Abstract
The aim of the present study was to evaluate the effects of sodium alginate (SA) coatings containing Mentha spicata essential oil (MSO; 0.5 and 1%) and cellulose nanoparticles (CN; 0.25 and 0.5%) on chemical (total volatile base nitrogen content and peroxide value), microbial (total viable count, psychrotrophic count, Pseudomonas spp., and Enterobacteriaceae), and sensory (odor, color, and overall acceptability) properties of raw silver carp fillets during 14 days of refrigerated storage. The MSO was mostly comprised of carvone (78.76%) and limonene (11.50%). SA + MSO 1% + CN 0.5% was most effective in extending the shelf life of silver carp fillets, followed by SA + MSO 1% + CN 0.25%, SA + MSO 1%, SA + MSO 0.5% + CN 0.5%, SA + MSO 0.5% + CN 0.25%, SA + MSO 0.5%, SA + CN 0.5%, SA +CN 0.25%, and SA. Incorporation of MSO 0.5% didn’t have any adverse effect on odor, color, and overall acceptability of treated samples.
Keywords: Sodium alginate, Mentha spicata essential oil, Cellulose nanoparticles, Silver carp fillets
Introduction
In Iran, the breeding of common silver carp (Hypophthalmichthys molitrix) species has increased by 47% since 2014 and currently estimated at 201,097 tons (Annual Agricultural Statistics of Iran, 2017). This upward trend is set to continue in the coming years due to its rapid growth rate, resistance to diseases, easy cultivation, high feed efficiency ratio, low feed demand, culinary taste, and the nutritional quality of its flesh (Kachele et al., 2017; Li et al., 2018). However, freshwater fish, including silver carp, are among the most easily perishable foods due to their inherent susceptibility to biological reactions and microbial activities, resulting in a short shelf life (Li et al., 2013; Ojagh et al., 2010). It has been reported that conventional food preservation methods (cooling, super chilling, and freezing) for preservation of fresh fish could not effectively inhibit the growth of spoilage microorganisms during prolonged storage (Wang et al., 2018). Therefore, the development of novel and effective approaches for improving the shelf-life and quality of fresh silver carp fillets is evidently crucial.
In the last two decades, edible coatings have been used as novel food packaging for preservation of fish and other seafood products by different researchers (Hao et al., 2017; Li et al., 2013; Ojagh et al., 2010). Sodium alginate (SA), a polysaccharide consisting of β-d-mannuronic acid and α-l-guluronic, is obtained from marine brown algae and frequently used as a thickening agent in the food industry (Hao et al., 2017). It has attracted interests as a potential food packaging material owing to its intriguing properties, such as good film forming potential, non-toxicity, biodegradability, biocompatibility, and edibility (Hamedi et al., 2017; Rezaei and Shahbazi, 2018). SA has been approved by the US Food and Drug Administration (US FDA) as Generally Recognized as Safe (GRAS) (Song et al., 2011). On the other hand, it has also been indicated that SA is a good carrier of bioactive compounds such as antioxidants, antimicrobials, organic acids, enzymes, and texture enhancers (Erbay et al., 2017). The incorporation of plant essential oils (EOs), extracts or their phenolic components might prove promising in retarding the microbial growth and lipid oxidation, in improving sensory quality and increasing the shelf life of fresh foods (Gómez-Estaca et al., 2010; Lekjing, 2016).
Numerous EOs or extracts such as grape seed (Kakaei and Shahbazi, 2016; Li et al., 2013), cinnamon (Ojagh et al., 2010), oregano, thyme (Jouki et al., 2014), marigold flower (Samsudin et al., 2014), and Ziziphora clinopodioides (Shavisi et al., 2017) have been incorporated into coatings/films to develop active edible packaging materials with antioxidant and antimicrobial properties. The leaves of Mentha spicata (spearmint) have been used since ancient times in food preparation as a natural flavoring agent and for the treatment of many gastrointestinal disorders (Govindarajan et al., 2012). It is well-documented that M. spicata essential oil (MSO) possesses larvicidal, antibacterial, antifungal, and antioxidant properties in food models and in vitro conditions, because of the high contents of phenolic compounds (Govindarajan et al., 2012; Shahbazi and Shavisi, 2016; Shahbazi et al., 2018). Moreover, cellulose nanoparticle (CN) has been recently proposed as an additive in the class of edible based bionanocomposite films/coatings. It has been known to possess good antibacterial and antifungal properties (Fortunati et al., 2012; Mohebi and Shahbazi, 2017; Shavisi et al., 2017).
Application of SA coating/film containing natural preservative compounds such as bamboo leaf, rosemary (Hao et al., 2017), Z. clinopodioides (Rezaei and Shahbazi, 2018), and tea polyphenols (Song et al., 2011) for the biopreservation and shelf life prolongation of fresh products has been recently evaluated. Nevertheless, to the best of our knowledge, there is no published data regarding the influence of SA coating enriched with MSO and CN on fresh silver carp fillet. Therefore, the aim of the present study was to evaluate the effects of SA coatings containing MSO (0.5 and 1%) and CN (0.25 and 0.5%) on the shelf life extension of raw silver carp fillets during 14 days of refrigerated storage condition.
