Abstract
This study was performed to investigate the efficacy of chemical sanitizers (viz., chlorine, chlorine dioxide, alcohol, and quaternary ammonium compound) against Bacillus cereus on five food contact materials under different conditions (smooth vs. scratched and with vs. without biofilms). After incubating materials in B. cereus suspension, cell adhesion on a smooth surface (10 cm2) was in the following ascending order: stainless steel (7.36 ± 0.08 log CFU), glass (7.51 ± 0.26 log CFU), polyethylene (7.66 ± 0.30 log CFU), polypropylene (7.76 ± 0.30 log CFU), and wood (8.02 ± 0.33 log CFU). The efficacy of sanitizers was dramatically reduced in the presence of a biofilm on all materials. Among four different chemical sanitizers, chlorine showed the best bactericidal activity against B. cereus on the surface with scratch and biofilm. Selection of adequate materials, maintenance of a smooth surface, and inhibition of biofilm formation are good practices for food safety.
Keywords: Bacillus cereus, Biofilm, Food contact surface, Sanitizing efficacy, Scratched surface
Introduction
Bacillus cereus is a Gram-positive endospore-forming pathogen that is ubiquitous in nature (Kotiranta et al., 2000). The bacterium causes a foodborne illness with emetic or diarrheal symptoms (Andersson et al., 1998). The emetic response is induced by toxic and heat-stable peptides secreted by B. cereus (Ehling-Schulz et al., 2004), whereas diarrheal symptoms are caused by enterotoxins produced by the bacterium (Granum, 1994). Because its natural habitat is soil, this pathogen commonly occurs on grains and vegetables during cultivation (Felske, 2004). Thus, foodborne outbreaks due to B. cereus are a major safety concern in Asian countries where consumption of grain and vegetable is high (Kramer et al., 1989; Pan et al., 1997). In addition, illnesses caused by B. cereus have gradually risen as a result of changes in the population’s eating behavior, such as an increased frequency of dining out and use of institutional food services (Lee et al., 2013). Furthermore, the pathogen is transmitted to foods and equipment during food preparation and processing, resulting in possible foodborne illness (Frank, 2001; Tauveron et al., 2006). Owing to its spore- and biofilm-forming characteristics, B. cereus is difficult to control in the food industry, especially in food services (Faille et al., 1997). Moreover, this species has an ability to form biofilms on various types of food contact surfaces, such as stainless steel (SUS) and wood (Jullien et al., 2003; Peng et al., 2002). Bacterial cells formed into a biofilm on food contact surfaces are extremely hard to remove since they are protected from outer stressors, such as cleaning, heat treatment, and chemical sanitization (Brooks and Flint, 2008; Giaouris et al., 2014).
Along with physical methods, such as heat and ultraviolet light treatments, chemical sanitizers are frequently used in the food industry because of their cost- and labor-saving benefits. In order to achieve effective sanitization in food industries and school food services, guidelines for the use of these chemical sanitizers have been suggested by the Korea Ministry of Food and Drug Safety (KMFDS). However, the KMFDS guidelines focus on the control of floating bacterial vegetative cells, but not on cells within biofilms or spores (Jahid and Ha, 2012; Kim et al., 2008). Thus, it is necessary to consider the presence of biofilms and spores in order to choose an adequate sanitizing method. The efficacy of sanitization differs depending on the presence of biofilms and the condition of the food contact surface (Kim et al., 2017).
Food contact surfaces such as SUS, glass (GL), polyethylene (PE), polypropylene (PP), and wood are characterized by their degrees of hydrophobicity and roughness, which affect cleanability of the surface. The cleanability of the surface materials is as follows in ascending order: wood, PP, PE, GL, and SUS (Boulané-Petermann, 1996). Materials with a hydrophobic surface except superhydrophobic surface like Teflon favor bacterial attachment and biofilm formation (Di Ciccio et al., 2015; Van Loosdrecht et al., 1987; Zhang et al., 2013). Moreover, the condition of any surface is changed continuously through washing and scrubbing, with the eventual formation of cracks and scratches on the food contact surface (Chaturongkasumrit et al., 2011). These changes in roughness of the surface provide a higher chance of bacterial attachment and biofilm formation (Moltz and Martin, 2005; Monk et al., 2004). Thus, the roughness of the food contact surface also lowers the effectiveness of cleaning and sanitization (Hilbert et al., 2003; Lomander et al., 2004).
Currently, guidelines for the chemical sanitization of food contact surfaces having formed biofilms and scratches are limited. It is important to assess the efficacy of chemical sanitizers for various types and roughness of food contact surface materials and in the presence of biofilms. Therefore, this study investigated the effects of several chemical sanitizers on the removal of B. cereus biofilm developed on food contact surfaces made from five different materials, with and without scratches.
Materials and methods
Bacillus cereus strains and culture conditions
Bacillus cereus ATCC 10,876, ATCC 13,061, BH09-3 (isolated from Perilla leaf), and BH09-5 (isolated from sprout) were obtained from the Food Microbiology Laboratory, Chung-Ang University (Anseong-si, Gyeonggi-do, Korea). The stock cultures were inoculated into 10 mL of tryptic soy broth (TSB; Difco, Detroit, MI, USA) and incubated at 37 °C. Three consecutive 24-h transfers were made before the experiments. Each strain was harvested by centrifugation at 8000×g for 5 min and washed twice with 0.1% peptone water (Difco). The harvested bacterial pellet of each strain was resuspended in 1% peptone water and mixed in an equal amount to prepare the B. cereus cocktail with 8–9 log colony forming units (CFU)/mL for the further experiments.
Coupon preparation
For the bacterial adhesion and biofilm formation experiments, coupons (2 × 5 cm2) were made with different materials: stainless steel (SUS), glass (GL), polyethylene (PE), polypropylene (PP), and wood. For the surface roughness experiment, the coupons were scrubbed with a multi-purpose sponge (Scotch-brite, 3 M Korea, Seoul, Korea) for 15 min, washed with soap, immersed in 70% ethanol for 2 h, and then dried in an oven (FO-600 M; Jeio Tech Co, Ltd, Deajeon, Korea). All the coupons, except that of PE, were autoclaved; the PE coupons were sanitized by 3 h of UV treatment (Bae and Lee, 2012). For the field emission-scanning electron microscopy (FE-SEM) experiment, 1 × 1 cm2 coupons were used.
