Abstract
This study aimed to evaluate the reduction of total, organic, and inorganic arsenic content in Hizikia fusiforme (hijiki). Initially, the six most common arsenic compounds in hijiki and its organs were evaluated, among which only arsenate and arsenobetaine were detected. Thereafter, the entire plant, including the stalk and leaves, was treated with heat and NaCl solution, individually and in combination. Heating at 90 °C for 5 min significantly reduced arsenic content in hijiki by approximately 33–80%. Treatment with NaCl solution significantly reduced arsenic content in hijiki, except for arsenobetaine content in the stalk. Combinatorial treatment further decreased arsenic content by more than 5–20%. In conclusion, consumption of hijiki after boiling at 90 °C and soaking in 2% NaCl solution reduces the intake of inorganic arsenic by consumers.
Keywords: Total arsenic, Inorganic arsenic, Organic arsenic, Soaking, Heating
Introduction
Arsenic is a naturally distributed element present in many minerals, soil, and air, in different oxidation states, forms, and species (Choong et al., 2007). Arsenic is classified into inorganic arsenic including arsenite (As(III)) and arsenate (As(V)) and organic arsenic including dimethylarsinic acid (DMA), monomethylarsonic acid (MMA), arsenobetaine (AsB), and arsenocholine (AsC). Arsenic toxicity differs on the basis of its chemical form, inorganic arsenic generally being more toxic than organic arsenic. Among inorganic arsenic forms, arsenite is more toxic than arsenate (Yang et al., 2016). The United States Environmental Protection Agency (EPA) reported that the LD50 values for arsenite, arsenate, MMA, DMA, AsC, and AsB are 15–42 mg/kg, 20–200 mg/kg, 700–1800 mg/kg, 1200–2600 mg/kg, 6500 mg/kg, and 10,000 mg/kg, respectively (Abernathy, 2003).
Arsenic is considered a hazardous material by numerous agencies worldwide. The International Agency for Research on Cancer (IARC, 1994) classified arsenic as a group 1 carcinogen, and the Agency for Toxic Substances and Disease Registry (ATSDR) ranked the element at the top of the substance priority list (ATSDR, 2018). Furthermore, arsenic is associated with cancers of the liver, bladder, lung, and skin in humans (Rose et al., 2007). Smith et al. (1992) reported that arsenic causes human kidney and bladder cancer.
Chronic exposure to arsenic affects the circulatory system, potentially triggering hypertension and cardiovascular disease (Jomova et al., 2011). Hall (2002) reported that chronic exposure to arsenic induced numerous disorders including palmar and plantar hyperkeratosis, gastrointestinal symptoms, anemia, and liver disease. A recent ATSDR publication (ATSDR, 2018) reported that inhalation and dermal exposure are considered minor routes of arsenic consumption in the general population. However, most of the arsenic exposure occurs orally via diet, indicating that the oral route is the predominant path of exposure (Chung et al., 2014). Arsenic contained in groundwater and surface water accumulates in agro-fishery products, thus entering the body (Yang et al., 2016). The Korea Food and Drug Administration (KFDA, 2018) reported that fish accounted for the highest proportion (35.2%) of the total arsenic exposure via food, followed by seaweed (20.0%), molluscs (10.1%), crustaceans (9.6%), grains (9.1%), shellfish (4.7%), processed food (3.7%), kimchi and pickled foods (2.8%), and fruits (1.5%). However, according to the Korean Ministry of Health and Welfare (KMHW, 2018), the daily per capita intake of seaweed ranked 13th among the 17 items with 22.4 g, whereas grains ranked 2nd with 293.7 g, and fish ranked 8th with 89.3 g. Although seaweed intake is lower than that of other food products, it greatly contributes to arsenic exposure.
Generally, the amounts of arsenic in vegetables, grains, and meats are 0.01–1.0 mg/kg, while that in seaweed is 10–60 mg/kg. Although seaweed contains relatively higher amounts of arsenic than vegetables and meats, most of the arsenic exists in the form of non-toxic arsenosugars. More than 20 arsenosugars have been identified in seaweed, most of which are dimethylarsinoylribosides (EFSA, 2009). Brown seaweeds, including Hizikia fusiforme (hijiki), accumulate arsenic as inorganic arsenic (EFSA, 2009; Hanaoka et al., 2001). Hijiki, family Phaeophyta (Katayama et al., 2008), is a brown seaweed commonly found along the Korean, Japanese, and Chinese coasts (Baek et al., 2015; Katayama et al., 2008). Hijiki is considered a healthy food product owing to its high dietary fiber content and essential minerals such as calcium, iron, and magnesium (Ryu et al., 2009). Rose et al. (2007) reported that among five common commercial varieties of seaweed (hijiki, arame, wakame, kombu, and nori), hijiki contains the highest total and inorganic arsenic levels.
Currently, no Korean and international statutory limits of arsenic in hijiki have been reported, although several countries advise against hijiki consumption. The Canadian Food Inspection Agency (CFIA, 2018) advised consumers to avoid consuming hijiki because of significantly higher levels of inorganic arsenic than other seaweeds. Similarly, the UK Food Standard Agency (UKFSA, 2018) advised against consuming hijiki. The Food Standards Australia-New Zealand (FSANZ, 2018) and the Hong Kong Centre for Food Safety (HKCFS, 2018) advised against the direct consumption of hijiki and suggested its use as a food ingredient, instead (Yokoi and Konomi, 2012).
