Abstract
In the present study, the effects of different ratios of milk phospholipids, cholesterol and phytosterols (Campesterol) powder (50–100%, 0–50%, and 0–50%, respectively) and sonication time (20, 25, 30, 35 and 40 min) were investigated to produce a new formulation of nanoliposomes for encapsulation of vitamin C. The results showed that increasing the time of sonication and decreasing the ratio of phospholipid to phytosterol significantly decreased nanoliposomes’ particle size (p < 0.05). The maximum encapsulation efficiency was obtained at 35 and 40 min of sonication time and 75–25 ratio of phospholipid: phytosterol. Also, reducing the sonication time in the same ratio of phospholipid/phytosterol caused to increase the controlled release. The highest stability of vitamin C during 20 days was obtained in the ratio of 75–25 (phospholipids: campesterol). The results showed a positive effect of cholesterol replacement with campesterol on encapsulation efficiency, control release and stability of vitamin C in nanoliposomes.
Keywords: Nanoliposomes, Milk phospholipids, Campesterol, Ultrasonic waves
Introduction
Nanotechnology includes the use of nano-carrier systems for the stability of biomaterials against a wide range of environmental and chemical changes, as well as improving nutritional properties of the food (Mozafari et al., 2008). The production of nanoliposomes is an effective technology for the encapsulation and control of the release of bio-compounds to enhance the stability and bioavailability of these materials (Farhang et al., 2012). Nanoliposome encapsulation has some advantages, like entrapment ability of substances with different solubility and use natural materials in industrial scale production. Lipid-based carriers can protect materials from damage of free radicals, metal ions, pH and enzymes. Nanoliposomes are nanoscale liposomes with colloidal structures are formed by introducing energy into a suitable mixture of compatible molecules (mainly phospholipids) in aqueous solution. Food-grade nanoliposomes are being increasingly used in food and beverage industries to encapsulate and deliver hydrophilic or lipophilic functional components such as vitamin C (Liu et al., 2017). Nanoliposome technology provides a special opportunity to control food in areas such as encapsulation and food control release, as well as increasing the availability, stability and shelf life of sensitive materials (Mozafari et al., 2008; Sekhon, 2010). Nanoliposomes have the same physical and thermodynamical properties with liposomes. The size of the liposomes is a critical parameter in many quantitative biophysical research (Richardson et al., 2007).
Although liposomes in recent years generally produce phospholipids from soy and eggs (Thompson et al., 2006), milk phospholipids have a higher quantity of sphingolipids and glycolipids than other phospholipids (Farhang et al., 2012). Milk phospholipids in compare with phospholipids prepared from soybean have high heat transfer, thick walls, and low membrane permeability. With any change in pH, milk phospholipid liposomes exhibit small changes in size. According to research, milk phospholipid liposomes have good stability when stored at temperatures in the range of 4–35 °C than soybean phospholipids (Farhang et al., 2012).
Ascorbic acid is a necessary nutrient that widely used as a food supplement, due to its antioxidant and regenerative properties (Wechtersbach et al., 2012). This vitamin has many therapeutic and biological functions. This vitamin increases collagen, protects against light, reduces melanin and free radicals and increases immunity (antiviral effects). However, this vitamin is unstable in air, moisture, light, heat, metal ions, oxygen and alkaline, and is simply degradable into inactive biological compounds such as oxalic acid (Yang et al., 2013). Ascorbic acid oxidizes to dehydroascorbic acid (DHA), which is synthetically unstable and subsequently hydrolyzes 2 and 3 di-ketogluconic acid (DKG), which does not have vitamin C activity (Wechtersbach et al., 2012). The mean value of vitamin C in cow’s milk is 2.1 mg/100 g. There is evidence that the concentration of vitamin C in cow’s milk and goat’s milk varies with seasonal changes (Fox et al., 2015).
Cholesterol is widely used to improve the liposomal delamination. This material improves membrane fluidity, stability of the layers and reduces the permeability of water-soluble molecules through membranes (Laouini et al., 2012). The presence of cholesterol in the lipid layers increases the stability and results in the formation of a hard membrane with similar liquid properties. Phytosterols are important from the nutritional and physiological point of view, because they interfere with cholesterol absorption, lowering cholesterol and LDL levels in the blood plasma (Quilez et al., 2003). Phytosterols not only reduce blood lipids, but also have a mechanism that protects the body from cardiovascular disease. These compounds are white powder, insoluble in water, and have a melting point of 100–215 °C, which, unlike the drugs, are not absorbed in the intestine and are excreted in combination with cholesterol (Ostlund, 2002). For food applications, probably the most important thing is food-grade material used in the production of liposomes (Barbosa-Canovas et al., 2009).