Bacterial adhesion on the coupons
Each coupon was placed into a 50-mL conical tube containing 30 mL of TSB and 1 mL of bacterial suspension (Ryu and Beuchat, 2005). Coupons were incubated for 4 h at room temperature to allow the bacterial cell adhesion. The coupon was then dried for 30 min on a clean bench (HS-CB-1600; Hansol Fine Lab., Seoul, Korea).
Biofilm formation on the coupons
For biofilm formation, a 100% relative humidity (RH) condition was first created by adding 0.8 mL of distilled water into an autoclaved 50-mL conical tube at 27 °C for 4 days (Ryu and Beuchat, 2005). Coupons with adhered bacterial cells were then placed into the conical tubes at 100% RH and incubated for 5 days (Ryu and Beuchat, 2005).
Sanitizing methods
In order to compare the effectiveness of the sanitizing methods, four chemical sanitizers commonly used in foodservice industry were used (Korea Ministry of Food and Drug Safety, 2017). Two hundred parts per million of two chlorine-based sanitizers were used: chlorine (LG, Household and Health Care Ltd., Seoul, Korea) and chlorine dioxide (Duozon, Shinwang Chemical Co. Ltd., Seoul, Korea.). The concentrations of chemical sanitizers were adjusted to comply with the hygiene and disinfection guidelines of the food industry (KMFDS, 2017). Seventy percent (v/v) ethanol (OCI Co. Ltd., Seoul, Korea) and 200 ppm quaternary ammonium compound (QAC; Quartz Plus, 3 M Co. Ltd., Seoul, Korea) were the other two sanitizers tested. To maximize the efficacy of disinfection, all four sanitizers were prepared freshly before each experiment, and the coupons were respectively immersed in 100 mL of each chemical for 3, 5, or 10 min at 25 °C.
Bacterial enumeration
The number of B. cereus cells adhered onto the coupons after sanitization was determined. The coupons were transferred into conical tubes containing 30 mL of sterile phosphate-buffered saline and 3 g of glass beads (2 mm in diameter; Glastechnique MFG, Lauda-Königshofen, Germany) and then vortexed at maximum speed for 1 min to detach the bacterial cells from the surface of the coupons (Kim et al., 2007). The suspensions were serially diluted in 9 mL of 0.1% peptone water, and the diluents were plated onto tryptic soy agar (Difco). Bacterial colonies were counted after 24–48 h of incubation of the plates at 37 °C.
FE-SEM analysis
The B. cereus cells attached to, and biofilms formed on, the surface of the coupons were visualized by field emission scanning electron microscopy (FE-SEM). After biofilm formation, the coupons were pre-fixed in 2.5% glutaraldehyde solution for 3–4 h at 4 °C, rinsed 5–6 times with 0.1 M phosphate buffer (pH 7.4 ± 0.1) at intervals of 10 min, and then incubated in fixation buffer (2:1:1 of 2% OsO4, H2O, and 0.2 M phosphate) for 2 h. After fixation, the coupons were dehydrated with an ethanol series (30–100%) and then dried twice with 100% isoamyl acetate solution, for 20 min each time, using a critical point drier (HCP-2; Hitachi Ltd., Tokyo, Japan). The dehydrated coupons were air-dried using an ion sputter (E-1030; Hitachi Ltd.) and then analyzed by FE-SEM (S-4200; Hitachi Ltd.).
Statistical analysis
Data were analyzed using SPSS Statistics (IBM SPSS Statistics ver. 19; SPSS Inc., Chicago, IL, USA). The t test was performed to compare significant differences between the mean values of bacterial cell attachment on coupons with different surface conditions and between those of bacterial cell survival after different sanitizer treatments (p < 0.05). One-way analysis of variance followed by Duncan’s multiple-range test was used to determine significant differences with a 95% confidence level.
Results and discussion
Bacillus cereus adherence and biofilm formation depending on the surface condition
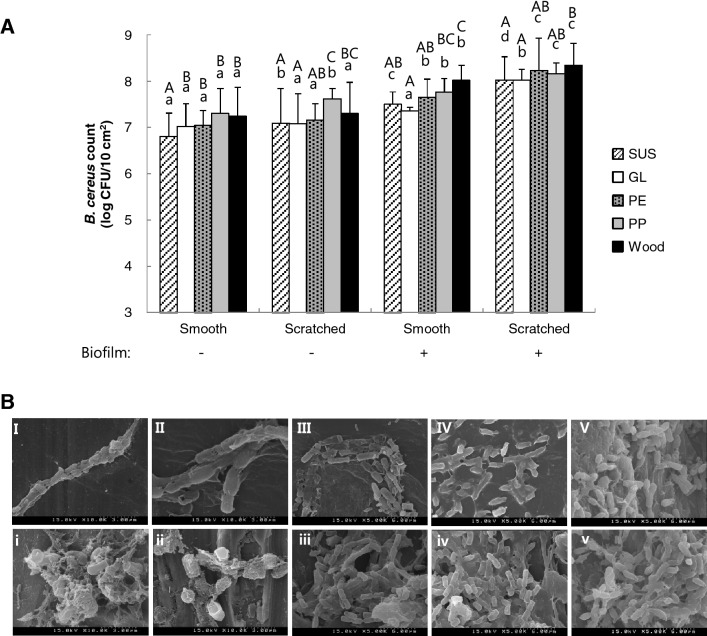
Differences in the bacterial adherence and biofilm formation were observed with regard to the condition and type of materials of the surfaces tested [Fig. 1(A)]. In the presence of scratches on the surface, polypropylene (PP) (7.62 ± 0.68 log CFU/10 cm2) and wood (7.30 ± 0.68 log CFU/10 cm2) showed significantly higher bacterial attachment than did stainless steel (SUS) (7.10 ± 0.74 log CFU/10 cm2) and glass (GL) (7.08 ± 0.65 log CFU/10 cm2). On a smooth surface with biofilm formation, the bacterial cell count on wood, PP, polyethylene (PE), SUS, and GL was 8.02 ± 0.33, 7.76 ± 0.30, 7.66 ± 0.30, 7.51 ± 0.26, and 7.36 ± 0.08 log CFU/10 cm2, respectively. The number of B. cereus cells on the scratched surfaces with biofilms tended to be significantly higher than that on the smooth ones regardless of material type.
