Abstract
Ultrasound is one of emerging technique’s which is being investigated extremely on food applications and extraction process. In this study, ultrasound-assisted solvent extraction was employed to extract vegetable oil from coriander (Coriandrum sativum L.) seeds. A response surface model was applied to determine the best condition of extraction concerning the independent factors (COY % and DPPH %). In addition, ultrasound variables were the sample solvent ratio, amplitude level, temperature and time. The best condition of extraction was obtained for sample solvent ratio of 1:13 (g/mL), amplitude level of 82 (%), temperature of 45 (°C) and extraction time of 9 (min), being the maximum point of oil yield and antioxidant activity (30.74–72.05%), respectively. Fatty acid profile of oil has been shown as a rich source of petroselinic acid (C18:1)-12, making up 76% of all fatty acids. TGA analyses revealed that 82% (by weight) of oil is thermally stable up to 224 °C.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0467-1) contains supplementary material, which is available to authorized users.
Keywords: Coriander seed oil, DPPH antioxidant activity, Response surface methodology (RSM), Petroselinic acid, TGA analysis
Introduction
Coriander (Coriandrum sativum L.) is an annual plant cultivated for its food applications and health benifits, grown in most agricultural areas, whose major producers are Bulgaria, Romania, Russia, Iran, Morocco, Canada, Australia, Ukraine, Canada, Russia, India, Hungary, Guatemala, Poland, Pakistan, Mexico, Turkey and Argentina (Evangelista et al., 2015; Lopez et al., 2008). The coriander seed has been used traditionally as medicine for various medical conditions like indigestion, rheumatism and pains in the articulations (Shahwar et al., 2012). The seed comprises of essential oil (0.3–1.2%) and a dark brownish green coloured fatty vegetable oil (19–21%). It is utilized for manufacture of detergents, emulsifiers, soaps and softeners (Reiter et al., 1998). It also prevents oxidation process in foods and was shown a high antioxidant activity (Ramadan and Moersel, 2006). The quality of oil mainly depends on extraction technique applied, variety of seed and ecological conditions of plant cultivated. From the literature studies, the following extraction techniques like solvent assisted extraction, supercritical fluid extraction (Mhemdi et al., 2011), screw press (Evangelista et al., 2015), single screw extrusion (Sriti et al., 2011) and double screw extrusion (Sriti et al., 2012) were used to obtain vegetable oil from coriander seeds. The complexity of the extraction equipment, longer time duration and high capital investment cost are the huge disadvantages of these techniques (Mhemdi et al., 2011; Uitterhaegen and Evon, 2017). On the other hand, ultrasound-assisted solvent extraction (UASE-hereinafter) is an adequate option for lipid extraction with simple equipment design, because it facilitates more efficacious interaction between solid and solvent because of increase in pressure and temperature (Pico, 2013). The raise in pressure favours penetration of solvent and transport of solute, while that of temperature improves the solubility and diffusivity. Other advantages include drastically reducing process time, consumption of less energy and reduced thermal degradation effects (Um et al., 2018). The higher efficiency of UASE is considered due to the breaking of cell walls, size reduction and improved mass transfer of the cell content by way of cavitation bubble collapse (Bimakr et al., 2012). A critical step in developing a successful process taking into account the effect of different variables like solvent sample ratio, amplitude, temperature and time, on the efficacy of UASE is the optimization of process conditions. Response surface model has been employed in many fields such as food, chemical and biological processes (Bimakr et al., 2012). This methodology incorporates mathematics with statistics for formulating a mathematical model to depict process, investigate the interaction between multiple process variables and generate a set of experimental combinations and optimize the process, thereby decreasing the number of experimental runs (Bimakr et al., 2013). The present study investigates on coriander seed oil yield and its antioxidant activity, obtained by UASE technique and optimizes its process variables using response surface model. The fatty acid composition, thermal stability and presence of functional groups of oil obtained at optimum condition are also examined.
Materials and methods
Sample preparation and chemicals
Coriander seeds were purchased from local market in Coimbatore (India). Seeds were dried at air temperature (30 °C) for 1 h, ground and sieved using mesh (No. 35-ASTM) for screening the particles ≤ 0.05 mm. The samples were stored in room temperature until the extraction process is carried out. Hexane was used as a solvent for oil extraction. In this work, all chemicals [Hexane, pure fatty acids methyl esters (FAME) and DPPH reagent] were used in analytical grade and purchased from Sigma-Aldrich (Bangalore, Karnataka, India).
Ultrasound assisted solvent extraction (UASE)
Ultrasonic Processor UP-400S, Hielscher (Teltow, Germany) has been used for UASE of oil from coriander seeds. A 400 W power of processor with H22 titanium sonotrode and tip diameter of 22 mm was used. The nominal frequency is 24 kHz, amplitude level can be change from 0 to 100% and a continuous cycle was applied for extraction process. Process variables of conditions were fixed based on the preliminary experiments. The top of the flask was covered with an aluminium foil in order to avoid the solvent loss during the time of extraction. The temperature of the extraction process was controlled using water bath. Using a rotary evaporator, the solvent was removed under vacuum at 50 °C. All experiments were duplicated.