Materials and methods
Materials
Fresh leaves from the spearmint plant was obtained from Kermanshah, Iran at full flowering stage. The plant material was identified as M. spicata by a taxonomist (Dr. Seyed Mohammad Masoumi, academic staff of Faculty of Agriculture, Razi University, Kermanshah, Iran). After air-drying the plant material at room temperature for 2 weeks, the extraction of MSO was performed based on hydrodistillation using a Clevenger-type apparatus for approximately 3 h and then the obtained MSO was stored in a dark container at refrigerated temperature (4 ± 1 °C) until further use. SA powder (viscosity: 15,000–20,000 cps, density: 1.261 g/cm3, and medium molecular weight: 80,000–120,000 Da) and commercial CN (diameter < 20 nm and purity > 99%) were purchased from Sigma Aldrich (Irvine, UK) and Nano Novin Polymer (Gorgan, Iran), respectively. All chemicals and culture media were supplied from Merck, Darmstadt, Germany.
Gas chromatography–mass spectrometry analysis of Mentha spicata essential oil
The result of gas chromatography–mass spectrometry (GC–MS) analysis of MSO was described in our previous study (Shahbazi et al., 2018).
Preparation of sodium alginate coatings
A stock solution of SA was prepared by dissolving 0.75 g SA powder in 100 mL of sterile distilled water and constant stirring on a hotplate/magnetic stirrer at 50 °C for 1 h to obtain complete dispersion of SA. Then, 0.75 mL of glycerol as a plasticizer and 0.25 mL of tween 80 as an emulsifier of MSO were added to the SA solution. Next, different concentrations of MSO (0.5 and 1%, v/v) alone and in combination with CN (0.25 and 0.5%, w/v) were added to the SA mixture. The resultant SA solution was then constantly stirred at 50 °C for 30 min and homogenized using a homogenizer (HG-15D, Wise Tis, Wonju, Korea) at 1300–1400×g for 5 min at room temperature (Rezaei and Shahbazi, 2018). The pure SA coating without MSO and CN was also prepared following the same procedure.
Preparation of silver carp fillets
Alive silver carps (H. molitrix) weighing 1550 ± 45 g were purchased from a local fish farm (Kermanshah, Iran) and immediately transferred to the laboratory within 1 h. Afterwards, the fishes were slaughtered, scaled, eviscerated, thoroughly washed using sterile distillated water, manually filleted using knives and left to drain on sterile stainless steel net for 5 min. The fillets were cut into samples weighing 100 g, randomly dipped into the designated SA coatings containing different concentrations of MSO (0.5 and 1%) alone and in combination with CN (0.25 and 0.5%) for 1 min, and then placed, as to be drained, on a sterile stainless steel net for 5 min at refrigerated temperature in order to form a coating. The control fillets were dipped into distilled water at 4 ± 1 °C for 1 min. All silver carp fillets were then packed in sterile bags, stored at refrigerated condition for 14 days for subsequent quality assessments including microbial, chemical, and sensory parameters on days 0, 2, 4, 6, 8, 10, 12, and 14 of storage.
Bacterial analysis
At each specified sampling day, 5 g of each fillet sample was aseptically put in a sterile stomacher bag containing 45 mL of 0.1% peptone water and homogenized using a stomacher blender (Interscience BagMixer 400 CC, Nom la Bretèche, France) for 3 min at room temperature to obtain a homogeneous suspension. For each sample, appropriate decimal dilutions (1:10) were then prepared in the test tubes containing 0.1% peptone water. Next, 100 µL aliquot of each serial dilution of homogenates was plated onto following culture media: plate count agar [total viable count (TVC) and psychrotrophic count (PTC)], Pseudomonas agar (Pseudomonas spp.), and Violet Red Bile Glucose (VRBG) (Enterobacteriaceae family) (Jouki et al., 2014). All bacterial counts were expressed as the logarithm of the colony forming units per gram (log CFU/g).
Chemical analysis
Total volatile base nitrogen (TVB-N) content and peroxide value (PV) of samples were examined based on the method proposed by Li et al. (2013) and Jouki et al. (2014), respectively. Results of TVB-N and PV were expressed as mg N/100 g fish muscle and meq peroxide/kg lipid, respectively.
Sensory analysis
Sensory attributes including odor, color, and overall acceptability of the uncoated/coated silver carp fillets were evaluated by ten trained panelists working in the laboratory (five females and five males, ranging from 22 to 32 years old). Panelists were asked to describe differences among samples using a ten-point hedonic scale [1 (dislike extremely) to 10 (like extremely)]. A score of 5 was considered as the lowest limit of acceptability for silver carp fillets.
Statistical analysis
All experiments were conducted in triplicate. Microsoft Windows Excel 2013 and SPSS software (Version 16, SPSS Inc., Chicago, USA) were employed for data analysis of different coating treatments over the storage period. Analysis of variance (ANOVA) and Duncan’s multiple range test were used to detect significant differences among designated treatments (p < 0.05).
Results and discussion
Chemical composition of Mentha spicata essential oil
The chemical compositions of MSO have been presented in our previous study (Shahbazi et al., 2018). The MSO was mainly composed of carvone (78.76%), limonene (11.50%), and β-bourbonene (1.23%).