Fig. 1.
The population count and morphology of Bacillus cereus on various food contact surface materials, with and without scratches and biofilms. (A) The food contact surfaces (10 cm2) were made of stainless steel (SUS), glass (GL), polyethylene (PE), polypropylene (PP), or wood. Values are expressed as the mean ± standard deviation (n = 3). Mean values with different letters are significantly different at p < 0.05 by Duncan’s multiple-range test. Statistical differences among the mean values within the same surface condition are indicated by the letters A–D, whereas those among the mean values within the same material are indicated by the letters a–d. (B) Morphology of Bacillus cereus on smooth (uppercase letters) or scratched (lowercase letters) surface materials with biofilm formation was visualized by field emission-scanning electron microscopy. Relatively hydrophilic surfaces are stainless steel with smooth (I) and scratched (i) surfaces, glass with smooth (II) and scratched (ii) surfaces. Relatively hydrophobic surfaces are polyethylene with smooth (III) and scratched (iii) surfaces, polypropylene with smooth (IV) and scratched (iv) surfaces; and wood with smooth (V), and scratched (v) surfaces
The B. cereus cells on the contact surface with the biofilm were observed using field emission scanning electron microscopy (FE-SEM). The food contact surface with the biofilm was divided into two groups according to the hydrophobicity of the material type: those with relatively low hydrophobicity (SUS and GL) and those with relatively high hydrophobicity (PE and PP). Both SUS and GL with biofilm formation showed more bacterial attachment on scratched surfaces than on smooth surfaces [Fig. 1(B)]. Similar results were observed on the surfaces with relatively high hydrophobicity.
The hydrophobic materials (PE and PP) showed higher attachment of bacterial populations than did the relatively hydrophilic ones (SUS and GL) (Fig. 1). Moreover, a scratched surface with biofilm formation had a synergistic effect on bacterial attachment to the coupon. B. cereus preferred a scratched surface with biofilm over the other surface conditions for attachment (Fig. 1). Consistent with our data, Arau´jo et al. (Araújo et al., 2009) reported that adherence of B. cereus on any food contact surface is affected by the hydrophobicity and roughness of the surface. The hydrophobicity contributes to adhesion of bacterial cells to surfaces since the water layer on a hydrophobic surface is easily removed owing to the lower interaction between the surface molecules and water molecules (Araújo et al., 2009).
Efficacy of chemical sanitizer treatment against B. cereus depending on the surface condition
The effects of different chemical sanitizers (chlorine, chlorine dioxide, 70% alcohol, and QAC) on the B. cereus cells and biofilm from different surface conditions and materials are shown in Tables 1, 2, 3, 4 and 5.
Table 1.
Survival of Bacillus cereus on stainless steel surface with different conditions (roughness and biofilm) by various chemical sanitizers
| Treatment | Time (min) | 200 ppm Chlorine | 200 ppm Chlorine dioxide | 70% Alcohol | 200 ppm Quaternary ammonium compound |
|---|---|---|---|---|---|
| Smooth | 0 | 6.81 ± 0.50Aa 1),2),3) | |||
| Scratched | 7.10 ± 0.74Ab | ||||
| Biofilm group smooth | 7.51 ± 0.26ABc | ||||
| Biofilm group scratched | 8.03 ± 0.50Ad | ||||
| Smooth | 3 | 4.79 ± 0.41Aa | 5.28 ± 0.10Ba | 4.69 ± 0.55Aa | 4.77 ± 0.53Aa |
| Scratched | 4.75 ± 0.87Aa | 5.30 ± 0.39Ba | 5.40 ± 0.05Bb | 4.83 ± 0.30Aa | |
| Biofilm group smooth | 6.34 ± 0.31ABb | 6.27 ± 0.40Ab | 6.70 ± 0.05BCc | 6.75 ± 0.10Cb | |
| Biofilm group scratched | 6.83 ± 0.07Ab | 6.41 ± 0.41Ab | 6.89 ± 0.63Ac | 6.99 ± 0.23Ab | |
| Smooth | 5 | 4.38 ± 0.66Aa | 4.92 ± 0.30Ba | 4.73 ± 0.45Aba | 4.48 ± 0.35Aa |
| Scratched | 4.88 ± 0.40ABb | 5.17 ± 0.03Ba | 5.24 ± 0.10Bb | 4.71 ± 0.64Aa | |
| Biofilm group smooth | 6.20 ± 0.54Ac | 6.27 ± 0.24Ab | 6.70 ± 0.08Ac | 6.59 ± 0.29Ab | |
| Biofilm group scratched | 6.62 ± 0.55Bc | 6.37 ± 0.38Ab | 6.87 ± 0.28Cd | 6.68 ± 0.12Cb | |
| Smooth | 10 | 4.04 ± 0.81Aa | 4.61 ± 0.51Aa | 4.67 ± 0.24Aa | 4.60 ± 0.39Aa |
| Scratched | 4.57 ± 0.37Aa | 4.87 ± 0.25Aa | 5.21 ± 0.07Aa | 4.54 ± 0.69Aa | |
| Biofilm group smooth | 5.57 ± 0.21Ab | 6.08 ± 0.28Bb | 6.62 ± 0.11Bb | 6.17 ± 0.45Bb | |
| Biofilm group scratched | 5.95 ± 1.13Ac | 6.13 ± 0.38ABb | 6.79 ± 0.14Bb | 6.39 ± 0.32Bb | |
1)Values are expressed as the mean ± standard deviation (n = 3)
2)ABCMeans followed by the different upper case letter within each row are significant different (p < 0.05)
3)abcMeans followed by the different lower case letter within the same treatment time are significant different (p < 0.05)
Table 2.