Coriander seed oil yield (COY %)
The coriander seed oil yield was determined by the ratio of amount of oil extracted to the initial amount of sample taken. The coriander seed oil yield was determined by the given formula (Bimakr et al., 2012):
| 1 |
DPPH scavenging activity (DPPH %)
The coriander fatty oil was attenuated (6 times) using n-hexane, for studying DPPH scavenging activity. One milli litre of 20 mmol prepared DPPH solution was added to 500 µL attenuated coriander oil samples. The incubation of the experimental samples was carried out for a period of 40 min in a dark place. The absorbance values of DPPH added oil samples were noted at the wavelength of 517 nm using a UV-spectrophotometer (Spectro UV–VIS double beam, Elico Ltd, Hyderabad, India). DPPH scavenging activity was determined as given below (Samaram et al., 2015):
| 2 |
Analysis of fatty acid composition
The presence of fatty acid components in the coriander seed oil was analyzed by gas chromatograph (GC-FID TRACE-1110, Thermo Fisher, India). The pure fatty acids methyl ester (FAME) was subjected to analysis which was used as standard sample. A silica column (50 m × 0.25 mm × 0.25 µm) was used and the process temperature was maintained at 185 °C for 4 min, then increased to 250 °C at the heating rate of 15 °C. Hydrogen gas was used as carrier gas with flow rate of 1.3 mL/min. The chromatogram of FAME was compared with that of the oil sample (obtained at optimum condition) for identification of fatty acid composition (expressed as percentage of fatty acid in 100% composition) using their retention time.
FT-IR analysis
The Fourier transform-infrared spectra of oil sample was obtained in a Jasco 6600 IR spectrometer (Japan) using an attenuated total reflectance (ATR) sampling accessory. Absorbance was measured in the wavelength range of 500–3500 cm−1.
TGA analysis
Thermo-gravimetric analyser-TGA (TA Instruments, Q-50, USA) was used to analyse the thermal stability of coriander oil. The sample was kept in aluminium pan, for a temperature range of 27–400 °C. Nitrogen at the rate of 50 mL/min, was used as an inert gas.
Statistical design and data analysis
The Response Surface Methodology (RSM) of Design Expert® software (version 8.0.7.1, Minneapolis, USA) was used to determine the best extraction conditions for UASE, focus on optimized the oil yield and the antioxidant content. A Face Centered-Central Composite Design (FCCD) with four independent factors, namely, sample-solvent ratio (X1), amplitude level (X2), temperature (X3) and time (X4) was applied. The 3 levels (− 1, 0 and + 1) coding of the independent variables for statistical calculations was determined using the given equation:
| 3 |
where xi, Xi, Xz and ∆Xi are the dimensionless value, real value, real value at the central point and the step change of the real value of an independent variable respectively. The independent process variables and its levels are shown in Table 1. A combination of 30 experiments (shown in Table 1) was generated by the given equation:
| 4 |
where K is number of independent variables and C0 is the number of central point. To study, the inter-relationship between the responses and independent variables, the second order polynomial equation was developed and its generalized form was given below (Teh et al., 2015).
| 5 |
Table 1.
Coded and uncoded values of FCCD design and experimental values of COY (%) and DPPH (%)
| Run order | Type | Sample-solvent ratio (g/mL) | Amplitude (%) | Temperature (°C) | Time (min) | COY (%) | DPPH (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | YExp | YPre | Residual | YExp | YPre | Residual | ||
| 1 | Axial | 0 (1:10) | 1 (90) | 0 (45) | 0 (10) | 27.52 | 27.30 | 0.22 | 66.65 | 66.76 | − 0.11 |
| 2 | Axial | 0 (1:10) | 0 (80) | − 1 (40) | 0 (10) | 27.3 | 27.46 | − 0.16 | 67.97 | 68.02 | − 0.05 |
| 3 | Axial | 0 (1:10) | 0 (80) | 1 (50) | 0 (10) | 27.52 | 27.57 | − 0.05 | 67.96 | 67.69 | 0.27 |
| 4 | Axial | 0 (1:10) | 0 (80) | 0 (45) | − 1 (5) | 27.32 | 27.22 | 0.10 | 66.92 | 66.84 | 0.08 |
| 5 | Axial | 1 (1:15) | 0 (80) | 0 (45) | 0 (10) | 29.98 | 29.04 | 0.94 | 72.05 | 71.93 | 0.12 |
