid growth of AAB population to log 5.8 CFU/mL. DGGE analysis showed the 14 bands, which identified each of the seven yeasts and seven bacterial species (Fig. 3). As expected, S. cerevisiae JBCC-21A inoculated at an early stage of AF dominated until day 27 (Fig. 3A), indicating that S. cerevisiae JBCC-21A adapted to BRV fermentation successfully. However, other yeasts, including Candida magnolia (CM), Botrytis aclada (BA), Denhamia viridissima (DV), and Saccharomycodes ludwigii (SL1, 2), also appeared in the initial (AAFi) and middle (AAFm) stages of static fermentation. However, those yeasts gradually disappeared without affecting the dominant species of S. cerevisiae JBCC-21A during the vinegar fermentation period. Previous studies have shown that non-Saccharomyces yeast species such as the genera Candida and Saccharomycodes appeared during the initial and middle stages of acetification for white wine vinegar, balsamic vinegar, wine vinegar and kombucha vinegar, often showing more beneficial effects with positive metabolic activities (Li et al., 2015). During AF (Fig. 3B), several ethanol-tolerant Acetobacter species such as Acetobacter nitrogenifigens (NR043086.1), A. indonesiensis (NR028626.1), A. tropicalis (NR036881.1), A. orleanensis (NR028614.1), A. malorum (NR025513.1), A. cerevisiae (NR025512.1), and A. estunensis (NR042113.1) could be detected due to the high ethanol concentration. After adding seed vinegar, A. aceti, which was the most dominant species in black raspberry seed vinegar (Song et al., 2016a), quickly disappeared, allowing A. pasteurianus to be the dominant bacterial species throughout the end stage of vinegar fermentation. A. pasteurianus was more successfully grown compared to A. aceti, which may be due to their different acetic acid tolerances. The increased stressful acidity and pH during AAF resulted in a more suitable environment for AAB, and yeasts were not detected during the final stage of the acetification process (AAFf). Interestingly, in our previous study (Song et al., 2016b), Acetobacter sp. was abundant in the initial and middle stages and Komagataeibacter xylinus was observed in the final stage of TMBV fermentation (Song et al., 2016b), whereas inoculation of indigenous S. cerevisiae JBCC-21A contributed to AAB succession. Although commercial wine yeast inoculation did not influence the AAB diversity or chemical composition of the vinegars in a previous study by Hidalgo et al. (2012), yeast inoculation during AF in our study may have affected AAB proliferation due to the rapidly-occurring and larger ethanol concentration (over 12%). Gluconacetobacter sp. can be easily dominated under harsh conditions such as high alcohol or high acidity conditions (Hidalgo et al., 2012), whereas A. pasteurianus also can grow quickly in blueberry wine vinegar (Hidalgo et al., 2013a), traditional wine vinegars (Vegas et al., 2010), traditional balsamic vinegar (Gullo et al., 2009), and Chilean wine vinegar (Ilabaca et al., 2008) due to their strong oxidizing activity for ethanol into acetic acid compared to glucose. Furthermore, A. pasteurianus was not only detected in vinegars with low concentrations of acetic acid (3–4%) (Haruta et al., 2006), but was also found as indigenous organisms in wine and fruit vinegars containing 5.5–6% acidity (Hidalgo et al., 2013b; Li et al., 2015; Vegas et al., 2010). Thus, the different AAB patterns (compared to the traditional method) observed in this study depend on the inoculated fermentation method, with A. pasteurianus continuously being the dominant species due to the higher alcohol and higher acidity conditions during BRV fermentation.
Fig. 3.
Yeast (A) and acetic acid bacteria (B) species monitoring by PCR/DGGE analysis during black raspberry vinegar fermentation using Saccharomyces cerevisiae JBCC-21A. AF, alcohol fermentation; AAFi, initial stage of acetic acid fermentation; AAFm, middle stage of acetic acid fermentation; AAFf, final stage of acetic acid fermentation. The closest relatives of the fragments sequenced (% identical nucleotides compared to sequences retrieved from the GenBank database) are described in separated table: (Top) (A) SC1, S. cerevisiae (99%, JX068683.1); CM, C. magnolia (97%, AJ749827.1); BA, B. aclada (99%, KC311471.1); DV, D. viridissima (97%, EU328648.1); SC2, S. cerevisiae (97%, JX068683.1); SL1, S. ludwigii (95%, JN248612.1); SL2, S. ludwigii (98%, JN248612.1); (B) AS, Acetobacter sp. (96%); UC, uncultured bacteria (100%, KC872586); AP1, A. pasteurianus (98%, JQ513826.1); AA, A. aceti (96%, AJ012542.1); AP2, A. pasteurianus (98%, JQ513826.1); UA, uncultured Acetobacter sp. (97%, JQ253455.1); AP3, A. pasteurianus (98%, JQ513826.1). (Bottom) SC3, S. cerevisiae (99%, JX068683.1); AP4, A. pasteurianus (99%, JQ513826.1); AP5, A. pasteurianus (99%, JQ513826.1); KX, Komagataeibacter xylinus (100%, NR_074338.1)
In this study, we suggested that indigenous starter yeast S. cerevisiae JBCC-21A with the seed vinegar of black raspberry was more effective for fast AF than the traditional black raspberry vinegar fermentation process. Our results indicated that the inoculated BRV fermentation process with the selected indigenous strain is an efficient approach for the fast AF step, therefore enhancing the overall BRV fermentation process without affecting the quality of traditional BRV. The native starter yeast, S. cerevisiae JBCC-21A inoculated with AF, showed higher viability and ethanol production than commercial yeast. Although the fungal and bacterial diversity were changed, S. cerevisiae JBCC-21A dominated while the A. pasteurianus species completed the acetification of BRV fermentation. Microbial succession was simpler compared to the traditional method, while showing similar or higher functional compounds and antioxidant activity. These results demonstrate the potential for industrial production of a stabilized and fast BRV fermentation process using indigenous yeast as a starter culture with seed vinegar.
