Abstract
Green tea is one of the most beverages with antioxidants and nutrients. As one of the major components of green tea, (-)-epicatechin gallate (ECG) was evaluated for its antioxidative properties in the present study. Cell proliferation assay, tube formation, cell migration, apoptosis, and autophagy were performed in human brain microvascular endothelial cells (HBMVECs) after oxygen-glucose deprivation/reoxygenation (OGD/R) to investigate potential anti-ischemia/reperfusion injury properties of ECG in vitro. Markers of oxidative stress as ROS, LDH, MDA, and SOD were further assayed in our study. Data indicated that ECG could affect neovascularization and promote cell proliferation, tube formation, and cell migration while inhibiting apoptosis and autophagy through affecting VEGF, Bcl-2, BAX, LC3B, caspase 3, mTOR, and Beclin-1 expression. All the data suggested that ECG may be protective for the brain against ischemia/reperfusion injury by promoting neovascularization, alleviating apoptosis and autophagy, and promoting cell proliferation in HBMVECs of OGD/R.
1. Introduction
Ischemic stroke is one of the most causes of mortality and disability worldwide which occurs as a result of an obstruction within a blood vessel supplying blood to the brain. Restoration of blood flow involved in the treatment of stroke may lead to reperfusion injury. Ischemia/reperfusion (I/R) often induces tissue damage. After a period of ischemia or lack of oxygen (anoxia or hypoxia), the absence of oxygen and nutrients from blood during the ischemic period creates a condition in which the restoration of circulation leads to oxidative damage and inflammation and through the induction of oxidative stress along with or rather than restoration of normal function. Reperfusion of ischemic tissues is often associated with microvascular injury, and activated endothelial cells produce more reactive oxygen species following reperfusion which results in a subsequent inflammatory response [1]. During the I/R, reactive oxygen species (ROS) lead to the oxidation of proteins, lipids, and DNA, which induce cell proliferation, apoptosis, and necrosis [2, 3]. Superoxide dismutase (SOD) serves a key antioxidant role in cells considering superoxide as one of the principal reactive oxygen species. Malondialdehyde (MDA) is a marker for oxidative stress which results from lipid peroxidation of polyunsaturated fatty acids. Dysfunction of SOD and MDA aggravate oxidative stress and I/R injury [3–5]. LDH is expressed extensively in body tissues and considered a marker of common injuries and disease as it is released during tissue damage. Autophagy is generally activated during I/R and causes autophagic cell death [6]. It is thought that inhibition of autophagy can reduce neurodegenerative damage after focal cerebral ischemia, which seems that the inhibition of autophagy may be a novel strategy to prevent ischemic brain injury [7–9]. Vascular endothelial growth factors (VEGFs) have been reported to participate in vessel repair [10, 11], angiogenesis [10, 12], postischemic brain [13, 14], and neuroprotection in experimental stroke [15], and VEGF signaling pathways are considered as important potential targets for the acute and chronic treatment of stroke [15].
As reported, green tea consumption may be correlated with a reduced risk of stroke [16, 17]. Most standardized green tea extracts are total polyphenols that contain several antioxidant compounds of polyphenols including (-)-epigallocatechin gallate (EGCG), (-)-epicatechin gallate (ECG), (-)-epigallocatechin (EGC), and (-)-epicatechin (EC) [18]. EGCG and ECG are the above two components of polyphenols extracted from green tea. The chemical structure of ECG is similar to that of EGCG (Figure 1). As previous studies reported, EGCG could scavenge the free radical ions and increase the activity of antioxidant enzymes [19] and reduce upregulation of MMP-9 activity and neuronal damage following transient focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO) in mice [20]. EGCG attenuates oxidative stress responses and promotes autophagy-dependent survival via influencing the balance of the mTOR-AMPK pathway upon endoplasmic reticulum stress [21]. Gundimeda et al. reported that green tea polyphenol precondition resisted cell death induced by oxygen-glucose deprivation (OGD), and the effect of ECG on cell death induced by OGD was consistent with EGCG [22]. ECG exhibited stronger inhibitory activity than did EGCG to oxidation-induced increase in secretory sphingomyelinase involved in many diseases caused by oxidative stress [23]. ECG showed to be more active, playing the important role in the formation of dityrosine (DT) cross-linkages in proteins which is one of the most widely used markers of oxidative stress [24]. In other studies, ECG also showed the maximum antioxidant property [25, 26] among the polyphenols extracted from green tea. On the contrary, the effect of ECG on cerebral I/R injury and its mechanism remain unclear.
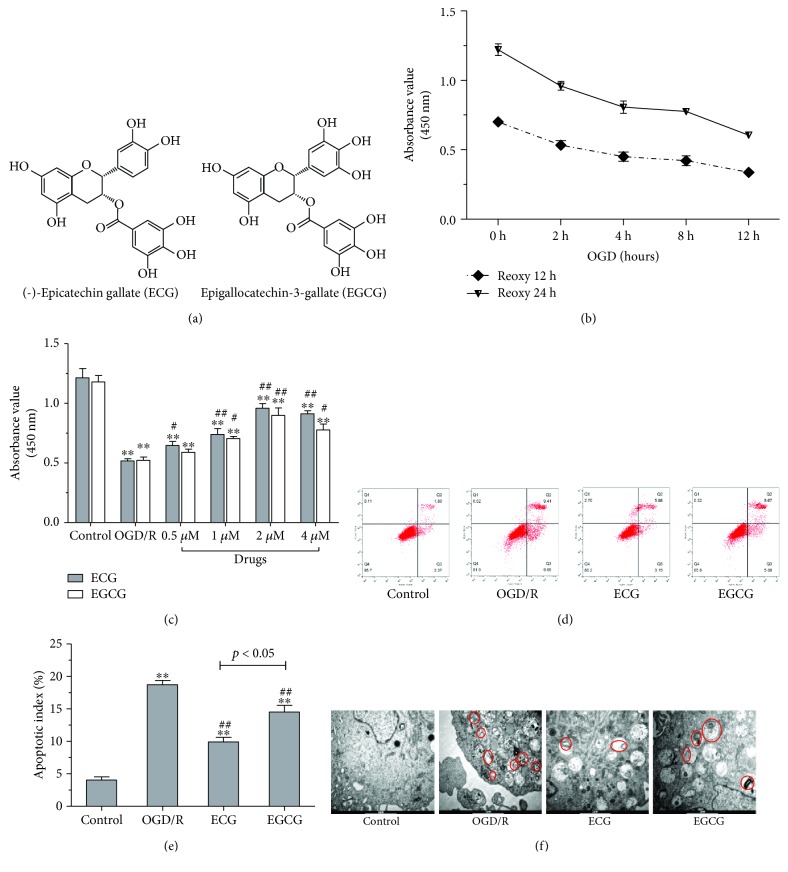
Figure 1.
Effect of ECG/EGCG on cell viability, apoptosis, and autophagy in HBMVECs of OGD/R. Chemical structures of ECG and EGCG are shown in (a). Cell viability was assayed by CCK-8 when HBMVECs were cultured in glucose-free culture and oxygen deprivation of 0.5% O2 + 5% CO2 + 94.5% N2 for 2 h, 4 h, 8 h, or 12 h and then reoxygenated for 12 or 24 h in normal medium (b). Cell viability of HBMVECs treated with 0.5, 1, 2, and 4 μM of ECG/EGCG in OGD/R measured by CCK-8 (c). Effect of ECG/EGCG on apoptosis induced by OGD/R, measured by flow cytometry, was explored (d and e). Autophagy was also observed using electron microscopy (f). Data was given as mean ± SD, n = 6. ∗ P < 0.05 and ∗∗ P < 0.01 compared with control, # P < 0.05 and ## P < 0.01 versus cells after OGD/R treatment with solvent (OGD/R or solvent control group).