In 1989, the provisional tolerable weekly intake (PTWI) of inorganic arsenic was set at 15 µg/kg bw/week by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2010). However, they noted that the PTWI of 15 µg/kg bw/week (equivalent to 2.1 µg/kg bw/day) is within the region of the threshold dose close to the lower confidence limit (BMDL0.5) of 3.0 µg/kg bw/day, which was determined in accordance with epidemiological studies and is no longer appropriate. Therefore, the JECFA withdrew the previous PTWI and has suggested no new PTWI value for inorganic arsenic. The JECFA noted that more accurate information regarding inorganic arsenic content of foods is needed owing to its consumption, to improve the assessment of dietary exposure to inorganic arsenic species (Yang et al., 2016). Therefore, the objective of this study was to develop a method to reduce inorganic arsenic content and measure total, inorganic, and organic arsenic content in hijiki.
Materials and methods
Materials and reagents
Hijiki was collected from Tong-yeong province, Korea, and separated into stalks and leaves. Total hijiki, separated stalks, and leaves were stored below 4 °C until use. All reagents were of analytical grade until otherwise specified.
Total arsenic analysis
Arsenic plasma emission standard (ICP) was purchased from AccuStandard (New Haven, CT, United States). Nitric acid (70%) was purchased from Dong Woo Fine Chem. Co. Ltd. (Pyeongtaek, Gyeonggido, Korea). Hydrogen peroxide (30%) was obtained from Junsei Chemical Co. (Chuo, Tokyo, Japan). Deionized water (18 MΩ cm) for the analysis was prepared using the ELGA purelab ultra water purification system (ELGA, High Wycombe, Buckinghamshire, United Kingdom).
Reagents for arsenic speciation analysis
Arsenic(III) oxide, sodium arsenate dibasic heptahydrate, arsenobetaine, and cacodylic acid were purchased from Sigma-Aldrich (St. Louis, MO, United States). Disodium methyl arsonate hexahydrate was purchased from Chem Service (West Chester, PA, United States). Arsenocholine bromide was purchased from Wako Pure Chemical Industries, Ltd. (Chuo, Osaka, Japan). Nitric acid (70%) was purchased from Dong Woo Fine Chem. Co. Ltd. Deionized water (18 MΩ cm) used for analysis was prepared using the ELGA purelab ultra water purification system.
Sample preparation
Total hijiki, separated stalks, and leaves were treated with or without heat, NaCl solution, or a combination of both. High-temperature heat treatment was briefly administered by placing samples in water (w/w = 1:10) at 90 °C for 5 min. Treatment with NaCl solution was administered by soaking the samples in 0%, 1%, and 2% NaCl solutions (w/w = 1:10) for 20 min. Combinatorial treatment was administered with heat treatment, followed by treatment with 2% NaCl solution. Thereafter, all the samples were freeze-dried, ground (Philips, HR2860, Amsterdam, Netherlands), and stored at 4 °C until use.
Analysis of total arsenic
The ground hijiki samples were subjected to microwave digestion in accordance with the Korea Food Code. Briefly, approximately 0.2 g of sample was weighed in a Teflon vessel. Subsequently, 7 mL of nitric acid and 0.5 mL of hydrogen peroxide were added. Microwave digestion was performed using a Start D microwave oven (Milestone, Sorisole, Italy) and the program is summarized in Table 1. The digests were cooled to ambient temperature, transferred to a volumetric flask, and diluted up to 20 mL with deionized water. All digests were filtered through a 0.45-µm syringe filter and analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES, OPTIMA 5300D, Perkin Elmer, Hopkinton, MA, United States) The conditions for ICP-OES were as follows: R.F. generator, 40 MHz; R.F. power, 1300 W; plasma gas flow, 15.0 L/min; auxiliary gas flow, 0.2 L/min; and nebulizer gas flow, 0.8 L/min; wavelength, 188.979 nm.
Table 1.
Contents of total arsenic, organic and inorganic arsenic in raw total hijiki and its parts (mg/kg, dry weight)
| Total As | Inorganic As | Organic As | |||||
|---|---|---|---|---|---|---|---|
| As(III) | As(V) | AsB | AsC | DMA | MMA | ||
| Total hijiki | 178.2±6.7 | N.D. | 106.9±13.7 | 44.3±2.8 | N.D. | N.D. | N.D. |
| hijiki stalks | 111.0±15.7 | N.D. | 58.8±3.8 | 27.0±3.9 | N.D. | N.D. | N.D. |
| hijiki leaves | 259.1±9.6 | N.D. | 164.7±7.5 | 37.2±7.2 | N.D. | N.D. | N.D. |
Values are expressed as mean ± standard deviation (n=3)
As means arsenic, AsB arsenobetaine, AsC arsenocholine, DMA dimethylarsinic acid, MMA monomethylarsonic acid
Analysis of arsenic speciation
Arsenic content was analyzed in accordance with the method of Lee et al. (2018) with a minor modification. Basically, 0.1 g of the hijiki samples were weighed in a 50-mL tube, and 50 mL of 1% nitric acid was added to each tube. All samples were vortexed for 30 s and sonicated for 30 min (Powersonic 610, Hwashin, Yeongcheongu, Gyeongsangbukdo, Korea), followed by vortex-mixing for 30 s. The samples were incubated in a rotary shaker water bath (BS-31, Lab. Companion, Geumcheongu, Seoul, Korea) at 60 °C and 150 rpm for 4 h. The samples were centrifuged at 4000 rpm for 15 min (1236R, Labogene, Lynge, Allerød, North Zealand, Denmark), and the supernatants were filtered through a 0.45-µm syringe filter and analyzed via HPLC (1200 series, Agilent, Santa Clara, CA, United States) combined with inductively coupled plasma mass spectrometry (ICP-MS, 7700 × series, Agilent).