| 6 | Axial | 0 (1:10) | 0 (80) | 0 (45) | 1 (15) | 26.36 | 26.68 | − 0.32 | 66.21 | 66.07 | 0.14 |
| 7 | Axial | 0 (1:10) | − 1 (70) | 0 (45) | 0 (10) | 24.45 | 24.89 | − 0.44 | 65.13 | 64.80 | 0.33 |
| 8 | Axial | − 1 (1:5) | 0 (80) | 0 (45) | 0 (10) | 19.65 | 20.81 | − 1.16 | 65.56 | 65.46 | 0.09 |
| 9 | Factorial | 1 (1:15) | 1 (90) | 1 (50) | 1 (15) | 19.09 | 18.68 | 0.41 | 58.09 | 58.44 | − 0.35 |
| 10 | Factorial | 1 (1:15) | 1 (90) | − 1 (40) | 1 (15) | 19.49 | 20.19 | − 0.7 | 60.12 | 59.76 | 0.36 |
| 11 | Factorial | 1 (1:15) | − 1 (70) | 1 (50) | 1 (15) | 19.04 | 19.07 | − 0.02 | 58.30 | 58.21 | 0.09 |
| 12 | Factorial | − 1 (1:5) | − 1 (70) | 1 (50) | − 1 (5) | 10.84 | 10.19 | 0.65 | 51.9 | 51.77 | 0.13 |
| 13 | Factorial | − 1 (1:5) | 1 (90) | − 1 (40) | − 1 (5) | 13.65 | 13.68 | − 0.02 | 55.18 | 54.79 | 0.39 |
| 14 | Factorial | 1 (1:15) | 1 (90) | − 1 (40) | − 1 (5) | 27.08 | 27.06 | 0.01 | 64.87 | 65.45 | − 0.58 |
| 15 | Factorial | 1 (1:15) | − 1 (70) | − 1 (40) | − 1 (5) | 19.81 | 19.52 | 0.29 | 59.81 | 59.39 | 0.42 |
| 16 | Factorial | − 1 (1:5) | 1 (90) | 1 (50) | 1 (15) | 12.93 | 13.28 | − 0.35 | 53.85 | 53.78 | 0.06 |
| 17 | Factorial | − 1 (1:5) | 1 (90) | − 1 (40) | 1 (15) | 13.79 | 13.16 | 0.63 | 52.31 | 52.34 | − 0.02 |
| 18 | Factorial | 1 (1:15) | 1 (90) | 1 (50) | − 1 (5) | 25.36 | 25.72 | − 0.36 | 62.65 | 62.02 | 0.63 |
| 19 | Factorial | − 1 (1:5) | − 1 (70) | 1 (50) | 1 (15) | 16.08 | 15.99 | 0.09 | 55.96 | 55.92 | 0.04 |
| 20 | Factorial | − 1 (1:5) | 1 (90) | 1 (50) | − 1(5) | 14.13 | 13.97 | 0.16 | 53.76 | 54.13 | − 0.37 |
| 21 | Factorial | 1 (1:15) | − 1 (70) | − 1 (40) | 1 (15) | 19.09 | 19.14 | − 0.04 | 58.03 | 58.20 | − 0.17 |
| 22 | Factorial | − 1 (1:5) | − 1 (70) | − 1 (40) | 1 (15) | 14.73 | 14.42 | 0.31 | 53.01 | 53.15 | − 0.14 |
| 23 | Factorial | 1 (1:15) | − 1 (70) | 1 (50) | − 1 (5) | 19.11 | 19.63 | − 0.52 | 56.77 | 57.28 | − 0.51 |
| 24 | Factorial | − 1 (1:5) | − 1 (70) | − 1 (40) | − 1 (5) | 8.15 | 8.45 | − 0.30 | 50.92 | 51.11 | − 0.19 |
| 25 | Center | 0 (1:10) | 0 (80) | 0 (45) | 0 (10) | 29.36 | 29.49 | − 0.13 | 70.81 | 70.73 | 0.08 |
| 26 | Center | 0 (1:10) | 0 (80) | 0 (45) | 0 (10) | 29.32 | 29.49 | − 0.17 | 70.98 | 70.73 | 0.25 |
| 27 | Center | 0 (1:10) | 0 (80) | 0 (45) | 0 (10) | 29.91 | 29.49 | 0.42 | 70.75 | 70.73 | 0.02 |
| 28 | Center | 0 (1:10) | 0 (80) | 0 (45) | 0 (10) | 30.73 | 29.49 | 1.24 | 70.12 | 70.73 | − 0.61 |
| 29 | Center | 0 (1:10) | 0 (80) | 0 (45) | 0 (10) | 29.04 | 29.49 | − 0.45 | 70.69 | 70.73 | − 0.03 |
| 30 | Center | 0 (1:10) | 0 (80) | 0 (45) | 0 (10) | 29.23 | 29.49 | − 0.26 | 70.37 | 70.73 | − 0.36 |
The predicted versus experimental plots, ANOVA, lack of fit, R2 and adjusted R2 were used to study the adequacy of the mathematical model developed by RSM. Three dimensional surface plots were generated from the statistical models to analyse the combined effect of process variables on the dependent variables. Derringer’s desirability function with numerical optimization technique was used to bring forth the optimum conditions having some specific desirability value. This optimization method depends on whether a response (Y) is to be maximized or minimized or targeted based on the necessity of the process, while the independent process variables are kept within the range. In this study, this technique was applied to determine the maximum oil yield and DPPH antioxidant activity.
Results and discussion
Experimental and FCCD analysis
The experimental and coded level of FCCD design analysis and the experimental results of thirty combinations, respective predicted values of FCCD design with residual values are shown in Table 1. Residual values of COY % (< 1.24) and DPPH % (< 0.63), indicates that the experimental values agree with the FCCD predicted values. From Table 1, results ranged from 8.15 to 30.73 for COY (%) and 50.92 to 72.05 for antioxidant activity (%). The variation of the values mainly depends on, individual and combined effects of independent variables on COY (%) and antioxidant activity (DPPH %).
