Abstract
We explored the effects of chitosan oligosaccharides (COS) on coronary heart disease (CHD) patients. The component of COS was measured by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). CHD patients were evenly assigned into the COS group (COG) and the placebo group (CG). The duration of treatment was 6 months and therapeutic results were explored by measuring left ventricular ejection fraction (LVEF) value, Lee scores, quality of life (QOL), blood urea nitrogen, and serum creatinine. The intestinal flora were determined by 16s rDNA sequencing. The circulating antioxidant levels and lipid profiles were compared between two groups. There were 7 different degrees of polymerization (DP4-10) in COS. Lee scores, QOL scores, and LVEF values in the COG group were higher than those in the CG group (P < 0.05). COS treatment improved blood urea nitrogen and serum creatinine when compared with controls (P < 0.05). Circulating antioxidant levels were higher in the COG group than in the CG group. COS consumption increased the serum levels of SOD and GSH and reduced the levels of ALT and AST (P < 0.05). Meanwhile, lipid profiles were improved in the COG group. COS consumption increased the abundance of Faecalibacterium, Alistipes, and Escherichia and decreased the abundance of Bacteroides, Megasphaera, Roseburia, Prevotella, and Bifidobacterium (P < 0.05). On the other hand, COS consumption increased the probiotic species Lactobacillus, Lactococcus, and Phascolarctobacterium. The increased species have been reported to be associated with antioxidant properties or lipid improvement. COS had similar effects with chitohexaose on the growth rate of these species. Therefore, COS ameliorate the symptoms of CHD patients by improving antioxidant capacities and lipid profiles via the increase of probiotics in the intestinal flora.
1. Introduction
Coronary heart disease (CHD) is mainly caused by circulating cholesterol accumulation on the artery walls, narrow arteries, and reduced blood flow to the heart [1]. CHD is a major cause of death worldwide and its prevalence is still increasing with population ageing [2]. CHD is difficultly diagnosed [3], and most medical treatment can cause side effects. Angiotensin-converting enzyme 2 (ACE2) is a regulator of the renin angiotensin system and has been widely used in the prevention of CHD development. Recent work showed that ACE2 treatment could increase the hazard of unwanted long-term cardiovascular outcomes in CHD patients [4]. Aspirin is also widely used for CHD therapy as anti-inflammatory pharmaceutical. Administration of aspirin may result in altered reproductive profiles and serum biochemistry [5]. Therefore, it is highly demanded to explore natural products with few side effects in the prevention of CHD risk and progression.
Chitosan is the second most abundant polysaccharide next to cellulose as a natural renewable resource. Chitosan oligosaccharides (COS) are effective antiatherosclerosis potential natural products [6] and have many valuable properties. COS exert obvious efficiency for preventing intestinal lipid absorption and improving liver lipid biosynthesis and accumulation [7] while lipid profile is an important risk factor of CHD [8]. Furthermore, COS have excellent biological properties presenting a promising prospect in antibacterial [9] and antioxidant biomaterials [10] with little cytotoxicity. Antioxidant therapy will be an effective way for CHD treatment since long-term hyperglycemia can result in the enhancement of oxidative stress [11]. Chitosan consumption can affect fecal microbiota and metabolites of humans [12], which may be associated with the changes of the intestinal flora. Intestinal flora disorder and disturbance also increase the CHD risk [13] and affect lipid metabolism [14] and antioxidant activities [15].
The above results suggest that COS are feasible and promising natural products for CHD patients. However, the effects of COS on CHD patients and the related molecular mechanisms remain unknown. Therefore, we explored the effects of COS on CHD patients and the changes of the intestinal flora. The improvement of the quality of life of CHD patients was compared with controls, and the levels of antioxidant and lipid profiles were measured.
2. Materials and Methods
2.1. Measurement of the Component of COS
Food-grade COS, chitohexaose hydrochloride (MW 1203.72), chitoheptaose hydrochloride (MW 1401.3), and chitooctaose hydrochloride (MW 1598.94) were purchased from Qingdao BZ Oligo Biotech Co. Ltd. (Qingdao, China). Thirty milligrams of COS or other oligosaccharides was dissolved in 1.0 mL ddH2O and transferred to a chromatography flask for the analysis of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Voyager DESTR type MALDI-TOF mass spectrometer was purchased from Applied Biosystems (Carlsbad, CA, USA). The following operating parameters were used: nitrogen laser (wavelength 337 nm, pulse width 3 ns), reflection mode vacuum 2.08 × 10−6 Torr, ion source acceleration voltage 20 kV, extraction voltage 92.1%, and the ion delay 125 s. The mass spectrometry signal was accumulated 50 times in a single scan, and the positive ion model was determined.
2.2. Participants
All procedures were approved by the human research ethical committee of China-Japan Union Hospital of Jilin University (Changchun, China) (the clinical register no. ChiCTR1900020902 at http://www.chictr.org.cn/searchprojen.aspx). All patients agreed to sign the written consent form. CHD patients were diagnosed by using electrocardiogram, myocardial enzymology markers, coronary angiography, and clinical manifestations.
2.3. Inclusion Criteria
The patients had clinical manifestations of typical pain, which mostly occurred in the early morning. Sudden and severe stern poststernal or precordial compression pain could be found but the cause was not obvious. Taking a rest or taking nitroglycerin tablets could not alleviate the symptoms. The patients were often upset, sweating, fearful, experienced chest tightness, or had a sense of death. Typical electrocardiogram showed that ST-segment elevation was arch-back-up with wide and deep Q wave (pathological Q wave) and T wave inversion. The levels of serum myocardial necrosis markers, myoglobin and troponin I (cTnI), or myocardial markers, such as cardiac troponin T (cTnT) and creatine kinase isoenzyme CK-MB, were significantly elevated.
2.4. Exclusion Criteria
The patients with the following condition were excluded: (1) gastrointestinal diseases, such as gastritis and gastric ulcer, and diarrhea in the past 4 weeks or a history of gastrointestinal surgery; (2) combined symptoms of heart failure; (3) cardiogenic shock; (4) previous coronary revascularization procedures (e.g., thrombolysis and PCI); (5) combined symptoms with autoimmune diseases; (6) other endocrine diseases such as thyroid disorder; (7) serious damage to organs such as the liver and kidney; (8) oral and intravenous antibiotic administration for nearly 1 week and adjustment of intestinal flora preparation and gastric mucosal protective agent for nearly 1 week; (9) hypertension, obesity, diabetes, and dyslipidemia; and (10) pregnant.
