Abstract
Patients with myotonia congenita suffer from muscle stiffness caused by muscle hyperexcitability. Although loss-of-function mutations in the ClC-1 muscle chloride channel have been known for 25 years to cause myotonia congenita, this discovery has led to little progress on development of therapy. Currently, treatment is primarily focused on reducing hyperexcitability by blocking Na+ current. However, other approaches such as increasing K+ currents might also be effective. For example, the K+ channel activator retigabine, which opens KCNQ channels, is effective in treating epilepsy because it causes hyperpolarization of the resting membrane potential in neurons. In this study, we found that retigabine greatly reduced the duration of myotonia in vitro. Detailed study of its mechanism of action revealed that retigabine had no effect on any of the traditional measures of muscle excitability such as resting potential, input resistance or the properties of single action potentials. Instead it appears to shorten myotonia by activating K+ current during trains of action potentials. Retigabine also greatly reduced the severity of myotonia in vivo, which was measured using a muscle force transducer. Despite its efficacy in vivo, retigabine did not improve motor performance of mice with myotonia congenita. There are a number of potential explanations for the lack of motor improvement in vivo including central nervous system side effects. Nonetheless, the striking effectiveness of retigabine on muscle itself suggests that activating potassium currents is an effective method to treat disorders of muscle hyperexcitability.
Keywords: myotonia, muscle, excitability, action potential, potassium channel, Kv7, retigabine, persistent inward current, sodium channel, muscle contraction
Introduction
Myotonia congenita is a muscle disease caused by loss-of-function mutations affecting the muscle chloride channel (ClC-1) (Koch, et al., 1992; Lipicky, et al., 1971; Steinmeyer, et al., 1991). Consequently, patients experience muscle stiffness due to hyperexcitability (Cannon, 2015; Lehmann-Horn, et al., 2008; Trivedi, et al., 2014). Despite the discovery that mutation of the ClCn1 gene causes muscle hyperexcitability in myotonia congenita, there has been little progress in therapy. The primary approach remains block of Na+ channels using drugs such as mexiletine, (Cannon, 2015; Lehmann-Horn, et al., 2008; Trivedi, et al., 2014). However, many patients suffer side effects or incomplete symptom resolution such that there is a need for improved therapy.
We recently discovered that a small, subthreshold Na+ current that lacks fast inactivation (Na persistent inward current, NaPIC), plays a central role in triggering myotonia (Hawash, et al., 2017). A theoretically attractive way to combat the depolarization triggered by activation of NaPIC is to increase resting membrane conductance. As NaPIC is small, a modest increase in resting conductance could be effective in lessening the depolarization triggered by its activation.
Normally 70%-80% of resting conductance is mediated by Cl− channels with the rest due to K+ channels (Palade and Barchi, 1977; Pedersen, et al., 2016). Both types of channels oppose depolarization such that they keep the membrane potential hyperpolarized (Hodgkin and Horowicz, 1957; Hodgkin and Horowicz, 1959). While Cl− conductance is reduced in myotonia congenita, K+ channels are unaffected such that opening K+ channels could partially normalize resting membrane conductance and hyperpolarize membrane potential. Consistent with this possibility, K-ATP channel openers have been shown to promote hyperpolarization and recovery from periodic paralysis, in vitro (Grafe, et al., 1990).
Retigabine opens KCNQ (Kv7) channels, which in neurons hyperpolarizes the resting membrane potential and decreases input resistance (Gunthorpe, et al., 2012; Yue and Yaari, 2004). These changes inhibit high frequency firing of action potentials (as occurs in epilepsy) by increasing hyperpolarization between action potentials without affecting single action potentials or firing at low frequencies (Gunthorpe, et al., 2012; Yue and Yaari, 2004). Kv7 channels are present in skeletal muscle (Iannotti, et al., 2010) such that treatment with retigabine would be expected to reduce muscle excitability. Consistent with this, a previous study found that retigabine reduced myotonia in a pharmacologic model of myotonia congenita (Su, et al., 2012). We examined the mechanism of retigabine’s efficacy against myotonia, its effect on in vivo myotonic muscle contraction, and its potential to treat myotonia congenita.
Materials and Methods
Animals
All animal procedures were performed in accordance with the policies of the Animal Care and Use Committee of Wright State University. Mice were bred from a colony of ClCn1adr-mto2J mice (a mouse model of myotonia congenita, ClCadr mice) that was established using mice purchased from Jackson Labs. Mice were genotyped by performing PCR on tail DNA using GGT GGG GGA AAG GGG TAC AG and CTG CCT AGC CTC ACA TCT CCT TAC as primers. The amplified DNA was sent for sequencing using GGT GGG GGA AAG GGG TAC AG as the primer. Mice with myotonia are homozygous for a deletion of CCCCCT to CCCCT, which causes a frame shift. We previously had identified mice with myotonia by inability to right themselves (Novak, et al., 2015). Using genotyping, we confirmed our ability to correctly identify 100% affected mice by phenotype. Most experimental mice were selected by phenotype without genetic confirmation. For some in vitro experiments, myotonia was induced by treatment of muscle from unaffected littermates (both heterozygous and wild type mice) with 100 μM 9-Anthracene-carboxylic acid (9AC, Sigma-Aldrich, catalogue # A-4678) to block Cl− channels. Both male and female mice were used from 2 months to 6 months of age. Symptomatic mice were supplied with moistened chow paste (Irradiated Rodent Diet; Harlan Teklad 2918) on the floor of the cage.
