Abstract
Extracellular Ca2+ (Ca2+o) is a crucial regulator of epidermal homeostasis and its receptor, the Ca2+-sensing receptor (CaSR), conveys the Ca2+o signals to promote keratinocyte adhesion, differentiation, and survival via activation of intracellular Ca2+ (Ca2+i) and E-cadherin-mediated signaling. Here, we took genetic loss-of-function approaches to delineate the functions of CaSR in wound re-epithelialization. Cutaneous injury triggered a robust CaSR expression and a surge of Ca2+i in epidermis. CaSR and E-cadherin were co-expressed at the cell-cell membrane between migratory keratinocytes in the nascent epithelial tongues. Blocking the expression of CaSR or E-cadherin in cultured keratinocytes markedly inhibited the wound-induced Ca2+i propagation and their ability to migrate collectively. Depleting CaSR also suppressed keratinocyte proliferation by down-regulating the E-cadherin/epidermal growth factor receptor (EGFR)/mitogen-activated protein kinase (MAPK) signaling axis. Blunted epidermal Ca2+i response to wounding and retarded wound healing were observed in the keratinocyte-specific CaSR knockout (EpidCasr−/−) mice, whose shortened neo-epithelia exhibited declined E-cadherin expression and diminished keratinocyte proliferation and differentiation. Conversely, stimulating endogenous CaSR with calcimimetic NPS-R568 accelerated wound re-epithelialization through enhancing the epidermal Ca2+i signals and E-cadherin membrane expression. These findings demonstrated a critical role for the CaSR in epidermal regeneration and its therapeutic potential for improving skin wound repair.
INTRODUCTION
Ca2+ maintains the normal homeostasis of mammalian epidermis by regulating keratinocyte adhesion, differentiation, and survival (Calautti et al., 2005; Yuspa et al., 1989), and growing evidence substantiate its involvement in wound repair. An elevation of Ca2+ concentration is detected in the wound bed and surrounding fluid within minutes after injury (Jungman et al., 2012; Lansdown et al., 1999), and elevated Ca2+ is the determining factor for wound fluid to stimulate cell motility in keratinocytes (Grzesiak and Pierschbacher, 1995). Increasing Ca2+ levels in the extracellular milieu of wound bed accelerates skin wound closure in mice (Kawai et al., 2011). Furthermore, mechanical or laser wounding triggers a rapid and transient increase in Ca2+i that spread from wound site to neighboring cell layers in epithelial sheets (Tsutsumi et al., 2013) and the epidermis of C. elegans (Xu and Chisholm, 2011) and embryos of Drosophila and Xenopus (Razzell et al., 2013; Soto et al., 2013), and blocking the Ca2+i propagation inhibits the ability of epithelial cells to close wound (Agle et al., 2010; Xu et al., 2012). These findings suggest that the surge of Ca2+i mobilization is one of the earliest signals produced at wound sites to trigger epithelial healing (Cordeiro and Jacinto, 2013; Wood, 2012).
The CaSR, a member of the family C G-protein coupled receptor, senses the changes in Ca2+o levels and initiates diverse cellular responses in epidermal keratinocytes. Activated CaSR instigates phospholipase C (PLC)-mediated Ca2+i accumulation and coordinates Ca2+i mobilizations from internal stores and across membrane channels through direct interactions of the receptor with various Ca2+i modulators, i.e. 1,4,5-trisphosphate receptor (IP3R), Ca2+-ATPase, and PLCγ1 (Tu et al., 2007). CaSR also activates the Rho GTPase-mediated signaling to facilitate the actin-cytoskeleton remodeling and the formation of E-cadherin/catenin adherens junction (AJ) (Tu et al., 2011; Tu and You, 2014), which play an obligatory role in transducing the outside-in signals by activating and integrating various intracellular signaling cascades. Assembly of AJs stimulates MAPK pathway through the recruitment and activation of EGFR to control cell proliferation and migration (Fedor-Chaiken et al., 2003; Pece and Gutkind, 2000) and engages phosphatidylinositol 3-kinase (PI3K) to activate Akt pathway to promote keratinocyte survival and differentiation (Calautti et al., 2005; Pang et al., 2005; Pece et al., 1999). Moreover, the E-cadherin/PI3K/phosphatidyl inositol 4-phosphate 5-kinase 1α (PIP5K1α) signaling complex activates PLC-γ1 via phosphatidylinositol 3,4,5-triphosphate to sustain elevated Ca2+i levels as keratinocytes differentiate (Xie and Bikle, 2007; Xie et al., 2009). CaSR-deficient keratinocytes display blunted Ca2+i response to Ca2+o due to depleted internal stores and aberrant Ca2+i influx and severely impaired intercellular adhesion (Tu et al., 2007; Tu et al., 2008). EpidCasr−/− mice, in which the Casr gene is deleted specifically in the keratinocytes, exhibit a delay in permeability barrier formation during embryonic development and the skins of adult mice manifest a loss of the epidermal Ca2+ gradient, impaired keratinocyte differentiation, abnormal sphingolipid metabolism, and defective permeability barrier (Tu et al., 2012).