2.5. Patient Grouping
From March 4, 2016, to April 28, 2017, a total of 528 CHD patients were screened. The first primary endpoint was mortality, stroke, and myocardial infarction and the endpoint was determined according to a one-month observation after randomization. Finally, a sample size with 120 subjects was determined. COS have been sold widely in China as healthy products. The dosage of COS (1-2 g/day) was provided according to product instructions. COS mixtures administered orally at doses between 50 and 1,000 mg/kg b.w. will not produce any significant change in the autonomic or behavioral responses in animal models [16]. To maintain the safety of COS, the lowest dose of COS (2 g daily) was used. All patients were selected and evenly assigned into the COG (received 2 g COS daily) and CG (placebo) groups. The therapeutic duration was half a year.
2.6. Specimen Collection
Fresh stools were collected on the first morning after admission, and stool samples from all subjects were collected in closed fecal storage boxes within two-hour defecation (subjects took hospital diet and normal control diet). The samples were immediately stored in a -80°C refrigerator.
2.7. Extraction of Total DNA
Two-gram feces was placed in a 2.0 mL Safe-Lock tube, and glass beads were added and one-milliliter PBS (50 mM, pH 7.0) and vortexed evenly. The mixture was water bathed at 95°C for 10 min, and 20 μL proteinase K was added and incubated at 55°C for 10 min. After centrifugation at 12000 rpm for 15 min, the supernatant was placed in a two-milliliter test tube. Genomic DNA was extracted and purified by using the kit from Promega (Madison, WI, USA). The quality of genomic DNA was determined by using Thermo NanoDrop 2000 Ultraviolet Micro Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and 1% agarose gel electrophoresis.
2.8. 16S rDNA Sequencing
The 16S rDNA amplification selection region is V3-V4 region, and a universal primer is used. The specific universal primers (forward primer: 5′-ACTCCTACGGGRSGCAGCAG-3′; reverse primer: 5′-GGACTACVVGGGTATCTAATC-3′) were used for 16S rDNA sequencing. The primers were completed by adding the index sequence and the linker sequence suitable for PE250 sequencing at the 5′ end of the primer. Using the diluted genomic DNA as a template, PCR was performed with KAPA HiFi HotStart ReadyMix PCR kit high-fidelity enzyme (Kapa Biosystems Inc., Boston, MA, USA). The PCR product was detected by 2% agarose gel electrophoresis, and the PCR product was recovered by gelatinization using an AxyPrep DNA Gel Recovery Kit (Axygen Scientific Co., CA, USA). After recovery, library quality checks were performed using a Thermo NanoDrop 2000 UV spectrophotometer and 2% agarose gel electrophoresis. PCR products were sequenced by using illumina HiSeq PE250 (Illumina, San Diego, CA, USA).
2.9. The Effects of COS on the Growth of Intestinal Flora
The strains Bacteroides thetaiotaomicron (CGMCC 1.5132, broken meat medium); Escherichia coli (CGMCC 1.12883, LB medium); Megasphaera elsdenii (CGMCC 1.2720, CGMCC medium 0288); and Bifidobacterium bifidum (CGMCC 1.5091, CGMCC medium 0244) were purchased from the China General Microbiological Culture Collection Center, Institute of Microbiology, Chinese Academy of Sciences (Beijing, China). The strains Faecalibacterium prausnitzii (ATCC 27768, ATCC medium: 2107 modified reinforced clostridial); Alistipes shahii (ATCC BAA-1179, ATCC medium 1490: modified chopped meat medium); and Prevotella bivia (ATCC 29303, ATCC medium 2107) were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The strain Roseburia intestinalis (DSM 14610, medium 143) was purchased from Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Inhoffenstraße, Braunschweig, Germany). Escherichia coli was cultured at a 200 rpm shaker at 37°C while other strains were cultured with anaerobic gas mixture, 80% N2, 10% CO2, and 10% H2 at 37°C with corresponding media and 10 μg/mL chitohexaose hydrochloride, chitoheptaose hydrochloride, chitooctaose hydrochloride, and mixed 80 μg/mL chitosan oligosaccharide. The group without chitosan oligosaccharides was used as a control. The growth rate of all strains was measured by using a Real-Time Cell Analyzer (xCELLigence™, Roche Inc., Indianapolis, IN, USA) within 24 hours.
2.10. Lipid Profile Analysis
Serum triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were examined by using an automatic biochemical analyzer (Dimension, Schererville, IN, USA).
2.11. Analyses of Circulating Oxidative Levels
2 mL blood was taken from individual patient. Circulating oxidative levels were examined by measuring the levels of superoxide dismutase (SOD), glutathione (GSH), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). ELISA kits were purchased from Beyotime Institute of Biotechnology (Beijing, China).
2.12. Measurement of Therapeutic Effects
The normal therapy of CHD included oxygen inhalation, angiotensin-converting enzyme inhibitors (ACE-in), and implantable cardioverter defibrillator (ICD). Ejection fraction (EF) value and scores of quality of life (QOL) were evaluated by using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) [17].
Left ventricular ejection fraction (LVEF) was detected by using radionuclide ventriculography with patients in the supine position [18]. Serum was separated from the blood sample via centrifugation at 4000 × g for 10 min. Blood urea nitrogen (BUN) was analyzed by using the above automatic biochemistry analyzer via a BUN kit (Beckman Coulter Inc., Brea, CA, USA). Serum creatinine was measured by a creatinine kit (Biosino Bio-Technology, Beijing, China).
2.13. Statistical Analysis
All data were presented as mean values ± S.D. and analyzed by using SPSS 20.0 statistical package. The t-test was used for the comparison of mean values between the two groups and count number was analyzed using the χ2 test. P < 0.05 was considered statistically significant.