Electrophysiology
Recordings were performed as previously described (Hawash, et al., 2017; Novak, et al., 2015). Briefly, prior to removal of extensor digitorum longus (EDL) muscle for recording, mice were killed by CO2 inhalation followed by cervical dislocation. Muscles were maintained and recorded from at 21-23 °C. The recording chamber was continually perfused with Ringer solution containing (in mM): 118 NaCl, 3.5 KCl, 1.5 CaCl2, 0.7 MgSO4, 26.2 NaHCO3, 1.7 NaH2PO4, 5.5 glucose, and maintained at pH 7.3-7.4 by aeration with 95% O2 and 5% CO2. To prevent contraction, muscles were loaded with 50μM BTS (N-benzyl-p-toluenesulfonamide, Tokyo Chemical Industry, Tokyo, Japan, catalogue #B3082) dissolved in DMSO for 45 minutes prior to recording.
Muscle was stained with 10 μM 4-(4-diethylaminostyrl)-N-methylpyridinium iodide (Molecular Probes, catalogue #D289 (discontinued)) for 3 minutes prior to recording and fibers were impaled with 2 sharp microelectrodes filled with 3 M KCl solution containing 1 mM sulforhodamine 101 (Sigma-Aldrich, Catalogue # S7635) to visualize the electrodes with epifluorescence. Electrode resistance was 15 to 25 MΩ. Retigabine was purchased from Cayman Chemicals (catalogue # 21449) and dissolved in DMSO. Total DMSO concentration was kept below 0.1%.
Capacitance compensation was optimized prior to recording. Fibers with resting potentials more depolarized than −74 mV were excluded from analysis. Action potential voltage threshold was defined as the voltage at which the dV/dt was 10 mV/ms. Input resistance was calculated by injecting hyperpolarizing current for 200 ms and measuring the steady state hyperpolarization. The amount of current injected was adjusted to keep the hyperpolarization under 15 mV. Altering the amplitude of current injection yielded the same value for input resistance when the hyperdepolarization was kept under 15 mV (data not shown). The time constant was measured as the time taken for the voltage to reach 63% of the steady state value. The mean slope of the afterdepolarization (AfD) was calculated as follows: the voltage difference between action potential threshold and the most negative potential reached during repolarization following each action potential was divided by the time from maximum repolarization to action potential threshold.
In vivo force recording
We employed an in vivo muscle force preparation that we used previously to study weakness (Nardelli, et al., 2016). Mice were anesthetized via isoflurane inhalation; then the distal tendon of the calf muscle (medial and lateral gastrocnemius, and soleus muscles) was surgically detached and attached to a force transduction motor. The common peroneal, sural and posterior tibial nerves were crushed. The sciatic nerve was then stimulated while isometric muscle force generation was measured. Muscle temperature was monitored with a laser probe and maintained between 29°C and 31°C with a heat lamp. The muscle was kept moist by applying mineral oil. Force recordings were performed before and 30 minutes following injection of 30 mg/kg retigabine. For injection, retigabine was dissolved in 20 ul of DMSO. This was added to 180 μl of sterile water, which caused the retigabine to precipitate. The solution with precipitate was then sonicated for 30s, which broke the precipitate into small pieces, and the solution was injected ip using a 27 gauge needle.
Behavioral Measurements
Nine ClCadr mice were treated with either retigabine or vehicle and motor performance was measured 30 min after ip injection, with the examiner blind to the treatment administered. Thirty minutes was chosen as it was the time of greatest efficacy of retigabine against myotonia in studies in which muscle force was measured. Motor function was analyzed using behavioral measures we have used previously to study the effect of therapy on severity of myotonia (Novak, et al., 2015). These included the mean time of three trials of the righting reflex and the mean of three trials on a modified rotarod test in which mice were placed on a stationary rod; then the rod was turned on to a single, intermediate speed. This modification was done to prevent warm up, which can occur as the speed of the rod is gradually increased. The averaged post-treatment righting times and the averaged post-treatment time on the rotarod were compared between vehicle and retigabine. As the same mice were used for all behavioral experiments, at least 2 days were given between studies to allow time for drug elimination. The plasma half-life for retigabine in mice is 7 hours (Zhou, et al., 2015) so 2 days represents more than 6 half-lives.
Statistical analysis.
For most experiments, a repeated measures ANOVA was run with either dose as a repeated factor or the mouse as a repeated factor. For the retigabine dose experiments, a random effect was included to account for the correlated measurements that can result from multiple comparisons with the control value. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for all ANOVA analyses. The “simulate” post hoc multiple comparison procedure was used for all post hoc multiple comparison. Origin software (OriginLab, Northhampton, MA) was used to perform paired t-tests for analysis of in vivo before and after retigabine measures of muscle force from the same mouse. All data are presented as means ± SEM. p < 0.05 was considered to be significant. Sample sizes were chosen based on the lab’s previous experiences in the calculation of experimental variability. The numbers of mice and fibers used are described in the corresponding figure legends and text. For statistical analysis n was the number of mice.