Bacterial analysis
The TVC and PTC values of the fish fillets stored at refrigerated condition are depicted in Fig. 1(A), (B), respectively. Fresh fish samples had the initial TVC and PTC of 3.27 and 2.85 log CFU/g, respectively. These values are similar to those reported by others for fresh silver carp fillet (Li et al., 2018; Rezaei and Shahbazi, 2018; Valipour Kootenaie et al., 2017). These results indicated the good hygiene condition of fresh silver carp during production. The TVC and PTC values of control and coated sample with straight SA reached 7 log CFU/g on day 4, which is the upper limit of microbiological acceptability for freshwater fish (Li et al., 2018). The bacterial growth for the uncoated sample was insignificantly higher than coated sample with pure SA solution (p > 0.05). Similarly, several studies had reported that pure SA coating didn’t have significant antibacterial effect on the microbial counts of chicken (Hamedi et al., 2017), rainbow trout (Hamzeh and Rezaei, 2012), and bighead carp (Heydari et al., 2015) fillets compared to control group. As shown in Fig. 1(A), (B), TVC and PTC values took 2 days longer to reach the maximum recommended limit of 7 log CFU/g if comparing the coated silver carp samples with SA + CN 0.25% and SA + CN 0.5% with the control group. There was no significant difference between coated samples with SA + CN 0.25% and SA + CN 0.5% (p > 0.05). Shavisi et al. (2017) examined the effect of incorporating CN 1% into polylactic acid (PLA) film on minced beef meat shelf life during 11 days of storage in refrigerated conditions. Their results demonstrated that the application of this active film retarded the growth of mesophilic and psychrotrophic bacterial population for up to 7 days and enhanced the shelf life of minced beef meat compared to the control. In another study (Dehnad et al., 2014), incorporation of CN 0.18% into chitosan film insignificantly decreased the bacterial population of packed raw beef meat compared to the unpacked sample, which is in good accordance with our findings. The results indicate that SA coatings with different concentrations of MSO (0.5 and 1%) separately and in combination with CN (0.25 and 0.5%) had obvious antimicrobial effects in retarding the TVC and PTC increase of silver carp fillets up to 14 days. The most effective bacterial inhibition was found for coated fishes with SA + MSO 1% + CN 0.5%. In this case, the TVC and PTC from 3.27 and 2.85 CFU/g reached 3.81 and 3.63 log CFU/g after 14 days of refrigerated storage, respectively. In a recent study (Shahbazi et al., 2018), authors found a reduction of 1.5–3.5 log CFU/g in TVC and PTC of treated minced camel meats with MSO (0.5, 1, and 1.5%) in comparison with the untreated group. In another study (Kanatt et al., 2008), the TVC of the treated salami with chitosan coating containing M. spicata extract 1% was significantly 2 log CFU/g lower than uncoated samples after 21 storage days in refrigerated conditions. Hamedi et al. (2017) investigated the effects of SA coatings enriched with Ziziphora persica EO (0.5 and 1%) on the microbiological quality of chicken fillets during cold storage and indicated that the designated coatings could successfully reduce the TVC and PTC of treated chicken fillets by 2–3 log CFU/g in comparison with the control group. Heydari et al. (2015) also reported lower TVC and PTC values of up to 2–4 log CFU/g in bighead carp fillets coated with SA containing Mentha longifolia EO (0.5 and 1%) compared to untreated fish fillets.
Fig. 1.
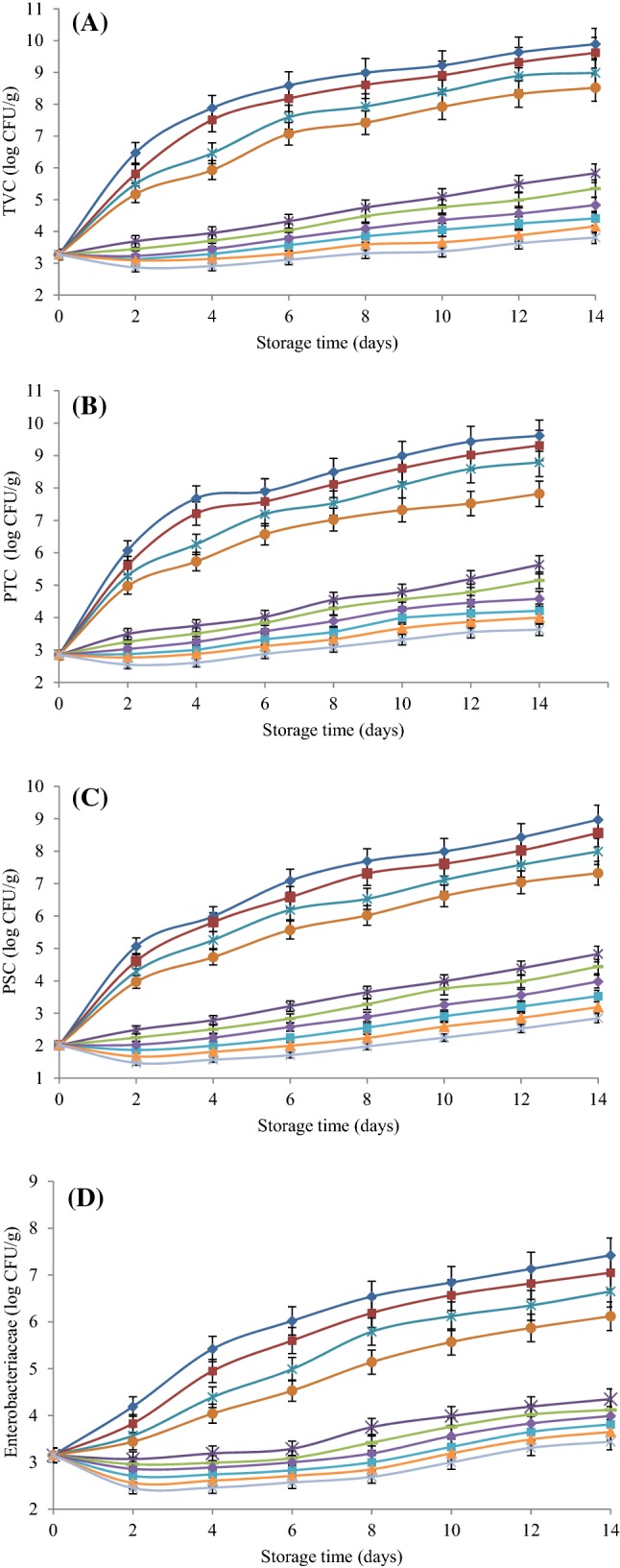
Changes in total viable count (TVC) (A), psychrotrophic bacteria (PTC) (B), Pseudomonas spp. (PSC) (C), and Enterobacteriaceae (D) of silver carp fillets coated with sodium alginate (SA) containing Mentha spicata essential oil (MSO) and cellulose nanoparticle (CN) during refrigerated storage.
 Control,