3. Results
3.1. The Main Components of COS
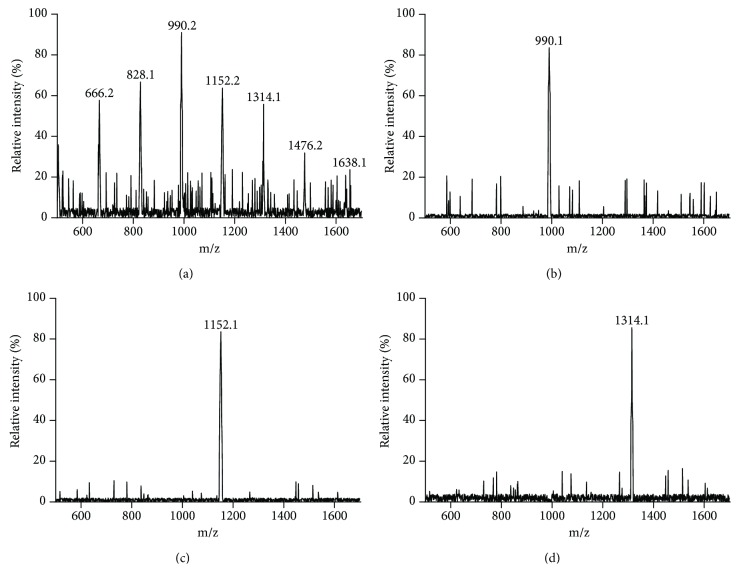
There were seven main kinds of chitosan oligosaccharides in food-grade COS from DP4 (666.2 Da) to DP10 (1638.1 Da) (Figure 1(a)). The molecular weight of chitohexaose (990.1 Da, Figure 1(b)), chitoheptaose (1152.1 Da, Figure 1(c)), and chitooctaose (1314.1 Da, Figure 1(d)) were also accordant with theoretical values in a positive mode.
Figure 1.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis of the main components of chitosan oligosaccharides (COS). (a) The main components of food-grade COS with different degrees of polymerization DP4-10. (b) Chitohexaose with molecular weight [M+H]+ = 990.1 Da. (c) Chitoheptaose with molecular weight [M+H]+ = 1152.1 Da. (d) Chitooctaose with molecular weight [M+H]+ = 1314.2 Da.
3.2. Clinical Characteristics
After the 6-month therapy, 4 and 6 patients left the COG and CG groups for other medical treatment, respectively. There was no significantly statistical differences for clinical characteristics of CHD patients between the COG and CG groups, including sex ratio, body mass index (BMI), age, diastolic blood pressure (DBP), and systolic blood pressure (SBP) (Table 1, P > 0.05). The cases for taking ACE-In, ARBS, beta-blockers, and diuretics were also comparable between the two groups (P > 0.05, Table 1).
Table 1.
Clinical characteristics between COS and placebo groups.
| Parameters | COS | Placebo | χ 2 and t value | P value |
|---|---|---|---|---|
| Gender (male/female) | 30/26 | 31/23 | 0.164 | 0.686 |
| Age (yr) | 39.29 ± 13.36 | 41.23 ± 12.98 | -1.307 | 0.189 |
| SBP (mm Hg) | 125.21 ± 11.62 | 128.54 ± 12.76 | -1.685 | 0.087 |
| DBP (mm Hg) | 87.23 ± 7.16 | 88.53 ± 7.38 | -1.290 | 0.157 |
| BMI | 24.93 ± 2.94 | 24.52 ± 2.68 | -1.564 | 0.198 |
| Cr (μmol/L) | 85.34 ± 13.58 | 87.24 ± 14.56 | -1.344 | 0.156 |
| HbA1C (%) | 8.47 ± 0.93 | 8.75 ± 0.96 | -0.664 | 0.256 |
| ACE-In | 7 | 8 | 2.47 | 0.48 |
| ARBS | 3 | 6 | ||
| Beta-blockers | 5 | 6 | ||
| Diuretics | 8 | 4 |
Chi-square test and t-test were used to compare the significant difference between the two groups. BMI: body mass index; ACE-In: angiotensin-converting enzyme; ARBS: angiotensin receptor blockers. All data were presented as mean value ± S.D. There were significantly statistical differences between two groups if P < 0.05.
3.3. Therapeutic Results
There was no significant difference (P > 0.05) in the mean values of BUN before COS therapy (P < 0.05, Table 2). After therapy, the values of mean BUN and serum creatinine were significantly reduced when compared with the placebo group (P < 0.05, Table 2). Before COS treatment, the statistical difference for Lee scores was insignificant between the COG and CG groups (Table 2, P > 0.05). After COS consumption, COS reduced more Lee scores than CG (Table 2, P < 0.05). Before COS consumption, the statistical difference for the QOL scores was insignificant between the COG and CG groups (Table 2, P > 0.05). After 6-month COS consumption, COS increased more QOL scores than CG (Table 2, P < 0.05). Before COS consumption, the statistical difference for LVEF volume was insignificant between the COG and CG groups (Table 2, P > 0.05). After 6-month COS consumption, the values of LVEF were improved in the COG group higher than in the CG group (Table 2, P < 0.05).
Table 2.
The therapeutic results of COS.
| Before treatment | After treatment | t values | P values | ||
|---|---|---|---|---|---|
| Blood urea nitrogen (mg/dL) | COS | 19.13 ± 6.85 | 15.33 ± 6.24 | 6.42 | 0.02b |
| Placebo | 18.73 ± 6.54 | 17.25 ± 5.98 | 1.16 | 0.23 | |
| t values | 0.39 | 3.21 | |||
| P values | 0.54 | 0.02a | |||
| Serum creatinine (mg/dL) | COS | 1.41 ± 0.32 | 1.04 ± 0.27 | 8.65 | 0.01b |
| Placebo | 1.35 ± 0.27 | 1.29 ± 0.22 | 0.35 | 0.24 | |
| t values | 0.25 | 4.37 | |||
| P values | 0.66 | 0.02a | |||
| Lee scores | COS | 4.52 ± 1.87 | 2.18 ± 0.43 | 5.38 | 0.01b |
| Placebo | 4.27 ± 1.79 | 4.19 ± 0.78 | 0.25 | 0.31 | |
| t values | 0.26 | 4.12 | |||
| P values | 0.68 | 0.02a | |||
| Quality-of-life (QOL) scores | COS | 43.61 ± 3.38 | 21.73 ± 4.12 | 13.40 | 0.01b |
| Placebo | 42.50 ± 3.25 | 39.39 ± 4.36 | 0.45 | 0.29 | |
| t values | 0.36 | 2.13 | |||
| P values | 0.75 | 0.02a | |||
| LVEF | COS | 29.06 ± 9.34 | 36.82 ± 10.43 | 3.03 | 0.02b |
| Placebo | 28.74 ± 8.15 | 30.73 ± 10.21 | 1.10 | 0.08 | |
| t values | 0.92 | 2.17 | |||
| P values | 0.36 | 0.03a |
Note: LVEF: left ventricular ejection fraction. n = 60 for each group. aP < 0.05 vs. the placebo group and bP < 0.05 vs. before treatment. There were significantly statistical differences between the two groups if P < 0.05.