Results
The mechanism underlying efficacy of retigabine against myotonia
Experiments were performed on mice with homozygous null mutation for the ClCn1 gene (ClCadr mice, adr stands for arrested development of righting response) (Hawash, et al., 2017; Novak, et al., 2015). A second model of myotonia was obtained by exposing muscle from unaffected littermates to 100 μM 9-Anthracene-carboxylic acid (9AC) to acutely block ClC-1 chloride channels, which is termed 9AC-induced myotonia (Palade and Barchi, 1977). Intracellular recordings were performed on single muscle fibers as previously described (Hawash, et al., 2017; Novak, et al., 2015). Following treatment with 9AC, stimulation of fibers by a 200 ms injection of current triggered myotonia in 100% of fibers (Fig 1). Muscles were treated with 5, 10 and 20 μM retigabine and the duration of myotonia following the 200 ms pulse was measured. While retigabine did not eliminate myotonia in any 9AC treated fibers, it reduced the duration of myotonia (Fig 1A,B). In ClCadr muscle, treatment with 20 μM retigabine eliminated myotonia in 16/30 fibers from four mice (Fig1C). The duration of myotonia in fibers in which there still was myotonia was shortened from 6.7 ± 1.0 s to 0.3 ± 0.1 s (Fig 2C, p < 0.01).
Fig 1.
Dose dependent reduction of the severity of myotonia by retigabine. A) Shown are three representative traces of myotonia in 9AC treated muscle following exposure to 0, 10, and 20 μM retigabine. B) Plot of the mean duration of myotonia following various doses of retigabine in 9AC treated muscle. ** = p < .01. n = 29 fibers from 5 mice for 0 μM retigabine, 20 fibers from 3 mice for 5 μM, 27 fibers from 3 mice for 10 μM and 22 fibers from 3 mice for 20 μM. C) Shown are traces from ClCadr muscle fibers in the absence and presence of 20 μM retigabine. No myotonia is present following treatment with 20 μM retigabine.
To determine the mechanism of action underlying the efficacy of retigabine against myotonia we measured passive membrane properties (resting membrane potential, membrane time constant and input resistance) by injecting a square hyperpolarizing current pulse into muscle fibers in the presence of 0, 5, 10, and 20 μM retigabine (Fig 2A and Table 1). While the input resistance trended higher in ClCadr muscle than in 9AC treated muscle, time constants were identical. These data suggest muscle fibers from ClCadr mice are smaller than in unaffected littermates. In both 9AC treated muscle and ClCadr muscle there were no changes in passive properties to explain the effects of retigabine (Table 1). In neurons, it has been shown that retigabine caused a hyperpolarization of the resting membrane potential and a decrease input resistance, indicating that retigabine had activated a K+ current (Brown and Passmore, 2009; Otto, et al., 2002). One reason for this difference from neurons may be the negative membrane potential of skeletal muscle, which we have found to be −80 to −85 mV (Novak, et al., 2015; Rich, et al., 1998). Activation of Kv7 channels by retigabine is voltage dependent such that the channels are inactive at the resting membrane potential of skeletal muscle but active at the more depolarized resting potential of neurons (−60 to −70 mV) (Gunthorpe, et al., 2012; Tatulian, et al., 2001; Wickenden, et al., 2000).
Fig 2.
Examples of traces used to measure passive properties and single action potentials. A) Representative trace used to measure input resistance and time constant. B) Properties of action potentials that were measured.
Table 1.
Electrical properties following treatment with retigabine.
| Dose (μM) |
Resting potential (mV) |
Input resistance (MΩ) |
Membrane time constant (ms) |
Action potential threshold (mV) |
Action potential peak (mV) |
Max depol dV/dt (mV/ms) |
Max repol dV/dt (mV/ms) |
|---|---|---|---|---|---|---|---|
| 9AC | |||||||
| 0 | −85.1 ± 0.5 | 0.58 ± 0.02 | 7.0 ± 0.2 | −66.7 ± 0.7 | 26.9 ± 0.8 | 216 ± 6 | −83 ± 3 |
| 5 | −83.9 ± 0.9 | 0.64 ± 0.02 | 8.1 ± 0.3 | −67.5 ± 1.0 | 22.7 ± 0.8* | 200 ± 2* | −71 ± 3* |
| 10 | −84.8 ± 0.5 | 0.55 ± 0.02 | 7.2 ± 0.3 | −68.0 ± 0.7 | 27.3 ± 0.9 | 212 ± 4 | −70 ± 2* |
| 20 | −83.4 ± 0.4 | 0.63 ± 0.02 | 7.9 ± 0.6 | −66.9 ± 0.7 | 27.4 ± 0.9 | 217 ± 5 | −80 ± 3 |
| ClCadr | |||||||
| 0 | −83.7 ± 0.6 | 0.83 ± 0.08 | 7.0 ± 0.6 | −63.3 ± 1.3 | 32.9 ± 2.3 | 227 ± 12 | −100 ± 7 |
| 20 | −85.2 ± 0.3 | 0.96 ± 0.08 | 7.7 ± 0.8 | −63.5 ± 1.1 | 34.6 ± 1.0 | 239 ± 5 | −99 ± 5 |
The top four rows present data from 9AC treated muscle. The bottom two rows present data from ClCadr muscle. Max depol dV/dt = the maximum rate of rise of the upstroke of the action potential. Max dV/dt down = the maximum rate of repolarization of the action potential. The standard error of the mean is given for each value. For 9AC treated muscle, n = 29 fibers from 5 mice for 0 uM retigabine, 20 fibers from 3 mice for 5 uM, 27 fibers from 3 mice for 10 uM and 22 fibers from 3 mice for 20 uM. For ClCadr muscle, n = 25 fibers from 4 mice for 0 uM retigabine and 30 fibers from 4 mice for 20 μM.