Recently, it has been shown that ultrasound can effectively control the release of a drug from liposomes by thermal or non-thermal effects. Ability to control the release of drugs and other molecules from liposomes using non-thermal effects of low-frequency ultrasound has been studied previously. It has been shown that ultrasound with low intensity, although it facilitates drug release, has no effect on the chemical accuracy of the drug or its biological potential (Schroeder et al., 2009).
The aim of this study was to replace cholesterol with phytosterol in the formulation of liposomes, using an oral solvent instead of organic solvent to produce nanoliposomes based on milk phospholipid for use in dairy product matrix.
Materials and methods
Chemicals
Phospholipid was prepared from Serva Feinbiochemica Heidelberg, Germany. Cholesterol, phytosterol, and vitamin C were purchased from Sigma-Aldrich (St Louis, MO, USA). Distilled water was purchased from Zolal (Tehran, Iran) as well as ethanol from the chemical industry of Ghadir (Tehran, Iran) and used as an oral solvent.
Nanoliposome production
In this study, one-step method of Alexander et al. (2012) was used with slight modifications. First, phospholipid, cholesterol and phytosterol powder were used in a ratio of 50–100%, 0–50%, and 0–50%, respectively, and in an Erlenmeyer containing an equal amount of distilled water and oral solvent were dissolved. Then, for the formation of multilayer liposomes, suspensions were placed on a hot plate at 600 rpm for 30 min. Subsequently, in other to the evaporation of solvent, the suspension was placed in a bath at a temperature of 75 °C. To reduce the size of the liposomal particles and encapsulation the vitamin C solution into the nanoliposomes, the suspension was placed under different sonication times (20, 25, 30, 35, 40 min) using a Soniprep 150 (MSE Scientific Instruments, Crawley, UK) with ultrasonic frequency 23 kHz, maximum power 150 W and amplitude 12 μm, equipped by probe 9.5 mm. To avoid increasing the temperature of the sonicator and suspension, the device switched on at 5 min’ intervals for 1 min. When the sonication was completed, the sedimentation of titanium particles isolated from the sonicator’s probe and multilayer large liposomes, as well as non-trapped material, was centrifuged at 7000×g for 10 min (Farhang et al., 2012). At the end, the samples were kept in the refrigerator at 4 °C for carrying out the relevant experiments.
Particle size determination
The average of particle diameter was measured by a particle size determination device based on laser light dispersion at ambient temperature using the laser diffraction particle size analyzer (SALD-2101, Shimadzu, Tokyo, Japan).
Encapsulation efficiency
Encapsulation efficiency was measured at ambient temperature with three replications using titration (AOAC Official Method 967.21, 2005; Alexander et al., 2012). At first, the dilutions of 10 mg/ml were diluted to 1 mg/ml, Then, 2 ml of the sample and 5 ml of meta-phosphoric acid solution poured into a volume of 50 ml and titrated with an indophenol solution until the appearance of pink. The efficiency of encapsulation defined as the ratio of the mass of the encapsulated substance to the mass of the same material used to prepare nanoparticles and calculated as follow:
Control release
Control release was carried out at ambient temperature with three replications. The suspensions were tested after one week of storage at 2–4 °C in order to measure the release rate of the encapsulated material according to Yang et al. (2003). At first, the samples were homogenized in a shaker incubator at 25 °C and 50 rpm for 15 min. Then, for titration, the samples were diluted to 1 mg/ml and 2 ml of the sample and 5 ml of methaphosphoric acid were poured into 50 ml Erlenmeyer, and titrated with the use of indophenol to reveal pink color (Yang et al., 2003).
Liposome stability
Stability of samples was measured at 25 °C in three replications according to Yang et al. (2003). At first, the samples were incubated for 20 days at a temperature of 42 °C and titrated at 10-day intervals for testing the stability of the encapsulated material. The samples were diluted to 1 mg/ml and then 2 ml of the sample and 5 ml of methaphosphoric acid was poured into a 50 ml Erlenmeyer and titrated with indophenol until pink color appearance (Yang et al., 2003).
Statistical analysis
In this study, a D-optimal combined design was used to study three formulation factors including ratio of milk phospholipid (50–100%): cholesterol (0–50%): phytosterol (0–50%) and one processing factor including sonication time (20–40 min) with 28 experimental runs. After collecting the data, the significance of the factors and their interactions were evaluated using Fisher’s distribution at ≥ 0.05. The experimental designs, statistical analysis and contour plots were done and drawn by Design-Expert Version 7 (Stat-Ease, Int. Co., Minneapolis, MN, USA).