Survival of Bacillus cereus on glass surface with different conditions (roughness and biofilm) by various chemical sanitizers
| Treatment | Time (min) | 200 ppm Chlorine | 200 ppm Chlorine dioxide | 70% Alcohol | 200 ppm Quaternary ammonium compound |
|---|---|---|---|---|---|
| Smooth | 0 | 7.02 ± 0.50Ba1),2),3) | |||
| Scratched | 7.08 ± 0.65Aa | ||||
| Biofilm group smooth | 7.36 ± 0.08Aa | ||||
| Biofilm group scratched | 8.02 ± 0.24Ab | ||||
| Smooth | 3 | 5.93 ± 0.95Aba | 6.42 ± 0.74Ba | 5.49 ± 1.18Aa | 5.45 ± 0.44Aa |
| Scratched | 6.08 ± 0.56Aa | 6.57 ± 0.67Aa | 6.10 ± 0.92Aab | 5.43 ± 0.71Aa | |
| Biofilm group smooth | 6.69 ± 0.08Ab | 6.70 ± 0.09Aa | 6.93 ± 0.04Bab | 7.02 ± 0.10Bb | |
| Biofilm group scratched | 6.79 ± 0.05Ab | 6.89 ± 0.05Aa | 7.18 ± 0.04Ab | 7.28 ± 0.64Ab | |
| Smooth | 5 | 4.50 ± 0.69Aa | 5.32 ± 0.96BCa | 4.84 ± 0.30Aba | 5.41 ± 0.91Ca |
| Scratched | 5.79 ± 0.60Bb | 6.04 ± 0.29Bb | 4.85 ± 0.23Aa | 5.22 ± 0.63ABa | |
| Biofilm group smooth | 6.31 ± 0.23Ab | 6.56 ± 0.33Ab | 6.69 ± 0.10Ab | 6.89 ± 0.18Ab | |
| Biofilm group scratched | 6.61 ± 0.42Ab | 6.72 ± 0.05Ab | 7.11 ± 0.05Ab | 6.92 ± 0.11Ab | |
| Smooth | 10 | 4.85 ± 0.51Aba | 5.18 ± 0.72Ba | 4.45 ± 0.45Aa | 4.73 ± 0.32Aba |
| Scratched | 4.87 ± 0.34Aa | 5.94 ± 0.35Bab | 4.98 ± 0.12Aa | 5.14 ± 0.44ABa | |
| Biofilm group smooth | 5.56 ± 0.48Aab | 6.28 ± 0.37Bab | 6.62 ± 0.12BCb | 6.72 ± 0.14Cb | |
| Biofilm group scratched | 6.42 ± 0.53Ab | 6.55 ± 0.20Ab | 6.97 ± 0.05Ab | 6.88 ± 0.12Ab | |
1)Values are expressed as the mean ± standard deviation (n = 3)
2)ABCMeans followed by the different upper case letter within each row are significant different (p < 0.05)
3)abcMeans followed by the different lower case letter within the same treatment time are significant different (p < 0.05)
Table 3.
Survival of Bacillus cereus on polyethylene surface with different conditions (roughness and biofilm) by various chemical sanitizers
| Treatment | Time (min) | 200 ppm Chlorine | 200 ppm Chlorine dioxide | 70% Alcohol | 200 ppm Quaternary ammonium compound |
|---|---|---|---|---|---|
| Smooth | 0 | 7.05 ± 0.32Ba1),2)3) | |||
| Scratched | 7.16 ± 0.36ABa | ||||
| Biofilm group smooth | 7.66 ± 0.39ABb | ||||
| Biofilm group scratched | 8.24 ± 0.69ABc | ||||
| Smooth | 3 | 5.00 ± 0.29Aa | 5.52 ± 1.06Ba | 5.29 ± 0.69Aba | 5.33 ± 0.49Aba |
| Scratched | 5.01 ± 0.41Aa | 6.12 ± 0.64Bab | 5.41 ± 0.57ABa | 5.46 ± 0.15ABa | |
| Biofilm group smooth | 6.28 ± 0.15Ab | 6.82 ± 0.32Bbc | 6.88 ± 0.11Bb | 6.90 ± 0.07Bb | |
| Biofilm group scratched | 6.69 ± 0.50Ab | 7.18 ± 0.35Bc | 7.27 ± 0.33Bc | 6.99 ± 0.04ABb | |
| Smooth | 5 | 4.71 ± 0.24Aa | 5.48 ± 1.02Ba | 5.07 ± 0.66Aba | 5.12 ± 0.23Aba |
| Scratched | 5.07 ± 0.25Aa | 5.77 ± 0.95Ca | 5.52 ± 0.17BCa | 5.24 ± 0.33ABa | |
| Biofilm group smooth | 6.26 ± 0.21Ab | 6.54 ± 0.12ABb | 6.84 ± 0.20Bb | 6.75 ± 0.12Bb | |
| Biofilm group scratched | 6.42 ± 0.30Ab | 7.00 ± 0.28Bc | 6.87 ± 0.47Bb | 6.96 ± 0.05Bb | |
| Smooth | 10 | 4.39 ± 0.13Aa | 4.76 ± 0.81Ba | 5.11 ± 0.44Ca | 4.87 ± 0.20Ba |
| Scratched | 4.45 ± 0.16Aa | 5.89 ± 0.70Cab | 5.47 ± 0.20BCa | 4.95 ± 0.40ABa | |
| Biofilm group smooth | 6.08 ± 0.11Ab | 6.36 ± 0.36ABb | 6.75 ± 0.15Bb | 6.66 ± 0.20Bb | |
| Biofilm group scratched | 6.14 ± 0.18Ab | 6.32 ± 0.41Ab | 6.96 ± 0.18Bb | 6.88 ± 0.01Bb | |
1)Values are expressed as the mean ± standard deviation (n = 3)
2)ABCMeans followed by the different upper case letter within each row are significant different (p < 0.05)
3)abcMeans followed by the different lower case letter within the same treatment time are significant different (p < 0.05)
Table 4.