= P < .05 vs 0 μM retigabine. Data from ClCadr muscle were not compared to 9AC-treated muscle and vice-versa.
We next measured the properties of single action potentials in the presence and absence of retigabine and found no consistent statistically significant differences (Table 1). Since the data from single action potentials did not easily explain the anti-myotonic effects of retigabine, we examined properties of action potentials during myotonia. In 9AC treated muscle, properties of the first myotonic action potential differed significantly from properties of single action potentials (Table 1 vs Table 2). Threshold was 20 mV depolarized relative to threshold of single action potentials (p < .01). The depolarized threshold was not due to damage of the fibers during the repetitive firing that triggered myotonia, as only fibers in which the resting potential returned to baseline following myotonia were analyzed. Instead, depolarization of threshold appeared to be due to inactivation of Na+ channels, as both the maximum rate of action potential depolarization and peak amplitude were significantly reduced (p < .01 for both comparisons vs baseline). The reason for the increase in Na+ channel inactivation was that the maximum repolarization before subsequent action potentials during myotonia was depolarized relative to the resting membrane potential (Fig 3, Table 2).
Table 2.
Properties of the first myotonic action potential and AfD with and without retigabine
| Dose (μM) |
Action potential threshold (mV) |
Max depol dV/dt (mV/ms) |
Action potential peak (mV) |
Max repol dV/dt (mV/ms) |
Max repolarization (mV) |
AfD slope (mV/s) |
|---|---|---|---|---|---|---|
| 9AC | ||||||
| 0 | −45.1 ± 1.0 | 87 ± 8 | 10.9 ± 1.5 | 37 ± 3 | −55.0 ± 1.1 | 1154 ± 65 |
| 20 | −47.4 ± 1.0 | 93 ± 7 | 14.5 ± 1.5 | 42 ± 2 | −59.9 ± 0.8 | 507 ± 47* |
| ClCadr | ||||||
| 0 | −52.3 ± 1.1 | 157 ± 5 | 23.3 ± 1.5 | 66 ± 4 | −64.2 ± 0.7 | 811 ± 79 |
| 20 | −52.6 ± 1.4 | 182 ± 7 | 28.3 ± 0.8 | 77 ± 3 | −69.9 ± 1.6 | 451 ± 43* |
The top two rows present data from 9AC treated muscle. The bottom two rows present data from ClCadr mice. The standard error of the mean is given for each value. For 9AC treated muscle, n = 29 fibers from 5 mice for 0 μM retigabine, and 22 fibers from 3 mice for 20 μM. For ClCadr muscle, n = 25 fibers from 4 mice for 0 μM retigabine and 14 fibers from 4 mice for 20 μM retigabine. Max depol dV/dt = the maximum rate of rise of the upstroke of the action potential. Max repol dV/dt = the maximum rate of repolarization of the action potential. AfD = afterdepolarization.
indicates p < 0.05 versus 0 μM retigabine. Data from ClCadr muscle were not compared to 9AC treated muscle and vice-versa.
Fig 3.
Retigabine lessens myotonia by reducing the afterdepolarization (AfD). Shown are two traces of myotonia from 9AC treated muscle. The top record is from a fiber in the absence of retigabine. The myotonia lasted for several seconds and continued beyond the time frame shown. The bottom record is from a fiber following treatment with 20 uM retigabine. The AfD peak fell below action potential threshold after only 3 myotonic action potentials. In the inset are shown the first two action potentials from myotonia in the absence and presence of 20 μM retigabine. The slope of the AfD is more than twice as steep in the absence of retigabine. AfD = after depolarization.
In muscle from ClCadr mice, differences between properties of the first myotonic action potential and single action potentials were less dramatic (Table 1 vs Table 2). One contributor to the reduction in difference was that the maximum repolarization between action potentials was greater than in 9AC treated muscle (p < .05). The greater repolarization allows for greater recovery of Na+ channels from inactivation such that action potential threshold, rate of rise and peak are all closer to values at baseline. These data suggest there is upregulation of K+ channel expression in ClCadr muscle.
Treatment with retigabine did not significantly alter any property of the first myotonic action potential in either 9AC-treated or ClCadr muscle, as the trends towards faster and greater repolarization following action potentials did not reach significance (Table 2). These data together with studies of single action potentials and passive membrane properties suggest that the mechanism underlying retigabine’s efficacy against myotonia is not detected by any of the normal measures used to assess excitability.
We previously found that efficacy of two treatments that eliminated myotonia correlated with reduction in the afterdepolarization (AfD), a slow depolarization of the membrane potential occurring over the 10 to 100 ms prior to each myotonic action potential (Hawash, et al., 2017). The AfD is mediated, in part, by activation of a Na persistent inward current (NaPIC) that lacks fast inactivation (Hawash, et al., 2017). If the AfD is large enough to reach action potential threshold, another action potential is triggered and myotonia continues. Retigabine might shorten runs of myotonia by activating Kv7 during myotonia to oppose the depolarizing effects of NaPIC. To test this hypothesis, we measured the initial slope of the AfD of myotonic action potentials in the presence and absence of 20 μM retigabine. The slope of the AfD between the first two myotonia action potentials was reduced by close 50% in both 9AC treated and ClCadr muscle (Fig 3, Table 2).