Results and discussion
Encapsulation efficiency
High percentage of EE shows the method application efficiency. As presented in Fig. 1A, by increasing sonication time and reducing the phospholipids to cholesterol ratio, efficient of cholesterol encapsulation increased. In the interval of 35–40 min of sonication, with the increase in cholesterol, the highest percentage of encapsulation achieved. Alexander et al. (2012) reported that increasing the amount of soy phospholipid from 100 to 250 mg/ml, the efficiency of ascorbic acid encapsulation increased from 15.8 to 32.7%. As presented in Fig. 1B, by increasing the time of sonication, and getting away from the ratio of 75–25 (phospholipid–phytosterol), the encapsulation efficiency reduced. At the time more than 35 min, in the ratio of 75–25 (phospholipid to phytosterol) maximum percentage of encapsulation achieved. Data analysis showed that the effect of phospholipid: phytosterol ratio and also the time of sonication on the encapsulation efficiency was significant at α = 0.05 (Table 1). By comparing Fig. 1A, B, replacement of cholesterol with phytosterol in the production of nanoliposomes using a one-step method and a low-power sonication device the percentage of encapsulation efficiency increased. The use of phytosterols instead of cholesterol may be very useful in producing liposomes because of its harmful effect on human health. In one study by Alexander et al. (2012), phytosterols used for nanoliposome producing by ethanol injection and high pressure homogenization method the particle size was below 200 nm and their distribution was limited. They also reported that with increasing phospholipid levels, the percentage of encapsulation efficiency also increased (Alexander et al., 2012). In accordance with the results of Wu et al. (2016), the encapsulation efficiency was increased with the increasing phosphatidylcholine to cholesterol ratio. Mohammadi et al. (2014), demonstrated that sterol made liposomes for vitamin D entrapment showed a higher vesicle size and encapsulation efficiency and lower size stability than sterol-free liposomes.
Fig. 1.
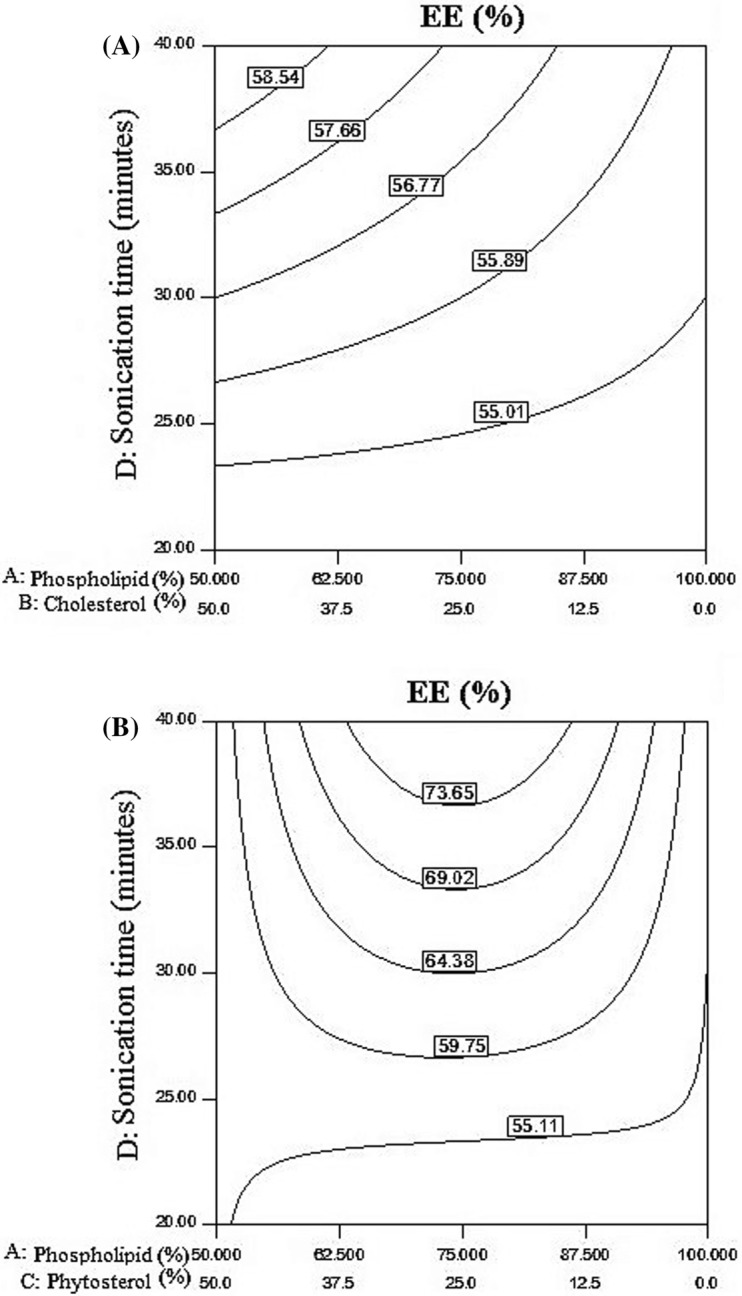
(A) The effect of phospholipid to cholesterol ratio and time of sonication on encapsulation efficiency, (B) Changes in encapsulation efficiency at the time of sonication and different ratios of phospholipid/phytosterol
Table 1.