Survival of Bacillus cereus on polypropylene surface with different conditions (roughness and biofilm) by various chemical sanitizers
| Treatment | Time (min) | 200 ppm Chlorine | 200 ppm Chlorine dioxide | 70% Alcohol | 200 ppm Quaternary ammonium compound |
|---|---|---|---|---|---|
| Smooth | 0 | 7.30 ± 0.54Ba1),2),3) | |||
| Scratched | 7.62 ± 0.22Cb1 | ||||
| Biofilm group smooth | 7.76 ± 0.30BCb | ||||
| Biofilm group scratched | 8.16 ± 0.24ABc | ||||
| Smooth | 3 | 5.38 ± 0.37Aa | 6.19 ± 0.80Aa | 5.99 ± 0.83Aa | 5.89 ± 0.74Aa |
| Scratched | 5.81 ± 0.78Aa | 6.44 ± 0.82Aa | 6.03 ± 0.78Aa | 6.20 ± 0.89Aab | |
| Biofilm group smooth | 6.30 ± 0.55Ab | 6.71 ± 0.05Bb | 6.89 ± 0.28Bb | 6.95 ± 0.15Bbc | |
| Biofilm group scratched | 6.66 ± 0.48Ab | 7.15 ± 0.12Bc | 7.12 ± 0.31Bc | 7.08 ± 0.06Bc | |
| Smooth | 5 | 5.28 ± 0.28Aa | 6.20 ± 0.92Ca | 5.75 ± 0.71Ba | 5.83 ± 0.85BCa |
| Scratched | 5.31 ± 0.59Aa | 6.36 ± 1.01Ba | 6.03 ± 0.58ABab | 6.16 ± 0.61ABab | |
| Biofilm group smooth | 5.94 ± 0.13Ab | 6.52 ± 0.41Ba | 6.72 ± 0.24Bbc | 6.80 ± 0.23Bbc | |
| Biofilm group scratched | 6.17 ± 0.30Ab | 6.70 ± 0.15ABa | 6.90 ± 0.53Bc | 6.90 ± 0.20Bc | |
| Smooth | 10 | 5.07 ± 0.35Aa | 5.67 ± 0.74Aba | 5.55 ± 0.75Aba | 5.88 ± 0.29Ba |
| Scratched | 5.28 ± 0.58Aa | 5.95 ± 0.35Aa | 5.65 ± 0.95Aa | 6.06 ± 0.61Aa | |
| Biofilm group smooth | 5.39 ± 0.72Aa | 6.20 ± 0.13ABab | 6.77 ± 0.36Bab | 6.29 ± 0.39Bab | |
| Biofilm group scratched | 5.75 ± 0.38Aa | 6.58 ± 0.40Bb | 6.84 ± 0.24Cb | 6.88 ± 0.27Cb | |
1)Values are expressed as the mean ± standard deviation (n = 3)
2)ABCMeans followed by the different upper case letter within each row are significant different (p < 0.05)
3)abcMeans followed by the different lower case letter within the same treatment time are significant different (p < 0.05)
Table 5.
Survival of Bacillus cereus on wood surface with different conditions (roughness and biofilm) by various chemical sanitizers
| Treatment | Time (min) | 200 ppm Chlorine | 200 ppm Chlorine dioxide | 70% Alcohol | 200 ppm Quaternary ammonium compound |
|---|---|---|---|---|---|
| Smooth | 0 | 7.25 ± 0.62Ba1),2),3) | |||
| Scratched | 7.30 ± 0.68BCa | ||||
| Biofilm group smooth | 8.02 ± 0.33Cb | ||||
| Biofilm group scratched | 8.35 ± 0.47Bc | ||||
| Smooth | 3 | 5.54 ± 0.31Aa | 6.20 ± 1.18Ba | 5.52 ± 0.28Aa | 6.18 ± 0.47Ba |
| Scratched | 6.02 ± 0.43Ab | 6.68 ± 0.92Ba | 5.95 ± 0.31Aa | 6.20 ± 0.30ABa | |
| Biofilm group smooth | 6.92 ± 0.72Ac | 7.63 ± 0.22Bb | 7.27 ± 0.41ABb | 7.32 ± 0.55Bb | |
| Biofilm group scratched | 7.34 ± 0.82Ac | 7.73 ± 0.33Ab | 7.49 ± 0.28Ab | 7.50 ± 1.10Ab | |
| Smooth | 5 | 5.17 ± 0.49Aa | 6.03 ± 0.97Ba | 5.32 ± 0.09Aa | 5.89 ± 0.21Ba |
| Scratched | 5.97 ± 0.89Ab | 6.56 ± 0.99Bab | 5.59 ± 0.16Aa | 6.03 ± 0.36Bab | |
| Biofilm group smooth | 6.87 ± 0.61Ac | 6.85 ± 0.39Abc | 6.52 ± 0.64Ab | 7.03 ± 0.56Abc | |
| Biofilm group scratched | 7.33 ± 0.59Ac | 7.49 ± 0.66Ac | 7.13 ± 0.55Ab | 7.15 ± 0.86Ac | |
| Smooth | 10 | 5.16 ± 0.09Aa | 5.73 ± 0.81Ba | 5.15 ± 0.29Aa | 5.74 ± 0.33Ba |
| Scratched | 5.82 ± 0.76ABab | 6.31 ± 0.75Bab | 5.18 ± 0.17Aa | 5.89 ± 0.39ABab | |
| Biofilm group smooth | 6.23 ± 0.92Abc | 6.85 ± 0.39Aab | 6.67 ± 0.22Ab | 6.08 ± 0.71Aab | |
| Biofilm group scratched | 7.00 ± 0.44Ac | 6.98 ± 0.66Ab | 6.81 ± 0.83Ab | 6.95 ± 0.77Ab | |
1)Values are expressed as the mean ± standard deviation (n = 3)
2)ABCMeans followed by the different upper case letter within each row are significant different (p < 0.05)
3)abcMeans followed by the different lower case letter within the same treatment time are significant different (p < 0.05)
Stainless steel
Table 1 shows the population of B. cereus cells on SUS surfaces after sanitizer treatment for 0, 3, 5, or 10 min. For the 3 min treatment, all sanitizers showed significantly higher activities against B. cereus cells on SUS without a biofilm than on SUS with a biofilm. All sanitizer treatments of over 3 min did not show any further reduction of B. cereus cells, regardless of the surface conditions. For the 5 min treatment, chlorine dioxide and QAC reduced the bacterial cells on the scratched surface to 4.92 ± 0.96 and 4.48 ± 0.35 log CFU/10 cm2, respectively. For the 10 min treatment, all four sanitizers significantly decreased the bacterial counts on the biofilm-formed groups relative to the initial population counts. Among them, chlorine showed the best bactericidal activity against B. cereus on the surface with scratch and biofilm. However, we could not observe any significant sanitizing activity on the non-biofilm groups, regardless of surface roughness.