Effect of extraction process variables on COY % and DPPH %
Effect of sample solvent ratio
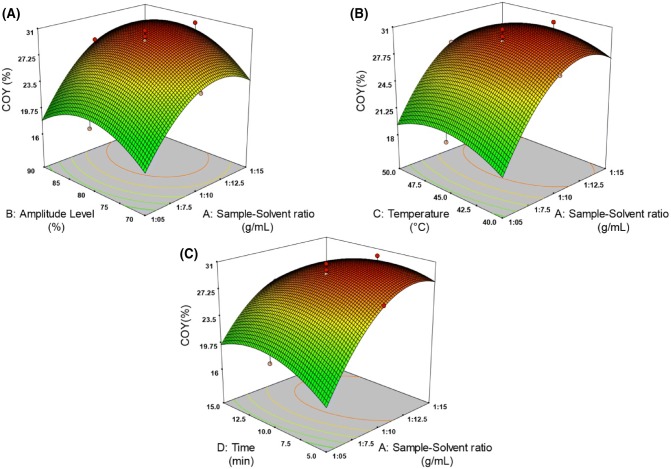
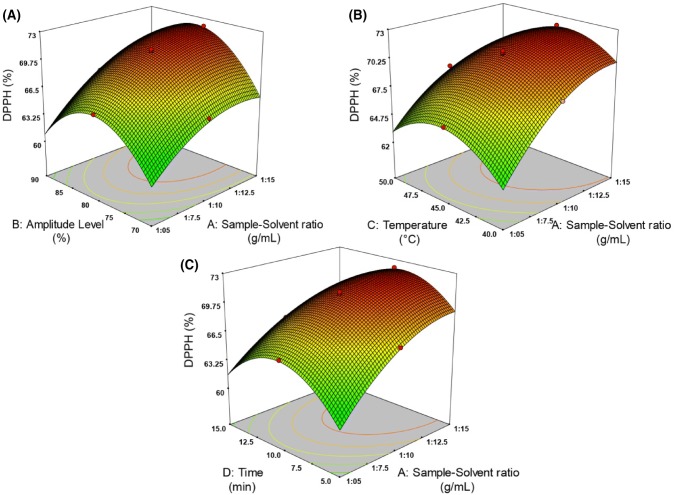
The interaction of UASE experimental variables on COY % and DPPH % were explained using the generated three dimensional surface plots. From Figs. 1 and 2, it can be seen that increasing the sample solvent ratio increases the oil yield and DPPH activity. This might be due to raising the sample solvent ratio, which leads to a heightened mass transfer, thereby increasing the oil yield and DPPH %. Nevertheless, the phenomena stops as the solvent saturates with the extracted oil making the concentration gradient zero (Wei et al., 2008). When higher levels of solvent to sample ratio was used, it can be seen that, the antioxidant activity of oil was increased as shown in Fig. 2. This might be due to the increased solubility of tocopherols (present in oil) in the solvent (Samaram et al., 2015; Wang and Weller, 2006). The current study results of, effect of sample solvent ratio on oil yield (%) and DPPH (%) agreed similarly with previous findings of Teh and Birch (2014) and Wei et al. (2008).
Fig. 1.
Effect of process variables on COY %: (A) sample solvent ratio versus amplitude level, (B) sample solvent ratio versus temperature, (C) sample solvent ratio versus time
Fig. 2.
Effect of process variables on DPPH %: (A) sample solvent ratio versus amplitude level, (B) sample solvent ratio versus temperature, (C) sample solvent ratio versus time
Effect of amplitude level
Increasing the amplitude level to 80%, leads to a noticeable increase in oil yield as shown in Fig. 1A. This might be due to the reason that increasing the amplitude level increases the agitation of sample and solvent (Santos et al., 2016; Tiwari, 2015) leading to a dispersion of oil from the sample matrix to the solvent phase (Khosravi et al., 2013). Similar behaviour was stated in the ultrasound-assisted extraction of vegetable oil from various oil seeds: tallow olein oil, sunflower, olive and sesame (Hosseini et al., 2015), tea seeds (Shalmashi, 2009), fennel seeds (Khosravi et al., 2013), grape seeds (Da Porto et al., 2013) and papaya seeds (Samaram et al., 2015). From the Fig. 2A, it can be revealed that DPPH activity increases with increasing the amplitude level of ultrasound. Klanjan and Preciat (2017) reported that DPPH scavenging activity increases for amplitude level up to 80%. Our present study also shows an increase in the coriander oil antioxidant activity while increasing the amplitude level (%) up to 80% (Fig. 2A). At maximum amplitude level (90%), the oil yield and DPPH % activity gets decreased, because high amplitude level reduces the formation of cavitation (Tiwari, 2015).
Effect of temperature
At elevated temperature, improvement of oil yield may be pertained to the increased solubility of oil with solvent. There are 2 marked trends observed with respect to the oil yield and its antioxidant activity (Figs. 1A, 2B). As the temperature increases from 40 to 45 °C, the oil yield and DPPH activity raises gradually and reaches their maximum value of 30.73% and 72.05% respectively. A further increase in temperature beyond 45 °C causes decrease in response. These two distinguished regions of responses can be considered as an effect of diffusion of oil in solvent (Sicaire, 2016). Moreover, at higher temperature of the extraction process may affect the bioactive compounds as the tocopherols content presence in the oil, which are heat sensitive compounds.
Effect of time
It is observed from Figs. 1C and 2C, the coriander seed powder which was subjected to extraction process, the oil yield (%) and DPPH (%) increases with increasing the time. During the action of ultrasonication, acoustic cavitational bubbles are generated. As time increases, these bubbles expands and collapses, there by disrupting the cell walls which results in the movement of solute into the solvent and hence gradually increasing the oil yield (%) and DPPH (%) gradually (Wei et al., 2008). However, the extraction of oil yield and DPPH % have negative effect when the sonication time rises from 10 to 15 min. Longer sonication time influence with other parameters, might cause volatilization of solvent.
Model fitting and analysis of variance (ANOVA)
The predicted responses (COY % and DPPH %) were tested through fit the model with generated second order polynomial equations and ANOVA test. The predicted models of generated ANOVA details are given in Table 2. From Table 2, p value (Prob > F) of model COY % and DPPH % are highly significant (< 0.0001). In this present study, for COY % response, X1, X2, X1X2, X1X3, X1X4, X2X3, X2X4, X21, X22, X23 and X24 were significant terms (p < 0.05). Similarly for DPPH % response, X1, X2, X4, X1X2, X1X3, X1X4, X2X3, X2X4, X3X4, X21, X22, X23 and X24 were significant terms (p < 0.05) (Table 2). So, the predictive models of second order polynomial equations for both responses are given below (Non significant terms were neglected):
| 6 |
| 7 |
Table 2.