The finding that the AfD slope is less in fibers treated with retigabine suggests that the peak of the AfD more rapidly falls below action potential threshold such that myotonia stops earlier. To explore this possibility, we measured the peak of the AfD after the brief runs of myotonia occurring following treatment with 20 μM retigabine and compared it to myotonic action potential threshold. The peak of the AfD was significantly more negative than action potential threshold during myotonia (Fig 3, −60.5 ± 0.8 mV vs −47.4 ± 1.0, p < .01, n = 22 fibers from 3 9AC treated muscles). These data suggest opening of Kv7 channels by retigabine prevents myotonia by preventing the AfD from driving the fiber to threshold.
Retigabine is effective against myotonia in vivo, but does not improve motor function
The efficacy of retigabine against myotonia in vitro encouraged us to test its efficacy against myotonia in vivo. To measure severity of myotonia in vivo, we used a muscle force preparation that we used previously in rats (Nardelli, et al., 2016). Mice were anesthetized via isoflurane inhalation and the distal tendon of the gastrocnemius and soleus muscles was dissected free and attached to a force transduction motor. The sciatic nerve was stimulated repetitively at 60 Hz for 2 s. In wild type muscle this resulted in full fusion of force during stimulation that was followed by immediate, and complete relaxation of force following termination of stimulation (Fig 4A).
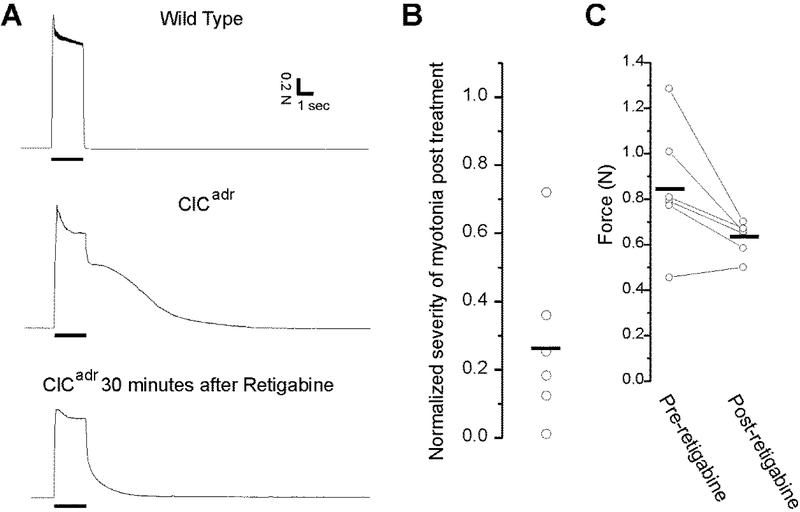
Fig 4.
Retigabine is effective in treating myotonia in vivo. A) Representative muscle force traces from a control and a ClCadr mouse before and after treatment with retigabine. Top trace: the response of muscle from a wild type mouse to 2 s of 60 Hz stimulation of the sciatic nerve. Force is fully fused and there is immediate, complete relaxation following termination of nerve stimulation. Some fatigue is present during the 2s of stimulation. Middle trace: in a ClCadr mouse, force generation during 60 Hz stimulation is normal, but relaxation following termination of stimulation is only partial and there is continued contraction secondary to myotonia for more than 5s. Bottom trace: force generation from the same myotonic gastrocnemius muscle shown in the middle trace 30 minutes after intraperitoneal injection of 30 mg/kg of retigabine. While myotonia is still present, it is reduced by close to 80%. B) A scatter plot of the integral of the post-stimulus muscle force*time relative to the normalized pre-treatment integral of a single twitch. Severity of myotonia was reduced by close to 75% following injection of retigabine (p < .01). The horizontal bar represents the mean. C) Scatter plot of peak muscle force in the 6 muscles recorded from before and 30 minutes after injection of retigabine. The horizontal bars represent the means before and after treatment with retigabine (p = 0.15).
Muscle contraction during 60 Hz stimulation in ClCadr mice appeared normal, but relaxation was greatly slowed due to myotonia (Fig 4A). Previous studies found negative effects on motor performance of retigabine at doses of 30 mg/kg or more (Hayashi, et al., 2014; Zagorchev, et al., 2016). We thus began by administering 20 mg/kg ip. However, this dose of retigabine only modestly reduced the severity of myotonia in the two mice studied (data not shown). We thus increased the dose to 30 mg/kg. At this dose, treatment with retigabine dramatically reduced the severity of myotonia (Fig 4A). Both amplitude and duration have been previously used to estimate severity of myotonia (van Lunteren, et al., 2011). To combine these into a single value we took the integral of force with respect to time. It has been reported that ex vivo treatment with retigabine can causes weakness (Zagorchev, et al., 2016). We did not want to mistakenly conclude that myotonia was reduced due to weakness caused by retigabine. To address this issue, the integral was normalized to the force generated by a single stimulus before and after administration of retigabine. Using this normalized measure of myotonia, the severity of myotonia was reduced by close to 75% following treatment with retigabine (Fig 4B, p < .01 vs pre-treatment).