The result of analysis of variance (ANOVA) for the regression model of EE% and RR%
| Source | Sum of squares | df | mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Prob > F | ||||||
| EE (%) | ||||||
| Model | 194.82 | 7 | 27.83 | 5.28 | 0.0018 | Significant |
| Linear mixture | 6.17 | 2 | 3.09 | 0.59 | 0.5665 | |
| AC | 97.89 | 1 | 97.89 | 18.57 | 0.0004 | |
| AD | 1.72 | 1 | 1.72 | 0.33 | 0.5747 | |
| BD | 27.67 | 1 | 27.67 | 5.25 | 0.0336 | |
| CD | 1.51 | 1 | 1.51 | 0.29 | 0.5984 | |
| ACD | 110.15 | 1 | 110.15 | 20.9 | 0.0002 | |
| Residual | 100.14 | 19 | 5.27 | |||
| Lack of fit | 68.96 | 14 | 4.93 | 0.79 | 0.6681 | Not significant |
| Pure error | 31.18 | 5 | 6.24 | |||
| Cor total | 294.96 | 26 | ||||
| RR (%) | ||||||
| Model | 1052.113 | 10 | 105.2113 | 6.366358 | 0.0010 | Significant |
| Linear mixture | 91.10301 | 2 | 45.5515 | 2.756331 | 0.0979 | |
| AB | 210.0737 | 1 | 210.0737 | 12.7116 | 0.0031 | |
| AC | 58.6082 | 1 | 58.6082 | 3.546394 | 0.0806 | |
| AD | 13.99071 | 1 | 13.99071 | 0.846581 | 0.3731 | |
| BC | 1.300631 | 1 | 1.300631 | 0.078701 | 0.7832 | |
| BD | 65.06671 | 1 | 65.06671 | 3.9372 | 0.0672 | |
| CD | 4.779008 | 1 | 4.779008 | 0.289179 | 0.5992 | |
| ACD | 326.444 | 1 | 326.444 | 19.75319 | 0.0006 | |
| BCD | 121.8098 | 1 | 121.8098 | 7.370733 | 0.0168 | |
| Residual | 231.3659 | 14 | 16.52614 | |||
| Lack of fit | 184.2922 | 9 | 20.47691 | 2.174984 | 0.2030 | Not significant |
| Pure error | 47.0737 | 5 | 9.41474 | |||
| Cor total | 1283.479 | 24 | ||||
Particle size
As showed in Fig. 2A phytosterol and cholesterol both exhibited an incremental effect on the particle size average, but the effect of cholesterol was less. Increasing phospholipid and reducing cholesterol and phytosterol led to decrease in the diameter average particle size of which was in contrast with the results of Alexander et al. (2012). It should be stated that increasing the level of cholesterol and phytosterol caused increases in the particle size, but the effect of phytosterol was higher than cholesterol. The results of Alexander et al. (2012) showed that the addition of phytosterol to phospholipid vesicles increased the diameter of vesicles. As presented in Fig. 2B, Increasing phospholipid ratio led to increase in the standard deviation of the particles, as well as increasing sonication time led to reduction in the standard deviation of the samples. In nanoliposomes with a high ratio of phytosterol to phospholipids and a sonication time of over 30 min, the lowest standard deviation obtained. Different and contradictory reports on the effect of cholesterol on liposomal size have been reported. Viriyaroj et al. (2009) observed that the use of cholesterol in the structure of lecithin led to a reduction in particle size from 72 to 63 nm. It seems that the effect of cholesterol on the size of particles depends on the production method and the type of used phospholipid, as well as showed that addition of cholesterol made the liposome structure harder and increased particle size. Wu et al. (2016) showed that after 5 min of ultrasound on samples, the particle size of lysozyme nanoliposomes changed significantly, and the release ratio on lysozyme nanoliposomes was low.
