Abstract
The objective of this research was to evaluate enzymatic glycerolysis–interesterification to synthesize structured lipids (SLs) containing high monoacylglycerol (MAG) and diacylglycerol (DAG) from a palm stearin–olein blend (PS–PO blend). The results showed that the optimum conditions for the solvent to fat ratio, glycerol to fat ratio, and enzyme concentration were 2:1 (v/w), 1.5:1, and 15% (w/w), respectively. The conversion rate of MAG and DAG decreased at a high glycerol to fat ratio, low solvent to fat ratio, and high enzyme concentration due to an increase in viscosity and low agitation effectiveness. The emulsion capacity and stability of the SLs were 60.19% and 96.80%, respectively. The hardness of the SLs increased about 3.1-fold. The MAG, DAG, and triacylglycerol conversion rates were 0.45, 0.48, and 1.02%/h, respectively. Thus, glycerolysis–interesterification of a PS–PO blend increased DAG and MAG concentrations and further improved the hardness, emulsion capacity, and emulsion stability of the SLs.
Keywords: Glycerolysis–interesterification, Diacylglycerol, Monoacylglycerol, Structured lipid, Palm stearin–olein blend
Introduction
Structured lipids (SLs) are lipids in which the physical, chemical, or nutritional properties have been modified for specific food and nutraceutical applications (Osborn and Akoh, 2002). SLs provide an effective alternative to produce tailor-made lipids with desired characteristics. They can be synthesized by blending or interesterification of two or more types of fats/oils with certain melting characteristics (Oliveira et al., 2017; Ornla-ied et al., 2016). SLs produced from blending vegetable fats/oils have a disadvantage, such as a lower melting profile and a soft texture (Biswas et al., 2017; Jahurul et al., 2013) because of low stearic acid content. Lauric fats/oils (Biswas et al., 2016; Sonwai et al., 2012) and hydrogenated fat (Abigor et al., 2003; Furlán et al., 2017) are used to increase hardness. However, a disadvantage of hydrogenated oils is that they may contain trans-oil/fat.
Interesterification of blends, based on low and high melting points such as a palm stearin and palm olein (PS–PO) blend, enhances the physicochemical properties of SLs (Soares et al., 2009). Their fatty acid and triacylglycerol composition are very versatile for producing a variety of SL products (Aini and Miskandar, 2007). However, SLs contain very low amounts of monoacylglycerol (MAG) and diacylglycerol (DAG), which prevents any emulsifier properties.
However, MAG and DAG have been used as emulsifiers in various emulsion-based foods, such as chocolate products and shortening (Bornscheuer, 1995; Feltes et al., 2013). They are synthesized by the triacylglycerol (TAG) hydrolysis method (Cheong et al., 2007) or esterification between glycerol and free fatty acids (Byun et al., 2007). A disadvantage of the TAG hydrolysis method is the occurrence of free fatty acids in the product, which are difficult to separate. Esterification between glycerol and free fatty acids produces water, which inhibits the esterification reaction (Feltes et al., 2013). MAG and DAG can also be synthesized through the glycerolysis reaction between TAG and glycerol (Krüger et al., 2010). The glycerolysis method may be more effective than other methods, as the reaction between TAG and glycerol produces MAG and DAG and results in the highest yield because each mole of TAG theoretically produces 3 mol of MAG and the reaction leads to the formation of DAG when there is no excess glycerol (Bornscheuer, 1995). In general, glycerolysis is performed on one type of fat/oil (Naik et al., 2014; Saberi et al., 2011) but it is not performed in a fat/oil blend.
The conversion rate of substrate to product is an important factor determining the effectiveness of the reaction. It depends on the solvent to fat ratio, glycerol to fat ratio, and enzyme concentration (Krüger et al., 2010; Naik et al., 2014; Wang et al., 2011).
In this research, SLs containing high MAG and DAG contents were synthesized by enzymatic glycerolysis–interesterification, which combines the interesterification method and glycerolysis method in one reaction system. It is expected that interesterification of the fat/oil blend will enhance the physicochemical properties of the products, while glycerolysis will provide MAG and DAG as emulsifiers. The reaction was initiated by adding Candida antarctica lipase, which was immobilized on a hydrophobic macroporous matrix. Factors, such as the solvent, the glycerol to fat ratio, and the enzyme concentration, were evaluated based on fatty acid composition, the conversion rate of MAG, DAG, and TAG; SL hardness; and emulsion capacity and stability.