Control,
 SA coating,
SA coating,
 SA + MSO 0.5%,
SA + MSO 0.5%,
 SA + MSO 1%,
SA + MSO 1%,
 SA + CN 0.25%,
SA + CN 0.25%,
 SA + CN 0.5%,
SA + CN 0.5%,
 SA + MSO 0.5% + CN 0.25%,
SA + MSO 0.5% + CN 0.25%,
 SA + MSO 0.5% + CN 0.5%,
SA + MSO 0.5% + CN 0.5%,
 SA + MSO 1% + CN 0.25%, and
SA + MSO 1% + CN 0.25%, and
 SA + MSO 1% + CN 0.5%. Each number is the mean of three samples taken from different experiments. Each sample was analyzed in triplicate
SA + MSO 1% + CN 0.5%. Each number is the mean of three samples taken from different experiments. Each sample was analyzed in triplicate
At the initial day of study (day 0), Pseudomonas spp. count (PSC) in the fresh silver carp fillets was found to be 2.02 log CFU/g (Fig. 1C). Although Pseudomonas spp. are not dominant in the initial microflora of fresh fish, they typically gain importance during refrigerated storage due to their ability to cause spoilage and formation of histamine in fish (Albertos et al., 2017). In the present study, the count of Pseudomonas spp. in the uncoated group reached up to 8.97 log CFU/g after 14 days of storage at refrigerated temperature. Such an increase in PSC has already been observed for fresh herring (Albertos et al., 2017) and rainbow trout (Jouki et al., 2014; Nowzari et al., 2013; Ojagh et al., 2010) fillets. According to the upper microbiological limit of 7 log CFU/g, the control, coated samples with straight SA, SA + CN 0.25%, and SA + CN 0.5% exceeded this threshold on days 6, 8, 10, and 12 by 7.09, 7.31, 7.11, and 7.04 log CFU/g, respectively. As it can be observed from Fig. 1(C), the incorporation of MSO (0.5 and 1%) into edible SA coatings caused a significant reduction of PSC (between 4.14 and 5.44 log CFU/g) in comparison with control (p < 0.05). Several studies reported that the growth of Pseudomonas spp. in fish and seafood products were inhibited/retarded in the presence of antimicrobial coatings containing natural plant EOs and extracts such as bamboo leaf or rosemary extracts (Hao et al., 2017), Ziziphora persica EO (Hamedi et al., 2017), Z. clinopodioides EO (Rezaei and Shahbazi, 2018), and Eucalyptus EO (Valipour Kootenaie et al., 2017), which is in accordance with our findings.
Enterobacteriaceae is considered by food manufacturers as a hygiene indicator and is usually related to the contaminated water and the delay in chilling for caught fish (Jay et al., 2005). In the present study, at the beginning of storage, Enterobacteriaceae count of fresh fish flesh was found to be 3.15 log CFU/g in fresh fillets (Fig. 1D). It was increased to 7.42 and 7.05 log CFU/g in the control group and the purely SA coated sample at the end of storage period, respectively. Among all designated coatings, the growth rate was significantly faster in the fish fillet batches stored with pure SA, followed by SA + CN 0.25%, and SA + CN 0.5%. Enterobacteriaceae counts of corresponding groups reached 7.42, 7.05, and 6.65 log CFU/g after 2 weeks of refrigerated storage, respectively. However, the increase in bacterial population of coated samples with MSO (0.5 and 1%) separately and in combination with CN (0.25 and 0.5%) were approximately 3.07–3.98 log CFU/g lower than the control group. Gómez-Estaca et al. (2010) also found that gelatin–chitosan films enriched with clove EO had good antimicrobial effects on the Enterobacteriaceae count of covered fish patties. Shahbazi et al. (2018) directly added MSO into minced camel meat, showing that the MSO 1.5% reduced the final count of Enterobacteriaceae by 3 log CFU/g compared to the control group, which is in good accordance with our findings. Jouki et al. (2014) also showed a reduction of Enterobacteriaceae count (between 1 and 3 log CFU/g) as a result of the treatments of rainbow trout fillets with quince seed mucilage based edible films containing four concentrations of oregano or thyme EOs (0, 1, 1.5, and 2%).