Fig. 2.
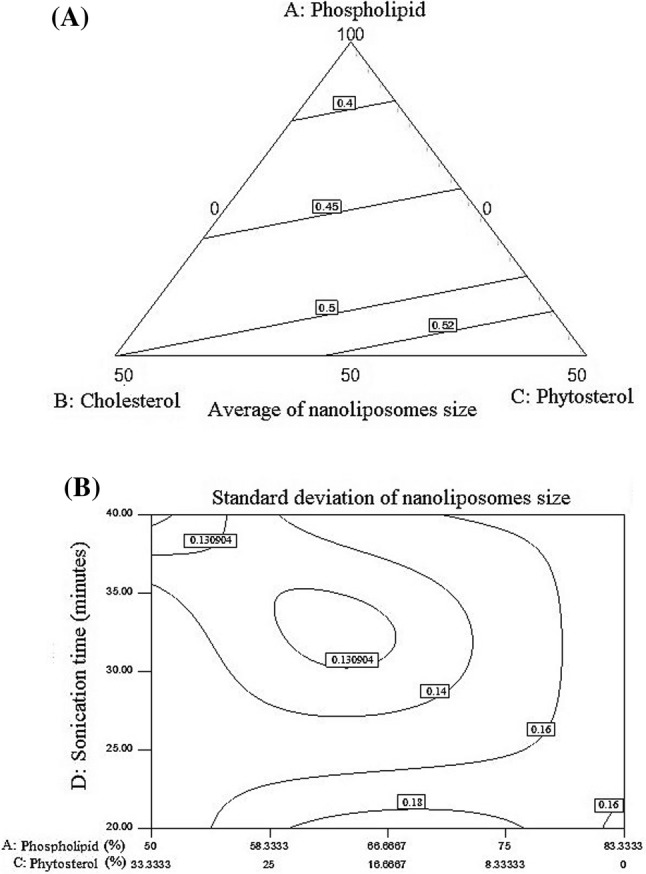
(A) The effect of three factors, phospholipid: cholesterol: phytosterol in the average of produced particle size, (B) The effect of the ratio of phospholipid: phytosterol and the time of sonication on the standard deviation of particle size
Liposome stability
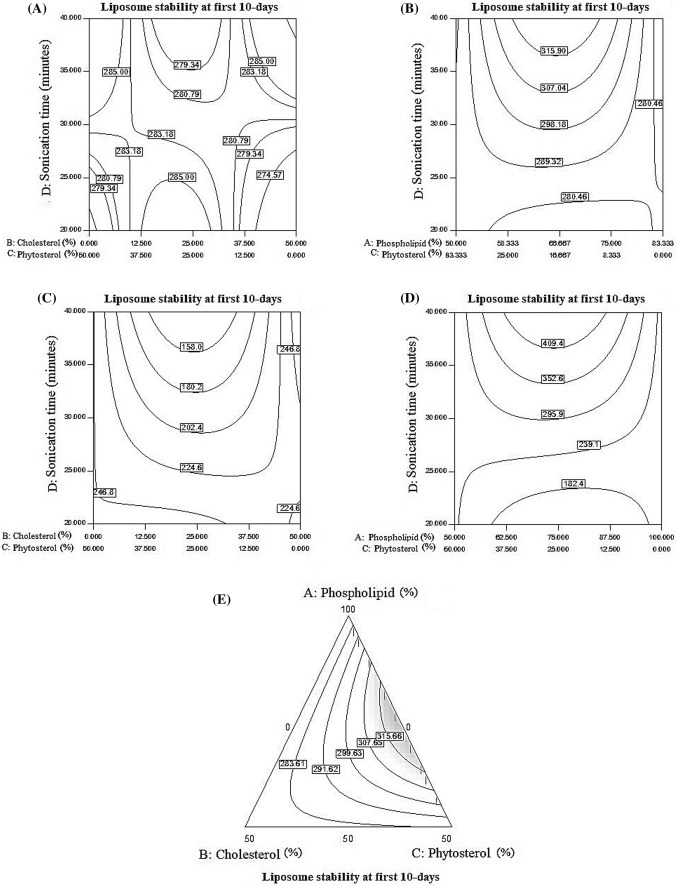
At the first day of nanoliposome storage, by 20–30 min of sonication, with the ratio of 25–25 (cholesterol–phytosterol) stability increased but any increased ratio of cholesterol to phytosterol had negative effect on stability. As showed in Fig. 3A, in the interval of 35–45 min, any increase in cholesterol ratio to phytosterol caused decrease in stability. In 20–30 min of sonication, in the ratio of 66.6–16.6 (phospholipid–phytosterol) stability increased, but increasing this ratio led to decrease in stability. As presented in Fig. 3B, in the ratio of 66.6–16.6 (phospholipid–phytosterol) and at a time of sonication greater than 35 min’ maximum stability obtained. Pezeshky et al. (2016) demonstrated that, addition of cholesterol had no significant effect on the particle size of liposomes and however, its effect was more noticeable on stability of the particle size of nanoliposomes.