Glass
Table 2 shows the effects of the four sanitizers against B. cereus on GL for 0, 3, 5, or 10 min. For the 3 min treatment, the populations of B. cereus on the smooth and scratched surfaces after chlorine dioxide treatment were 6.42 ± 0.74 and 6.57 ± 0.67 log CFU/10 cm2, respectively. Similarly, alcohol and QAC treatments lowered the bacterial cell number to 5.49 ± 1.18 and 5.45 ± 0.44 log CFU/10 cm2, respectively. For the 3 min treatments, all sanitizers effectively reduced B. cereus in the non-biofilm groups especially chlorine and QAC. Chlorine treatment for 10 min on glass surface with biofilm showed statistically significant reduction of B. cereus compared to other sanitizers but the difference in B. cereus count was less than 1 log CFU/10 cm2.
Polyethylene
The efficacy of the sanitizers against B. cereus on PE with various surface conditions is shown in Table 3. The lowest mean populations of B. cereus after sanitizer treatment for 3 min were observed on the smooth surfaces, being 5.0, 5.52, 5.29, and 5.33 log CFU/10 cm2 for the chlorine, chlorine dioxide, alcohol, and QAC treatments, respectively. All sanitizer treatments for 5 min effectively killed B. cereus cells in the non-biofilm groups compared with the biofilm group. For 5 and 10 min treatment, chlorine showed a significantly higher activity than alcohol and QAC in biofilm group with or without scratches.
Polypropylene
Table 4 summarizes the sanitizing activity of the chemical agents on PP. Similar to that observed on PE, chlorine showed the highest bactericidal activity against B. cereus on PP. All sanitizer treatments on smooth and scratched surface without biofilm showed similar reduction of B. cereus. However, for surface with biofilm, chlorine treatment exhibited significant effects on B. cereus compared with the other sanitizers. The remaining bacterial cell counts on smooth surfaces with biofilms after chlorine and chlorine dioxide treatments for 3 min were 6.30 ± 0.55 and 6.71 ± 0.05 log CFU/10 cm2, respectively. On the other hand, bacterial populations treated with alcohol and QAC for 3 min numbered 6.89 ± 0.28 and 6.95 ± 0.15 log CFU/10 cm2, respectively. Chlorine treatment for 10 min significantly reduced B. cereus on surface with biofilm, approximately 5 log CFU/10 cm2, which was about < l log CFU/10 cm2 than other sanitizer treatments. Sanitizer treatment even for 10 min on PP was not enough to eliminate B. cereus cells.
Wood
Table 5 shows the changes in bacterial counts on wood surfaces after the various sanitizer treatments. Significant differences in the population of B. cereus between the non-biofilm and biofilm groups were observed with all sanitizer treatments for 3 and 5 min. Similar to that observed on PP, all four chemical sanitizers failed to reduce the counts of B. cereus on the wood surfaces with scratches and biofilms by over a 2 log CFU/10 cm2 reduction, despite up to 10 min of treatment.
The presence of a biofilm is the most pivotal factor determining the efficacy of chemical sanitizers (Tables 1–5). Consistent with the bacterial cell attachment results, the efficacy of each chemical sanitizer was dramatically suppressed in the presence of a biofilm on the food contact surface. It is also known that the efficacy of chemical sanitizers is much greater on adhered bacterial cells than on biofilm of five types of foodborne pathogens; namely, Escherichia coli O157:H7, Listeria monocytogenes, Salmonella Typhimurium, Staphylococcus aureus, and Cronobacter sakazakii (Bae et al., 2012). In our current study, the 100% RH condition used contributes greatly to the generation of biofilms. Bae et al. (Bae et al., 2012) showed that the RH is positively correlated with biofilm formation, and those generated under 100% RH survived longer and were more highly resistant to sanitizer treatment than those formed under RHs of 23, 43, 68, and 85%. It is worth noting that a 100% RH represents the condition of a hot and humid summer season, when foodborne illnesses most often occur. Therefore, adequate cleaning and sanitization practices to prevent biofilm formation are critical in the food safety system. In addition, more careful safety management is required during a hot and rainy season.
It seems B. cereus is relatively more resistant than other foodborne bacteria to chemical sanitizer treatment. According to our results, the B. cereus population was never below the critical limit after chemical sanitizer treatment (Tables 1–5). Even after 10 min of treatment with the sanitizers tested, the reduction of bacterial cells was < 2 log CFU/10 cm2 (Tables 1–5). However, we previously reported that chemical sanitizers, such as 70% alcohol and chlorine, could reduce the population of S. aureus below the detection limit of < 2.48 log CFU/cm2 (Kim et al., 2017). Moreover, the resistance of B. cereus by chemical sanitizer treatment was supported by the fact that time did not increase sanitizer efficacy after 3 min. In the case of S. aureus, however, the bactericidal activity of the sanitizers increased in a time-dependent manner (Kim et al., 2017). Therefore, the establishment of sanitization guidelines specific to B. cereus is required, based on the scientific evidence obtained.