Acknowledgements
This research was supported by “Research Base Construction Fund Support Program” funded by Chonbuk National University in 2017 and the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) and Establishment of Infrastructure for Industrialization of Korean Useful Microbes (R0004073).
References
- Barbosa C, Lage P, Vilel A, Mendes-Faia A, Mendes-Ferreira A. Phenotypic and metabolic traits of commercial Saccharomyces cerevisiae yeasts. AMB Express. 2014;4:39. doi: 10.1186/s13568-014-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale M, Abajo MJS, Sáiz JMG, Pizarro C, Forina M. Study of the aging and oxidation processes of vinegar samples from different origins during storage by near-infrared spectroscopy. Anal. Chim. Acta. 2006;557:360–366. doi: 10.1016/j.aca.2005.10.063. [DOI] [Google Scholar]
- Davis WB. Determination of flavanones in citrus fruits. Anal. Chem. 1947;19:476–478. doi: 10.1021/ac60007a016. [DOI] [Google Scholar]
- De Vero L, Gala E, Gullo M, Solieri L, Landi S, Giudici P. Application of denaturing gradient gel electrophoresis (DGGE) analysis to evaluate acetic acid bacteria in traditional balsamic vinegar. Food Microbiol. 2006;23:809–813. doi: 10.1016/j.fm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Di Maro E, Ercolini D, Coppola S. Yeast dynamics during spontaneous wine fermentation of the Catalanesca grape. Int. J. Food. Microbiol. 2007;117:201–210. doi: 10.1016/j.ijfoodmicro.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Folin O, Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912;12:239–243. [Google Scholar]
- Frezier V, Dubourdieu D. Ecology of yeast strain Saccharomyces cerevisiae during spontaneous fermentation in a Bordeaux winery. Am. J. Enology Vitic. 1992;43:375–380. [Google Scholar]
- Giudici P, Gullo M, Solieri L, Falcone PM. Technological and microbiological aspects of traditional balsamic vinegar and their influence on quality and sensorial properties. Adv. Food Nutr. Res. 2009;58:137–182. doi: 10.1016/S1043-4526(09)58004-7. [DOI] [PubMed] [Google Scholar]
- Gullo M, De Vero L, Giudici P. Succession of selected strains of Acetobacter pasteurianus and other acetic acid bacteria in traditional balsamic vinegar. Appl. Environ. Microbiol. 2009;75:2585–2589. doi: 10.1128/AEM.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta S, Ueno S, Egawa I, Hashiguchi K, Fujii A, Nagano M, Ishii M, Igarashi Y. Succession of bacterial and fungal communities during a traditional pot fermentation of rice vinegar assessed by PCR-mediated denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2006;109:79–87. doi: 10.1016/j.ijfoodmicro.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, García D, Romero J, Mas A, Torija MJ, Mateo E. Acetobacter strains isolated during the acetification of blueberry (Vaccinium corymbosum L.) wine. Lett. Appl. Microbiol. 2013;57:227–232. doi: 10.1111/lam.12104. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Mateo E, Cerezo AB, Torija MJ, Mas A. Technological process for production of persimmon and strawberry vinegars. Int. J. Wine Res. 2010;2010:55–61. [Google Scholar]
- Hidalgo C, Mateo E, Mas A, Torija MJ. Identification of yeast and acetic acid bacteria isolated from the fermentation and acetification of persimmon (Diospyros kaki) Food Microbiol. 2012;30:98–104. doi: 10.1016/j.fm.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Torija MJ, Mas A, Mateo E. Effect of inoculation on strawberry fermentation and acetification processes using native strains of yeast and acetic acid bacteria. Food Microbiol. 2013;34:88–94. doi: 10.1016/j.fm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Hong SM, Kang MJ, Lee JH, Jeong JH, Kwon SH, Seo KI. Production of vinegar using Rubus coreanus and its antioxidant activities. Korean J. Food Preserv. 2012;19:594–603. doi: 10.11002/kjfp.2012.19.4.594. [DOI] [Google Scholar]