3.4. The Effects of COS Consumption on Intestinal Flora of CHD Patients
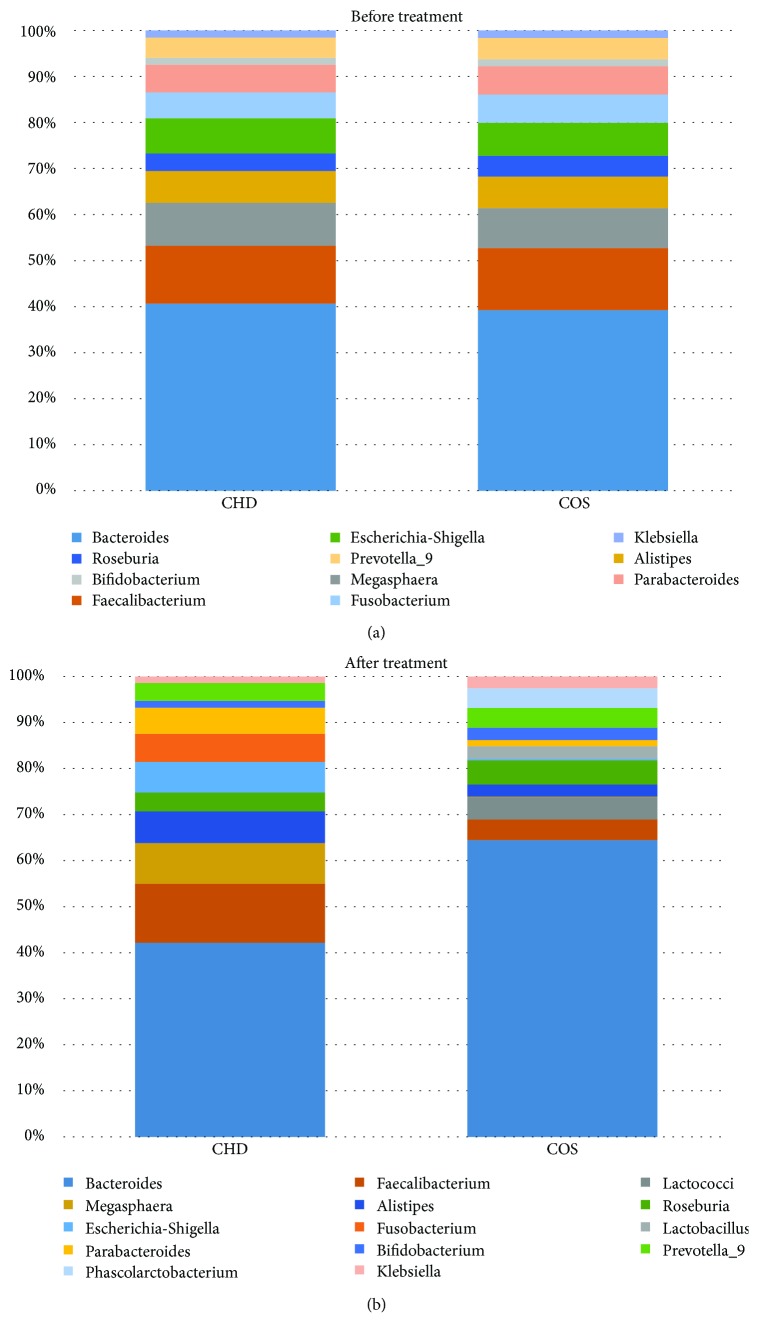
The statistical difference for the abundance of intestinal flora was insignificant between the two groups before therapy (Figure 2(a)P > 0.05). After 6-month therapy, the abundance of Faecalibacterium, Alistipes, and Escherichia was reduced, while the abundance of Bacteroides, Megasphaera, Roseburia, Prevotella, and Bifidobacterium was increased when compared with the CG group (Figure 2(b), P < 0.05). On the other hand, COS consumption increased the probiotic species Lactobacillus, Lactococcus, and Phascolarctobacterium. The results suggest that COS consumption can inhibit the abundance of harmful bacteria and increase the abundance or species of probiotics in CHD patients.
Figure 2.
The effects of COS on intestinal flora. (a) The abundance of intestinal flora before COS treatment. (b) The abundance of intestinal flora after 6-month treatment.
3.5. The Effects of COS on the Growth Rate of Intestinal Flora
In vitro test showed that COS mixture and chitooctaose (DP8) treatment inhibited the growth rate of Escherichia coli (Figure 3(a)), Megasphaera elsdenii (Figure 3(b)), and Faecalibacterium prausnitzii (Figure 3(c)) and promoted the growth of Alistipes shahii (Figure 3(d)), Prevotella bivia (Figure 3(e)), Roseburia intestinalis (Figure 3(f)), Bacteroides thetaiotaomicron (Figure 3(g)), and Bifidobacterium bifidum (Figure 3(h)). Meanwhile, chitooctaose had similar results with mixed COS. Comparatively, chitohexaose and chitoheptaose had no effects on these bacteria. The results suggest that COS affect intestinal flora via chitooctaose.
Figure 3.
Real-time analysis of the effects of COS on the growth of intestinal flora. (a) The effects of COS on the growth of Escherichia coli. (b) The effects of COS on the growth of Megasphaera elsdenii. (c) The effects of COS on the growth of Faecalibacterium prausnitzii. (d) The effects of COS on the growth of Alistipes shahii. (e) The effects of COS on the growth of Prevotella bivia. (f) The effects of COS on the growth of Roseburia intestinalis. (g) The effects of COS on the growth of Bacteroides thetaiotaomicron. (h) The effects of COS on the growth of Bifidobacterium bifidum. Mix: food-grade COS, DP4-10 chitosan oligosaccharides; DP6: chitohexaose hydrochloride (MW 1203.72); DP7: chitoheptaose hydrochloride (MW1401.3); and DP8: chitooctaose hydrochloride (MW1598.94). ∗P < 0.05 vs. the control group without COS.