Statistical analysis
All experimental data were evaluated using one-way analysis of variance and significant differences among means from triplicate analysis at (p < 0.05 indicating statistical significance) were determined using Duncan’s multiple range test and an SPSS software program (SPSS Inc., Chicago, IL, United States).
Results and discussion
Analysis of arsenic compounds in raw hijiki, stalks, and leaves
The hijiki and its organs were treated with or without hot water, salt solution, and a combination of both treatments, followed by analysis of total arsenic treatment and arsenic speciation. Among the six common types of arsenic content investigated herein, arsenate and arsenobetaine were detected.
Total arsenic, organic, and inorganic arsenic concentrations in raw total hijiki and its organs are presented in Table 1. Arsenate was the predominant arsenic species in hijiki accounting for approximately 60% of the total arsenic content in total hijiki and leaves, whereas hijiki stalks contained slightly greater than 50% of total arsenic. However, arsenobetaine in total hijiki and stalks constituted approximately 25% of the total arsenic, whereas hijiki leaves contained approximately 24% of the total arsenic. Thus, hijiki stalks contain a higher ratio of arsenobetaine compared with that of leaves. Conversely, hijiki leaves contained a higher ratio of arsenate than stalks.
The total arsenic content in raw total hijiki was 178.2 mg/kg, while that in raw stalks and leaves were 111.0 mg/kg and 259.1 mg/kg, respectively. By contrast, Rose et al. (2007) reported that the total arsenic content in hijiki in the United Kingdom is 95–124 mg/kg. Furthermore, Almela et al. (2006) reported that the total arsenic content in hijiki is 68.3–149 mg/kg. Although the total arsenic content in the present study was slightly higher than that reported previously, the present results are consistent, being typical of this type of seaweed. The high reported were approximately 179 mg/kg (Almela et al., 2002; Hanaoka et al., 2001; Laparra et al., 2004; Van Netten et al., 2000; Watanabe et al., 1979; Yasui et al., 1983).
However, Hanaoka et al. (2001) reported that the total arsenic content in the stalks is 91.2 mg/kg and that in the leaves is 231.0 mg/kg. Katayama et al. (2001) reported that the total arsenic concentration is higher in the leaves than in stalks. By contrast, Ichikawa et al. (2006) reported that the total arsenic content is higher in stalks than in leaves. Furthermore, two inorganic and four organic arsenic compounds were evaluated. Only arsenate and arsenobetaine were detected in total hijiki, stalks, and leaves. None of the other four arsenic compounds (arsenite, arsenocholine, dimethylarsinic acid, and monomethylarsonic acid) was detected in the samples.
In accordance with Raab et al. (2005) and Ryu et al. (2009), most of the arsenic species in hijiki were inorganic arsenic. Arsenate was the predominant arsenic species, accounting for approximately 56–80% of the total arsenic content. The arsenate content in raw total hijiki was 106.9 mg/kg, accounting for 60% of the total arsenic content. The arsenate content in raw stalks and leaves was 58.8 mg/kg and 164.7 mg/kg, accounting for 53.0% and 63.6% of total arsenic content, respectively. By contrast, Almela et al. (2006) reported that inorganic arsenic content was 41.6–117 mg/kg.
In case of organic arsenic, the arsenobetaine content in raw total hijiki, stalks, and leaves was 44.3 mg/kg, 27.0 mg/kg, and 37.2 mg/kg, respectively. These levels are similar to those reported by Katayama et al. (2008), who investigated the behavior of the arsenic compounds eluted during the soaking of dried hijiki. The eluted arsenic compounds were mostly arsenate and arsenobetaine-like compounds. However, Ryu et al. (2009) reported that arsenic forms such as MMA, DMA, AsC, and AsB were not detected in 30 samples of hijiki. Therefore, only arsenate and arsenobetaine were selected for further study.
Effect of heat treatment on arsenic content
Transient high-temperature treatment was administered to mimic cooking conditions, since hijiki is not consumed in the raw form. The effect of heat treatment on total arsenic, arsenate, and arsenobetaine in total hijiki, stalks, and leaves is shown in Fig. 1. Total arsenic, arsenate, and arsenobetaine contents were significantly reduced (p < 0.05) upon heat treatment in total hijiki (Fig. 1).
Fig. 1.