In the present study, we first explored the effect of ECG on cell proliferation and apoptosis induced by oxygen-glucose deprivation/reoxygenation (OGD/R) in HBMVECs. Oxidative stress detection, angiogenesis, and cell migration experiments were carried out to evaluate the action of ECG, and the role of ECG in autophagy induced by OGD/R was investigated in our study.
2. Materials and Methods
2.1. Cells, Reagents, and Antibodies
Human brain microvascular endothelial cells (HBMVECs) were supplied by Angio-Proteomie (MA, USA) and cultured in endothelial cell medium (ECM) (CA, ScienCell) or glucose-free ECM supplemented with endothelial cell growth supplement (ECGS) and 5% fetal bovine serum (FBS). Cells were incubated at 37°C and in an atmosphere of 5% CO2 and 95% of air.
(-)-Epicatechin gallate (ECG) (E3893) and epigallocatechin-3-gallate (EGCG) (E4143) were purchased from Sigma (E3893, Sigma-Aldrich, MO, USA). BCA protein assay kit and enhanced chemiluminescence (ECL) were provided by Thermo Scientific (Shanghai, China). Antibodies of GAPDH, VEGF, Bcl-2, LC3B, and FITC goat anti-rabbit antibody used for apoptosis assay were supplied by Abcam (UK). Antibodies of BAX, Caspase 3, mTOR, and Beclin-1 were purchased from Cell Signaling Technology (CST, USA). Secondary antibodies conjugated with horseradish peroxidase were obtained from Abbkine Scientific Co. Ltd (CA, USA).
2.2. Oxygen-Glucose Deprivation/Reoxygenation (OGD/R) Model and Treatment
To establish the OGD/R model, HBMVECs were incubated in glucose-free culture under hypoxic conditions of oxygen deprivation of 0.5% O2 + 5% CO2 + 94.5% N2 for 2 h, 4 h, 8 h, or 12 h and then reoxygenated for 12 or 24 h in normal medium. 0.5, 1, 2, and 4 μM of ECG/EGCG were added to the cells prior to hypoxia.
2.3. Cell Proliferation Assay
HBMVECs were plated in 96-well plates at a density of 0.5 × 104 cells/well and incubated overnight and subsequently in glucose-free culture under hypoxia oxygenation or treated with various concentrations of ECG/EGCG followed by reoxygenation in complete medium. Cell Counting Kit 8 (CCK-8) (Dojindo, Japan) was utilized to detect cell viability of HBMVECs according to the instructions. In short, 20 μL of CCK-8 solution was added to HBMVECs and incubated at 37°C for 2 h; finally, formazan products were quantified by absorbance at 450 nm detected by a microplate reader (Thermo, MA, USA).
2.4. Detection of Apoptosis by Flow Cytometry
We measured cell apoptosis by flow cytometry. HBMVECs after OGD/R and treatment were collected and double-stained with Annexin V-FITC and PI. Apoptosis of cells was measured and analyzed by BD FASAria Cell Sorter flow cytometer (Becton Dickinson) with BD Accuri C6 Software (Becton Dickinson).
2.5. Observation of Autophagy by Electron Microscopy
After OGD/R and treatment, HBMVECs were fixed using 2.5% glutaraldehyde and then 1% osmium tetroxide (Sigma-Aldrich, USA) for 30 minutes. Dehydration via acetone and embedding via epoxy embedding medium (Sigma-Aldrich, USA) were performed at room temperature. 1 μm ultrathin sections were made and stained by uranyl acetate (Tianfu Chemical Co. Ltd, China). Autophagy was observed by transmission electron microscopy (FEI, Netherlands).
2.6. ROS, LDH, MDA, and SOD Assay
After OGD/R and treatment with ECG, HBMVECs were harvested and cell culture medium was collected. Reactive oxygen species (ROS) of cells was detected using ROS Assay Kit purchased from Beyotime Biotechnology (Shanghai, China) according to the kit instructions of the manufacturer. Lactated hydrogenase (LDH) levels of cell culture medium and malondialdehyde (MDA) and superoxide dismutase (SOD) activity of cells were assayed using assay kits purchased from Jiancheng Co. (Nanjing, China) according to the kit instructions of the manufacturer, and data was calculated using standard curves.
2.7. Transwell Migration Assay
To measure cell migration ability, transwell migration assay was performed as previously reported [27, 28]. Briefly, 0.5 × 104 HBMVECs suspended in 200 μL of culture medium with/without drugs were seeded in the upper chamber of transwell (BD) followed by OGD/R and then placed in 24-well plates, and the lower chamber was filled with fresh culture medium. After 48 hours of incubation at 37°C, the filters were taken out gently and cells on the upper surface were removed using cotton swabs. The cells on the underside of transwell filters were stained by 1% of crystal violet (Genemed, USA) for 10 minutes, and photographs were taken (Leica DC 100). Migrating cells stained with crystal violet were collected and measured at 570 nm for quantitative assessment per filter.
2.8. Tube Formation Assay
We performed tube formation assay to investigate the effect of ECG on HBMVECs. 50 μL of ice-cold matrigel (BD) and serum-free culture medium were mixed and added to 96-well plates. After treatment and OGD/R, HBMVECs with 1 × 104/well in 200 μL of medium were added to the plates and incubated for 5 h, and the tube networks were photographed and quantified by the number of tube formations using ImageJ software [29].
2.9. RNA Extraction and Real-Time Quantitative PCR (qRT-PCR)
Total RNA of cells was extracted using RNA isolation reagent (Invitrogen, USA) and then was reverse-transcribed using One Step RT-qPCR Kit (Sangon Biotech, China) according to the manufacturer's protocol. Next, equal amounts of cDNA were used for RT-qPCR. PCR reaction and real-time detection were performed using iQ5 Real-time Quantitative PCR (Bio-Rad, USA). As shown in Table 1, the appropriate forward and reverse real-time PCR primers were used for GAPDH, VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1. The real-time PCR cycles included predenaturation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 20 sec, annealing at 60°C for 30 sec, and extension at 72°C for 30 sec. 2−ΔΔCt (ΔΔCt = (CmRNA–CtGAPDH) − (control − CtGAPDH)) was used to quantify the relative expression of target mRNA.
Table 1.