Materials and methods
Materials
Palm olein (PO) and palm stearin (PS) were obtained from PT Smart (Tbk, Indonesia). Macroporous Amberlite IRA-96 free base, C. antarctica lipase B, and molecular sieves were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2-Phenylpropionaldehyde, glycerol, tert-butanol, and hexane were obtained from Merck KGaA (Darmstadt, Germany).
Immobilization of lipase on macroporous hydrophobic matrix
The lipase was immobilized on the macroporous hydrophobic matrix according to our previous study (Hilmanto et al., 2016). Adsorption was performed at 30 °C in a shaker water bath at 150 strokes/min for various times (1, 2, 3, 4, and 5 h). The adsorbed protein was calculated as follows.
| 1 |
Effect of solvent to substrate ratio on conversion of MAG and DAG
PS was added to PO at a ratio of 60:40 (w/w) in a batch stirred tank reactor (BSTR). Glycerol was then added to the mixture at a glycerol to fat molar ratio 1.5:1. Tert-butanol was added at various ratios of solvent to substrate (1.5:1, 2:1, and 3:1 v/w). The reaction was initiated by adding the immobilized lipase (10% w/w), and the mixture was incubated at 50 °C (Naik et al., 2014; Wang et al., 2011). A column containing a molecular sieve (12% of the total reactants) was connected to the BSTR. After the reaction, the immobilized lipase was separated and the filtrate was stored for further analysis. The conversion rates of MAG, DAG, and TAG were determined as the slope of the linear part of product concentration versus reaction time curve.
Effect of glycerol to fat ratio on conversion of MAG and DAG
A mixture of palm stearin–palm olein was prepared at a ratio of 60:40 (w/w). Glycerol was added to the fat at a glycerol to fat molar ratios of 1:1, 1.5:1, 2:1, and 2.5:1. Tert-butanol was added to the substrate at a solvent to substrate ratio of 2:1 (v/w). The reaction was initiated by adding the immobilized lipase (10%) to the BSTR at 50 °C for 24 h. A column containing a molecular sieve was connected to the BSTR. After the reaction, the immobilized lipase was separated, and the filtrate was stored for further analysis. The conversion rates of MAG, DAG, and TAG were determined as the slope of the linear part of product concentration versus reaction time curve.
Effect of lipase concentration on conversion of MAG and DAG
A mixture of palm stearin–palm olein was prepared at a ratio of 60:40 (w/w). Glycerol was added to the fat at a glycerol to fat molar ratio 1.5:1. Tert-butanol was added to the substrate at a solvent to substrate ratio of 2:1 (v/w). The reaction was initiated by adding various concentrations of the immobilized lipase (10, 15, and 20%). It was performed in a BSTR at 50 °C for 24 h. A column containing a molecular sieve was connected to the BSTR. After the reaction, the immobilized lipase was separated and the filtrate was stored for further analysis. The conversion rates of MAG, DAG, and TAG were determined as the slope of the linear part of product concentration versus reaction time curve.
Determination of fatty acid composition
Fatty acid composition was analyzed by gas chromatography (GC) after methylation of the fatty acids to fatty acid methyl esters (FAMEs). About 200 µl of fat was methylated by adding 400 µl of BF3-methanol complex in a sealed flask. The mixture was heated for 2 h at 90°C. The FAME residues were extracted with 500 µl hexane. The FAMEs were analyzed by a Shimadzu GC-2010 equipped with a focused silica column of CP Sil 8 CB (30 m length, 0.25 mm diameter, 0.25 μm film thickness), according to the AOCS Official Method Ce 1-62 (AOCS, 2004). All samples were analyzed in duplicate.
Determination of slip melting point and melting point
The AOCS method Cc. 3.25 (AOCS, 1997) was used to determine the slip melting point (SMP), and the AOCS official method Cc 1-25 (AOCS, 1997) was used to determine the melting point (MP).
Determination of hardness
Hardness was determined according to Biswas et al. (2017) using a TA.XT Plus texture analyzer. The fats were stored at 5°C for 24 h and the hardness measurements were performed at 25°C. Hardness was determined at a distance of 4 mm and speed of 1 mm/s for 4 s. The samples were measured in triplicate.
Determination of emulsion capacity and stability
Emulsion capacity and emulsion stability were determined according to Cano-Medina et al. (2011). Heating and centrifugation were used to determine emulsion stability.