Fig. 3.
(A) Vitamin C stability during sonication and different ratios of cholesterol to phytosterol, (B) Stability changes during sonication and different ratios of phospholipid to phytosterol, (C) Effect of sonication time and cholesterol/phytosterol ratio on vitamin C stability after 10 days, (D) Time of Sonication and the ratio of phospholipid to phytosterol in vitamin C stability after 10 days, (E) The effect of three factors phospholipid: cholesterol: phytosterol on the stability of vitamin C after 10 days
As showed in Figs. 3C and 4D, after 10 days of storage, increasing the time of sonication has a negative effect on vitamin C stability. In interval of 20–25 min of sonication, any change in the ratio of cholesterol to phytosterol has not effect on stability, but at time higher than 25 min, in the ratio of 25–25 (cholesterol–phytosterol) stability increased, However, in higher cholesterol to phytosterol ratios, the increase in sonication time, stability was in high level. At 20–30 min of sonication and ratio of 0–50 (cholesterol–phytosterol), the highest stability was observed which indicating the effectiveness of the replacement of cholesterol with phytosterol in the formulation of nanoliposomes. The results of Alexander et al. (2012) revealed that the use of cholesterol and phytosterols, potentially increases the order of phospholipid as well as membrane tightness, and prevents release of active substances. However, based on the results of present study, during 10 days of storage, by increasing phytosterol level, vitamin C stability increased, but cholesterol had a negative effect on stability. From 20 to 30 min of sonication, increasing the ratio of phospholipid to phytosterol has no effect on stability. Within sonication time more than 30 min, by increasing phospholipid to phytosterol ratio (75–25) stability also increased. By increasing the ratio of phospholipid to phytosterol (reduction of phytosterol), stability decreased. Alexander et al. (2012) stated that the stability of liposomes containing phytosterol was reduced in comparison to cholesterol-based formulations, and phase separation observed over the time, but liposomes containing cholesterol had a good stability during storage. Cholesterol has a negative effect on the phospholipid stability during 10 days of storage, however, the phospholipid stability increased in the presence of phytosterols. Figure 3E indicate that replacing low levels of phytosterol with high levels of cholesterol can increase stability, phytosterol also has many benefits for the body. The results showed that phospholipid, phytosterol and sonication time effects were significant at α = 0.05 level on vitamin C stability after 10 days of storage. Liu et al. (2017) results demonstrated that the polyelectrolytes layer can increase average diameter and pH, but reduce absolute zeta potential value and promote aggregation of vesicle in a 90-days storage period at 4 °C. However, the degree of lipid peroxidation and vitamin C release of Alginate–chitosan nanoliposome were lower than traditional nanoliposome, which implied the core (nanoliposome) was protected by the outer shell to some extent.
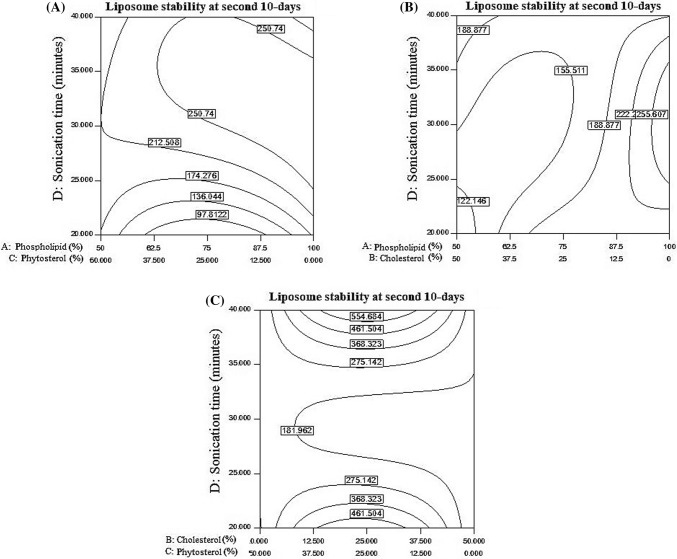
Fig. 4.