As previously described, application of SA coatings enriched with different concentrations of MSO (0.5 and 1%) alone and in combination with CN (0.25 and 0.5%) for treatment of silver carp fillets could successfully retard the growth of bacterial spoilage (Fig. 1A–D). In general, SA + MSO 1% + CN 0.5% had the most effect on shelf life extension of silver carp fillets, followed by SA + MSO 1% + CN 0.25%, SA + MSO 1%, SA + MSO 0.5% + CN 0.5%, SA + MSO 0.5% + CN 0.25%, SA + MSO 0.5%, SA + CN 0.5%, SA + CN 0.25%, and SA. Some previous in vitro studies confirmed that MSO had antimicrobial activity against a wide range of target microorganisms including Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Staphylococcus epidermidis, Salmonella typhimurium, Escherichia coli O157:H7, Vibrio cholera, V. vulnificus, V. parahaemolyticus, V. alginolyticus, Aspergillus niger, Mucor mucedo, and Candida albicans (Hussain et al., 2010; Shahbazi, 2015; Shahbazi and Shavisi, 2016; Snoussi et al., 2015). Some studies have focused on the application of Mentha species in real food models to improve the shelf life and decrease the growth of pathogens of food products (Heydari et al., 2015; Kanatt et al., 2008; Shahbazi et al., 2018). It can be concluded that the major compounds of MSO including carvone and limonene oxygenated unsaturated fatty acids, compromised the genetic material of bacteria and also interacted with anionic lipids on the cytoplasmic membrane of bacterial cells. This interaction causes the formation of transient pores in the plasma membrane, depletion of the ATP pool, amino acids, collapse of crucial ion gradients and ultimately leads to cell death (Burt, 2004). In recent years, the same results about the inhibitory effects of active films enriched with CN on bacterial growth in food models and in-vitro were obtained in the studies of Dehnad et al. (2014), Fortunati et al. (2012), Mohebi and Shahbazi (2017), and Shavisi et al. (2017), which are similar to our findings. In this study, the highest antimicrobial effects were found for treated groups with SA + MSO 1% + CN 0.5% and SA + MSO 1% + CN 0.25%. Previous studies found that the combination of EOs with other antimicrobial compounds had higher effects than the EOs alone did against microbial growth in food models (Lv et al., 2011; Shahbazi and Shavisi, 2016; Shavisi et al., 2017). It has been shown that synergistic effects of antimicrobial compounds affect microbial cells by various antimicrobial mechanisms, including sequential inhibition of a common biochemical pathway, disruption of protective enzymes, and increasing the number and size of pores created in the phospholipid bilayer of the cell membrane (Lv et al., 2011).
Chemical analysis
As shown in Fig. 2(A), TVB-N content of fresh silver carp progressively increased from 9.04 mg N/100 g to a final value of 45.3 mg N/100 g in the control group (p < 0.05). According to the highest acceptable level of TVB-N (25 mg N/100 g fish muscle) (Rezaei and Shahbazi, 2018), the control, fish fillets coated with pure SA, SA + CN 0.25%, and SA + CN 0.5% were spoiled on days 4, 4, 6, and 6, respectively. The increase of TVB-N is probably due to the growth of microbial spoilage and the subsequently increase of microbial degradation products including ammonia, primary, secondary, and tertiary amines (Li et al., 2018). Based on our findings, TVB-N contents were significantly in accordance with the microbial growth of untreated and treated samples with SA coatings containing MSO and CN (p < 0.05). The TVB-N values of coated samples with SA + MSO 1% + CN 0.5%, SA + MSO 1% + CN 0.25%, and SA + MSO 1% were found to be 13.1, 14.3, and 15 mg N/100 g fish muscle at the end of study period, respectively. Lower TVB-N in treated samples during refrigerated storage may be due to the presence of bioactive phenolic compounds in MSO, which could remarkably delay oxidative deamination of non-protein nitrogen compounds of silver carp fillets by microbial action (Jouki et al., 2014). Similarly, Li et al. (2013) reported that application of chitosan coatings containing grape seed extract (0.2%) and tea polyphenols (0.2%) could effectively inhibit the increase of TVB-N refrigerated red drum (Sciaenops ocellatus) fillets. Ojagh et al. (2010) also found lower TVB-N contents in treatments of rainbow trout fillets with chitosan coating containing 1.5% cinnamon EO compared to control group.
Fig. 2.