To determine whether the dose of retigabine effective against myotonia caused weakness, we measured peak force generated during 60 Hz stimulation before and after treatment with retigabine in 6 ClCadr mice. While there was a trend towards lower force generation, it was not significant (Fig 4C).
The efficacy of retigabine against myotonia in vivo suggested it had the potential to improve motor function. Motor function of myotonic mice was measured using the time of righting and a modified rotarod test similar to one we used previously (Novak, et al., 2015). In the modified rotarod test a single speed rather than a gradually increasing speed was used so as not to allow time for warm-up. Blinded, vehicle-control studies of motor performance in nine ClCadr mice were performed on six different days. There was an increase in the time of righting suggesting worse motor function following treatment with retigabine (Fig 5). No significant difference was present in rotarod performance (Fig 5).
Fig 5:
Lack of motor improvement following treatment with retigabine. Shown are scatter plots of the mean time of righting and mean time on the rotarod for 9 ClCadr mice treated with either vehicle or retigabine. Each point represents the mean of 3 different treatment trials on different days. Error bars are not shown for clarity. The horizontal bars represent the means before and after treatment with retigabine. There was worsening of the time of righting following treatment with retigabine (p = 0.03). There was no difference in the time on the rotarod.
Discussion
The mechanism underlying efficacy of retigabine in treating myotonia
The goal of therapy in patients with myotonia is to reduce excitability to prevent muscle stiffness. Currently, the mechanism of action of drugs used to treat myotonia congenita (ie mexiletine) is block of Na+ channels (Cannon, 2015; Lehmann-Horn, et al., 2008; Trivedi, et al., 2014). However, this approach is not optimal as many patients suffer side effects or have incomplete response. An alternative approach to therapy is to replace the missing chloride current with another current that stabilizes the membrane potential and thus reduces excitability. Such an approach could be combined with block of Na+ channels to improve the response to therapy and lessen side effects.
Both the current study and a previous study found that treatment of myotonic muscle with retigabine to open KCNQ (Kv7) channels, lessened myotonia (Su, et al., 2012). Retigabine has also been shown to improve motor function in R6/2 transgenic Huntington’s disease mice (Cao, et al., 2015). In addition to altering the activity of striatal output neurons (Cao, et al., 2015), retigabine may improve motor function by reducing the skeletal muscle hyperexcitability in the R6/2 mice (Miranda, et al., 2017; Waters, et al., 2013). However, the mechanism underlying efficacy of retigabine against myotonia was not apparent when classic measures of muscle excitability such as resting membrane potential, input resistance or properties of single action potentials were measured.
The likely explanation for this lack of effect of retigabine on traditional measures of muscle excitability is the relatively negative membrane potential of muscle. The way in which retigabine increases Kv7 current is a hyperpolarized shift in the voltage dependence of Kv7 channel activation and speeding of the rate of activation (Gunthorpe, et al., 2012; Main, et al., 2000; Tatulian, et al., 2001; Wickenden, et al., 2000). However, the voltage dependence of Kv7 channels is depolarized enough that even after treatment with retigabine, few Kv7 channels may be opened at the resting membrane potential of −85 mV in muscle (Gunthorpe, et al., 2012; Tatulian, et al., 2001; Wickenden, et al., 2000). Furthermore, as Kv7 channels gate slowly, they may not open quickly enough to affect properties of single action potentials (Main, et al., 2000; Yue and Yaari, 2004).
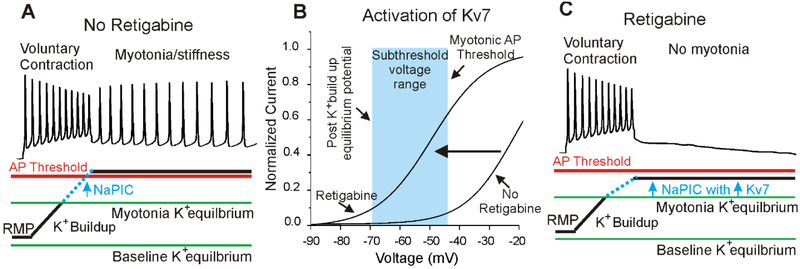
Our hypothesis as to how retigabine lessens myotonia is shown in Fig 6. We recently found that a Na persistent inward current (NaPIC), present normally in muscle, plays a central role in triggering myotonia (Hawash, et al., 2017). In muscle from mice with myotonia congenita, repetitive firing leads to depolarization secondary to build-up of K+ in t-tubules. This depolarization activates NaPIC to trigger repetitive firing (myotonia) (Hawash, et al., 2017) (Fig 6A). The hyperpolarized shift in the voltage dependence of Kv7 channel activation and the speeding of activation caused by retigabine (Fig 6B) causes Kv7 channels to open during myotonia to oppose the depolarizing effect of NaPIC (Fig 6C). In this way retigabine opposes myotonia while having no measurable effect on properties of muscle fibers at rest or during firing of single action potentials.