Primers sequences, size of the amplification product, and NCBI reference sequence.
| Gene | Sequence | AMPL. size | NCBI ref. seq. | |
|---|---|---|---|---|
| GAPDH | Fw: | 5′- GGAGCGAGATCCCTCCAAAAT -3′ | 197 bp | NM_001256799 |
| Rev: | 5′- GGCTGTTGTCATACTTCTCATGG -3′ | |||
| VEGF | Fw: | 5′- AGGGCAGAATCATCACGAAGT -3′ | 75 bp | NM_001171627 |
| Rev: | 5′- AGGGTCTCGATTGGATGGCA -3′ | |||
| Bcl-2 | Fw: | 5′- GGTGGGGTCATGTGTGTGG-3′ | 89 bp | NM_000657 |
| Rev: | 5′- CGGTTCAGGTACTCAGTCATCC-3′ | |||
| BAX | Fw: | 5′- CCCGAGAGGTCTTTTTCCGAG-3′ | 155 bp | NM_138763 |
| Rev: | 5′- CCAGCCCATGATGGTTCTGAT-3′ | |||
| LC3B | Fw: | 5′- GATGTCCGACTTATTCGAGAGC-3′ | 167 bp | NM_022818 |
| Rev: | 5′- TTGAGCTGTAAGCGCCTTCTA-3′ | |||
| Caspase 3 | Fw: | 5′- CATGGAAGCGAATCAATGGACT-3′ | 139 bp | NM_004346 |
| Rev: | 5′- CTGTACCAGACCGAGATGTCA-3′ | |||
| mTOR | Fw: | 5′- ATGCTTGGAACCGGACCTG-3′ | 173 bp | NM_004958 |
| Rev: | 5′- TCTTGACTCATCTCTCGGAGTT-3′ | |||
| Beclin-1 | Fw: | 5′- CCATGCAGGTGAGCTTCGT-3′ | 215 bp | NM_003766 |
| Rev: | 5′- GAATCTGCGAGAGACACCATC-3′ | |||
Fw = forward; Rev = reverse.
2.10. Western Blotting
After OGD/R and treatment with ECG, HBMVECs were harvested and cell extracts were prepared. Briefly, cells were lysed in lysis buffer (20 mM Tris-pH 7.5, 150 mM NaCl, 1% Triton X-100) followed by centrifugation for 3 min at 10,000 × g at 4°C. The supernatant was collected and transferred electrophoretically to a polyvinylidene fluoride membrane (PVDF) (Millipore, Shanghai, China). The membranes were blocked with 5% dry milk and subsequently incubated with primary antibodies against GAPDH (Abcam, UK), VEGF (Abcam, UK), Bcl-2 (Abcam, UK), BAX (CST, USA), Caspase 3 (CST, USA), mTOR (CST, USA), LC3B (Abcam, UK), and Beclin-1 (CST, USA) overnight at 4°C. The membranes were subsequently washed and incubated with secondary antibodies conjugated with horseradish peroxidase. The immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Scientific, Shanghai, China) and analyzed by automatic chemiluminescence image analysis system (Tanon, China). The results were normalized to GAPDH.
2.11. Statistical Analysis
All experiments were repeated at least three times, and all data are shown as means ± standard deviations (SD) of six independent samples. The significant difference in two groups was evaluated using the Mann–Whitney test or nonparametric test, and a P value of 0.05 or less is considered statistically significant.
3. Results
3.1. Effect of ECG/EGCG on Cell Viability, Apoptosis, and Autophagy of HBMVECs Undergoing OGD/R
The effect of ECG/EGCG on cell viability, autophagy, and apoptosis in HBMVECs induced by OGD/R was explored in our study. The OGD/R model in HBMVECs was first made, and the effect of ECG/EGCG was evaluated. It is shown in Figure 1(b) that cell viability was decreased time-dependently during OGD/R. Cell viability of HBMVECs induced by 12 h-OGD combined with 12 h reperfusion or 12 h OGD combined with 24 h reperfusion was decreased to 48.1% and 49.6% of control cells individually. We choose OGD/R of 12 h OGD and 12 h reperfusion for the following study. Cell viability of HBMVECs after OGD/R treated with ECG/EGCG showed a dose-dependent increase in doses of 0.5 μM to 4 μM compared with the solvent control group (OGD/R) (Figure 1(c)). It was 0.5 μM ECG not EGCG which showed a significant effect on promoting cell viability compared with the solvent control group (OGD/R) (P < 0.05). 2 μM ECG/EGCG showed the most significant promotion of cell viability effect. Cell viability in 4 μM ECG/EGCG-treated cells showed a decreasing trend, and EGCG was more obvious. We chose 2 μM of ECG/EGCG for further apoptosis and autophagy experiments. As shown in Figures 1(d) and 1(e), both ECG and EGCG showed inhibition of OGD/R-induced apoptosis. In ECG-treated cells, the rate of apoptosis was significantly decreased compared with the EGCG-treated group. OGD/R-induced autophagy was observed by transmission electron microscopy, and the effect of ECG on autophagy was similar to that on apoptosis (Figure 1(f)). ECG was chosen for our further study.
3.2. Effect of ECG on ROS, LDH, MDA, and SOD of HBMVECs after OGD/R
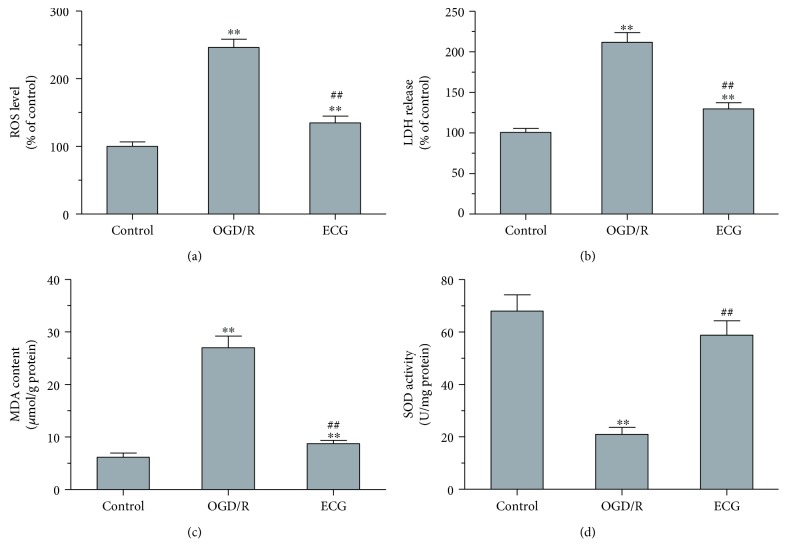
To evaluate the antioxidant activity of ECG, ROS, LDH, MDA, and SOD assay was performed in our study. OGD/R induced an ROS level about 2.5-fold to control (normal), whereas ECG downregulated ROS significantly compared with the OGD/R group (P < 0.01) (Figure 2(a)). OGD/R induced oxidative stress, and the LDH level was used to assess cell death. ECG treatment significantly inhibited LDH leakage induced by OGD (P < 0.01) (Figure 2(b)). As shown in Figure 2(c), OGD/R upregulated intracellular MDA levels compared with control which was significantly decreased by ECG treatment (Figure 2(c)). As MDA was considered an indicator of lipid peroxidation, ECG may show inhibition of lipid peroxidation in HBMVECs that underwent OGD/R. In the OGD/R group, the SOD activities were significantly decreased compared with the control group (Figure 2(d)). ECG treatment significantly increased SOD activity (P < 0.01).
Figure 2.
ECG affected ROS, LDH, MDA, and SOD in HBMVECs after OGD/R. Levels of ROS (a) and MDA (c), SOD activity (d) in cells and LHD releasing level (b) were detected using assay kits correspondingly. Data was given as mean ± SD, n = 6. ∗ P < 0.05 and ∗∗ P < 0.01 compared with control, # P < 0.05 and ## P < 0.01 versus cells after OGD/R treatment with solvent (OGD/R or solvent control group).