3.6. COS Consumption Improved Lipid Profiles of CHD Patients
Before COS consumption, the statistical difference was insignificant between the two groups (P > 0.05, Table 3). After 6-month therapy, the serum levels of TG, TC, and LDL-c were reduced while HDL-c was increased when compared with the control group (P < 0.05, Table 3). The results suggest that COS consumption can improve lipid profiles of CHD patients.
Table 3.
Lipid profiles between two groups.
| COS | Placebo | t values | P values | ||
|---|---|---|---|---|---|
| Before therapy | TC (mmol/L) | 5.42 ± 0.63 | 5.70 ± 0.81 | -0.621 | 0.284 |
| TG (mmol/L) | 2.34 ± 0.81 | 2.17 ± 0.92 | -2.108 | 0.129 | |
| LDL-C (mmol/L) | 2.11 ± 0.62 | 2.31 ± 0.81 | -1.834 | 0.167 | |
| HDL-C (mmol/L) | 1.83 ± 0.42 | 1.65 ± 0.38 | -2.609 | 0.094 | |
| After therapy | TC (mmol/L) | 4.89 ± 0.87 | 5.81 ± 0.72 | -1.982 | 0.013 |
| TG (mmol/L) | 2.01 ± 0.65 | 2.24 ± 0.83 | -2.696 | 0.035 | |
| LDL-C (mmol/L) | 1.81 ± 0.54 | 2.40 ± 0.75 | -1.992 | 0.031 | |
| HDL-C (mmol/L) | 2.13 ± 0.40 | 1.71 ± 0.46 | -2.852 | 0.009 |
Note: there were significant statistical differences between two groups if P < 0.05.
3.7. COS Consumption Increased Antioxidant Properties of CHD Patients
The statistical difference for the biomarkers of antioxidant and oxidative stress was insignificant between the two groups before therapy (Table 4, P > 0.05). After 6-month therapy, the circulating levels of SOD and GSH were increased while the levels of ALT and AST were reduced in the COG group when compared with the CG group (P < 0.05, Table 4). The results suggest that COS consumption can increase antioxidant properties of CHD patients.
Table 4.
Antioxidant levels between two groups.
| COS | Placebo | t values | P values | ||
|---|---|---|---|---|---|
| Before therapy | SOD (U/mL) | 12.25 ± 4.06 | 11.30 ± 4.21 | 0.79 | 0.46 |
| GSH (U/mL) | 9.20 ± 2.99 | 9.35 ± 2.73 | 0.12 | 0.81 | |
| ALT (U/mL) | 68.79 ± 9.03 | 65.52 ± 8.76 | 0.35 | 0.47 | |
| AST (U/mL) | 198.21 ± 27.21 | 190.36 ± 20.38 | 0.83 | 0.12 | |
| After therapy | SOD (U/mL) | 21.34 ± 3.78 | 13.49 ± 4.71 | 8.77 | 0.01 |
| GSH (ng/L) | 15.26 ± 3.12 | 10.59 ± 2.01 | 6.25 | 0.01 | |
| ALT (U/L) | 61.03 ± 12.27 | 66.49 ± 8.24 | 4.31 | 0.02 | |
| AST (U/L) | 147.43 ± 21.73 | 188.52 ± 23.62 | 7.04 | 0.01 |
Note: there were significant statistical differences between two groups if P < 0.05.
4. Discussion
Chitosan has been widely used for CHD therapy as biomaterials of the drug delivery system [19, 20] and coronary artery bypass graft [21]. However, the direct effects of chitosan on CHD have seldom been reported. This study showed that COS consumption increased the values of LVEF in the COG group higher than in the CG group (P < 0.05). Medicine combined with COS effectively ameliorated CHD patients with lower LVEF. Lee scores and QOL scores were also increased in the COG group (P < 0.05, Table 2). Meanwhile, lipid profiles (Table 3) and antioxidant properties (Table 4) were also improved in the COG group better than the CG group (P < 0.05). The results suggest that COS are effective to ameliorate symptoms of CHD patients by improving biochemical indices and the living ability of CHD patients.
LVEF is an important predicator for heart failure hospitalization and mortality in ambulatory adults with CHD [22]. CHD patients with low-level LVEF had a poor prognosis [23]. The present findings demonstrated that COS consumption improved LVEF values significantly when compared with controls (Table 2, P < 0.05). COS have been reported to have the clinical effects of pain relief [24], which will be beneficial for CHD patients with chest pain or chronic back pain [25, 26]. Chitosan microspheres were used for chronotherapy of chronic stable angina in an animal model [27] while angina is the common symptom of CHD patients [28, 29]. Fatigue is a prevalent and disabling symptom associated with CHD [30] while COS have been proved to delay fatigue in animal models [31]. All these results suggest that COS may have direct or indirect effect on improving CHD symptoms.
On the other hand, intestinal flora total load was found to be associated with CHD risk in obese patients [13]. Intestinal flora can produce short-chain fatty acids (SCFA) [32, 33] and bile acids [34, 35] involved in various metabolic pathways, endotoxin secretion and circulation, dopamine, the fasting-induced adipose factor (FIAF) [36], and adenosine monophosphate-activated protein kinase (AMPK) [37], which affect CHD risk factors such as hypertension [38], obesity [39], diabetes [40], and dyslipidemia [41]. Adjusting the structure and function of the flora via probiotics, antibiotics or diet can provide ideal methods for the prevention of CHD. Therefore, the effects of COS on intestinal flora were explored in CHD patients.