(A) The effects of the phospholipid to phytosterol ratio and the time of sonication on vitamin C stability after 20 days of storage, (B) Impact of sonication time and the ratio of phospholipid to cholesterol on the stability of vitamin C after 20 days, (C) The effects of changes in cholesterol/phytosterol ratio and sonication time on vitamin C stability after 20 days
As showed in Fig. 4A, by increasing sonication time, the amount of stored vitamins has also increased. The highest amount of stored vitamins in nanoliposomes obtained in the time more than 30 min and as well as in the ratio of 75–25 (phospholipids–phytosterol). As presented in Fig. 4B, with increasing sonication time and the ratio of phospholipid to cholesterol, the amount of retained vitamin C also increased. In low cholesterol levels, vitamin C levels are high. As presented in Fig. 4C, from 20 to 25 min of sonication, increasing cholesterol to phytosterol ratio led to decrease in the stability of vitamin C, which indicating negative effect of sonication time and the level of cholesterol on stability. From 25 to 35 min of sonication, increasing the ratio of cholesterol to phytosterol did not affect stability and there was no change. At a time, higher than 35 min, the stability of vitamin C increased by increasing sonication time, but in the ratio of 25–25 (cholesterol–phytosterol), the maximum stability was observed.
Control release
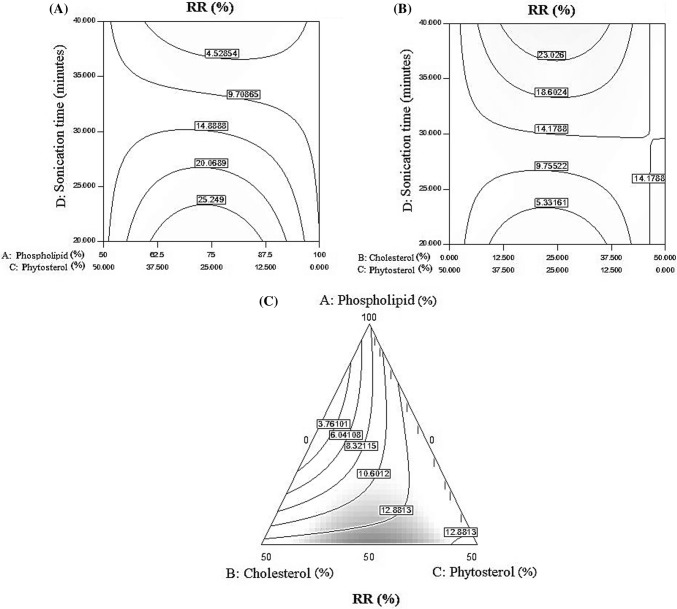
The control release is the amount of vitamin C release from nanoliposomes after one week of storage in the refrigerator, calculated as the release ratio percentage (RR%). As presented in Fig. 5A, the highest amount of control release obtained at a ratio of 75–25 (phospholipids–cholesterol) and at a time below 25 min sonication. Increasing sonication time had a negative effect on control release and led to a decrease in RR percentage. As presented in Fig. 5B, the highest percentage of RR observed after 35 min’ sonication and in the equal ratio of cholesterol to phytosterol. Sonication time showed a synergetic effect on the controlling of vitamin C release which led to an increase in RR percentage. As presented in Fig. 5C, in the middle amount of phospholipid and as well as in the equal amount of cholesterol to phytosterol the best result obtained for release ratio percentage. Mohammadi et al. (2014) showed that, with increasing phospholipid level and decreasing cholesterol and phytosterol level, control release level also decreased. Compared to hydrophilic compounds, lipophilic compounds exhibit higher encapsulation efficiency and they are more stable against hydrolytic degradation, oxidation and show slower release rate of ingredients. The results (Table 1) indicated that the amount of phospholipid and cholesterol together with phospholipid, phytosterol and sonication time, as well as cholesterol, phytosterol and sonication time had significant effect on control release (P ≥ 0.05).
Fig. 5.
(A) The ratio of phospholipid to cholesterol, and the effect of time of sonication on control release, (B) The effects of cholesterol to phytosterol ratio and sonication time on RR%, (C) The effects of three phospholipids, cholesterol and phytosterol factors on control of vitamin C release in samples
Acknowledgements
The authors are grateful to the Biotech center of Urmia University for allowing them to use that center’s laboratory facilities and equipment during this research.