3.3. ECG Prompted Migration and Tube Formation of HBMVECs after OGD/R
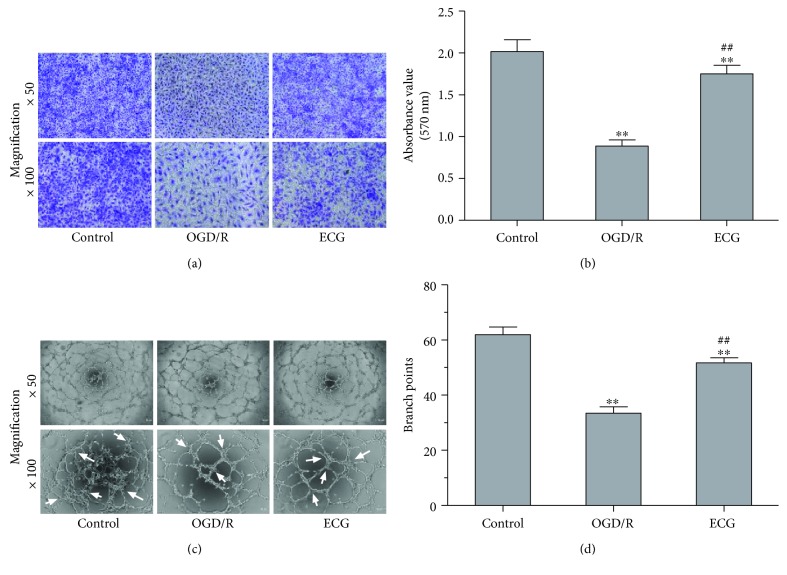
To explore ECG on neovascularization in vitro, migration assay and tube formation were performed. OGD/R induced HBMVEC migration ability decreasing significantly (P < 0.01), and ECG prompted cell migration significantly (P < 0.01) even though it did not reverse OGD/R-induced inhibition of migration (Figures 3(a) and 3(b)). Cell tube formation capacity assayed by branch point number [30] was significantly decreased in the OGD/R group (P < 0.01) (Figures 3(c) and 3(d)). The number of tube formation in the ECG-treated group was about 1.55-fold of that in the OGD/R group.
Figure 3.
ECG prompted migration and tube formation of HBMVECs after OGD/R. Cell migration of HBMVECs after OGD/R and treated with ECG was assayed using the Transwell method (a), and migrating cells were stained with crystal violet and measured at 570 nm for quantitative analysis (b). Tube formation of HBMVECs after OGD/R and treatment with ECG was performed (c), and tubes were quantified as analysis branch points as indicated by white arrows using ImageJ software (d). Data was given as mean ± SD, n = 6. ∗ P < 0.05 and ∗∗ P < 0.01 compared with control, # P < 0.05 and ## P < 0.01 versus cells after OGD/R treatment with solvent (OGD/R or solvent control group).
3.4. Effect of ECG on mRNA and Protein Expression of VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1
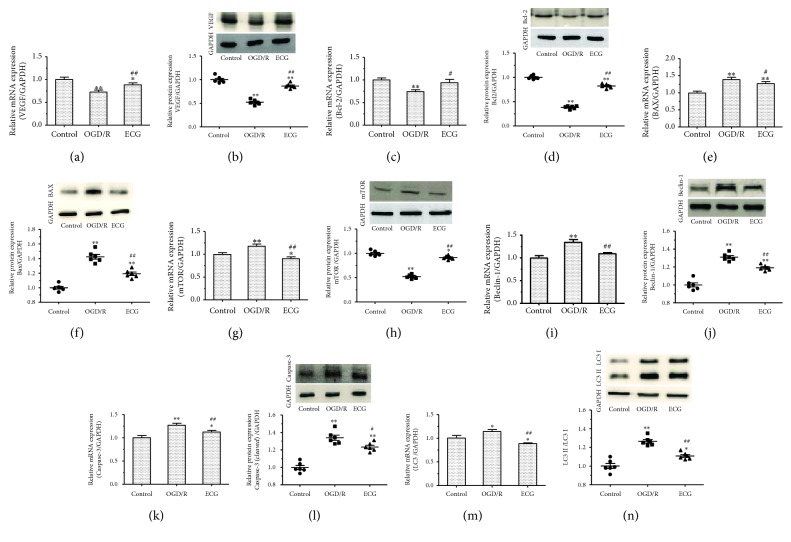
mRNA and protein expression of vascular endothelial growth factor of VEGF, cell proliferation, and apoptosis associated Bcl-2, BAX, and Caspase 3, as well as LC3B, mTOR, and Beclin-1 which is related to autophagy was measured in our study. Compared with control, mRNA expression of VEGF (Figure 4(a)) and Bcl-2 (Figure 4(c)) decreased significantly (P < 0.01), whereas BAX (Figure 4(e)), mTOR (Figure 4(g)), Beclin-1 (Figure 4(i)), Caspase 3 (Figure 4(k)), and LC3B (Figure 4(m)) mRNA expression increased significantly (P < 0.01) in the OGD/R group. ECG tends to inhibit downregulation of VEGF and Bcl2 as well as upregulation of BAX, mTOR, Beclin-1, Caspase 3, and LC3B induced by OGD/R.
Figure 4.
The effect of ECG on mRNA and protein expression of VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1 in HBMVEs after OGD/R. (a), (c), (e), (g), (i), (k), and (m) represent relative mRNA expression of VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1 measured by qPCR. Protein expression of VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1 determined by western blotting is shown in (b), (d), (f), (h), (j), (l), and (n) correspondingly. Data was given as mean ± SD, n = 6. ∗ P < 0.05 and ∗∗ P < 0.01 compared with control, # P < 0.05 and ## P < 0.01 versus cells after OGD/R treatment with solvent (OGD/R or solvent control group).
To investigate the effect of ECG on the expression of OGD/R-related proteins and the mechanism involved, we also measured protein expressions of VEGF, Bcl2, BAX, mTOR, Beclin-1, cleaved Caspase 3, and LC3B. The expression of VEGF (Figure 4(b)), Bcl2 (Figure 4(d)), and mTOR (Figure 4(h)) in the OGD/R group was 0.52-, 0.38-, and 0.51-fold to that in the control group. As for BAX (Figure 4(f)), Beclin-1 (Figure 4(j)), and cleaved Caspase 3 (Figure 4(l)) protein expression, there was a 1.42-, 1.31-, and 1.34-fold expression to the control group, respectively, in OGD/R group. Relative content of LC3II to LC3I was significantly upregulated in the OGD/R group compared with control (P < 0.01) (Figure 4(n)). ECG treatment attenuated regulation, up or down, induced by OGD/R (P < 0.05 or P < 0.01).
4. Discussion
Green tea is one of the most beverages with antioxidants and nutrients that have powerful effects on the body which include improving brain function [31–33], fat loss [34], lower risk of cancer [35, 36], and many other impressive benefits [37, 38]. There are many reports that green tea or its extract has protective effects on ischemia-reperfusion injury of the brain [39, 40], heart [41, 42], kidney [43], liver [44, 45], and intestine [46]. Polyphenols exhibit antioxidative and anti-inflammation effects in a lot of studies in vivo and in vitro [47, 48]. Green tea and its extracts are rich in polyphenols. EGCG and ECG are the major two polyphenols included in green tea [49]. The chemical structure of ECG is similar to EGCG (Figure 1(a)). Cerebral microcirculation plays an important role in substance exchange and oxygen supply, and reperfusion of ischemic tissues is often associated with microvascular injury.