Determination of hydrolytic and esterification activities of immobilized lipase
Esterification and hydrolytic activities of the immobilized lipase were determined according to Hilmanto et al. (2016).
Analysis of acylglycerols
Thin layer chromatography (TLC) was used to analyze the composition of acylglycerols (MAG, DAG, and TAG) (Fuchs et al., 2011). The samples were applied to an activated TLC plate and developed using a mixture of hexane: ethyl ether: acetic acid (80:20:2 v/v/v) as the mobile phase. The TLC plate was subsequently dried. Coomassie Blue R-350 (0.02%, w/v) in a mixture containing water: methanol: acetic acid (6:3:1; v/v/v) was used to stain the samples. A Camag TLC Scanner III, which was equipped with winCATS Planar Chromatography software was used to quantify the results.
Protein assay
Protein analysis was determined according to the Lowry method (Lowry et al., 1951). Bovine serum albumin was used as the standard.
Statistical analysis
The data were analyzed by one-way analysis of variance. Tukey’s test was applied to detect the differences. P values < 0.05 were considered significant.
Results and discussion
Effect of adsorption time on immobilized C. antarctica lipase activity
The adsorbed protein did not increase significantly as adsorption time increased from 1 to 5 h (Fig. 1). However, hydrolytic and esterification activities increased 2.84 times and 1.45 times as adsorption time increased from 1 to 4 h, respectively. Further increases in adsorption time resulted in a decrease in hydrolytic and esterification activities of 1.21 times and 1.28 times, respectively. These results suggest that the adsorption of lipase on the matrix surface was very quick (< 1 h). Increasing the adsorption time provided more chances for hydrophobic interactions between the active sites of lipase and the surface of the hydrophobic matrix. As a result, the structure of the enzyme changed from closed to open. The hydrophobic matrix also improved the microenvironment for the enzymatic reactions (Chen et al., 2012), which increased immobilized enzyme activity (Fig. 1). Further increasing the adsorption time decreased lipase activity, which may have been due to a decrease in enzyme stability. Thus the optimum conditions for immobilized lipase were reached at an adsorption time of 3 h.
Fig. 1.
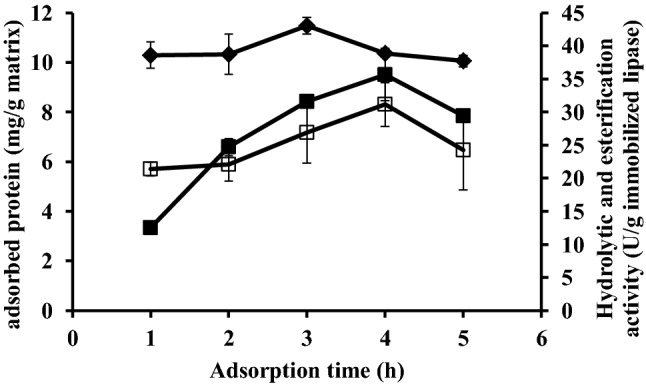
Effect of adsorption time on the adsorbed protein (black diamond symbols), esterification activity (white square symbols), and hydrolytic activity (black square symbols) of the immobilized Candida antarctica lipase on the macroporous hydrophobic matrix. Adsorption was performed at 30 °C, and the initial enzyme concentration was 12 mg/mL
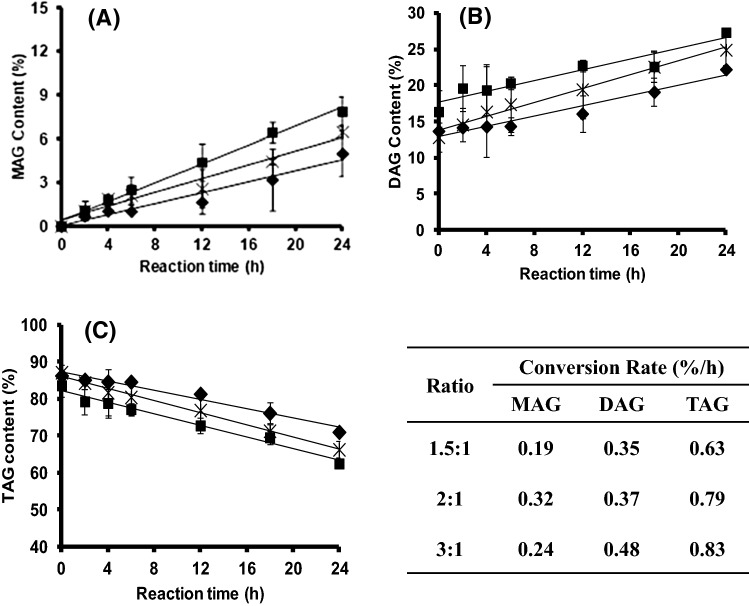
Effect of solvent to substrate ratio on conversion of MAG and DAG