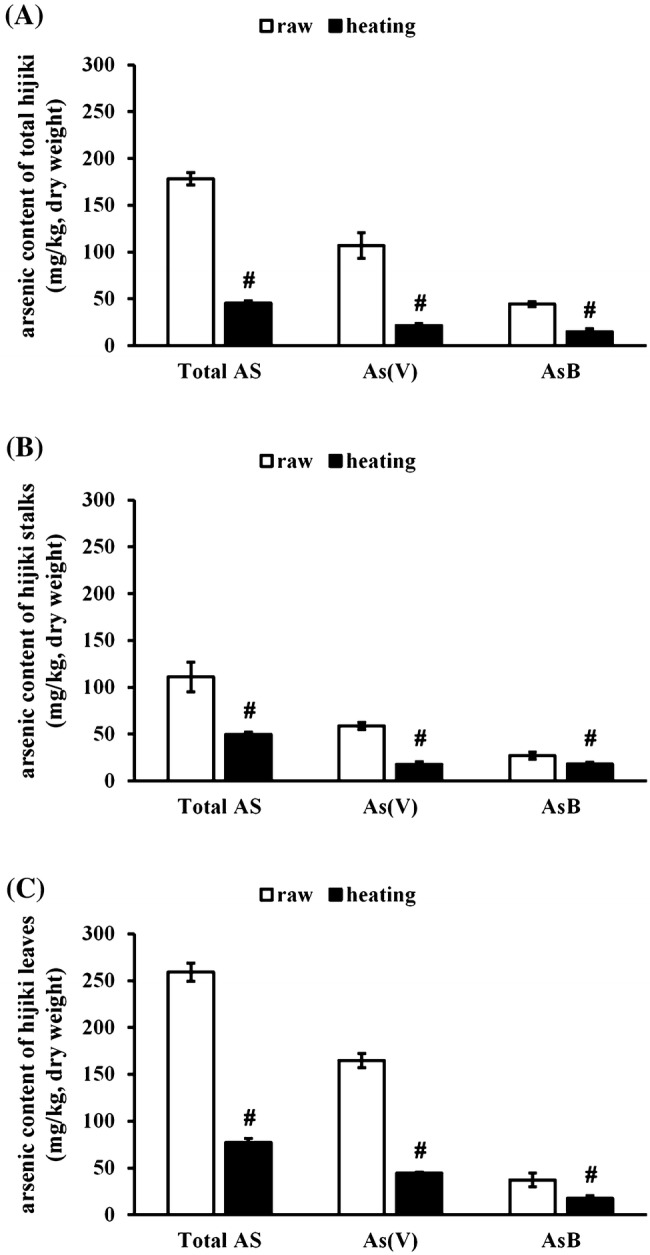
Effect of heat treatment on arsenic content. Total hijiki, stalks, and leaves were obtained. All samples were heated in water (w/w = 1:10) at 90 °C for 5 min. Total arsenic, arsenate, and arsenobetaine content was determined in total hijiki (A), stalks (B), and leaves (C). Untreated raw hijiki samples were used as the control.# indicates significant differences at p < 0.05 against untreated raw control
The amount of total arsenic, arsenate, and arsenobetaine were 178.2 mg/kg, 106.9 mg/kg, and 44.3 mg/kg before heat treatment and 45.3 mg/kg, 21.5 mg/kg, and 14.9 mg/kg, respectively, after heat treatment. Furthermore, heat treatment significantly reduced (p < 0.05) the total arsenic, arsenate, and arsenobetaine content in hijiki stalks (Fig. 1B). The level of total arsenic, arsenate, and arsenobetaine were 111.0 mg/kg, 58.8 mg/kg, and 27.0 mg/kg before heat treatment and 49.5 mg/kg, 17.6 mg/kg, and 18.0 mg/kg, respectively, after heat treatment. Moreover, heat treatment significantly reduced (p < 0.05) the total arsenic, arsenate and arsenobetaine content in hijiki leaves (Fig. 1C). The content of total arsenic, arsenate, and arsenobetaine were 259.1 mg/kg, 164.7 mg/kg, and 37.2 mg/kg before heat treatment and 77.1 mg/kg, 44.4 mg/kg, and 17.5 mg/kg, respectively, after heat treatment.
Overall, the reduction of total arsenic content in total hijiki, stalks, and leaves were 74.6%, 55.1%, and 70.2%, respectively. Furthermore, the reduction of arsenate of total hijiki, hijiki stalks and leaves were 79.9%, 70.1%, and 73.0%, while that of arsenobetaine of total hijiki, hijiki stalks and leaves were 66.4% 33.3%, and 53.0%, respectively. The results showed that, with the exception of arsenobetaine content in hijiki stalks, arsenic contents in hijiki and its organs were reduced more than twofold. These results suggest that even brief heat exposure is adequate to reduce the arsenic content in hijiki.
Arsenic reduction in the present study was greater than that reported by Laparra et al. (2004), who suggested that boiling at 100 °C for 20 min significantly reduced inorganic arsenic content from 46 to 50%. Hanaoka et al. (2001) reported that soaking resulted in a greater loss of inorganic arsenic in hijiki leaves than in stalks, whereas elimination of organic arsenic was not significantly different between stalks and leaves. Arsenobetaine-like compounds appear stable and are not extracted via boiling (Katayama et al., 2008).
Effect of NaCl treatment on arsenic content in hijiki
NaCl solution was utilized to mimic the basic flavor during cooking. Total arsenate levels in all samples were significantly decreased (p < 0.05) in a concentration-dependent manner. The effect of NaCl treatment on total arsenic, arsenate, and arsenobetaine in total hijiki, stalks, and leaves is shown in Fig. 2. Overall, with the exception of arsenobetaine in hijiki stalks, soaking in water significantly reduced (p < 0.05) the amount of total arsenic, arsenate, and arsenobetaine in total hijiki, stalks, and leaves.
Fig. 2.