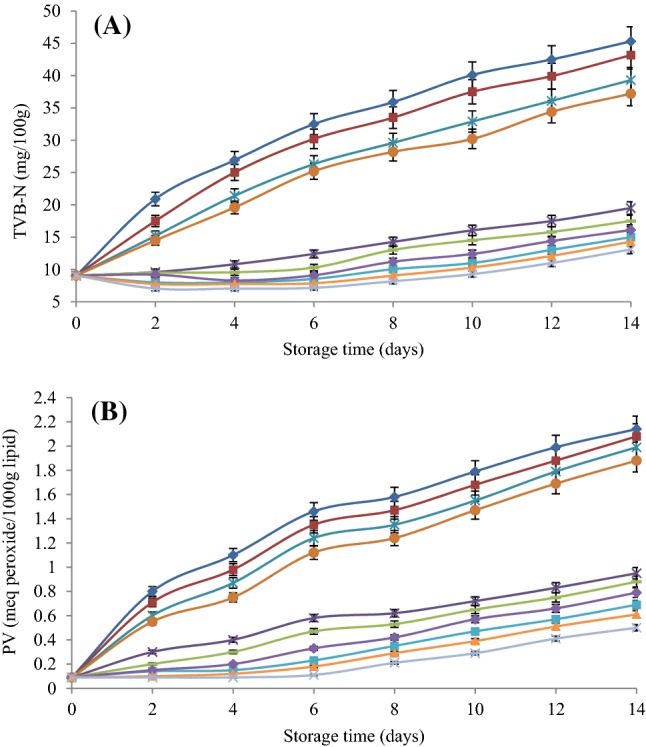
Changes in total volatile base nitrogen (TVB-N) (A) and peroxide value (PV) (B) of silver carp fillets coated with sodium alginate (SA) containing Mentha spicata essential oil (MSO) and cellulose nanoparticle (CN) during refrigerated storage. Each number is the mean of three samples taken from different experiments.
 Control,
Control,
 SA coating,
SA coating,
 SA + MSO 0.5%,
SA + MSO 0.5%,
 SA + MSO 1%,
SA + MSO 1%,
 SA + CN 0.25%,
SA + CN 0.25%,
 SA + CN 0.5%,
SA + CN 0.5%,
 SA + MSO 0.5% + CN 0.25%,
SA + MSO 0.5% + CN 0.25%,
 SA + MSO 0.5% + CN 0.5%,
SA + MSO 0.5% + CN 0.5%,
 SA + MSO 1% + CN 0.25%, and
SA + MSO 1% + CN 0.25%, and
 SA + MSO 1% + CN 0.5%. Each sample was analyzed in triplicate
SA + MSO 1% + CN 0.5%. Each sample was analyzed in triplicate
PV has been traditionally used as an indicator of primary lipid oxidation (hydroperoxides) of foods containing lipid (Jay et al., 2005). The results of the present study revealed that the PV of the control group was increased from 0.09 meq peroxide/kg lipid to 2.14 meq peroxide/kg lipid after 14 days of refrigerated storage (Fig. 2B). Moreover, our findings showed that the PVs of all designated treatments continuously increased with increasing storage time (p < 0.05), but the increasing rate varied with different treatments. At the end of study period, there was no significant difference between the control group and sample coated with the straight SA (p > 0.05). It has been suggested that the increase in PV of the refrigerated food corresponds with the increase in the Psychrotrophic bacteria specially Pseudomonas spp. (Jouki et al., 2014). In the present study, a significant correlation was found between the extent of lipid oxidation and Pseudomonas spp. population (p < 0.05). The lowest PV was found for coated samples with SA + MSO 1% + CN 0.5%, SA + MSO 1% + CN 0.5%, and SA + MSO 1% by 0.50, 0.61, and 0.69 meq peroxide/kg lipid, respectively (p < 0.05). These results are possibly due to the antioxidant activity of MSO. The antioxidant activity of MSO was evaluated by Hussain et al. (2010) who determined the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radicals scavenging ability of MSO with ultraviolet visible spectroscopy. According to their results, MSO had a good antioxidant property (IC50 = 13.3 µg/mL). Moreover, Snoussi et al. (2015) found that the IC50 value for MSO was 13 µg/mL in comparison to 11.5 µg/mL for BHT as a standard compound. Other authors have similarly reported that EOs or their phenolic compounds have strong antioxidant activity to retard the lipid oxidation of stored fish during refrigerated conditions (Jouki et al., 2014; Ojagh et al., 2010).
Sensory analysis
The results of the sensory evaluation including odor, color, and overall acceptability of the silver carp fillets are shown in Fig. 3. Incorporation of MSO 0.5% didn’t have any adverse effect on odor, color, and overall acceptability of treated samples. The control and coated sample with pure SA solution had the lowest sensory attributes during storage under refrigerated conditions due to high microbial and chemical spoilage. Based on our findings, groups treated with SA + MSO 1% separately and in combination with CN (0.25 and 0.5%) had received lower sensory scores when compared to coated samples containing MSO 0.5%. In addition, our preliminary study showed that the sensory properties (odor, color, and overall acceptability) of treated fillets with SA coating containing MSO 2% were negatively affected (data not shown). Our findings indicated that there was significant correlation between the results of the sensory analysis and microbial counts (p < 0.05).
Fig. 3.