Adding a solvent to the substrate affected the MAG and TAG conversion rates (Fig. 2). The conversion rates increased with an increase in the solvent to substrate ratio from 1.5:1 to 2:1 (Fig. 2A, C). The increased conversion rates of MAG and TAG were about 1.78 times and 1.25 times, respectively. The DAG conversion rate was not significantly different. The reactant system was more viscous at the low solvent ratio. Therefore, molecular diffusion and mass transfer were low. Adding more solvent increased the solubility of the substrates, mixture homogeneity and stability, and substrate diffusivity due to the change in the polarities of the fat and glycerol mixture. Therefore, mass transfer increased due to lowering of the viscosity (Naik et al., 2014). However, a further increase in the solvent to substrate ratio (3:1) increased the DAG and TAG conversion rates, but it decreased the MAG conversion rate (Fig. 2). Thus, a 2:1 the solvent to substrate ratio was chosen for further experiments.
Fig. 2.
Effect of the solvent to substrates ratio, 1.5:1 (black diamond symbols), 2:1 (black square symbols), and 3:1 (cross symbols) on monoacylglycerol (MAG) content (A); diacylglycerol (DAG) content (B); and triacylglycerol (TAG) content (C) in the product mixture. Glycerolysis–interesterification was performed at palm stearin–olein (PS–PO) ratio of 60:40 (w/w), a glycerol to fat molar ratio of 1.5:1, and 10% immobilized lipase at 50°C for 24 h
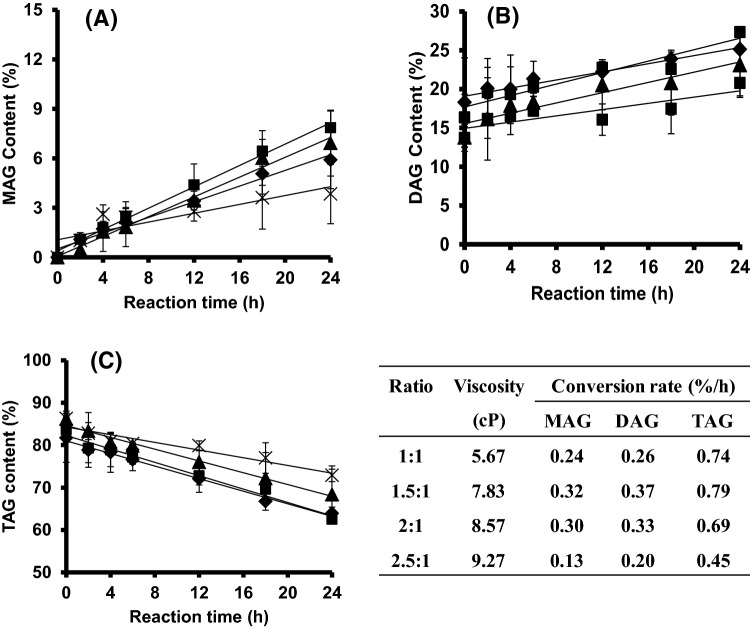
Effect of glycerol to fat ratio on conversion of MAG and DAG
Synthesis of SLs by the glycerolysis–interesterification reaction requires glycerol to convert TAG to MAG and DAG. The MAG and TAG conversion rates increased as the glycerol to fat ratio was increased from 1:1 to 1.5:1 (Fig. 3A, C). The MAG and TAG conversion rates increased about 1.33 times and 1.25 times, respectively. However, the DAG conversion rate was not significantly different. An increase in the glycerol fraction from 1:1 to 1.5:1 lead to an increase in the reactant equilibrium and the MAG and TAG conversion. However, a further increase in the glycerol to fat ratio (2:1) did not have a significant effect on the MAG, DAG, or TAG conversion rates (Fig. 3). The conversion rates decreased at a higher glycerol to fat ratio (2.5:1). This was due to an increase in the reactant mixture viscosity at a higher glycerol fraction. The viscosity of the reaction mixture increased about 1.6 times as the glycerol to fat molar ratio was increased from 1:1 to 2.5:1 (Fig. 3). An increase in viscosity affected the mass transfer of the substrates to the enzymes. Glycerol is hydrophilic; hence, it can interfere with the reaction system because the fat and immobilized lipase are favor in hydrophobic. Thus, a high amount of glycerol formed a coating around the enzyme, which inhibited enzyme activity, and lowered the conversion rates of MAG, DAG, and TAG (Cheong et al., 2007; Naik et al., 2014). Thus, the glycerol to fat molar ratio of 1.5:1 was chosen for further experiments.