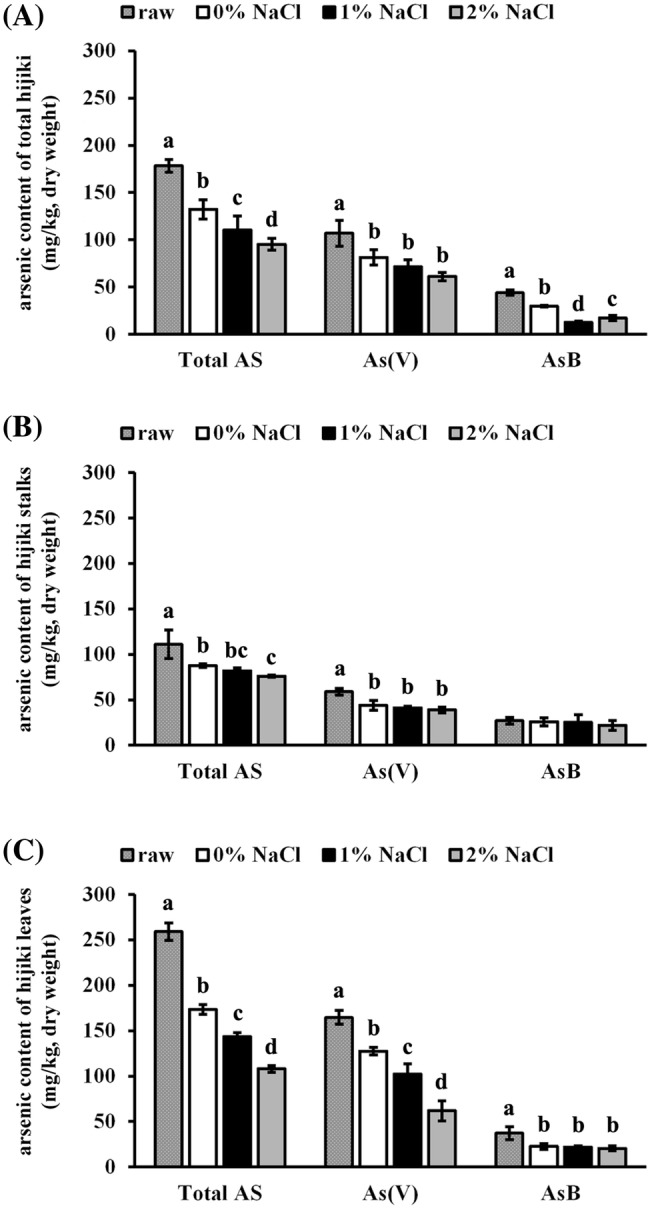
Effect of NaCl treatment on arsenic content. Total hijiki, stalks, and leaves were obtained. All samples were soaked in 0%, 1%, and 2% NaCl solution (w/w = 1:10) for 20 min. Total arsenic, arsenate, and arsenobetaine content were determined in total hijiki (A), stalks (B), and leaves (C). Untreated raw hijiki samples were used as the control. Different letters indicate significant differences at p < 0.05
Total arsenic, arsenate, and arsenobetaine content in total hijiki soaked in 0%, 1%, and 2% NaCl are shown in Fig. 2(A). Total arsenic, arsenate, and arsenobetaine content was significantly reduced (p < 0.05) in a concentration-dependent manner upon NaCl treatment compared with those upon water treatment. The amount of total arsenic was 132.2 mg/kg, 110.4 mg/kg, and 95.2 mg/kg; arsenate, 81.4 mg/kg, 71.3 mg/kg, and 61.3 mg/kg; arsenobetaine, 29.7 mg/kg, 12.6 mg/kg, and 17.2 mg/kg, respectively. Total arsenic, arsenate, and arsenobetaine in hijiki stalks soaked in 0%, 1%, and 2% NaCl are shown in Fig. 2(B). The total arsenic content of hijiki stalks was significantly reduced (p < 0.05) in a concentration-dependent manner upon NaCl treatment compared with that upon water treatment, whereas arsenate and arsenobetaine content did not differ significantly. The amount of total arsenic was 87.4 mg/kg, 81.9 mg/kg, and 76.0 mg/kg; arsenate, 43.7 mg/kg, 41.1 mg/kg, and 38.8 mg/kg; arsenobetaine, 25.8 mg/kg, 25.1 mg/kg, and 22.0 mg/kg, respectively. Total arsenic, arsenate, and arsenobetaine in hijiki leaves soaked in 0%, 1%, and 2% NaCl are shown in Fig. 2(C). NaCl treatment significantly reduced (p < 0.05) the total arsenate and arsenate content in hijiki leaves in a concentration-dependent manner, whereas arsenobetaine content did not differ significantly. The amount of total arsenic was 173.3 mg/kg, 143.4 mg/kg, and 107.9 mg/kg; arsenate, 127.5 mg/kg, 102.1 mg/kg, and 61.9 mg/kg; arsenobetaine, 22.5 mg/kg, 21.9 mg/kg, and 20.5 mg/kg, respectively.