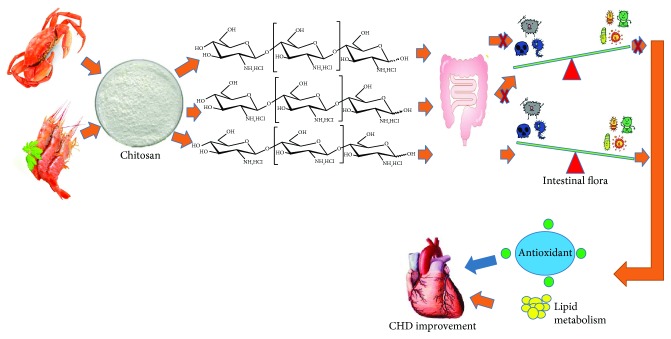
After COS consumption, the abundance of Faecalibacterium, Alistipes, and Escherichia was reduced, while the abundance of Bacteroides, Megasphaera, Roseburia, Prevotella, and Bifidobacterium was increased (Figure 2(b)). Furthermore, COS consumption increased the probiotic species Lactobacillus, Lactococcus, and Phascolarctobacterium. Faecalibacterium was found to be linked with type 2 diabetes mellitus or other risk factors of heart disease [42]. Conversely, Faecalibacterium numbers were also found to be decreased in the patients with chronic heart disease [43]. Cardiac abnormalities were reported to be caused by bacterial myocarditis resulting from E. coli infection [44, 45]. A high-fiber diet and supplementation with the SCFA can increase the number of Bacteroides acidifaciens and prevent cardiovascular disease [46]. Roseburia reduced microbially derived, proinflammatory secondary bile acids and LDL-c, which are associated with heart disease [47]. Bifidobacterium exerted beneficial effects on the serum cholesterol metabolism by reducing the levels of TC and LDL-c in the patients with dyslipidemia [48]. Bifidobacterium had strong antioxidant properties by scavenging 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical, superoxide anion, and hydroxyl radical [49]. However, Prevotella was reported to be a potential risk bacterium of heart disease [50]. All the growth of these species could be affected by COS while COS had similar results with chitooctaose but not chitohexaose and chitoheptaose (Figure 3). Thus, the COS from crab and shrimp may ameliorate CHD by affecting intestinal flora, which improve lipid profiles and antioxidant activities of CHD patients (Figure 4).
Figure 4.
COS show health-promoting properties for coronary heart disease by affecting intestinal flora via chitooctaose. COS increase the levels of probiotics, which exert antioxidant properties and improve lipid profiles. All the function will be beneficial in the prevention of CHD.
More importantly, the increased probiotic species Lactobacillus and Lactococcus show health-promoting activities for CHD. Lactobacillus showed strong antioxidant function by upregulating the expression of glutathione reductase, glutathione S-transferase, glutamate-cysteine ligase catalytic subunit, and NAD(P)H quinone oxidoreductase 1 [51]. Lactococcus acidophilus was found to prevent the progression of arteriosclerosis and coronary heart disease by affecting lipid profiles and increasing the antioxidant abilities of hyperlipidemia animal model [52].
The lipid metabolism may be correlated with not only intestinal flora but also intestinal enzymes. Previous work showed that intestinal disaccharidases (such as sucrase and maltase) were significantly decreased animals being fed with COS [53]. COS were proved to have antihyperglycemia ability for inhibiting carbohydrate hydrolysis enzymes, such as sucrase and glucoamylase [54]. Based on these findings, further clinical trials are highly demanded to prove the mechanism.
There were some limitations to the present work. The effects of chitosan oligosaccharides DP4-5 and DP9-10 on intestinal flora were not investigated although the mixed COS had similar results with chitooctaose for the growth rate of intestinal flora. The effects of COS on the increased probiotic species Lactobacillus, Lactococcus, and Phascolarctobacterium were not measured and reason remained unknown. Considering the short time of the present study, the small sample size, and other influencing factors, further work is highly demanded to confirm the present results.
5. Conclusions
COS combined with conventional treatment improved the LVEF values, QOL scores, and Lee scores. COS consumption increased the types and numbers of probiotic species of intestinal flora, which may improve lipid profiles and antioxidant properties of CHD patients. COS had similar effects with chitohexaose on the growth rate of these species. Therefore, COS improve the symptoms of CHD patients by improving antioxidant capacities via the increase of probiotics in intestinal flora.
Contributor Information
Jixue Zhao, Email: jixuezhao0431@yeah.net.
Xin Liu, Email: liuxinccjlu@126.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There are no investigator conflicts of interest to declare on the part of any of the investigators.
Authors' Contributions
TJ and XX designed the study and drafted the manuscript. LZ, ZL, and JZ conducted the study and analyzed the data. JZ and XL commented and revised the manuscript. Tiechao Jiang and Xiaohong Xing contributed equally to this work.
References
- 1.Lawler P. R., Akinkuolie A. O., Ridker P. M., et al. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women. Clinical Chemistry. 2017;63(4):870–879. doi: 10.1373/clinchem.2016.264515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBlanc A. J., Kelm N. Q., George M. Current themes in myocardial and coronary vascular aging. Current Opinion in Physiology. 2018;1:27–33. doi: 10.1016/j.cophys.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimoch W. J., Kubler P., Kosowski M., et al. Pacjenci z nasilonymi zwapnieniami w naczyniu odpowiedzialnym za niedokrwienie, poddawani zabiegom angioplastyki wieńcowej z powodu zawału serca cechują się złym rokowaniem w obserwacji odległej. Kardiologia Polska. 2017;75(9):859–867. doi: 10.5603/KP.a2017.0093. [DOI] [PubMed] [Google Scholar]
- 4.Ramchand J., Patel S. K., Srivastava P. M., Farouque O., Burrell L. M. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13(6, article e0198144) doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vyas A., Ram H., Purohit A., Jatwa R. Adverse effects of subchronic dose of aspirin on reproductive profile of male rats. Journal of Pharmaceutics. 2016;2016:9. doi: 10.1155/2016/6585430.6585430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y., Luo T., Liu S., et al. Chitosan oligosaccharides attenuate atherosclerosis and decrease Non-HDL in ApoE-/- mice. Journal of Atherosclerosis and Thrombosis. 2015;22(9):926–941. doi: 10.5551/jat.22939. [DOI] [PubMed] [Google Scholar]
- 7.Liu S. H., Chiu C. Y., Shi C. M., Chiang M. T. Functional comparison of high and low molecular weight chitosan on lipid metabolism and signals in high-fat diet-fed rats. Marine Drugs. 2018;16(8) doi: 10.3390/md16080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maleki M. H., Mousavi M., Kazemi T., Azdaki N., Sharifabad A. R., Hoshyar R. Comparison of atherogenic index and lipid profiles in candidates for coronary artery bypass graft surgery versus normal people. Pakistan Journal of Pharmaceutical Sciences. 2018;31(5):1899–1902. [PubMed] [Google Scholar]