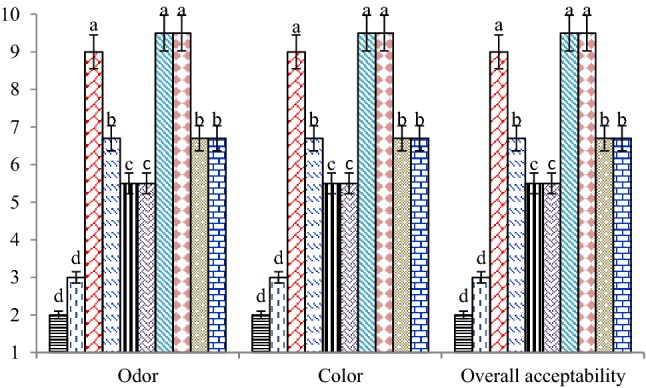
Sensory attributes (odor, color, and overall acceptability) of silver carp fillets coated with sodium alginate (SA) containing Mentha spicata essential oil (MSO) and cellulose nanoparticle (CN) during refrigerated storage. Each number is the mean of three samples taken from different experiments.
 Control,
Control,
 SA coating,
SA coating,
 SA + MSO 0.5%,
SA + MSO 0.5%,
 SA + MSO 1%,
SA + MSO 1%,
 SA + CN 0.25%,
SA + CN 0.25%,
 SA + CN 0.5%,
SA + CN 0.5%,
 SA + MSO 0.5% + CN 0.25%,
SA + MSO 0.5% + CN 0.25%,
 SA + MSO 0.5% + CN 0.5%,
SA + MSO 0.5% + CN 0.5%,
 SA + MSO 1% + CN 0.25%, and
SA + MSO 1% + CN 0.25%, and
 SA + MSO 1% + CN 0.5%. Each sample was analyzed in triplicate. a–d For each attribute, different lowercase letters indicate significant differences (p < 0.05)
SA + MSO 1% + CN 0.5%. Each sample was analyzed in triplicate. a–d For each attribute, different lowercase letters indicate significant differences (p < 0.05)
In conclusion, the results of the present study indicate that MSO present a good source of bioactive compounds including carvone (78.76%) and limonene (11.50%). Our findings show that the shelf life of silver carp fillets using active SA coatings containing MSO (0.5 and 1%) separately and in combination with CN (0.25 and 0.5%) can be significantly improved for up to 14 days compared to the control group. In detail, the designated treatments successfully retarded spoilage microbial growth (TVC, PTC, PSC, and Enterobacteriaceae) and chemical changes (TVB-N and PV) of fresh silver carp fillets. Based on our findings, the development of these novel active coatings can be good alternatives in extending the shelf life of silver carp fillets and reducing chemical preservatives and synthetic materials in food packaging.
Acknowledgements
The author acknowledges Razi University for providing facilities and instrumentations.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This paper does not contain any studies with human or animal subject.
References
- Albertos I, Martin-Diana A, Cullen P, Tiwari B, Ojha KS, Bourke P. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. Technol. in press (2017)
- Annual Agricultural Statistics of Iran . Consumption of seafood in Iran, Ministry of Agriculture. Tehran: Statistical Centre of Iran; 2017. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Dehnad D, Mirzaei H, Emam-Djomeh Z, Jafari SM, Dadashi S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014;109:148–154. doi: 10.1016/j.carbpol.2014.03.063. [DOI] [PubMed] [Google Scholar]
- Erbay EA, Dağtekin BBG, Türe M, Yeşilsu AF, Torres-Giner S. Quality improvement of rainbow trout fillets by whey protein isolate coatings containing electrospun poly (ε-caprolactone) nanofibers with Urtica dioica L. extract during storage. LWT-Food Sci. Technol. 2017;78:340–351. doi: 10.1016/j.lwt.2017.01.002. [DOI] [Google Scholar]
- Fortunati E, Peltzer M, Armentano I, Torre L, Jiménez A, Kenny J. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012;90:948–956. doi: 10.1016/j.carbpol.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Gómez-Estaca J, De Lacey AL, López-Caballero M, Gómez-Guillén M, Montero P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27:889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Govindarajan M, Sivakumar R, Rajeswari M, Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitol. Res. 2012;110:2023–2032. doi: 10.1007/s00436-011-2731-7. [DOI] [PubMed] [Google Scholar]
- Hamedi H, Kargozari M, Shotorbani PM, Mogadam NB, Fahimdanesh M. A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: the antioxidant and antimicrobial activity, and application in food model. Food Hydrocoll. 2017;72:35–46. doi: 10.1016/j.foodhyd.2017.05.014. [DOI] [Google Scholar]
- Hamzeh A, Rezaei M. The effects of sodium alginate on quality of rainbow trout (Oncorhynchus mykiss) fillets stored at 4 ± 2 °C. J. Aquat. Food Prod. Technol. 2012;21:14–21. doi: 10.1080/10498850.2011.579384. [DOI] [Google Scholar]
- Hao R, Liu Y, Sun L, Xia L, Jia H, Li Q. Sodium alginate coating with plant extract affected microbial communities, biogenic amine formation and quality properties of abalone (Haliotis discus hannai Ino) during chill storage. LWT-Food Sci. Technol. 2017;81:1–9. doi: 10.1016/j.lwt.2017.03.031. [DOI] [Google Scholar]
- Heydari R, Bavandi S, Javadian SR. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015;3:188–194. doi: 10.1002/fsn3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AI, Anwar F, Shahid M, Ashraf M, Przybylski R. Chemical composition, and antioxidant and antimicrobial activities of essential oil of spearmint (Mentha spicata L.) from Pakistan. J. Essent. Oil Res. 2010;22:78–84. doi: 10.1080/10412905.2010.9700269. [DOI] [Google Scholar]