In the present study, we first established an OGD/R model in HBMVECs in vitro. Cell viability decreased time-dependently during OGD/R, and therefore, we choose time conditions of OGD/R of 12 h OGD and 12 h reperfusion, under which cell viability decreased to about 50% to control and OGD/R in the shortest time simultaneously. The cell viability of HBMVECs after OGD/R treatment with 0.5 μM to 4 μM of ECG/EGCG showed a dose-dependent increase compared to the solvent control group (P < 0.05). 0.5 μM of ECG showed a significant effect on promoting cell viability compared to the solvent control group, and no significant effect of 0.5 μM of EGCG showed. 2 μM ECG/EGCG showed the most significant promoting cell viability effect, which was similar to Gundimeda et al.'s report [22], and 4 μM ECG/EGCG-treated cells tend to show a decreasing cell viability. 2 μM of ECG/EGCG was chosen for the followed apoptosis and autophagy experiments. ECG and EGCG both showed inhibition of OGD/R-induced apoptosis and autophagy. Compared with the solvent control group (OGD/R), the apoptosis rate in the ECG-treated group decreased to its 53.5 percent and 25.2 percent decrease in the EGCG-treated group. The apoptosis rate of EGCG-treated cells was about 1.4-fold higher than that of ECG-treated ones. It is similar to apoptosis; autophagic bodies in ECG- and EGCG-treated cells were significantly less than those in the solvent control group, and EGCG-treated cells produced even more autophagic bodies than did ECG-treated cells. During ischemia/reperfusion injury, oxidative stress may induce epithelial cell apoptosis [50–53] and/or autophagy [51, 54, 55]. Autophagy is a widely existing metabolic and strictly regulated process, and excessive or deficient autophagy may contribute to pathogenesis [56]. Autophagy exerts dual roles in cell death or survival during an ischemic insult or preconditioning during which autophagy is activated [57]. Autophagy may be triggered by preconditioning or lethal ischemia and then interacts with apoptotic and necrotic signaling pathways to regulate cell death. In addition, autophagy may also maintain cell function by removing protein aggregates or damaged mitochondria. Autophagy is also a double-edged sword in angiogenesis. Induction autophagy could inhibit retinal neovascularization in vitro and in vivo to improve oxygen-induced retinopathy [58]. ECG showed a more antagonistic effect on OGD/R-induced apoptosis and autophagy than did EGCG. The effect of EGCG on I/R-induced apoptosis and autophagy was consistent to previous studies [59, 60]. We firstly evaluated the effects of ECG/EGCG on cell viability, apoptosis, and autophagy of HBMVECs after OGD/R. And our results suggested that ECG may show a more significant effect than EGCG. Considering that ECG exhibited the maximum antioxidant property [25, 26] among the polyphenols extracted from green tea, we chose ECG for our following study. The chemical structure of ECG is similar to EGCG; however, it seemed that ECG acts better than EGCG on cerebral ischemia/reperfusion injury in vitro. Ghosh et al. reported that aromatic interactions, hydrophobic interactions, the radical scavenging activity, and autoxidation of polyphenols are likely to be the major reasons for ECG being the most effective inhibitor of fibrillation [26]. To evaluate the scavenging effects of ECG and EGCG, the results of the previous study of Kondo et al. indicated that ECG can be converted to an anthocyanin-like compound after cleavage of the gallate moiety; however, EGCG can be converted to an anthocyanin-like compound followed by cleavage of the gallate moiety by oxidation [25]. Active oxygen including superoxide (O2−) would not be produced in EGCG, but can be produced in ECG [25]. The radical scavenging property of green tea polyphenols lies in the order of ECG > EGCG, which accounts for better protective activity of ECG from ischemia/reperfusion injury than EGCG [26]. Data of further studies for ECG showed that ECG has antioxidant activity that upregulation of ROS, LDH, and MDA as well as downregulation of SOD induced by OGD/R were almost reversed by treatment of ECG. Results on the investigation of ECG effect on neovascularization in vitro indicated that ECG could promote HBMVECs undergoing OGD/R migration and tube formation significantly (P < 0.01). We further measured mRNA and protein expression of vascular endothelial growth factor of VEGF, cell proliferation, and apoptosis associated Bcl-2, BAX, and Caspase 3, as well as LC3B, mTOR, and Beclin-1 associated with autophagy. It was found that mRNA and protein expression of VEGF and Bcl-2 decreased and BAX, Beclin-1, and Caspase 3 increased significantly after OGD/R; however, ECG treatment alleviated even eliminated the influence of OGD/R on HBMVECs. OGD/R also induced mRNA of LC3B and relative protein expression of LC3II to LC3I increase, all of which was downregulated by ECG. It is worth noting that the tendency of mRNA and protein expression of mTOR change was inconsistent in the OGD/R group (solvent control group) or ECG-treated group. Different regulation mechanisms as synthesis and degradation rates, acting on both the synthesized mRNA and the synthesized protein, affect the amount of the two molecules differentially, and the relation between mRNA and protein maybe not strictly linear, but has a more intrinsic and complex dependence [61, 62]. The OGD/R group induced protein expression of mTOR decrease while ECG antagonized OGD/R induction generally.
ECG is one of the major polyphenolic components of green tea only second to EGCG which was reported to have protective effects on ischemia-reperfusion injury by attracting cell proliferation, apoptosis, and autophagy in vitro and in vivo. It has been shown that a high concentration (more than 10 μM) of EGCG causes a significant decrease in the number of viable cells and induces human embryonic kidney cells (HEK293T) apoptosis and autophagy [21]; however, low concentrations (2 μM) of ECG and EGCG enhance the viability of PC12 cells undergoing OGD/R [22]. In another study, 2 μM of EGCG and ECG showed an effect against radical oxidation in aqueous media [25]. Therefore, the doses of 0.5, 1, 2, and 4 μM of ECG/EGCG for treatment were involved in our study. Our results suggested that ECG tends to play a more important role than does EGCG in OGD/R-induced HBMVECs in cell proliferation, apoptosis, and autophagy. In our study, we have also investigated the effect of ECG on neovascularization. Our results indicated that ECG could promote HBMVEC migration and tube formation in the OGD/R-induced group, and VEGF expression was upregulated in the ECG-treated OGD/R group compared with solvent control. Although ECG showed an effect on I/R injury in vitro in our study, we would evaluate the role of ECG in vivo against I/R injury in our further study. Considering that the content of ECG is less than that of EGCG and treatment of a lower dose of 0.2 μM combined with other polyphenolic components of green tea showed about equivalent effects on protection cells from I/R injury-induced cell death, which was mentioned in the previous study [22], exploring the effect of ECG combined with EGCG on I/R injury in vitro and in vivo will be involved in our further study too.
In conclusion, the results from the present study indicated that ECG could promote cell proliferation, intervene apoptosis and autophagy, and promote cell migration & tube formation and expression of VEGF to affect neovascularization in protection HBMVECs from I/R injury. ECG also showed oxidation resistance through affecting OGD/R-induced ROS, LDH, MDA, and SOD change and antagonized OGD/R induction to mRNA and protein expression of cell proliferation and apoptosis associated Bcl-2, BAX, and Caspase 3, as well as LC3B, mTOR, and Beclin-1 related to autophagy. ECG may protect HBMVECs from OGD/R and offer a promising therapeutic approach for the treatment of I/R injury.