Fig. 3.
Effect of the glycerol to fat molar ratio, 1:1 (black diamond symbols), 1.5:1 (black square symbols), 2:1 (black triangle symbols), and 2.5:1 (cross symbols) on monoacylglycerol (MAG) content (A); diacylglycerol (DAG) content (B); and triacylglycerol (TAG) content (C) in the product mixture. Reaction was performed at palm stearin–olein (PS–PO) ratio of 60:40 (w/w), a solvent to substrate ratio of 2:1 (v/w), and 10% immobilized lipase at 50°C for 24 h
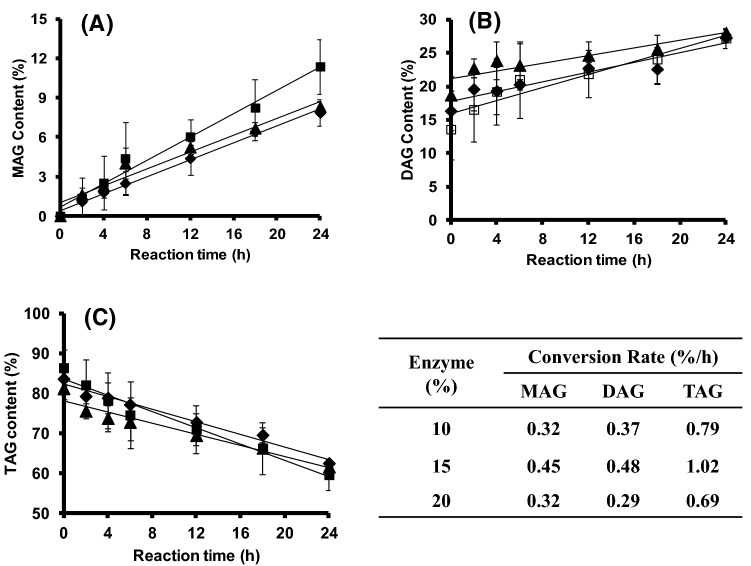
Effect of lipase concentration on conversion of MAG and DAG
The conversion rates of TAG, MAG, and DAG increased as enzyme concentration was increased from 10 to 15% (Fig. 4). The increases in the MAG, DAG, and TAG conversion rates were about 1.40 times, 1.29 times, and 1.29 times, respectively. However, a further increase in the enzyme concentration (20%) resulted in a decrease in the MAG, DAG, and TAG conversion rates. In general, the MAG, DAG, and TAG conversion rates increased as enzyme loading increased. Nonetheless, the product conversion decreased at a 20% enzyme concentration (Fig. 4), suggesting that agitation was less effective due to high solid particle concentrations in the reaction system; therefore the conversion rate decreased. This result is in agreement with Krüger et al. (2010), who showed that yield does not increase with an increase in enzyme concentration above a certain amount. This was due to poorer mixing of the reaction mixture, which affected the mass-transfer limitations. Thus, a 15% enzyme concentration was chosen for enzymatic glycerolysis of the PS–PO blend in the BSTR at a solvent to substrate ratio of 2:1 (v/w) and a glycerol to fat molar ratio of 1.5:1 at 50°C for 24 h. These conditions resulted in MAG, DAG, and TAG conversion rates of 0.45, 0.48, and 1.02%/h, respectively (Fig. 4). The MAG, DAG, and TAG concentrations were 11.39%, and 27.24%, and 59.60%, respectively.
Fig. 4.