Analysis of variance (ANOVA) for COY % and DPPH %
| Source | COY (%) | DPPH (%) | ||||
|---|---|---|---|---|---|---|
| Sum of squares | Coefficient |
P value Prob > F |
Sum of squares | Coefficient |
P value Prob > F |
|
| Model | 1320.81 | 29.49 | < 0.0001HS | 1405.78 | 70.73 | < 0.0001HS |
| X1-sample-solvent ratio | 305.045 | 4.12 | < 0.0001HS | 188.43 | 3.24 | < 0.0001HS |
| X2-amplitude level | 26.25 | 1.21 | < 0.0001HS | 17.306 | 0.98 | < 0.0001HS |
| X3-temperature | 0.0566 | 0.056 | 0.7311 | 0.493 | − 0.17 | 0.1150 |
| X4-time | 1.306 | − 0.27 | 0.1134 | 2.645 | − 0.38 | 0.0015S |
| X1X2 | 5.37 | 0.58 | 0.0039S | 5.65 | 0.59 | < 0.0001HS |
| X1X3 | 2.665 | − 0.41 | 0.0298S | 7.65 | − 0.69 | < 0.0001HS |
| X1X4 | 40.35 | − 1.59 | < 0.0001HS | 10.44 | − 0.81 | < 0.0001HS |
| X2X3 | 2.095 | − 0.36 | 0.0500S | 1.749 | − 0.33 | 0.0066S |
| X2X4 | 42.08 | − 1.62 | < 0.0001HS | 20.22 | − 1.12 | < 0.0001HS |
| X3X4 | 0.0315 | − 0.044 | 0.7976 | 4.441 | 0.53 | 0.0002S |
| X21 | 54.05 | − 4.57 | < 0.0001HS | 10.68 | − 2.03 | < 0.0001HS |
| X22 | 29.91 | − 3.40 | < 0.0001HS | 63.38 | − 4.95 | < 0.0001HS |
| X23 | 10.08 | − 1.97 | 0.0003S | 21.35 | − 2.87 | < 0.0001HS |
| X24 | 16.75 | − 2.54 | < 0.0001HS | 47.26 | − 4.27 | < 0.0001HS |
| Residual | 6.936 | 2.64 | ||||
| Lack of fit | 4.976 | 0.4183 | 2.14 | 0.207 | ||
| SD | 0.68 | SD | 0.42 | |||
| CV % | 3.09 | CV % | 0.67 | |||
| R2 | 0.9948 | R2 | 0.9981 | |||
| Adj R2 | 0.9899 | Adj R2 | 0.9964 | |||
| Pred R2 | 0.9758 | Pred R2 | 0.9877 | |||
| Adeq. pre | 43.75 | Adeq. pre | 70.15 | |||
HS highly significant, S significant
The fitness of the models were assessed by the determination coefficient (R2), adjusted determination co-efficient (adj R2), predicted determination co-efficient (pred R2) and co-efficient of variance (CV %) and standard deviation (Prakash Maran et al., 2015). The R2 values (0.9948 for COY % and 0.9981 for DPPH %) revealed the quality of the models and statistical significance of models could be check with p value and only small standard deviations (0.68 for COY % and 0.42 for DPPH %) shows that experimental values were agreed with predictive model. The value of pred R2 (0.9758 for COY % and 0.9877 for DPPH %) is in reasonable agreement with the value of adj R2 (0.9899 for COY % and 0.9964 for DPPH %) and also shows that the form of the model chosen to explain the relationship between the factors and the response is well correlated. The lack of fit determines whether the selected model is adequate to explain the experimental data, or whether another model should be reselected. The value of lack of fit test (0.4183 of COY % and 0.207 of DPPH %) is higher than 0.05, which is not significant relative to the pure error and indicates that the fitting model is adequate to describe the experimental data. Lower CV values (3.09 for COY % and 0.67 for DPPH %) clearly stated that, the deviations between experimental and predicted values are low and also showed a high degree of precision and reliability of the conducted experiments. The ratio of response to deviation is called adequate precision and its value can be predicted by statistical analysis. Generally, the ratio greater than 4.0 is desirable. In this study adequate precision values was found to be > 43 for COY % and > 70 for DPPH %, which indicates an adequate signal and confirm that, the models are statistically significant.
Optimization
Optimization of process variables for the higher oil yield (COY %) and its strong DPPH (%) activity was determined using numerical optimization method. The optimal process condition of UASE method was found to be: sample solvent ratio of 1:12.48 (g/mL), amplitude level of 81.5 (%), temperature of 44.5 (°C) and time of 8.9 (min) respectively. Under this optimized process condition, the predicted values were determined as oil yield (30.94%) and DPPH activity (72.12%). Based on the operability of experimental condition, the optimal process condition was modified as sample solvent ratio of 1:13 g/mL, amplitude level of 82%, temperature of 45 °C and extraction time of 9 min and the experiments were triplicate with the modified condition. The average coriander oil yield and DPPH activity (30.75 ± 0.76% and 72.04 ± 0.09%) were well correlated with predicted value. The ultrasound assisted solvent extraction (UASE) results were slightly higher than the previous techniques used to obtain coriander vegetable oil such as soxhlet apparatus (Khodadadi et al., 2016; Msaada et al., 2009), solvent assisted extraction (Shahwar et al., 2012) and supercritical extraction (Mhemdi et al., 2011). The variation in the results might be due to the operating variables of extraction techniques, choice of solvents and the ecological conditions of raw material cultivated. The present study Coriander seed oil has high DPPH antioxidant activity than pumpkin seed oil (65%), grape seed oil (13%), rice bran oil (24%), flax seed oil (20%) and sunflower oil (24%) (Siger et al., 2008).