In addition to biofilm presence, the type of material of the contact surface is also an important factor affecting the efficacy of chemical sanitizers, and is generally characterized by its hydrophobicity and roughness (Bernardes, 2010; Fernandes et al., 2014). The reduction of B. cereus on wood with rough surface by the sanitizer treatments for 3 min was relatively less than that on other materials tested. Although our data showed that chlorine was the most effective chemical sanitizer tested for surface with biofilm, the log reduction between the initial population and the population after 10 min of chlorine treatment decreased with the increasing hydrophobicity of the surface materials. It is well known that a higher hydrophobicity of the food contact surface lowers the efficacy of chemical sanitizers, possibly by increasing bacterial adhesion to the food contact surface and formation of the biofilm (Joseph et al., 2001; Ren and Frank, 1993). In addition, the roughness of the food contact surface reduces the sanitization efficacy (Hilbert et al., 2003; Lomander et al., 2004). Chemical sanitizer treatment was not efficient to inactivate B. cereus on the surface of PP and wood owing to the inherent features of these materials. Because of the hydrophobicity of PP and the naturally existing pores in wood, B. cereus can attach easily onto the surface and form a biofilm. In particular, wood is the most difficult surface from which to remove bacterial cells by chemical sanitizers because of its low surface charge and naturally existing surface roughness (Adetunji and Isola, 2011).
The results from this study suggest that general sanitizing methods may not be effective depending on the type and condition of the food contact surface. Therefore, it is necessary to select cleanable materials for the food contact surface, and to use smooth surfaces in order to enhance the effectiveness of sanitizing methods. Since the presence of biofilm on the food contact surface significantly suppresses the sanitizer activity, it is necessary to avoid circumstances favorable for biofilm generation. In practice, adequate cleaning with proper detergents and brushes should be done before sanitization to inhibit biofilm formation.
Abbreviations
- FE-SEM
The field emission-scanning electron microscopy
- GL
Glass
- KMFDS
The Korea Ministry of Food and Drug Safety
- PE
Polyethylene
- PP
Polypropylene
- RH
Relative humidity
- SUS
Stainless steel
- TSA
Tryptic soy agar
- TSB
Tryptic soy broth
- QAC
Quaternary ammonium compound
References
- Adetunji VO, Isola TO. Crystal violet binding assay for assessment of biofilm formation by Listeria monocytogenes and Listeria spp on wood, steel and glass surfaces. Glob Vet. 2011;6(1):6–10. [Google Scholar]
- Andersson A, Granum PE, Rönner U. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int J Food Microbiol. 1998;39:93–99. doi: 10.1016/S0168-1605(97)00121-9. [DOI] [PubMed] [Google Scholar]
- Araújo EA, Bernardes PC, Andrade NJ, Fernandes PE, Sá JPN. Gibbs free energy of adhesion of Bacillus cereus isolated from dairy plants on different food processing surfaces evaluated by the hydrophobicity. Int J Food Sci Technol. 2009;44:2519–2525. doi: 10.1111/j.1365-2621.2009.02078.x. [DOI] [Google Scholar]
- Bae Y-M, Baek S-Y, Lee S-Y. Resistance of pathogenic bacteria on the surface of stainless steel depending on attachment form and efficacy of chemical sanitizers. Int J Food Microbiol. 2012;153:465–473. doi: 10.1016/j.ijfoodmicro.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Bae Y-M, Lee S-Y. Inhibitory effects of UV treatment and a combination of UV and dry heat against pathogens on stainless steel and polypropylene surfaces. J Food Sci. 2012;77(1):M61–M64. doi: 10.1111/j.1750-3841.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- Bernardes PC. Andrade NJd, Ferreira SO, Sá JPNd, Araújo EA, Delatorre DMZ, Luiz LMP. Assessment of hydrophobicity and roughness of stainless steel adhered by an isolate of Bacillus cereus from a dairy plant. Braz. J Microbiol. 2010;41:984–992. doi: 10.1590/S1517-838220100004000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulané-Petermann L. Processes of bioadhesion on stainless steel surfaces and cleanability: a review with special reference to the food industry. Biofouling. 1996;10:275–300. doi: 10.1080/08927019609386287. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Flint SH. Biofilms in the food industry: problems and potential solutions. Int J Food Sci Technol. 2008;43:2163–2176. doi: 10.1111/j.1365-2621.2008.01839.x. [DOI] [Google Scholar]
- Chaturongkasumrit Y, Takahashi H, Keeratipibul S, Kuda T, Kimura B. The effect of polyesterurethane belt surface roughness on Listeria monocytogenes biofilm formation and its cleaning efficiency. Food Control. 2011;22:1893–1899. doi: 10.1016/j.foodcont.2011.04.032. [DOI] [Google Scholar]
- Di Ciccio P, Vergara A, Festino AR, Paludi D, Zanardi E, Ghidini S, Ianieri A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control. 2015;50:930–936. doi: 10.1016/j.foodcont.2014.10.048. [DOI] [Google Scholar]
- Ehling-Schulz M, Fricker M, Scherer S. Identification of emetic toxin producing Bacillus cereus strains by a novel molecular assay. FEMS Microbiol Lett. 2004;232:189–195. doi: 10.1016/S0378-1097(04)00066-7. [DOI] [PubMed] [Google Scholar]
- Faille C, Lebret V, Gavini F, Maingonnat J-F. Injury and lethality of heat treatment of Bacillus cereus spores suspended in buffer and in poultry meat. J Food Prot. 1997;60:544–547. doi: 10.4315/0362-028X-60.5.544. [DOI] [PubMed] [Google Scholar]
- Felske, A. 2004. Ecology of Bacillus species in soil. Bacterial spore formers-probiotics and emerging applications. Horizon. Biosci. Norfolk 35–44.