Effect of enzyme concentration, 10% (black diamond symbols), 15% (black square symbols), 20% (black triangle symbols) on monoacylglycerol (MAG) content (A); diacylglycerol (DAG) content (B); and triacylglycerol (TAG) content (C) in the product mixture. Reaction was performed at palm stearin–olein (PS–PO) ratio of 60:40 (w/w), a solvent to substrate ratio of 2:1 (v/w), and a glycerol to fat molar ratio of 1.5:1, at 50 °C for 24 h
Fatty acid compositions of MAG, DAG, and TAG
The fatty acid composition affected the physical properties of fat. MAG and DAG in the SLs had different fatty acid compositions compared with those of TAG and the SLs (Table 1). MAG and DAG contained higher total saturated fatty acid contents, particularly palmitic and stearic acids, than TAG and SLs. Total unsaturated fatty acids, especially oleic acid on MAG and DAG, was lower than TAG and the SLs. This was because C. antarctica lipase has higher specificity toward saturated fatty acids than unsaturated fatty acids (Kirk et al., 1992). Accordingly, these fatty acids reacted with glycerol to produce MAG and DAG; therefore, the saturated fatty acids in MAG and DAG were higher in content than TAG and the SLs.
Table 1.
Fatty acid composition in the structured lipids (SLs), MAG, DAG, and TAG
| Fatty acid | Structured lipids (%) | MAG(1) (%) | DAG (%) | TAG (%) |
|---|---|---|---|---|
| C14:0 | 1.02 ± 0.07 | Not detected | Not detected | Not detected |
| C16:0 | 49.05 ± 0.15b(2) | 56.46 ± 0.12d | 47.64 ± 1.84a | 51.65 ± 0.36c |
| C18:0 | 5.12 ± 0.11a | 11.32 ± 0.60c | 15.75 ± 4.25d | 7.49 ± 2.17b |
| C18:1 | 44.81 ± 0.11d | 32.22 ± 0.72a | 36.61 ± 2.41b | 40.86 ± 1.80c |
(1)MAG monoacylglycerol, DAG diacylglycerol, TAG triacylglycerol
(2)Different letters indicated significantly different values (p < 0.05)
Physical properties of structured lipids in palm stearin–olein blend
SLs had a higher SMP and MP compared to the PS–PO blend due to high MAG and DAG content (Table 2). MAG and DAG have a higher MP than TAG (Lo et al., 2008; Zhang et al., 2014). Therefore, fats containing high MAG and DAG contents have a higher MP than those with lower concentrations of MAG and DAG. MAG and DAG contained higher saturated fatty acid content than TAG (Table 1). The MAG and DAG saturated fatty acids contributed to SMP and MP. The increase in SMP and MP resulted in a 3.1-fold higher hardness of the SLs than the PS–PO blend (Table 2).
Table 2.
Physical properties of the structured lipids
| Sample | PS–PO blend | Structured lipids |
|---|---|---|
| Monoacylglycerol (%) | Not detected | 8.59 ± 2.31 |
| Diacylglycerol (%) | 15.03 ± 3.75a(1)) | 26.90 ± 1.92b |
| Triacylglycerol (%) | 84.97 ± 3.75b | 64.51 ± 0.47a |
| Slip melting point (°C) | 38.17 ± 0.29a | 38.67 ± 0.29b |
| Melting point (°C) | 44.00 ± 1.00a | 44,67 ± 0.58a |
| Hardness (N) | 1.82 ± 0.31a | 5.63 ± 0.59b |
| Emulsion capacity (%) | 38.48 ± 1.70a | 60.19 ± 4.17b |
| Emulsion stability (%) | Not detected | 96.80 ± 0.23 |
| Hydrophilic–lipophilic balance | 4.67 ± 0.25a | 8.05 ± 0.44b |
(1)Different letters indicated significantly different values (p < 0.05)
Glycerolysis–interesterification of the PS–PO blend resulted in an increase in emulsion capacity of the product (Table 2), which was about 1.56 times higher than that of the PS–PO blend. The emulsion stability value was 96.80%. The increase in emulsion capacity was due to an increase in MAG and DAG concentrations (Table 2). MAG and DAG have hydroxyl groups, so they can act as emulsifiers. The HLB value of the SLs was 8.05. Emulsifiers, which have a low HLB are usually used for water-in-oil emulsions, whereas high HLB emulsifiers are used for oil-in-water emulsions (Losada-Barreiro et al., 2013). The SLs formed an emulsion, which was relatively stable to heat and centrifugation.
In summary, the optimum conditions for the solvent to fat ratio, glycerol to fat ratio, and enzyme concentration were 2:1 (v/w), 1.5:1, and 15% (w/w), respectively. The conversion rate of MAG and DAG decreased at a high glycerol to fat ratio, low solvent to fat ratio, and high enzyme concentration. Finally, glycerolysis–interesterification of a PS–PO blend increased DAG and MAG concentrations and further improved the hardness, emulsion capacity, and stability of the SLs.