Analysis of fatty acid composition
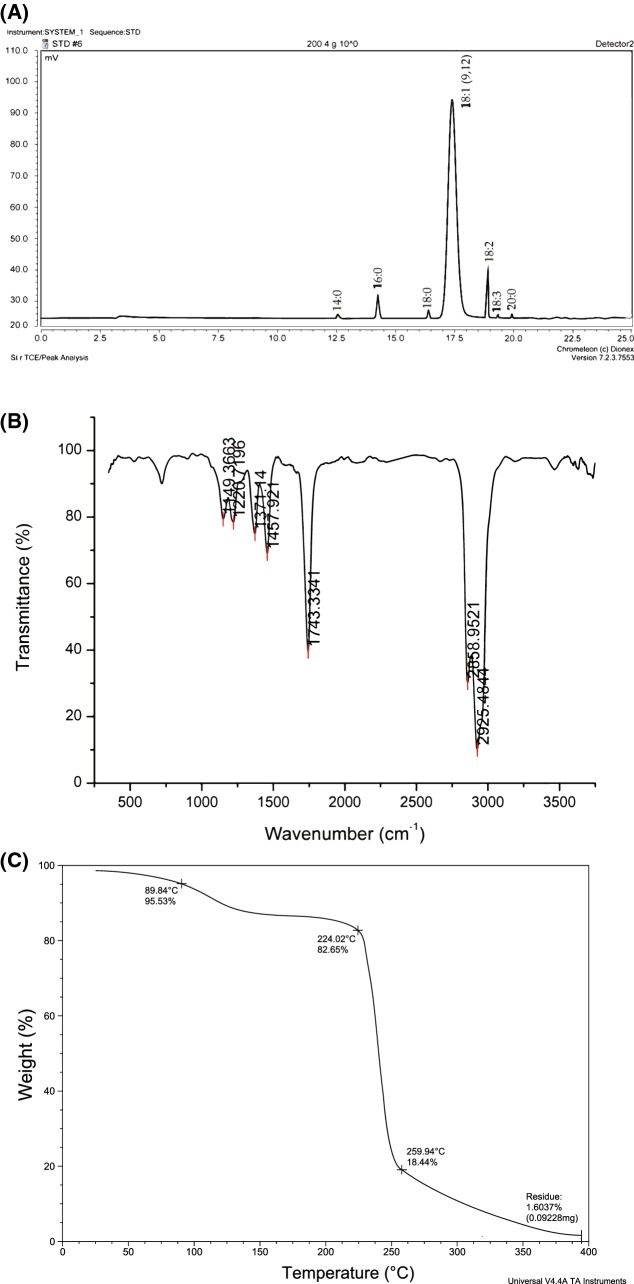
The GC-FID chromatogram of coriander seed oil obtained at optimum condition is shown in Fig. 3A. From Table 3, it can be seeing that coriander seed oil has 95% of unsaturated fatty acid which is spitted in 84% of monounsaturated (MUFA) (omega-9 fatty acids) and 11% of polyunsaturated (PUFA). Petroselinic acid (18:1)-12, was the major fatty acid in total composition of coriander seed oil (Table 3), is an isomer of oleic acid. The present study value of petroselinic acid is in accordance with the previous results reported by Sriti et al. (2012) and Uitterhaegen et al. (2016). In addition, similar levels were obtained for the other fatty acid. Noticeably, palmitoleic acid (16:1) and gadoleic acid (20:1) are not detectable in this study, this might be due to extraction conditions of ultrasound which might have decomposed these two fatty acid functional groups. Parsley and celery oil, with identical fatty acid composition to coriander seed oil, are also similarly rich in composition of petroselinic acid (75% and 64%, respectively) (Ngo-Duy et al., 2009). The presence of rich amount of omega-9 fatty acids (MUFA) can lower the risk of heart disease and stroke, increase the good cholesterol (HDL) and decrease the bad cholesterol (LDL) (Hernandez, 2016).
Fig. 3.
Analysis of oil obtained at optimum condition: (A) GC–FID chromatogram, (B) FTIR spectroscopy, (C) thermo-gravimetric analysis
Table 3.
Fatty acid composition of coriander seed oil
| S. no. | Fatty acid | Composition % |
|---|---|---|
| 1 | Myristic acid (14:0) | 0.38 |
| 2 | Palmitic acid (16:0) | 3.35 |
| 3 | Palmitoleic acid (16:1) | ND |
| 4 | Stearic acid (18:0) | 0.88 |
| 5 | Petroselinic acid (18:1)-12 | 76.22 |
| 6 | Oleic acid (18:1)-9 | 7.93 |
| 7 | Linoleic acid (18:2) | 10.91 |
| 8 | Linolenic acid (18:3) | 0.10 |
| 9 | Arachidic acid (20:0) | 0.19 |
| 10 | Gadoleic acid (20:1) | ND |
| Total saturated fatty acid (SFA) | 4.80 | |
| Total monounsaturated fatty acid (MUFA) | 84.15 | |
| Total polyunsaturated fatty acid (PUFA) | 11.01 |
ND not detectable
FT-IR spectroscopy
The FT-IR spectra of coriander seed oil obtained at optimum condition is shown in Fig. 3B. It can be see that the broad bands in 2500–3000 cm−1 region are assignable to C–H symmetric and asymmetric stretching vibrations. The peak at 1748 cm−1 indicates the presence of –C=O stretching modes of carboxylic acids free ester. C–H scissoring and CH3 bending vibrations exists at the band region of 1300–1480 cm−1. The peaks existing in the region of 1100–1250 cm−1 confirms the presence ester groups by C–O stretching vibrations (Ali et al., 2015). The present study FT-IR result matches well with vegetable oil band stretching of FT-IR spectrum as given by Rohman and Che Man (2011).