- Ilabaca C, Navarrete P, Mardones P, Romero J, Mas A. Application of culture culture-independent molecular biology based methods to evaluate acetic acid bacteria diversity during vinegar processing. Int. J. Food Microbiol. 2008;126:245–249. doi: 10.1016/j.ijfoodmicro.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Jang M, Min JW, Yang DU, Jung SK, Kim SY, Yang DC. Ethanolic fermentation from red ginseng extract using Saccharomyces cerevisiae and Saccharomyces carlsbergensis. Food Sci. Biotechnol. 2011;20:131–135. doi: 10.1007/s10068-011-0018-5. [DOI] [Google Scholar]
- Kim EK, Kim IY, Ko JY, Yim SB, Jeong YH. Physicochemical characteristics and acceptability of commercial low-priced French wines. J. Korean Soc. Food Sci. Nutr. 2010;39:1666–1671. doi: 10.3746/jkfn.2010.39.11.1666. [DOI] [Google Scholar]
- Lee SJ, Ahn BM. Changes in physicochemical characteristics of black raspberry wines from different regions during fermentation. Korean J. Food Sci. Technol. 2009;41:662–667. [Google Scholar]
- Lee SJ, Lee JE, Kim HW, Kim SS, Koh KH. Development of Korean red wines using Vitis labrusca varieties: instrumental and sensory characterization. Food Chem. 2006;94:385–393. doi: 10.1016/j.foodchem.2004.11.035. [DOI] [Google Scholar]
- Lee SW, Yoon SR, Kim GR, Woo SM, Jeong YJ, Yeo SH, Kim KS, Kwon JH. Effect of nuruk and fermentation method on organic acid and volatile compounds in brown rice vinegar. Food Sci. Biotechnol. 2012;21:453–460. doi: 10.1007/s10068-012-0057-6. [DOI] [Google Scholar]
- Li S, Li P, Feng F, Luo LX. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biotechnol. 2015;99:4997–5024. doi: 10.1007/s00253-015-6659-1. [DOI] [PubMed] [Google Scholar]
- Lim JW, Jeong JT, Shin CS. Component analysis and sensory evaluation of Korean black raspberry (Rubus coreanus Mique) wines. Int. J. Food Sci. Technol. 2012;47:918–926. doi: 10.1111/j.1365-2621.2011.02922.x. [DOI] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Mills DA, Johannsen EA, Cocolin L. Yeast diversity and persistence in botrytis-affected wine fermentations. Appl. Environ. Microbiol. 2002;68:4884–4893. doi: 10.1128/AEM.68.10.4884-4893.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JS, Kim Y, Jang KI, Cho KJ, Yang SJ, Yoon GM, Kim SY, Han NS. Analysis of biogenic amines in fermented fish products consumed in Korea. Food Sci. Biotechnol. 2010;19:1689–1692. doi: 10.1007/s10068-010-0240-6. [DOI] [Google Scholar]
- Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal D, Colomer B, Piña B. Molecular polymorphism distribution in phenotypically distinct populations of wine yeast strains. Appl. Environ. Microbiol. 1996;62:1944–1950. doi: 10.1128/aem.62.6.1944-1950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solieri L, Landi S, De Vero L, Giudici P. Molecular assessment of indigenous yeast population from traditional balsamic vinegar. J. Appl. Microbiol. 2006;101:63–71. doi: 10.1111/j.1365-2672.2006.02906.x. [DOI] [PubMed] [Google Scholar]
- Song NE, Cho HS, Baik SH. Bacteria isolated from Korean black raspberry vinegar with low biogenic amine production in wine. Braz. J. Microbiol. 2016;47:452–460. doi: 10.1016/j.bjm.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song NE, Cho SH, Baik SH. Microbial community, and biochemical and physiological properties of Korean traditional black raspberry (Robus coreanus Miquel) vinegar. J. Sci. Food Agric. 2016;96:3723–3730. doi: 10.1002/jsfa.7560. [DOI] [PubMed] [Google Scholar]
- Soufleros E, Barrios ML, Bertrand A. Correlation between the content of biogenic amines and other wine compounds. Am. J. Enology Vitic. 1998;49:266–278. [Google Scholar]
- Tu YY, Xia HL, Watanabe N. Changes in catechins during the fermentation of green tea. Appl. Biochem. Microbiol. 2005;41:574–577. doi: 10.1007/s10438-005-0104-7. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Hidalgo C, Torija MJ, Mas A, Troncoso AM, Morales ML. Evaluation of antioxidant activity and total phenols index in persimmon vinegars produced by different processes. LWT Food Sci. Technol. 2011;44:1591–1596. doi: 10.1016/j.lwt.2011.03.001. [DOI] [Google Scholar]
- Vegas C, Mateo E, González Á, Jara C, Guillamón JM, Poblet M, Torija MJ, Mas A. Population dynamics of acetic acid bacteria during traditional wine vinegar production. Int. J. Food Microbiol. 2010;138:130–136. doi: 10.1016/j.ijfoodmicro.2010.01.006. [DOI] [PubMed] [Google Scholar]