References
- Abigor RD, Marmer WN, Foglia TA, Jones KC, DiCiccio RJ, Ashby R, Uadia PO. Production of cocoa butter-like fats by the lipase-catalyzed interesterification of palm oil and hydrogenated soybean oil. J. Am. Oil Chem. Soc. 2003;80:1193–1196. doi: 10.1007/s11746-003-0841-7. [DOI] [Google Scholar]
- Aini IN, Miskandar MS. Utilization of palm oil and palm products in shortenings and margarines. Eur. J. Lipid Sci. Technol. 2007;109:422–432. doi: 10.1002/ejlt.200600232. [DOI] [Google Scholar]
- AOCS. Official methods and recommended practices of the American Oil Chemists’ Society. American Oil Chemist’s Society Press, Champaign, IL, USA (2004)
- AOCS. Official and Tentative Methods of the American Oil Chemist’s Society, 7th Ed. American Oil Chemist’s Society Press, Champaign, IL, USA (1997)
- Biswas N, Cheow YL, Tan CP, Siow LF. Physical, rheological and sensorial properties, and bloom formation of dark chocolate made with cocoa butter substitute (CBS) LWT-Food Sci. Technol. 2017;82:420–428. doi: 10.1016/j.lwt.2017.04.039. [DOI] [Google Scholar]
- Biswas N, Cheow YL, Tan CP, Siow LF. Blending of palm mid-fraction, refined bleached deodorized palm kernel oil or palm stearin for cocoa butter alternative. J. Am. Oil Chem. Soc. 2016;93:1415–1427. doi: 10.1007/s11746-016-2880-z. [DOI] [Google Scholar]
- Bornscheuer UT. Lipase-catalyzed syntheses of monoacylglycerols. Enzyme Microb. Technol. 1995;17:578–586. doi: 10.1016/0141-0229(94)00096-A. [DOI] [Google Scholar]
- Byun H-G, Eom T-K, Jung W-K, Kim S-K. Lipase catalyzed production of MAG by the esterification of fish oil FA with glycerol. Biotechnol. Bioprocess Eng. 2007;1:491–496. doi: 10.1007/BF02931345. [DOI] [Google Scholar]
- Cano-Medina A, Jiménez-Islas H, Dendooven L, Herrera RP, González-Alatorre G, Escamilla-Silva EM. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res. Int. 2011;44:684–692. doi: 10.1016/j.foodres.2010.12.015. [DOI] [Google Scholar]
- Chen B, Yin C, Cheng Y, Li W, Cao ZA, Tan T. Using silk woven fabric as support for lipase immobilization: The effect of surface hydrophilicity/hydrophobicity on enzymatic activity and stability. Biomass and Bioenergy. 2012;39:59–66. doi: 10.1016/j.biombioe.2010.08.033. [DOI] [Google Scholar]
- Cheong LZ, Tan CP, Long K, Affandi Yusoff MS, Arifin N, Lo SK, Lai OM. Production of a diacylglycerol-enriched palm olein using lipase-catalyzed partial hydrolysis: Optimization using response surface methodology. Food Chem. 2007;105:1614–1622. doi: 10.1016/j.foodchem.2007.03.070. [DOI] [Google Scholar]
- Feltes MMC, de Oliveira D, Block JM, Ninow JL. The production, benefits, and applications of monoacylglycerols and diacylglycerols of nutritional interest. Food Bioprocess Technol. 2013;6:17–35. doi: 10.1007/s11947-012-0836-3. [DOI] [Google Scholar]
- Fuchs B, Süß R, Teuber K, Eibisch M, Schiller J. Lipid analysis by thin-layer chromatography-A review of the current state. J. Chromatogr. A. 2011;1218:2754–2774. doi: 10.1016/j.chroma.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Furlán LTR, Baracco Y, Lecot J, Zaritzky N, Campderrós ME. Influence of hydrogenated oil as cocoa butter replacers in the development of sugar-free compound chocolates: Use of inulin as stabilizing agent. Food Chem. 2017;217:637–647. doi: 10.1016/j.foodchem.2016.09.054. [DOI] [PubMed] [Google Scholar]
- Hilmanto H, Hidayat C, Hastuti P. Surface Modification of Macroporous Matrix for Immobilization of Lipase for Fructose Oleic Ester Synthesis. Bull. Chem. React. Eng. Catal. 2016;11:339–345. doi: 10.9767/bcrec.11.3.573.339-345. [DOI] [Google Scholar]