- 9.Wongpreecha J., Polpanich D., Suteewong T., Kaewsaneha C., Tangboriboonrat P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydrate Polymers. 2018;199:641–648. doi: 10.1016/j.carbpol.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 10.Tan W., Zhang J., Mi Y., Dong F., Li Q., Guo Z. Synthesis, characterization, and evaluation of antifungal and antioxidant properties of cationic chitosan derivative via azide-alkyne click reaction. International Journal of Biological Macromolecules. 2018;120(Part A):318–324. doi: 10.1016/j.ijbiomac.2018.08.111. [DOI] [PubMed] [Google Scholar]
- 11.Liang W., Zhao Y. J., Yang H., Shen L. H. Effects of antioxidant system on coronary artery lesions in patients with abnormal glucose metabolism. Aging Clinical and Experimental Research. 2017;29(2):141–146. doi: 10.1007/s40520-016-0564-z. [DOI] [PubMed] [Google Scholar]
- 12.Inoguchi S., Ohashi Y., Narai-Kanayama A., Aso K., Nakagaki T., Fujisawa T. Effects of non-fermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. International Journal of Food Sciences and Nutrition. 2012;63(4):402–410. doi: 10.3109/09637486.2011.630992. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Li C. Analysis of changes in intestinal flora and intravascular inflammation and coronary heart disease in obese patients. Experimental and Therapeutic Medicine. 2018;15(5):4538–4542. doi: 10.3892/etm.2018.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukushima M., Nakano M. Effects of the lipid-saccharide complex and unsaponifiable matter from sunflowers on liver lipid metabolism and intestinal flora in rats. Bioscience, Biotechnology, and Biochemistry. 1995;59(5):860–863. doi: 10.1271/bbb.59.860. [DOI] [PubMed] [Google Scholar]
- 15.Yang X. W., Wang N., Li W., Xu W., Wu S. Biotransformation of 4,5-O-dicaffeoylquinic acid methyl ester by human intestinal flora and evaluation on their inhibition of NO production and antioxidant activity of the products. Food and Chemical Toxicology. 2013;55:297–303. doi: 10.1016/j.fct.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes J. C., Spindola H., de Sousa V., et al. Anti-inflammatory activity of chitooligosaccharides in vivo. Marine Drugs. 2010;8(6):1763–1768. doi: 10.3390/md8061763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogle J., Buck H., Zambroski C., Alvaro R., Vellone E. Cross‐validation of the Minnesota Living With Heart Failure Questionnaire. Journal of Nursing Scholarship. 2017;49(5):513–520. doi: 10.1111/jnu.12318. [DOI] [PubMed] [Google Scholar]
- 18.Naar J., Málek F., Lang O., et al. Assessment of left ventricular diastolic function by radionuclide ventriculography in patients with chronic heart failure and reduced ejection fraction. Vnitr̆ní Lékar̆ství. 2014;60:110–113. [PubMed] [Google Scholar]
- 19.Gao J. Q., Zheng J. P., Jin H. G., et al. A new rapamycin-abluminally coated chitosan/heparin stent system accelerates early re-endothelialisation and improves anti-coagulant properties in porcine coronary artery models. Clinical and Investigative Medicine. 2014;37(6):E395–E402. doi: 10.25011/cim.v37i6.22244. [DOI] [PubMed] [Google Scholar]
- 20.Meng S., Liu Z., Shen L., et al. The effect of a layer-by-layer chitosan–heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials. 2009;30(12):2276–2283. doi: 10.1016/j.biomaterials.2008.12.075. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo E. P., Retarekar R., Raghavan M. L., Kumar V. Mechanical properties of cellulose: chitosan blends for potential use as a coronary artery bypass graft. Journal of Biomaterials Science, Polymer Edition. 2013;24(3):239–252. doi: 10.1080/09205063.2012.690273. [DOI] [PubMed] [Google Scholar]
- 22.Ristow B., Ali S., Whooley M. A., Schiller N. B. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) The American Journal of Cardiology. 2008;102(1):70–76. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dopheide J. F., Knopf P., Zeller G. C., et al. Low IL-10/TNFα ratio in patients with coronary artery disease and reduced left ventricular ejection fraction with a poor prognosis after 10 years. Inflammation. 2015;38(2):911–922. doi: 10.1007/s10753-014-0053-5. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto Y., Kawakami K., Miyatake K., Morimoto M., Shigemasa Y., Minami S. Analgesic effects of chitin and chitosan. Carbohydrate Polymers. 2002;49(3):249–252. doi: 10.1016/S0144-8617(01)00316-2. [DOI] [Google Scholar]
- 25.Schweier R., Grande G., Richter C., Riedel-Heller S. G., Romppel M. In-depth statistical analysis of the use of a website providing patients’ narratives on lifestyle change when living with chronic back pain or coronary heart disease. Patient Education and Counseling. 2018;101(7):1283–1290. doi: 10.1016/j.pec.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Kim M. N., Cho D., Kim S. A., et al. PS 05-42 the usefulness of heart rate and blood pressure recovery in diagnosis of coronary artery disease in hypertensive women with suspected coronary artery disease: from Korean women’s chest pain registry (KoROSE) Journal of Hypertension. 2016;34:E152–E153. doi: 10.1097/01.hjh.0000500303.01572.d3. [DOI] [Google Scholar]
- 27.Jose S., Prema M. T., Chacko A. J., Thomas A. C., Souto E. B. Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids and Surfaces B: Biointerfaces. 2011;83(2):277–283. doi: 10.1016/j.colsurfb.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Kimble L. P. A randomized clinical trial of the effect of an angina self-management intervention on health outcomes of patients with coronary heart disease. Rehabilitation Nursing. 2018;43(5):275–284. doi: 10.1097/rnj.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Jones P., Weintraub W. S., et al. Predicting the benefits of percutaneous coronary intervention on 1-year angina and quality of life in stable ischemic heart disease: risk models from the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) Circulation: Cardiovascular Quality and Outcomes. 2018;11(5, article e003971) doi: 10.1161/CIRCOUTCOMES.117.003971. [DOI] [PubMed] [Google Scholar]