Fig 6:
The mechanism underlying efficacy of retigabine against myotonia. A) Firing of action potentials during voluntary movement causes build-up of K+ in t-tubules, which depolarizes the K+ equilibrium potential. This causes depolarization of the muscle membrane potential, which activates Na+ persistent inward current (NaPIC) and depolarizes the membrane potential to above action potential threshold. The result is involuntary repetitive firing (myotonia) that is responsible for muscle stiffness. B) Shown is a plot of the voltage dependence of activation of Kv7 channels in the presence and absence of retigabine (based on the work of (Tatulian, et al., 2001)). Retigabine causes a hyperpolarized shift in the voltage dependence of opening of Kv7 channels such that the channels activate in the subthreshold voltage range (light blue) between the K+ equilibrium potential and action potential threshold during runs of myotonia. With no retigabine, the voltage dependence of activation of Kv7 channels is relatively depolarized such that the channels are not significantly activated during myotonia. C) Following treatment with retigabine, Kv7 channels activate over the same voltage range as NaPIC and prevent NaPIC from depolarizing the membrane potential to action potential threshold. RMP = resting membrane potential, AP = action potential.
The potential for treatment of myotonia with drugs that activate Kv7 channels
Despite its efficacy against myotonia in vivo, retigabine did not cause significant improvement in motor function of mice with myotonia congenita. There are a number of potential explanations for the lack of in vivo efficacy of retigabine.
One explanation is that the in vivo dose of retigabine administered was too low. It is difficult to relate the in vitro concentration of retigabine used to the concentration in plasma during in vivo experiments due to issues such as volume of distribution and protein binding. While it is possible that the dose was too low, the 30 mg/kg dose we used was one that has previously been shown to cause worsening of motor performance (Hayashi, et al., 2014; Zagorchev, et al., 2016) and caused worsened performance in the time of righting in our study. Thus, increasing the dose of retigabine in an effort to improve motor performance does not appear to be a therapeutic option.
Another explanation for the lack of behavioral effects is that different muscle groups have differential sensitivity to the effects of retigabine. We only tested the efficacy of retigabine against myotonia in the triceps surae muscle group. The righting reflex involves a number of truncal muscles that might express different K+ channels than limb muscles.
Finally, it is possibility that side effects of retigabine hurt motor performance and cancel out its beneficial effect of reducing myotonia. One potential side effect of retigabine is weakness. It has been reported that ex vivo treatment with retigabine causes significant weakness that is associated with worse motor performance in vivo (Zagorchev, et al., 2016). However, while we did see a trend towards weakness, we did not find clear evidence of severe weakness following treatment with retigabine. Thus, if retigabine causes weakness in myotonia congenita, the weakness is relatively modest and thus seems unlikely to fully explain the lack of motor improvement. One important difference between our study and that of Zagorachev et. al. is that we measured the effect of retigabine on force generation of pathologically excitable muscle whereas the previous study used wild type muscle.
Another potential side effect is depression of the CNS. As retigabine was designed to treat epilepsy, it has relatively high CNS penetrance (Gunthorpe, et al., 2012). A study of the efficacy of retigabine against pain demonstrated that motor side effects of retigabine could be prevented by intra-ventricular injection of a blocker of Kv7 channels (Hayashi, et al., 2014). This study suggests that central nervous system side effects may negatively impact motor performance following treatment with retigabine. One reason that retigabine may preferentially reduce CNS excitability is that different subtypes of Kv7 channels have differential sensitivity to retigabine. One of the Kv7 isoforms expressed at highest levels in mouse skeletal muscle is Kv7.4, which appears to have lower drug sensitivity to retigabine compared to Kv7.2 and Kv7.3 channels often expressed in neurons (Iannotti, et al., 2010; Tatulian, et al., 2001). It thus remains possible that if an activator of Kv7 channels in muscle could be identified that does not cross the blood brain barrier as readily, it might be effective in improving motor function of patients with myotonia.
Conclusion
Our study suggests that retigabine is effective in treating myotonia by activating Kv7 channels during myotonia to prevent the afterdepolarization from triggering firing of myotonic action potentials. Despite its efficacy against myotonia, treatment with retigabine did not improve motor function. The lack of motor improvement could be due to differential sensitivity to retigabine of various muscle groups, weakness or central nervous system side effects. It thus remains possible that if an activator of Kv7 channels could be identified that had low central nervous system penetrance, it might provide effective therapy for myotonia congenita.
Highlights.
Retigabine is effective in treating myotonia both in vitro and in vivo.
Retigabine lessens myotonia without affecting normal measures of excitability.
Despite its efficacy against myotonia, retigabine does not improve motor function.
Acknowledgements
We would like to thank Nicklaus Brown for assistance with behavioral measures.
Funding
This work was supported in part by MDA grant 378033. Additional support was provided by NIH grants NS082354 (M.M.R.) and NS099850 (A.A.V.).