References
- Alexander M, Acero Lopez A, Fang Y, Corredig M. Incorporation of phytosterols in soy phospholipids nanoliposomes: Encapsulation efficiency and stability. LWT-Food Sci. Technol. 2012;47:427–436. doi: 10.1016/j.lwt.2012.01.041. [DOI] [Google Scholar]
- AOAC. Official Method of Analysis of AOAC Intl. 18th ed. Method 967.21. Association of Official Analytical Chemists, Arlington, VA, USA (2005)
- Barbosa-Canovas GV, Mortimer A, Lineback D, Spiess W, Buckle K, Colonna P. Global issues in food science and technology. Pullman, WA, USA: Academic Press; 2009. pp. 411–424. [Google Scholar]
- Farhang B, Kakuda Y, Corredig M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci. Technol. 2012;92:353–366. doi: 10.1007/s13594-012-0072-7. [DOI] [Google Scholar]
- Fox PF, Uniacke-Lowe T, McSweeney PLH, O’Mahony JA. Dairy Chemistry and Biochemistry. 2. Cham: Springer; 2015. pp. 145–239. [Google Scholar]
- Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012;1:147–168. doi: 10.1166/jcsb.2012.1020. [DOI] [Google Scholar]
- Liu W, Tian M, Kong Y, Lu J, Li N, Han J. Multilayered vitamin C nanoliposomes by self-assembly of alginate and chitosan: Long-term stability and feasibility application in mandarin juice. LWT-Food Sci. Technol. 2017;75:608–615. doi: 10.1016/j.lwt.2016.10.010. [DOI] [Google Scholar]
- Mohammadi M, Ghanbarzadeh B, Hamishehkar H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014;4:569–575. doi: 10.5681/apb.2014.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 2008;18:309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- Ostlund RE. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- Pezeshky A, Ghanbarzadeh B, Hamishehkar H, Moghadam M, Babazadeh A. Vitamin A palmitate-bearing nanoliposomes: preparation and characterization. Food Biosci. 2016;13:49–55. doi: 10.1016/j.fbio.2015.12.002. [DOI] [Google Scholar]
- Quilez J, Garcia-Lorda P, Slas-Salvado J. Potential uses and benefits of phytosterols in diet: present situation and future directions. Clin. Nutr. 2003;22:343–351. doi: 10.1016/S0261-5614(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Richardson ES, Pitt WG, Woodbury DJ. The role of cavitation in liposome formation. Biophys. J. 2007;93:4100–4107. doi: 10.1529/biophysj.107.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem. Phys. Lipids. 2009;162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Sekhon BS. Food nanotechnology–an overview. Nanotechnol. Sci. Appl. 2010;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Hindmarsh JP, Haisman D, Rades T, Singh H. Comparison of the structure and properties of liposomes prepared from milk fat globule membrane and soy phospholipids. J. Agric. Food Chem. 2006;54:3704–3711. doi: 10.1021/jf052859b. [DOI] [PubMed] [Google Scholar]
- Viriyaroj A, Ngawhirunpat T, Sukma M, Akkaramongkolporn P, Ruktanonchai U, Opanasopit P. Physicochemical properties and antioxidant activity of gamma-oryzanol-loaded liposome formulations for topical use. Pharm. Dev. Technol. 2009;14:665–671. doi: 10.3109/10837450902911937. [DOI] [PubMed] [Google Scholar]
- Wechtersbach L, Poklar Ulrih N, Cigić B. Liposomal stabilization of ascorbic acid in model systems and in food matrices. LWT-Food Sci. Technol. 2012;45:43–49. doi: 10.1016/j.lwt.2011.07.025. [DOI] [Google Scholar]
- Wu Z, Guan R, Lyu F, Liu M, Gao J, Cao G. Optimization of preparation conditions for lysozyme nanoliposomes using response surface methodology and evaluation of their stability. Molecules. 2016;21:741–753. doi: 10.3390/molecules21060741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Lee SY, Han YS, Park KC, Choy JH. Efficient transdermal penetration and improved stability of L-ascorbic acid encapsulated in an inorganic nanocapsule. Bull. Korean Chem. Soc. 2003;24:499–503. doi: 10.5012/bkcs.2003.24.4.499. [DOI] [Google Scholar]
- Yang S, Liu C, Liu W, Yu H, Zheng H, Zhou W, Hu Y. Preparation and Characterization of Nanoliposomes Enencapsulation Medium-Chain Fatty Acids and Vitamin C by Lyophilization. Int. J. Mol. Sci. 2013;4:19763–19773. doi: 10.3390/ijms141019763. [DOI] [PMC free article] [PubMed] [Google Scholar]