- 30.Eckhardt A. L., Devon H. A., Piano M. R., Ryan C. J., Zerwic J. J. Fatigue in the presence of coronary heart disease. Nursing Research. 2014;63(2):83–93. doi: 10.1097/NNR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Wen Z., Xiong J. Chitosan oligosaccharides reduces the fatigue in a rat model. Journal of Zhanjiang Teachers College. 2005;26(1):53–55. [Google Scholar]
- 32.Kao L., Liu T. H., Tsai T. Y., Pan T. M. Beneficial effects of the commercial lactic acid bacteria product, Vigiis 101, on gastric mucosa and intestinal bacterial flora in rats. Journal of Microbiology, Immunology and Infection. 2018;(18):30195–30196. doi: 10.1016/j.jmii.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Ben X. M., Zhou X. Y., Zhao W. H., et al. Supplementation of milk formula with galacto-oligosaccharides improves intestinal micro-flora and fermentation in term infants. Chinese Medical Journal. 2004;117(6):927–931. [PubMed] [Google Scholar]
- 34.Balasso A., Fritzsche M., Liepsch D., Prothmann S., Kirschke J. S., Sindeev S., et al. High-frequency wall vibrations in a cerebral patient-specific aneurysm model. Biomedical Engineering/Biomedizinische Technik. 2018 doi: 10.1515/bmt-2017-0142. [DOI] [PubMed] [Google Scholar]
- 35.Sidira M., Galanis A., Ypsilantis P., et al. Effect of probiotic-fermented milk administration ongastrointestinal survival of Lactobacillus casei ATCC 393 and modulation of intestinal microbial flora. Journal of Molecular Microbiology and Biotechnology. 2010;19(4):224–230. doi: 10.1159/000321115. [DOI] [PubMed] [Google Scholar]
- 36.Alex S., Lichtenstein L., Dijk W., Mensink R. P., Tan N. S., Kersten S. ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochemistry and Cell Biology. 2014;141(4):383–391. doi: 10.1007/s00418-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 37.Li Q., Wu T., Liu R., Zhang M., Wang R. Soluble dietary fiber reduces trimethylamine metabolism via gut microbiota and co-regulates host AMPK pathways. Molecular Nutrition & Food Research. 2017;61(12) doi: 10.1002/mnfr.201700473. [DOI] [PubMed] [Google Scholar]
- 38.Zhai C., Shi W., Feng W., et al. Activation of AMPK prevents monocrotaline-induced pulmonary arterial hypertension by suppression of NF-κB-mediated autophagy activation. Life Sciences. 2018;208:87–95. doi: 10.1016/j.lfs.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Thomas E. C., Hook S. C., Gray A., et al. Isoform-specific AMPK association with TBC1D1 is reduced by a mutation associated with severe obesity. Biochemical Journal. 2018;475(18):2969–2983. doi: 10.1042/bcj20180475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desjardins E. M., Steinberg G. R. Emerging role of AMPK in brown and beige adipose tissue (BAT): implications for obesity, insulin resistance, and type 2 diabetes. Current Diabetes Reports. 2018;18(10):p. 80. doi: 10.1007/s11892-018-1049-6. [DOI] [PubMed] [Google Scholar]
- 41.Yuan E., Duan X., Xiang L., et al. Aged oolong tea reduces high-fat diet-induced fat accumulation and dyslipidemia by regulating the AMPK/ACC signaling pathway. Nutrients. 2018;10(2):p. 187. doi: 10.3390/nu10020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Q., Lin S. L., Kwok M. K., Leung G. M., Schooling C. M. The roles of 27 genera of human gut microbiota in ischemic heart disease, type 2 diabetes mellitus, and their risk factors: a Mendelian randomization study. American Journal of Epidemiology. 2018;187(9):1916–1922. doi: 10.1093/aje/kwy096. [DOI] [PubMed] [Google Scholar]
- 43.Cui X., Ye L., Li J., et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Scientific Reports. 2018;8(1):p. 635. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiwanitkit V. E. coli outbreak and myocarditis: a story in cardiology. The Anatolian Journal of Cardiology. 2011;11(8):p. 746. doi: 10.5152/akd.2011.202. [DOI] [PubMed] [Google Scholar]
- 45.Uribarri A., Martinez-Selles M., Yotti R., Perez-David E., Fernandez-Aviles F. Acute myocarditis after urinary tract infection by Escherichia coli. International Journal of Cardiology. 2011;152(2):e33–e34. doi: 10.1016/j.ijcard.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 46.Marques F. Z., Nelson E., Chu P. Y., et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 47.Holscher H. D., Guetterman H. M., Swanson K. S., et al. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. The Journal of Nutrition. 2018;148(6):861–867. doi: 10.1093/jn/nxy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K., Yu X., Li Y., et al. Bifidobacterium bifidum TMC3115 can characteristically influence glucose and lipid profile and intestinal microbiota in the middle-aged and elderly. Probiotics and Antimicrobial Proteins. 2018 doi: 10.1007/s12602-018-9441-8. [DOI] [PubMed] [Google Scholar]
- 49.Wang B. G., Xu H. B., Xu F., Zeng Z. L., Wei H. Efficacy of oral Bifidobacterium bifidum ATCC 29521 on microflora and antioxidant in mice. Canadian Journal of Microbiology. 2016;62(3):249–262. doi: 10.1139/cjm-2015-0685. [DOI] [PubMed] [Google Scholar]
- 50.Kelly T. N., Bazzano L. A., Ajami N. J., et al. Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa Heart Study participants. Circulation Research. 2016;119(8):956–964. doi: 10.1161/CIRCRESAHA.116.309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin X., Xia Y., Wang G., et al. Lactobacillus plantarum AR501 alleviates the oxidative stress of D-galactose-induced aging mice liver by upregulation of Nrf2-mediated antioxidant enzyme expression. Journal of Food Science. 2018;83(7):1990–1998. doi: 10.1111/1750-3841.14200. [DOI] [PubMed] [Google Scholar]
- 52.Chen J., Wu Y., Yang C., Xu X., Meng Y. Antioxidant and hypolipidemic effects of soymilk fermented via Lactococcus acidophilus MF204. Food & Function. 2017;8(12):4414–4420. doi: 10.1039/C7FO00701A. [DOI] [PubMed] [Google Scholar]
- 53.Yao H. T., Huang S. Y., Chiang M. T. A comparative study on hypoglycemic and hypocholesterolemic effects of high and low molecular weight chitosan in streptozotocin-induced diabetic rats. Food and Chemical Toxicology. 2008;46(5):1525–1534. doi: 10.1016/j.fct.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Kim J. G., Jo S. H., Ha K. S., et al. Effect of long-term supplementation of low molecular weight chitosan oligosaccharide (GO2KA1) on fasting blood glucose and HbA1c in db/db mice model and elucidation of mechanism of action. BMC Complementary and Alternative Medicine. 2014;14(1):p. 272. doi: 10.1186/1472-6882-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.