Together, with the exception of arsenobetaine in stalks, soaking total hijiki, stalks, and leaves in water without salt (0% NaCl) significantly reduced (p < 0.05) the level of total arsenic, arsenate, and arsenobetaine compared to that in raw samples. The reduction of total arsenic, arsenate, and arsenobetaine content in total hijiki, stalks, and leaves was approximately 20–40% that in raw samples. Soaking in 2% NaCl solution yielded the greatest reduction in total arsenic, arsenate, and arsenobetaine content in total hijiki, stalks and leaves, by approximately 30–70% that in raw samples. Therefore, even a small amount of common salt may reduce arsenic content in hijiki.
Overall, although soaking in water in the present study showed slightly lower values than those of Hanaoka et al. (2001) and Ichikawa et al. (2006), the overall tendency was consistent. Hwang (2010) reported that NaCl concentration is directly associated with the reduction in water content in vegetables. Arsenic is fairly water-soluble (Rose et al., 2007), thereby reducing arsenic levels following dissolution in water.
Effect of combinatorial treatment of NaCl and heat on arsenic content in hijiki
Based on previous results, combining heat treatment with soaking in 2% NaCl solution resulted in highly significant differences in arsenic levels of total hijiki, stalks, and leaves. Combinatorial treatment of heating at 90 °C and soaking in 2% NaCl solution significantly reduced (p < 0.05) the content of total arsenic, arsenate, and arsenobetaine in total hijiki, stalks, and leaves compared with heating or NaCl treatment alone, with the exception of arsenobetaine levels in total hijiki (Fig. 3).
Fig. 3.
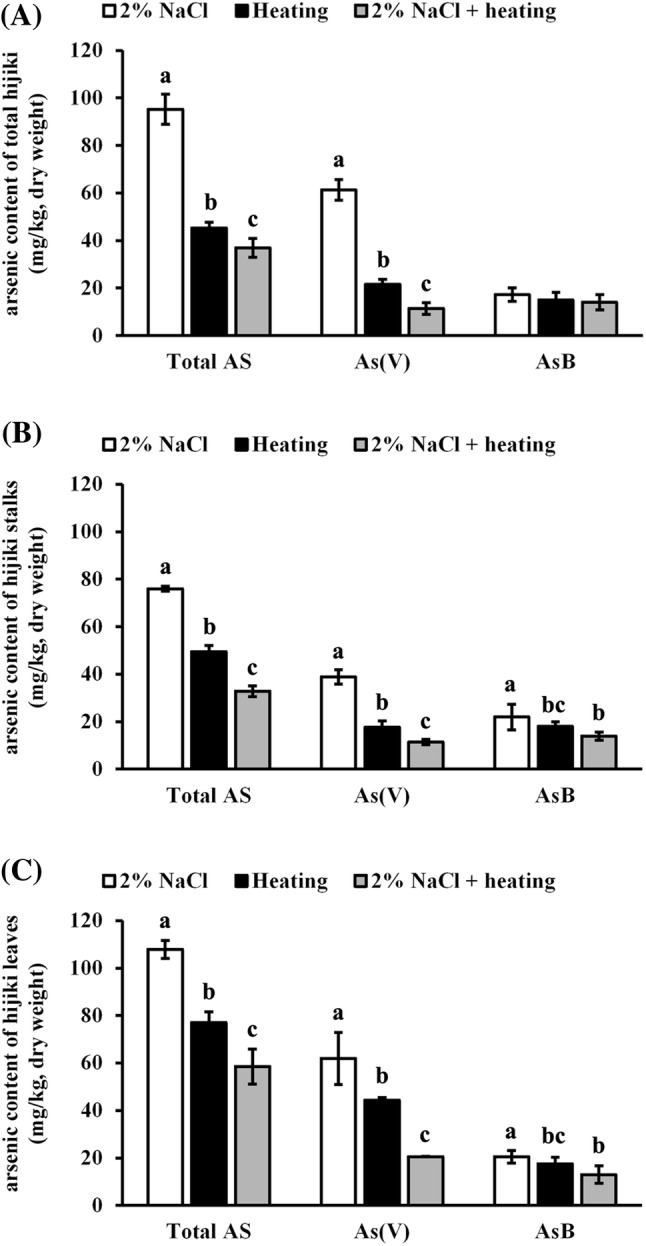
Effect of combinatorial treatment of heat and NaCl on arsenic content. Total hijiki, stalks, and leaves were obtained. All samples were heated in water (w/w = 1:10) at 90 °C for 5 min and soaked in 0%, 1%, and 2% NaCl solution (w/w = 1:10) for 20 min. Total arsenic, arsenate, and arsenobetaine content was determined in total hijiki (A), stalks (B), and leaves (C). Untreated raw hijiki samples were used as the control. Different letters indicate significant differences at p < 0.05
The levels of total arsenic, arsenate, and arsenobetaine in total hijiki administered combinatorial treatment were 36.9 mg/kg, 11.4 mg/kg, and 14.0 mg/kg (Fig. 3A); stalks, 32.8 mg/kg, 11.7 mg/kg, and 13.9 mg/kg (Fig. 3B); leaves, 58.5 mg/kg, 20.5 mg/kg, and 13.0 mg/kg, respectively (Fig. 3C). Total arsenic and arsenate content in total hijiki, stalks, and leaves subjected to combinatorial treatment were significantly lower (p < 0.05) than those upon individual 2% NaCl and heat treatment; furthermore, heat treatment alone significantly reduced (p < 0.05) total arsenic and arsenate levels compared to that with 2% NaCl alone. Interestingly, there were no significant differences in arsenobetaine levels in total hijiki treated with 2% NaCl, heat, and combinatorial treatments. Additionally, arsenobetaine levels in stalks and leaves after combinatorial treatment were significantly lower (p < 0.05) than those after 2% NaCl treatment. However, combinatorial treatment slightly reduced arsenobetaine levels than did heat treatment alone.