- Fernandes PE, Sao Jose JFB, Zerdas ERMA, Andrade NJ, Fernandes CM, Silva LD. Influence of the hydrophobicity and surface roughness of mangoes and tomatoes on the adhesion of Salmonella enterica serovar Typhimurium and evaluation of cleaning procedures using surfactin. Food Control. 2014;41:21–26. doi: 10.1016/j.foodcont.2013.12.024. [DOI] [Google Scholar]
- Frank JF. Microbial attachment to food and food contact surfaces. Adv Food and Nutr Res. 2001;43:319–370. doi: 10.1016/S1043-4526(01)43008-7. [DOI] [PubMed] [Google Scholar]
- Giaouris E, Heir E, Hébraud M, Chorianopoulos N, Langsrud S, Møretrø T, Habimana O, Desvaux M, Renier S, Nychas G-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014;97:298–309. doi: 10.1016/j.meatsci.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Granum, P,E. 1994. Bacillus cereus and its toxins. J. Appl. Microbiol.76. [PubMed]
- Hilbert LR, Bagge-Ravn DB, Kold J, Gram L. Influence of surface roughness of stainless steel on microbial adhesion and corrosion resistance. Int Biodeterior Biodegradation. 2003;52:175–185. doi: 10.1016/S0964-8305(03)00104-5. [DOI] [Google Scholar]
- Jahid IK, Ha S-D. A review of microbial biofilms of produce: future challenge to food safety. Food Sci Biotechnol. 2012;21:299–316. doi: 10.1007/s10068-012-0041-1. [DOI] [Google Scholar]
- Joseph B, Otta S, Karunasagar I, Karunasagar I. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int J Food Microbiol. 2001;64:367–372. doi: 10.1016/S0168-1605(00)00466-9. [DOI] [PubMed] [Google Scholar]
- Jullien C, Bénézech T, Carpentier B, Lebret V, Faille C. Identification of surface characteristics relevant to the hygienic status of stainless steel for the food industry. J Food Eng. 2003;56:77–87. doi: 10.1016/S0260-8774(02)00150-4. [DOI] [Google Scholar]
- Kim, C.Y., Ryu, G.J., Park, H.Y. and Ryu, K. 2017. Resistance of Staphylococcus aureus on food contact surfaces with different surface characteristics to chemical sanitizers. J. Food Safe.
- Kim H, Ryu J-H, Beuchat LR. Effectiveness of disinfectants in killing Enterobacter sakazakii in suspension, dried on the surface of stainless steel, and in a biofilm. Appl Environ Microbiol. 2007;73(4):1256–1265. doi: 10.1128/AEM.01766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-I, Jeon D-H, Yoon H-J, Choi H-C, Eom M-O, Sung J-H, Park N-Y, Won S-A, Kim N-Y, Lee Y-J. Evaluation of the efficacy of sanitizers on food contact surfaces using a surface test method. J Food Hyg Safe. 2008;23:291–296. [Google Scholar]
- Korea Ministry of Food and Drug Safety. 2017. Adapted standard and specifications regarding foods in limited time, Noticifcation No. 2017-36.
- Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes and Infection. 2000;2:189–198. doi: 10.1016/S1286-4579(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Kramer, J., Gilbert, R. and Doyle, M. 1989. Foodborne bacterial pathogens ( Ed. M.P. Doyle), Marcel Dekker, Inc., New York and Basel. pp 22–70.
- Lee Y-D, Yoo H-L, Park J-H. Biocontrol of biofilm-forming Bacillus cereus by using organic acid, ethanol, and sodium chloride. Korean J. Food Sci. Technol. 2013;45:120–125. doi: 10.9721/KJFST.2013.45.1.120. [DOI] [Google Scholar]
- Lomander A, Schreuders P, Russek-Cohen E, Ali L. Evaluation of chlorines’ impact on biofilms on scratched stainless steel surfaces. Bioresour Technol. 2004;94:275–283. doi: 10.1016/j.biortech.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Moltz AG, Martin SE. Formation of biofilms by Listeria monocytogenes under various growth conditions. J Food Prot. 2005;68:92–97. doi: 10.4315/0362-028X-68.1.92. [DOI] [PubMed] [Google Scholar]
- Monk IR, Cook GM, Monk BC, Bremer PJ. Morphotypic conversion in Listeria monocytogenes biofilm formation: biological significance of rough colony isolates. Appl Environ Microbiol. 2004;70:6686–6694. doi: 10.1128/AEM.70.11.6686-6694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T-M, Wang T-K, Lee C-L, Chien S-W, Horng C-B. Food-borne disease outbreaks due to bacteria in Taiwan, 1986 to 1995. J Clin Microbiol. 1997;35:1260–1262. doi: 10.1128/jcm.35.5.1260-1262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J-S, Tsai W-C, Chou C-C. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int J Food Microbiol. 2002;77:11–18. doi: 10.1016/S0168-1605(02)00060-0. [DOI] [PubMed] [Google Scholar]
- Ren T-J, Frank JF. Susceptibility of starved planktonic and biofilm Listeria monocytogenes to quaternary ammonium sanitizer as determined by direct viable and agar plate counts. J Food Prot. 1993;56:573–576. doi: 10.4315/0362-028X-56.7.573. [DOI] [PubMed] [Google Scholar]
- Ryu J-H, Beuchat LR. Biofilm formation and sporulation by Bacillus cereus on a stainless steel surface and subsequent resistance of vegetative cells and spores to chlorine, chlorine dioxide, and a peroxyacetic acid–based sanitizer. J Food Prot. 2005;68:2614–2622. doi: 10.4315/0362-028X-68.12.2614. [DOI] [PubMed] [Google Scholar]
- Tauveron G, Slomianny C, Henry C, Faille C. Variability among Bacillus cereus strains in spore surface properties and influence on their ability to contaminate food surface equipment. Int J Food Microbiol. 2006;110:254–262. doi: 10.1016/j.ijfoodmicro.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Van Loosdrecht M, Lyklema J, Norde W, Schraa G, Zehnder A. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang L, Levänen E. Superhydrophobic surfaces for the reduction of bacterial adhesion. Rsc Adv. 2013;3:12003–12020. doi: 10.1039/c3ra40497h. [DOI] [Google Scholar]