Thermal stability
The thermal stability of coriander seed oil was studied by thermo-gravimetric analysis (TGA). The weight loss percentage of oil with increase in temperature, is shown in Fig. 3C. The initial weight loss of 18% occurs due to water desorption and other impurities associated with the sample. Sudden weight loss reaches to 18% of total weight of the sample, when the temperature increases beyond 224 °C as shown in Fig. 3C. This could be due to the degradation of major fatty acid [Petroselinic acid (18:1)-12] which has boiling point of 238 °C and other minor fatty acids [linoleic (18:2), linolenic (18:3) and myristic acids(14:0)] whose boiling point lies below 260 °C (Li et al., 2015). The presence of oleic acid could be the reason for the stability of oil beyond 260 °C.
An UASE method was successfully employed for the extraction of vegetable oil from coriander seeds and optimized using RSM. Face centered-central composite design was used to assess and optimize the process variables (sample solvent ratio, amplitude level, temperature and time) for the coriander oil yield and its antioxidant activity (DPPH %). The results show that the independent variables are statistically significant and a high correlation of developed second order polynomial model is satisfactory and precise to predict the oil yield and DPPH activity. The optimized conditions are as follows: sample solvent ratio of 1:13 g/mL, amplitude level of 82%, temperature of 45 °C and extraction time of 9 min. Under these conditions, the experimental values are 30.75% for COY and 72.04% for DPPH activity; which agreed closely with the predicted values. The GC-chromatogram analysis shows that coriander oil has rich amount (84.15%) of mono unsaturated fatty acids (MUFA), in which petroselinic acid has dominant fatty acid about 76.22%. The thermal stability analysis confirmed that 82% (weight basis) of oil can withstand up to 224 °C. It can be comprehended from the study that UASE technique gives a better oil yield with 84% MUFA and good thermal stability under shorter extraction time and low solvent consumption.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Ali MA, Al-Hattab TA, Al-Hydary IA. Extraction of date palm seed oil (Phoenix dactylifera) by soxhlet apparatus. Int. J. Adv. Eng. Technol. 2015;8:261–271. [Google Scholar]
- Bimakr M, Abdul Rahman R, Taip FS, Adzahan NM, Sarker MZ, Ganjloo A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules. 2012;17:11748–11762. doi: 10.3390/molecules171011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimakr M, Abdul Rahman R, Taip FS, Adzahan NM, Sarker MZ, Gajloo A. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules. 2013;18:997–1014. doi: 10.3390/molecules18010997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Porto C, Porretto E, Decorti D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013;20:1076–1080. doi: 10.1016/j.ultsonch.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Evangelista RL, Evangelista MH, Cermak SC, Isbell TA. Dehulling of coriander fruit before oil extraction. Ind. Crop Prod. 2015;69:378–384. doi: 10.1016/j.indcrop.2015.02.057. [DOI] [Google Scholar]
- Hernandez EM. Specialty oils: functional and nutraceutical properties. 1. TX, USA: Woodhead Publishing; 2016. pp. 69–101. [Google Scholar]
- Hosseini S, Gharachorloo M, Tarzi BG, Ghavami M, Bakhoda H. Effects of ultrasound amplitude on the physicochemical properties of some edible oils. J. Am. Oil Chem. Soc. 2015;92:1717–1724. doi: 10.1007/s11746-015-2733-1. [DOI] [Google Scholar]
- Khodadadi M, Dehghani H, Javaran MJ, Christopher JT. Fruit yield, fatty and essential oils content genetics in coriander. Ind. Crop Prod. 2016;94:72–81. doi: 10.1016/j.indcrop.2016.08.030. [DOI] [Google Scholar]
- Khosravi M, Mortazavi SA, Karimi M, Sharayie P, Armin M. Comparison of ultrasound assisted and kelavenger extraction methods on efficiency and antioxidant properties of Fennel’s oil essence and its optimization by response surface methodology. Int. J. Agric. Crop Sci. 2013;5:2521–2528. [Google Scholar]
- Klanjan MG, Preciat MT. Optimization of the ultrasound-assisted extraction of phenolic compounds from Brosimum alicastrum leaves and the evaluation of their radical-scavenging activity. Molecules. 2017;22:1286. doi: 10.3390/molecules22081286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HZ, Zhang ZJ, Hou TY, Li XJ, Chen T. Optimization of ultrasound-assisted hexane extraction of perilla oil using response surface methodology. Ind. Crop Prod. 2015;76:18–24. doi: 10.1016/j.indcrop.2015.06.021. [DOI] [Google Scholar]
- Lopez PA, Widrlechner MP, Simon PW, Rai TD, Isbell TA, Bailey TB, Gardner C, Wilson LA. Assessing phenotypic biochemical, and molecular diversity in coriander (Coriandrum sativum L.) germplasm. Genet. Resour. Crop Evol. 2008;55:247–275. doi: 10.1007/s10722-007-9232-7. [DOI] [Google Scholar]
- Mhemdi H, Rodier E, Kechaou N, Fages J. A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. J. Food Eng. 2011;105:609–616. doi: 10.1016/j.jfoodeng.2011.03.030. [DOI] [Google Scholar]