Together, with the exception of arsenobetaine in total hijiki, combinatorial treatment yielded the greatest reduction in total arsenic content, followed by heat treatment, and finally 2% NaCl treatment was the highest among three treatments. The present study shows that the reduction in total arsenic content after heat treatment was significantly higher than that after soaking in water, concurrent with the results of Ichikawa et al. (2006), albeit with slightly higher values. Furthermore, concurrent with the results of Katayama et al. (2008) study, the higher the temperature of the soaking water, greater the extraction of arsenic from hijiki. The reduction of metal content in the seafood during cooking may be related to the release of these metals as free salts in water (Bryan and Hummerstone, 1971).
The combination of both heating and salt treatment significantly reduced total arsenic content in hijiki compared with individual treatments. Total arsenic content eliminated from raw samples via combinatorial treatment was approximately 20–45% more than the that upon salt treatment alone, but approximately 5–15% greater than that upon heat treatment alone. Yamashita (2014) reported that total arsenic could be reduced by 86–92% via boiling in seawater multiple times. Although individual salt and heat treatments eliminated a lower percentage of arsenic content, upon combinatorial treatment, the estimated elimination of total arsenic was similar to that reported by Yamashita (2014).
Because arsenic is fairly water-soluble and water content decreased with an increase in NaCl concentration during soaking, total arsenic levels decreased. Arsenobetaine stability prevented its extraction during boiling. In conclusion, the present results suggest that the consumption of hijiki after heating in water at 90 °C, followed by soaking in 2% NaCl solution may reduce the intake of inorganic arsenic by consumers.
Acknowledgements
This research was supported by the Grant (15162MFDS077) from Ministry of Food and Drug Safety and Chung-Ang University Graduate Research Scholarship in 2017.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abernathy CO. Bioaccumulation of arsenic (As) in fish & toxicity of as species. Washington DC, USA: US Environmental Protection Agency; 2003. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Public health statement for arsenic. Available from: https://www.atsdr.cdc.gov/spl/resources/index.html. Accessed June 27, 2018
- Almela C, Algora S, Benito V, Clemente MJ, Devesa V, Súñer MA, Vélez D, Montoro D. Heavy metal, total arsenic, and inorganic arsenic contents of algae food products. J. Agric. Food Chem. 2002;50(4):918–923. doi: 10.1021/jf0110250. [DOI] [PubMed] [Google Scholar]
- Almela C, Clemente M, Vélez M, Montoro R. Total arsenic, inorganic arsenic, lead and cadmium contents in edible seaweed sold in Spain. Food Chem. Toxicol. 2006;44:1901–1908. doi: 10.1016/j.fct.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Baek HI, Kim SW, Ha KC, Kim HM, So BO, Choi EK, Park EO, Jeon BJ, Park BH, Nam TJ, Kim IH, Chae SW. Effectiveness of Hizikia fusiformis extract on erosive gastritis: A4-week, randomized, double-blind and placebo-controlled trial. Int. J. Pharmacol. 2015;11(1):719–725. [Google Scholar]
- Bryan GW, Hummerstone LG. Adaption of the polychaete, Nereis diverscolor to sediments containing high concentration of heavy metals. J. Mar. Biol. Assoc. U. K. 1971;51:857–863. doi: 10.1017/S0025315400018014. [DOI] [Google Scholar]
- Canadian Food Inspection Agency. Inorganic Arsenic and Hijiki Seaweed Consumption. Available from: http://www.inspection.gc.ca/food/information-for-consumers/fact-sheets-and-infographics/products-and-risks/chemical-hazards/inorganic-arsenic/eng/1332268146718/1332268231124. Accessed June 28, 2018
- Choong JY, Chuah TG, Robiah Y, Gregory Koay FL, Azni I. Arsenic toxicity, health hazards and removal techniques from water: an overview. J. Prev. Med. Public Health. 2007;47:253–257. doi: 10.3961/jpmph.14.036. [DOI] [Google Scholar]
- Chung JY, Yu SD, Hong YS. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health. 2014;47:253–257. doi: 10.3961/jpmph.14.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority Scientific opinion on arsenic in food. EFSA Journal. 2009;7(10):1351. doi: 10.2903/j.efsa.2009.1351. [DOI] [Google Scholar]
- Food Standards Australia New Zealand. Survey of Inorganic Arsenic in Seaweed and Seaweed-containing Products Available in Australia. Available from: http://www.foodstandards.gov.au/science/surveillance/pages/surveyofinorganicars5773.aspx. Accessed June 27, 2018.
- Hanaoka K, Yosida K, Tomano M, Kuroiwa T, Kaise T, Maeda S. Arsenic in the prepared edible brown alga hijiki. Hizikia fusiforme. Appl. Organomet. Chem. 2001;15:561–565. doi: 10.1002/aoc.195. [DOI] [Google Scholar]
- Hall AH. Chronic arsenic poisoning. Toxicol. Lett. 2002;128:69–72. doi: 10.1016/S0378-4274(01)00534-3. [DOI] [PubMed] [Google Scholar]
- Hong Kong Centre for Food Safety (2018). Food Safety Focus (42nd Issue, January 2010)—Food for Thought. Available from: http://www.cfs.gov.hk/english/multimedia/multimedia_pub/multimedia_pub_fsf_42_04.html. Accessed June 27, 2018.