Acknowledgments
This work was supported by Lianyungang Science and Technology Social Development Project (SH1538) and Construction of Neurology Project (SH1602).
Abbreviations
- ECG:
(-)-Epicatechin gallate
- EGCG:
Epigallocatechin-3-gallate
- OGD/R:
Oxygen-glucose deprivation/reoxygenation
- HBMVECs:
Human brain microvascular endothelial cells
- LDH:
Lactated hydrogenase
- ROS:
Reactive oxygen species
- MDA:
Malondialdehyde
- SOD:
Superoxide dismutase
- I/R:
Ischemia/reperfusion
- VEGF:
Vascular endothelial growth factor
- mTOR:
Mammalian target of rapamycin.
Data Availability
All the tables and figures used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Supplementary Materials
Western blotting after OGD/R and treatment with ECG. HBMVECs were harvested, and cell extracts were prepared. Briefly, cells were lysed in lysis buffer (20 mM Tris-pH 7.5, 150 mM NaCl, and 1% Triton X-100) followed by centrifugation for 3 min at 10,000 × g at 4°C. The supernatant was collected and transferred electrophoretically to a polyvinylidene fluoride membrane (PVDF) (Millipore, Shanghai, China). The membranes were blocked by 5% dry milk and subsequently incubated with primary antibodies against GAPDH (Abcam, UK), VEGF (Abcam, UK), Bcl-2 (Abcam, UK), BAX (CST, USA), Caspase 3 (CST, USA), mTOR (CST, USA), LC3B (Abcam, UK), and Beclin-1 (CST, USA) overnight at 4°C. The membranes were subsequently washed and incubated with secondary antibodies conjugated with horseradish peroxidase. The immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Scientific, Shanghai, China) and analyzed by automatic chemiluminescence image analysis system (Tanon, China). The results were normalized to GAPDH. Results on the effect of ECG on mRNA and protein expression of VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1 mRNA and protein expression of vascular endothelial growth factor of VEGF, cell proliferation, and apoptosis associated Bcl-2, BAX, and Caspase 3, as well as LC3B, mTOR, and Beclin-1 which is related to autophagy were measured in our study. Compared with control, mRNA expression of VEGF (Figure 4(a)) and Bcl-2 (Figure 4(c)) decreased significantly (P < 0.01), whereas BAX (Figure 4(e)), mTOR (Figure 4(g)), Beclin-1 (Figure 4(i)), Caspase 3 (Figure 4(k)), and LC3B (Figure 4(m)) mRNA expression increased significantly (P < 0.01) in the OGD/R group. ECG tends to inhibit downregulation of VEGF and Bcl2 as well as upregulation of BAX, mTOR, Beclin-1, Caspase 3, and LC3B induced by OGD/R. To investigate the effect of ECG on expression of OGD/R related proteins and the mechanism involved, we also measured protein expression of VEGF, Bcl2, BAX, mTOR, Beclin-1, cleaved Caspase 3, and LC3B. The expression of VEGF (Figure 4(b)), Bcl2 (Figure 4(d)), and mTOR (Figure 4(h)) in the OGD/R group was 0.52-, 0.38-, and 0.51-fold to that in the control group. As for BAX (Figure 4(f)), Beclin-1 (Figure 4(j)), and cleaved Caspase 3 (Figure 4(l)) protein expression, there was a 1.42-, 1.31-, and 1.34-fold expression to the control group, respectively, in the OGD/R group. The relative content of LC3II to LC3I was significantly upregulated in the OGD/R group compared with control (P < 0.01) (Figure 4(n)). ECG treatment attenuated up- or downregulation of protein expression induced by OGD/R (P < 0.05 or P < 0.01).
References
- 1.Carden D. L., Granger D. N. Pathophysiology of ischaemia-reperfusion injury. The Journal of Pathology. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Jung J. E., Kim G. S., Chen H., et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Molecular Neurobiology. 2010;41(2-3):172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P., Zhao H., Wang R., et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46(2):513–519. doi: 10.1161/STROKEAHA.114.007482. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z., Wang G., Zhai X., et al. Selective inhibition of protein kinase C β2 attenuates the adaptor P66Shc-mediated intestinal ischemia–reperfusion injury. Cell Death & Disease. 2014;5(4, article e1164) doi: 10.1038/cddis.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venardos K. M., Perkins A., Headrick J., Kaye D. M. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Current Medicinal Chemistry. 2007;14(14):1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 6.Feng D., Wang B., Wang L., et al. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. Journal of Pineal Research. 2017;62(3) doi: 10.1111/jpi.12395. [DOI] [PubMed] [Google Scholar]
- 7.Puyal J., Vaslin A., Mottier V., Clarke P. G. H. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Annals of Neurology. 2009;66(3):378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 8.Xing S., Zhang Y., Li J., et al. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy. 2012;8(1):63–76. doi: 10.4161/auto.8.1.18217. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G., Zhang W., Li L., Wu S., Du G. Pinocembrin protects the brain against ischemia-reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules. 2014;19(10):15786–15798. doi: 10.3390/molecules191015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi J. J., Yi L. Effects of integrins and integrin αvβ3 inhibitor on angiogenesis in cerebral ischemic stroke. Journal of Huazhong University of Science and Technology [Medical Sciences] 2014;34(3):299–305. doi: 10.1007/s11596-014-1274-4. [DOI] [PubMed] [Google Scholar]
- 11.Tajiri N., Lau T., Glover L. E., et al. Cerebral aneurysm as an exacerbating factor in stroke pathology and a therapeutic target for neuroprotection. Current Pharmaceutical Design. 2012;18(25):3663–3669. doi: 10.2174/138161212802002724. [DOI] [PubMed] [Google Scholar]
- 12.Ueta T., Mori H., Kunimatsu A., Yamaguchi T., Tamaki Y., Yanagi Y. Stroke and anti-VEGF therapy. Ophthalmology. 2011;118(10):2093–2093.e2. doi: 10.1016/j.ophtha.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Miyake H., Nakagawa I., Takeshima Y., et al. Post-ischemic administration of vascular endothelial growth factor inhibitor in a rat model of cerebral venous infarction. Neurologia Medico-Chirurgica (Tokyo) 2013;53(3):135–140. doi: 10.2176/nmc.53.135. [DOI] [PubMed] [Google Scholar]
- 14.Zechariah A., ElAli A., Hagemann N., et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(7):1561–1567. doi: 10.1161/ATVBAHA.112.300749. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg D. A., Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cellular and Molecular Life Sciences. 2013;70(10):1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C., Qin Y. Y., Wei X., Yu F. F., Zhou Y. H., He J. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. European Journal of Epidemiology. 2015;30(2):103–113. doi: 10.1007/s10654-014-9960-x. [DOI] [PubMed] [Google Scholar]
- 17.Larsson S. C. Coffee, tea, and cocoa and risk of stroke. Stroke. 2014;45(1):309–314. doi: 10.1161/STROKEAHA.113.003131. [DOI] [PubMed] [Google Scholar]
- 18.Golden E. B., Lam P. Y., Kardosh A., et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009;113(23):5927–5937. doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]
- 19.Chakrawarti L., Agrawal R., Dang S., Gupta S., Gabrani R. Therapeutic effects of EGCG: a patent review. Expert Opinion on Therapeutic Patents. 2016;26(8):907–916. doi: 10.1080/13543776.2016.1203419. [DOI] [PubMed] [Google Scholar]