- Jahurul MHA, Zaidul ISM, Norulaini NAN, Sahena F, Jinap S, Azmir J, Sharif KM, Mohd Omar AK. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J. Food Eng. 2013;117:467–476. doi: 10.1016/j.jfoodeng.2012.09.024. [DOI] [Google Scholar]
- Kirk O, Björkling F, Godtfredsen SE, Larsen TO. Fatty acid specificity in lipase-catalyzed synthesis of glucoside esters. Biocatal. Biotransformation. 1992;6:127–134. [Google Scholar]
- Krüger RL, Valério A, Balen M, Ninow JL, Oliveira JV, de Oliveira D, Corazza ML. Improvement of mono and diacylglycerol production via enzymatic glycerolysis in tert-butanol system. Eur. J. Lipid Sci. Technol. 2010;112:921–927. doi: 10.1002/ejlt.200900253. [DOI] [Google Scholar]
- Lo SK, Tan CP, Long K, Yusoff MSA, Lai OM. Diacylglycerol oil-properties, processes and products: A review. Food Bioprocess Technol. 2008;1:223–233. doi: 10.1007/s11947-007-0049-3. [DOI] [Google Scholar]
- Losada-Barreiro S, Sanchez-Paz V, Bravo-Diaz C. Effects of emulsifier hydrophile-lipophile balance and emulsifier concentration on the distributions of gallic acid, propyl gallat, and a-tocopherol in corn oil emulsions. J. Colloid Interface Sci. 2013;389:1–9. doi: 10.1016/j.jcis.2012.07.036. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the follin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Naik MK, Naik SN, Mohanty S. Enzymatic glycerolysis for conversion of sunflower oil to food based emulsifiers. Catal. Today. 2014;237:145–149. doi: 10.1016/j.cattod.2013.11.005. [DOI] [Google Scholar]
- Oliveira PD, Rodrigues AMC, Bezerra CV, Silva LHM. Chemical interesterification of blends with palm stearin and patawa oil. Food Chem. 2017;215:369–376. doi: 10.1016/j.foodchem.2016.07.165. [DOI] [PubMed] [Google Scholar]
- Ornla-ied P, Sonwai S, Lertthirasuntorn S. Trans-free margarine fat produced using enzymatic interesterification of rice bran oil and hard palm stearin. Food Sci. Biotechnol. 2016;25:673–680. doi: 10.1007/s10068-016-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn HT, Akoh CC. Structured lipids – novel fats with medical, nutraceutical, and food applications. Compr. Rev. Food Sci. Food Saf. 2002;1:93–103. doi: 10.1111/j.1541-4337.2002.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Saberi AH, Kee BB, Oi-Ming L, Miskandar MS. Physico-chemical properties of various palm-based diacylglycerol oils in comparison with their corresponding palm-based oils. Food Chem. 2011;127:1031–1038. doi: 10.1016/j.foodchem.2011.01.076. [DOI] [PubMed] [Google Scholar]
- Soares FASDM, Claro da Silva R, Caroline Guimarães da Silva K, Bertolessi Lourenço M, Ferreira Soares D, Antonio Gioielli L. Effects of chemical interesterification on physicochemical properties of blends of palm stearin and palm olein. Food Res. Int. 2009;42:1287–1294. doi: 10.1016/j.foodres.2009.03.022. [DOI] [Google Scholar]
- Sonwai S, Kaphueakngam P, Flood A. Blending of mango kernel fat and palm oil mid-fraction to obtain cocoa butter equivalent. J. Food Sci. Technol. 2012;51:2357–2369. doi: 10.1007/s13197-012-0808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li T, Ning Z, Wang Y, Yang B, Yang X. Production of extremely pure diacylglycerol from soybean oil by lipase-catalyzed glycerolysis. Enzyme Microb. Technol. 2011;49:192–196. doi: 10.1016/j.enzmictec.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang Y, Ma X, Wang E, Liu M, Yan R. Characterisation and oxidation stability of monoacylglycerols from partially hydrogenated corn oil. Food Chem. 2014;173:70–79. doi: 10.1016/j.foodchem.2014.09.155. [DOI] [PubMed] [Google Scholar]