- Msaada K, Hosni K, Taarit MB, Chahed T, Hammami M, Marzouk B. Changes in fatty acid composition of coriander (Coriandrum sativum L.) fruit during maturation. Ind. Crop. Prod. 2009;29:269–274. [Google Scholar]
- Ngo-Duy C, Destaillats F, Keskitalo M, Arul J, Angers P. Triacylglycerols of apiaceae seed oils: composition and regiodistribution of fatty acids. Eur. J. Lipid. Sci Tech. 2009;111:164–169. doi: 10.1002/ejlt.200800178. [DOI] [Google Scholar]
- Pico Y. Ultrasound-assisted extraction for food and environmental samples. TrAC-Trend. Anal Chem. 2013;43:84–99. doi: 10.1016/j.trac.2012.12.005. [DOI] [Google Scholar]
- Prakash Maran J, Sivakumar V, Thirugnanasambandham K, Sridhar R. Extraction of natural anthocyanin and colors from pulp of jamun fruit. J. Food Sci. Tech. Mys. 2015;52:3617–3626. doi: 10.1007/s13197-014-1429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan MF, Moersel JT. Screening of the antiradical action of vegetable oils. J. Food Compos. Anal. 2006;19:838–842. doi: 10.1016/j.jfca.2006.02.013. [DOI] [Google Scholar]
- Reiter B, Lechner M, Lorbeer E. The fatty acid profiles including petroselinic and cis-vaccenic acid of different umbelliferae seed oils. Eur. J. Lipid Sci. Tech. 1998;100:498–502. [Google Scholar]
- Rohman A, Che Man YB. Determination of extra virgin olive oil in quaternary mixture using FTIR spectroscopy and multivariate calibration. Spectros. 2011;26:203–211. doi: 10.1155/2011/471376. [DOI] [Google Scholar]
- Samaram S, Mirhosseini H, Tan CP, Ghazali HM, Bordbar S, Serjouie A. Optimisation of ultrasound assisted extraction of oil from papaya seed by response surface methodology: oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015;172:7–17. doi: 10.1016/j.foodchem.2014.08.068. [DOI] [PubMed] [Google Scholar]
- Santos BH, Miranda JR, Lara EH, Uco JT, Garcia RC, Barrientos JJ, Zamudio RC, Sanchez CM. Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo) Ultrason. Sonochem. 2016;31:429–436. doi: 10.1016/j.ultsonch.2016.01.029. [DOI] [PubMed] [Google Scholar]
- Shahwar MK, El-Ghorab AH, Anjum FM, Butt MS, Hussain S, Nadeem M. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: volatile and non volatile extracts. Int. J. Food Prop. 2012;15:736–747. doi: 10.1080/10942912.2010.500068. [DOI] [Google Scholar]
- Shalmashi A. Ultrasound-assisted extraction of oil from tea seeds. J. Food Lip. 2009;16:465–474. doi: 10.1111/j.1745-4522.2009.01159.x. [DOI] [Google Scholar]
- Sicaire AG. Abert vain M, Fine F, Carre P, Tostain S, Chemat F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016;31:319–329. doi: 10.1016/j.ultsonch.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Siger A, Nogala-kalucka M, Lampart-Szczapa E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids. 2008;15:137–149. doi: 10.1111/j.1745-4522.2007.00107.x. [DOI] [Google Scholar]
- Sriti J, Talou T, Faye M, Vilarem G, Marzouk B. Oil extraction from coriander fruits by extrusion and comparison with solvent extraction processes. Ind. Crops Prod. 2011;33(3):659–664. doi: 10.1016/j.indcrop.2011.01.005. [DOI] [Google Scholar]
- Sriti J, Msaada K, Talou T, Faye M, Kartika IA, Marzouk B. Extraction of coriander oil by twin-screw extruder: screw configuration and operating conditions effect. Ind. Crops Prod. 2012;40:355–360. doi: 10.1016/j.indcrop.2012.03.034. [DOI] [Google Scholar]
- Teh S, Birch EJ. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014;21:346–353. doi: 10.1016/j.ultsonch.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Teh S, Niven BE, Bekhit AE, Carne A, Birch J. Optimization of polyphenol extraction and antioxidant activities of extracts from defatted flax seed cake (Linum usitatissimum L.) using microwave-assisted and pulsed electric field (PEF) technologies with response surface methodology. Food Sci. Biotechnol. 2015;24(5):1649–1659. doi: 10.1007/s10068-015-0214-9. [DOI] [Google Scholar]
- Tiwari BK. Ultrasound: a clean, green extraction technology. TrAC-Trend. Anal Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- Uitterhaegen E, Sampaio KA, Delbeke E, De Greyt W, Cerny M, Evon P, Merah O, Talou T, Stevens CV. Characterization of French coriander oil as source of petroselinic acid. Molecules. 2016;21:1202. doi: 10.3390/molecules21091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitterhaegen E, Evon P. Twin-screw extrusion technology for vegetable oil extraction: a review. J. Food Eng. 2017;212:190–200. doi: 10.1016/j.jfoodeng.2017.06.006. [DOI] [Google Scholar]
- Um M, Han TH, Lee JW. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa thunb) fruit. Food Sci. Biotechnol. 2018;27:375–382. doi: 10.1007/s10068-017-0247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends. Food Sci. Tech. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- Wei F, Gao G, Wang X, Dong X, Li P, Hua W, Wang X, Wu XM, Chen H. Quantitative determination of oil content in small quantity of oilseed rape by ultrasound-assisted extraction combined with gas chromatography. Ultrason. Sonochem. 2008;15:938–942. doi: 10.1016/j.ultsonch.2008.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.