- 20.Park J. W., Hong J. S., Lee K. S., Kim H. Y., Lee J. J., Lee S. R. Green tea polyphenol (-)-epigallocatechin gallate reduces matrix metalloproteinase-9 activity following transient focal cerebral ischemia. The Journal of Nutritional Biochemistry. 2010;21(11):1038–1044. doi: 10.1016/j.jnutbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Holczer M., Besze B., Zambo V., Csala M., Banhegyi G., Kapuy O. Epigallocatechin-3-gallate (EGCG) promotes autophagy-dependent survival via influencing the balance of mTOR-AMPK pathways upon endoplasmic reticulum stress. Oxidative Medicine and Cellular Longevity. 2018;2018:15. doi: 10.1155/2018/6721530.6721530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundimeda U., McNeill T. H., Elhiani A. A., Schiffman J. E., Hinton D. R., Gopalakrishna R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cϵ. Journal of Biological Chemistry. 2012;287(41):34694–34708. doi: 10.1074/jbc.M112.356899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K., Ishizaki Y., Kojo S., Kikuzaki H. Strong inhibition of secretory sphingomyelinase by catechins, particularly by (-)-epicatechin 3-O-gallate and (-)-3'-O-methylepigallocatechin 3-O-gallate. Journal of Nutritional Science and Vitaminology. 2016;62(2):123–129. doi: 10.3177/jnsv.62.123. [DOI] [PubMed] [Google Scholar]
- 24.Roy P., Dinda A. K., Chaudhury S., Dasgupta S. β-Cyclodextrin encapsulated polyphenols as effective antioxidants. Biopolymers. 2018;109(1) doi: 10.1002/bip.23084. [DOI] [PubMed] [Google Scholar]
- 25.Kondo K., Kurihara M., Miyata N., Suzuki T., Toyoda M. Scavenging mechanisms of (-)-epigallocatechin gallate and (-)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radical Biology & Medicine. 1999;27(7-8):855–863. doi: 10.1016/S0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh S., Pandey N. K., Dasgupta S. (-)-Epicatechin gallate prevents alkali-salt mediated fibrillogenesis of hen egg white lysozyme. International Journal of Biological Macromolecules. 2013;54:90–98. doi: 10.1016/j.ijbiomac.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Bertolini F., Casarotti G., Righi L., et al. Human renal angiomyolipoma cells of male and female origin can migrate and are influenced by microenvironmental factors. PLoS One. 2018;13(6, article e0199371) doi: 10.1371/journal.pone.0199371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh M., Lee J., Kim Y. J., Rhee W. J., Park J. H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. International Journal of Molecular Sciences. 2018;19(6) doi: 10.3390/ijms19061715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Zhu C., An B., et al. Indirubin inhibits cell proliferation, migration, invasion and angiogenesis in tumor-derived endothelial cells. OncoTargets and Therapy. 2018;11:2937–2944. doi: 10.2147/OTT.S157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor A. C., Seltz L. M., Yates P. A., Peirce S. M. Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina. Microvascular Research. 2010;79(2):93–101. doi: 10.1016/j.mvr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalaf A. A., Moselhy W. A., Abdel-Hamed M. I. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology. 2012;33(3):280–289. doi: 10.1016/j.neuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Flôres M. F., Martins A., Schimidt H. L., et al. Effects of green tea and physical exercise on memory impairments associated with aging. Neurochemistry International. 2014;78:53–60. doi: 10.1016/j.neuint.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Li H., Wu X., Wu Q., et al. Green tea polyphenols protect against okadaic acid-induced acute learning and memory impairments in rats. Nutrition. 2014;30(3):337–342. doi: 10.1016/j.nut.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Huang J., Wang Y., Xie Z., Zhou Y., Zhang Y., Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. European Journal of Clinical Nutrition. 2014;68(10):1075–1087. doi: 10.1038/ejcn.2014.143. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Zhang W., Sun L., et al. Green tea drinking and risk of pancreatic cancer: a large-scale, population-based case-control study in urban Shanghai. Cancer Epidemiology. 2012;36(6):e354–e358. doi: 10.1016/j.canep.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X. J., Zeng X. T., Duan X. L., Zeng H. C., Shen R., Zhou P. Association between green tea and colorectal cancer risk: a meta-analysis of 13 case-control studies. Asian Pacific Journal of Cancer Prevention. 2012;13(7):3123–3127. doi: 10.7314/APJCP.2012.13.7.3123. [DOI] [PubMed] [Google Scholar]
- 37.Mansour-Ghanaei F., Hadi A., Pourmasoumi M., Joukar F., Golpour S., Najafgholizadeh A. Green tea as a safe alternative approach for nonalcoholic fatty liver treatment: a systematic review and meta-analysis of clinical trials. Phytotherapy Research. 2018;32(10):1876–1884. doi: 10.1002/ptr.6130. [DOI] [PubMed] [Google Scholar]
- 38.Xi J., Ge S., Zuo L., Zhu Y., Wang L., Xie Q. Protective role of green tea polyphenols in intestinal mucosal barrier function of mice with colitis induced by TNBS through inhibiting JAK2/STAT3 pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi= Chinese Journal of Cellular and Molecular Immunology. 2018;34(3):237–241. [PubMed] [Google Scholar]
- 39.Schimidt H. L., Vieira A., Altermann C., et al. Memory deficits and oxidative stress in cerebral ischemia-reperfusion: neuroprotective role of physical exercise and green tea supplementation. Neurobiology of Learning and Memory. 2014;114:242–250. doi: 10.1016/j.nlm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Akinrinmade O., Omoruyi S., Dietrich D., Ekpo O. Long-term consumption of fermented rooibos herbal tea offers neuroprotection against ischemic brain injury in rats. Acta Neurobiologiae Experimentalis. 2017;77(1):94–105. doi: 10.21307/ane-2017-040. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty M., Kamath J. V. Pharmacodynamic interaction of green tea extract with hydrochlorothiazide against ischemia-reperfusion injury-induced myocardial infarction. Journal of Advanced Pharmaceutical Technology & Research. 2014;5(3):134–139. doi: 10.4103/2231-4040.137428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liou Y. M., Hsieh S. R., Wu T. J., Chen J. Y. Green tea extract given before regional myocardial ischemia-reperfusion in rats improves myocardial contractility by attenuating calcium overload. Pflügers Archiv - European Journal of Physiology. 2010;460(6):1003–1014. doi: 10.1007/s00424-010-0881-6. [DOI] [PubMed] [Google Scholar]
- 43.Lv J., Feng M., Zhang L., et al. Protective effect of epigallocatechin gallate, a major constituent of green tea, against renal ischemia-reperfusion injury in rats. International Urology and Nephrology. 2015;47(8):1429–1435. doi: 10.1007/s11255-015-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang R., Nickkholgh A., Kern M., et al. Green tea extract ameliorates reperfusion injury to rat livers after warm ischemia in a dose-dependent manner. Molecular Nutrition & Food Research. 2011;55(6):855–863. doi: 10.1002/mnfr.201000643. [DOI] [PubMed] [Google Scholar]