Footnotes
Conflict of Interest
The authors declare that they have no competing financial interests or other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown DA, and Passmore GM, 2009. Neural KCNQ (Kv7) channels. Br J Pharmacol 156, 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC, 2015. Channelopathies of skeletal muscle excitability. Compr Physiol 5, 761–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bartolome-Martin D, Rotem N, Rozas C, Dellal SS, Chacon MA, Kadriu B, Gulinello M, Khodakhah K, and Faber DS, 2015. Rescue of homeostatic regulation of striatal excitability and locomotor activity in a mouse model of Huntington's disease. Proc Natl Acad Sci U S A 112, 2239–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P, Quasthoff S, Strupp M, and Lehmann-Horn F, 1990. Enhancement of K+ conductance improves in vitro the contraction force of skeletal muscle in hypokalemic periodic paralysis. Muscle Nerve 13, 451–457. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Large CH, and Sankar R, 2012. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 53, 412–424. [DOI] [PubMed] [Google Scholar]
- Hawash AA, Voss AA, and Rich MM, 2017. Inhibiting persistent inward sodium currents prevents myotonia. Ann Neurol 82, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Iwata M, Tsuchimori N, and Matsumoto T, 2014. Activation of peripheral KCNQ channels attenuates inflammatory pain. Mol Pain 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, and Horowicz P, 1957. Effects of K+ and Cl on the membrane potential of isolated muscle fibres. J Physiol 137, 30P. [PubMed] [Google Scholar]
- Hodgkin AL, and Horowicz P, 1959. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol 148, 127–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti FA, Panza E, Barrese V, Viggiano D, Soldovieri MV, and Taglialatela M, 2010. Expression, localization, and pharmacological role of Kv7 potassium channels in skeletal muscle proliferation, differentiation, and survival after myotoxic insults. J Pharmacol Exp Ther 332, 811–820. [DOI] [PubMed] [Google Scholar]
- Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, and Jentsch TJ, 1992. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 257, 797–800. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Jurkat-Rott K, and Rudel R, 2008. Diagnostics and therapy of muscle channelopathies--Guidelines of the Ulm Muscle Centre. Acta Myol 27, 98–113. [PMC free article] [PubMed] [Google Scholar]
- Lipicky RJ, Bryant SH, and Salmon JH, 1971. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. Journal of Clinical Investigation 50, 2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, and Burbidge SA, 2000. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol 58, 253–262. [DOI] [PubMed] [Google Scholar]
- Miranda DR, Wong M, Romer SH, McKee C, Garza-Vasquez G, Medina AC, Bahn V, Steele AD, Talmadge RJ, and Voss AA, 2017. Progressive Cl- channel defects reveal disrupted skeletal muscle maturation in R6/2 Huntington's mice. J Gen Physiol 149, 55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli P, Vincent JA, Powers R, Cope TC, and Rich MM, 2016. Reduced motor neuron excitability is an important contributor to weakness in a rat model of sepsis. Exp Neurol 282, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KR, Norman J, Mitchell JR, Pinter MJ, and Rich MM, 2015. Sodium channel slow inactivation as a therapeutic target for myotonia congenita. Ann Neurol 77, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto JF, Kimball MM, and Wilcox KS, 2002. Effects of the anticonvulsant retigabine on cultured cortical neurons: changes in electroresponsive properties and synaptic transmission. Mol Pharmacol 61, 921–927. [DOI] [PubMed] [Google Scholar]
- Palade PT, and Barchi RL, 1977. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol 69, 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade PT, and Barchi RL, 1977. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol 69, 879–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Riisager A, de Paoli FV, Chen TY, and Nielsen OB, 2016. Role of physiological ClC-1 Cl- ion channel regulation for the excitability and function of working skeletal muscle. J Gen Physiol 147, 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ, Kraner SD, and Barchi RL, 1998. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Annals of Neurology 43, 171–179. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Klocke R, Ortland C, Gronemeier M, Jockusch H, Grunder S, and Jentsch TJ, 1991. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature 354, 304–308. [DOI] [PubMed] [Google Scholar]
- Su TR, Zei WS, Su CC, Hsiao G, and Lin MJ, 2012. The Effects of the KCNQ Openers Retigabine and Flupirtine on Myotonia in Mammalian Skeletal Muscle Induced by a Chloride Channel Blocker. Evid Based Complement Alternat Med 2012, 803082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, and Brown DA, 2001. Activation of expressed KCNQ potassium currents and Native neuronal M-type potassium currents by the anticonvulsant drug retigabine. J Neurosci 21, 5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi JR, Cannon SC, and Griggs RC, 2014. Nondystrophic myotonia: challenges and future directions. Exp Neurol 253, 28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunteren E, Spiegler SE, and Moyer M, 2011. Fatigue-inducing stimulation resolves myotonia in a drug-induced model. BMC Physiol 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CW, Varuzhanyan G, Talmadge RJ, and Voss AA, 2013. Huntington disease skeletal muscle is hyperexcitable owing to chloride and potassium channel dysfunction. Proc Natl Acad Sci U S A 110, 9160–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden AD, Yu W, Zou A, Jegla T, and Wagoner PK, 2000. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol 58, 591–600. [DOI] [PubMed] [Google Scholar]
- Yue C, and Yaari Y, 2004. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci 24, 4614–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorchev P, Apostolova E, Kokova V, and Peychev L, 2016. Activation of KCNQ channels located on the skeletal muscle membrane by retigabine and its influence on the maximal muscle force in rat muscle strips. Naunyn Schmiedebergs Arch Pharmacol 389, 439–446. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zhang Y, Xu H, Chen F, Chen X, Li X, Pi X, Wang L, Zhan L, Na+n F, and Gao Z, 2015. P-retigabine: an N-propargyled retigabine with improved brain distribution and enhanced antiepileptic activity. Mol Pharmacol 87, 31–38. [DOI] [PubMed] [Google Scholar]