- Hwang E-S. Changes in myrosinase activity and total glucosinolate levels in Korean Chinese cabbages by salting conditions. Korean J. Food Cookery Sci. 2010;26(1):104–109. [Google Scholar]
- Ichikawa S, Kamoshida M, Hanaoka K, Hamano M, Maitani T, Kaise T. Decrease of arsenic in edible brown algae Hijikia fusiforme by the cooking process. Appl. Organomet. Chem. 2006;20(9):585–590. doi: 10.1002/aoc.1102. [DOI] [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans: some industrial chemicals. 60: 389–433 (1994) [PMC free article] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives . Summary and conclusions of seventy-second meeting (JECFA/72/SC) Geneva: Switzerland; 2010. [Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Katayama M, Sakiyama C, Nakano Y, Sugawa-Katayama Y. Distribution of accumulated arsenic in the seaweed hijiki, Hizikia fusiforme okam (1) Trace Nutrients Res. 2001;18:29–34. [Google Scholar]
- Katayama M, Sugawa-Katayama Y, Yamaguchi Y, Murakami K, Hirata S. Effect of temperature on the extraction of various arsenic compounds from dried hijiki, Sargassum fusiforme by water-soaking as a pre-cooking proecess. Trace Nutrients Res. 2008;2008(25):134–138. [Google Scholar]
- Korea Food and Drug Administration. Food and heavy metals. 16–17. 2011. http://www.nifds.go.kr/brd/m_18/view.do?seq=4998&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=19. Accessed October 29, 2018
- Korea Ministry of Health and Welfare. National health and nutrition examination survey in Korea. Available from: https://www.khidi.or.kr/kps/dhraStat/result5?menuId=MENU01656&year. Accessed June 27, 2018
- Laparra J, Velez D, Montoro R, Barbera R, Farre R. Bioaccessibility of inorganic arsenic species in raw and cooked Hizikia fusiforme seaweed. Appl. Organometal. Chem. 2004;18(12):662–669. doi: 10.1002/aoc.732. [DOI] [Google Scholar]
- Lee SG, Kim DH, Lee YS, Cho SY, Chung MS, Cho M, Kang Y, Kim H, Kim D, Lee KW. Monitoring of arsenic contents in domestic rice and human risk assessment for daily intake of inorganic arsenic in Korea. J. Food Composit. Anal. 2018;69:25–32. doi: 10.1016/j.jfca.2018.02.004. [DOI] [Google Scholar]
- Raab A, Fecher P, Feldmann J. Determination of arsenic in algae–results of an interlaboratory trial: determination of arsenic species in the water-soluble fraction. Microchim. Acta. 2005;151(3):153–166. doi: 10.1007/s00604-005-0395-7. [DOI] [Google Scholar]
- Rose M, Lewis J, Langford N, Baxter M, Origgi S, Barber M, MacBain H, Thomas K. Arsenic in seaweed-Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007;45:1263–1267. doi: 10.1016/j.fct.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Ryu KY, Shim SL, Hwang IM, Jung MS, Jun SN, Seo HY, Park JS, Kim HY, Om AS, Park KS, Kim KS. Arsenic speciation and risk assessment of hijiki (hizikia fusiforme) by HPLC-ICP-MS. Korean J. Food Sci. Technol. 2009;41(1):1–6. [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Food Standard Agency. Hijiki and Arsenic. Available from: http://www.cfs.gov.hk/english/programme/programme_rafs/programme_rafs_fc_02_08.html. Accessed June 27, 2018
- Van Netten C, Cann SH, Morley D, Van Netten J. Elemental and radioactive analysis of commercially available seaweed. Sci. Total Environ. 2000;255(1):169–175. doi: 10.1016/S0048-9697(00)00467-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hirayama T, Takahashi T, Kokubo T, Ikeda M. Toxicological evaluation of arsenic in edible seaweed. Hizikia species. Toxicology. 1979;14(1):1–22. doi: 10.1016/0300-483x(79)90088-x. [DOI] [PubMed] [Google Scholar]
- Yamashita Y. Method of removing arsenic from dried hijiki seaweed products. Nippon Suisan Gakk. 2014;80(6):973–978. doi: 10.2331/suisan.80.973. [DOI] [Google Scholar]
- Yang SH, Park JS, Cho MJ, Choi H. Risk analysis of inorganic arsenic in foods. J. Food Hyg. Saf. 2016;31(4):227–249. doi: 10.13103/JFHS.2016.31.4.227. [DOI] [Google Scholar]
- Yasui A, Tsutsumi C, Toda S. Some Characters of Water-soluble Arsenic Compounds in Marine Brown Algae, HIJIKI (Hijikia fusiforme) and ARAME {Eisenia bicyclis. Agric. Biol. Chem. 1983;47(6):1349–1351. [Google Scholar]
- Yokoi K, Konomi A. Toxicity of so-called edible hijiki seaweed (Sargassum fusiforme) containing inorganic arsenic. Regul. Toxicol. Pharmacol. 2012;63:291–297. doi: 10.1016/j.yrtph.2012.04.006. [DOI] [PubMed] [Google Scholar]