- 45.Tao J., Shen X., Ai Y., Han X. Tea polyphenols protect against ischemia/reperfusion-induced liver injury in mice through anti-oxidative and anti-apoptotic properties. Experimental and Therapeutic Medicine. 2016;12(5):3433–3439. doi: 10.3892/etm.2016.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdeen S. M., Mathew T. C., Dashti H. M., Asfar S. Protective effects of green tea on intestinal ischemia-reperfusion injury. Nutrition. 2011;27(5):598–603. doi: 10.1016/j.nut.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Y. C., Sheen J. M., Hu W. L., Hung Y. C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxidative Medicine and Cellular Longevity. 2017;2017:16. doi: 10.1155/2017/8526438.8526438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng A., Yan H., Han C., Wang W., Tian Y., Chen X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. International Journal of Biological Macromolecules. 2014;69:382–387. doi: 10.1016/j.ijbiomac.2014.05.071. [DOI] [PubMed] [Google Scholar]
- 49.El-Missiry M. A., Othman A. I., El-Sawy M. R., Lebede M. F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. International Journal of Radiation Biology. 2018;94(9):798–808. doi: 10.1080/09553002.2018.1492755. [DOI] [PubMed] [Google Scholar]
- 50.Li F., Liang J., Tang D. Brahma-related gene 1 ameliorates the neuronal apoptosis and oxidative stress induced by oxygen-glucose deprivation/reoxygenation through activation of Nrf2/HO-1 signaling. Biomedicine & Pharmacotherapy. 2018;108:1216–1224. doi: 10.1016/j.biopha.2018.09.144. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y., Zhang Y., Tang J., et al. Oxymatrine inhibits homocysteine-mediated autophagy via MIF/mTOR signaling in human umbilical vein endothelial cells. Cellular Physiology and Biochemistry. 2018;45(5):1893–1903. doi: 10.1159/000487912. [DOI] [PubMed] [Google Scholar]
- 52.Yang X., Zheng T., Hong H., et al. Neuroprotective effects of Ginkgo biloba extract and ginkgolide B against oxygen-glucose deprivation/reoxygenation and glucose injury in a new in vitro multicellular network model. Frontiers in Medicine. 2018;12(3):307–318. doi: 10.1007/s11684-017-0547-2. [DOI] [PubMed] [Google Scholar]
- 53.Liao L. X., Zhao M. B., Dong X., Jiang Y., Zeng K. W., Tu P. F. TDB protects vascular endothelial cells against oxygen-glucose deprivation/reperfusion-induced injury by targeting miR-34a to increase Bcl-2 expression. Scientific Reports. 2016;6(1, article 37959) doi: 10.1038/srep37959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun H., Zhong D., Wang C., Sun Y., Zhao J., Li G. MiR-298 exacerbates ischemia/reperfusion injury following ischemic stroke by targeting Act1. Cellular Physiology and Biochemistry. 2018;48(2):528–539. doi: 10.1159/000491810. [DOI] [PubMed] [Google Scholar]
- 55.Li H., Gao A., Feng D., et al. Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Translational Stroke Research. 2014;5(5):618–626. doi: 10.1007/s12975-014-0354-x. [DOI] [PubMed] [Google Scholar]
- 56.Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. The Journal of Clinical Investigation. 2015;125(1):1–4. doi: 10.1172/JCI78652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheng R., Qin Z. H. The divergent roles of autophagy in ischemia and preconditioning. Acta Pharmacologica Sinica. 2015;36(4):411–420. doi: 10.1038/aps.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen N., Zhang R., Zhang H. R., et al. Inhibition of retinal angiogenesis by gold nanoparticles via inducing autophagy. International Journal of Ophthalmology. 2018;11(8):1269–1276. doi: 10.18240/ijo.2018.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang T., Yang D., Fan Y., Xie P., Li H. Epigallocatechin-3-gallate enhances ischemia/reperfusion-induced apoptosis in human umbilical vein endothelial cells via AKT and MAPK pathways. Apoptosis. 2009;14(10):1245–1254. doi: 10.1007/s10495-009-0391-1. [DOI] [PubMed] [Google Scholar]
- 60.Xuan F., Jian J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. International Journal of Molecular Medicine. 2016;38(1):328–336. doi: 10.3892/ijmm.2016.2615. [DOI] [PubMed] [Google Scholar]
- 61.de Sousa Abreu R., Penalva L. O., Marcotte E. M., Vogel C. Global signatures of protein and mRNA expression levels. Molecular BioSystems. 2009;5(12):1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui K., Yang Z., Darwish H., et al. Molecular cloning and characterization of the β-catenin gene from fine-wool sheep. Gene. 2014;546(2):277–282. doi: 10.1016/j.gene.2014.05.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blotting after OGD/R and treatment with ECG. HBMVECs were harvested, and cell extracts were prepared. Briefly, cells were lysed in lysis buffer (20 mM Tris-pH 7.5, 150 mM NaCl, and 1% Triton X-100) followed by centrifugation for 3 min at 10,000 × g at 4°C. The supernatant was collected and transferred electrophoretically to a polyvinylidene fluoride membrane (PVDF) (Millipore, Shanghai, China). The membranes were blocked by 5% dry milk and subsequently incubated with primary antibodies against GAPDH (Abcam, UK), VEGF (Abcam, UK), Bcl-2 (Abcam, UK), BAX (CST, USA), Caspase 3 (CST, USA), mTOR (CST, USA), LC3B (Abcam, UK), and Beclin-1 (CST, USA) overnight at 4°C. The membranes were subsequently washed and incubated with secondary antibodies conjugated with horseradish peroxidase. The immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Scientific, Shanghai, China) and analyzed by automatic chemiluminescence image analysis system (Tanon, China). The results were normalized to GAPDH. Results on the effect of ECG on mRNA and protein expression of VEGF, Bcl-2, BAX, LC3B, Caspase 3, mTOR, and Beclin-1 mRNA and protein expression of vascular endothelial growth factor of VEGF, cell proliferation, and apoptosis associated Bcl-2, BAX, and Caspase 3, as well as LC3B, mTOR, and Beclin-1 which is related to autophagy were measured in our study. Compared with control, mRNA expression of VEGF (Figure 4(a)) and Bcl-2 (Figure 4(c)) decreased significantly (P < 0.01), whereas BAX (Figure 4(e)), mTOR (Figure 4(g)), Beclin-1 (Figure 4(i)), Caspase 3 (Figure 4(k)), and LC3B (Figure 4(m)) mRNA expression increased significantly (P < 0.01) in the OGD/R group. ECG tends to inhibit downregulation of VEGF and Bcl2 as well as upregulation of BAX, mTOR, Beclin-1, Caspase 3, and LC3B induced by OGD/R. To investigate the effect of ECG on expression of OGD/R related proteins and the mechanism involved, we also measured protein expression of VEGF, Bcl2, BAX, mTOR, Beclin-1, cleaved Caspase 3, and LC3B. The expression of VEGF (Figure 4(b)), Bcl2 (Figure 4(d)), and mTOR (Figure 4(h)) in the OGD/R group was 0.52-, 0.38-, and 0.51-fold to that in the control group. As for BAX (Figure 4(f)), Beclin-1 (Figure 4(j)), and cleaved Caspase 3 (Figure 4(l)) protein expression, there was a 1.42-, 1.31-, and 1.34-fold expression to the control group, respectively, in the OGD/R group. The relative content of LC3II to LC3I was significantly upregulated in the OGD/R group compared with control (P < 0.01) (Figure 4(n)). ECG treatment attenuated up- or downregulation of protein expression induced by OGD/R (P < 0.05 or P < 0.01).
Data Availability Statement
All the tables and figures used to support the findings of this study are included within the article.