- Jay JM, Loessner MJ, Golden DA. Modern Food Microbiology. 7. New York: Springer; 2005. pp. 245–276. [Google Scholar]
- Jouki M, Yazdi FT, Mortazavi SA, Koocheki A, Khazaei N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014;174:88–97. doi: 10.1016/j.ijfoodmicro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Kachele R, Zhang M, Gao Z, Adhikari B. Effect of vacuum packaging on the shelf-life of silver carp (Hypophthalmichthys molitrix) fillets stored at 4 °C. LWT-Food Sci. Technol. 2017;80:163–168. doi: 10.1016/j.lwt.2017.02.012. [DOI] [Google Scholar]
- Kakaei S, Shahbazi Y. Effect of chitosan–gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT-Food Sci. Technol. 2016;72:432–438. doi: 10.1016/j.lwt.2016.05.021. [DOI] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Chitosan and mint mixture: a new preservative for meat and meat products. Food Chem. 2008;107:845–852. doi: 10.1016/j.foodchem.2007.08.088. [DOI] [Google Scholar]
- Lekjing S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016;111:192–197. doi: 10.1016/j.meatsci.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Li D, Zhang J, Song S, Feng L, Luo Y. Influence of heat processing on the volatile organic compounds and microbial diversity of salted and vacuum-packaged silver carp (Hypophthalmichthys molitrix) fillets during storage. Food Microbiol. 2018;72:73–81. doi: 10.1016/j.fm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Li T, Li J, Hu W, Li X. Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem. 2013;138:821–826. doi: 10.1016/j.foodchem.2012.11.092. [DOI] [PubMed] [Google Scholar]
- Lv F, Liang H, Yuan Q, Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011;44:3057–3064. doi: 10.1016/j.foodres.2011.07.030. [DOI] [Google Scholar]
- Mohebi E, Shahbazi Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: a novel functional wrapping design. LWT-Food Sci. Technol. 2017;76:108–116. doi: 10.1016/j.lwt.2016.10.062. [DOI] [Google Scholar]
- Nowzari F, Shábanpour B, Ojagh SM. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013;141:1667–1672. doi: 10.1016/j.foodchem.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Rezaei F, Shahbazi Y. Shelf-life extension and quality attributes of sauced silver carp fillet: a comparison among direct addition, edible coating and biodegradable film. LWT-Food Sci. Technol. 2018;87:122–133. doi: 10.1016/j.lwt.2017.08.068. [DOI] [Google Scholar]
- Samsudin H, Soto-Valdez H, Auras R. Poly(lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control. 2014;46:55–66. doi: 10.1016/j.foodcont.2014.04.045. [DOI] [Google Scholar]
- Shahbazi Y. Chemical composition and in vitro antibacterial activity of Mentha spicata essential oil against common food-borne pathogenic bacteria. J. Pathogens. 2015;2015:1–5. doi: 10.1155/2015/916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi Y, Karami N, Shavisi N. Effect of Mentha spicata essential oil on chemical, microbial, and sensory properties of minced camel meat during refrigerated storage. J. Food Saf. 2018;38:e12375. doi: 10.1111/jfs.12375. [DOI] [Google Scholar]
- Shahbazi Y, Shavisi N. Interactions of Ziziphora clinopodioides and Mentha spicata essential oils with chitosan and ciprofloxacin against common food-related pathogens. LWT-Food Sci. Technol. 2016;71:364–369. doi: 10.1016/j.lwt.2016.04.011. [DOI] [Google Scholar]
- Shavisi N, Khanjari A, Basti AA, Misaghi A, Shahbazi Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017;124:95–104. doi: 10.1016/j.meatsci.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Snoussi M, Noumi E, Trabelsi N, Flamini G, Papetti A, De FeoV. Mentha spicata essential oil: chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of Vibrio spp. strains. Molecules. 2015;20:14402–14424. doi: 10.3390/molecules200814402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Liu L, Shen H, You J, Luo Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala) Food Control. 2011;22:608–615. doi: 10.1016/j.foodcont.2010.10.012. [DOI] [Google Scholar]
- Valipour Kootenaie F, Ariaii P, Khademi Shurmasti D, Nemati M. Effect of chitosan edible coating enriched with Eucalyptus essential oil and α-tocopherol on silver carp fillets quality during refrigerated storage. J. Food Saf. 2017;37:1–8. doi: 10.1111/jfs.12295. [DOI] [Google Scholar]
- Wang Q, Lei J, Ma J, Yuan G, Sun H. Effect of chitosan-carvacrol coating on the quality of Pacific white shrimp during iced storage as affected by caprylic acid. Int. J. Biol. Macromol. 2018;106:123–129. doi: 10.1016/j.ijbiomac.2017.07.180. [DOI] [PubMed] [Google Scholar]