Our previous studies show that restricting dietary calcium or deleting CaSR exacerbate the deficit in wound healing in mice caused by deletion of the vitamin D receptor (Vdr) from keratinocytes (Oda et al., 2016; Oda et al., 2017), implicating the interaction of calcium and vitamin D signaling in regulating repair processes. Wound re-epithelialization is mostly affected by the concurrent deletion of Vdr and Casr genes due to perturbance of a number of pathways relevant to wound healing, including β-catenin and AJ signaling (Oda et al., 2017). As recent studies show that VDR controls the renewal and activation of epidermal stem cells during wound repair via the β-catenin pathway (Oda et al., 2018), the role of calcium in wound healing remains indistinguishable in the models examined. To surely identify the mechanism underlying the calcium action in wound healing, we deleted CaSR in keratinocytes in vivo and in vitro in this study. Importantly, our results demonstrated the requirement for CaSR in the initiation of acute epidermal Ca2+i signaling and the formation of the E-cadherin-containing AJ involved in the proliferation and migration of the keratinocytes to re-epithelialize the wound and their subsequent differentiation to regenerate the epidermis.
RESULTS
Ablation of CaSR Delays Wound Repair
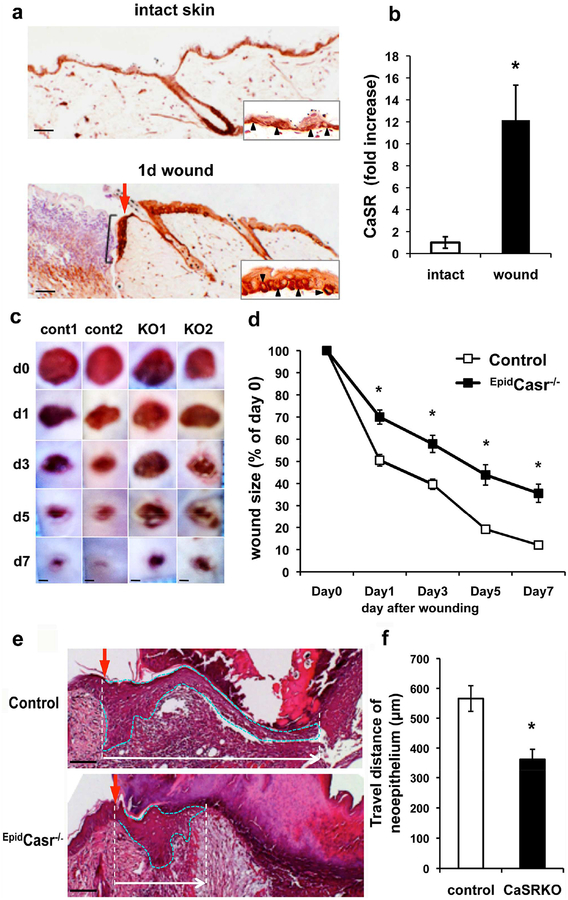
As a first step to define the role of CaSR in wound repair, we compared the CaSR expression in intact and puncture-wounded skin. Immunohistochemistry showed that the CaSR was present in all epidermal cell layers in the undamaged skin with predominant expression in the basal keratinocytes of the interfollicular epidermis (Figure 1a, upper panel). CaSR expression was significantly increased one day after injury in the thickened epidermis at the wound margins and in the nascent epithelial tongue above the wound bed (Figure 1a, lower panel). QPCR analyses of RNA extracted from the wounds confirmed an increase (12-fold) in CaSR message level (Figure 1b) compared to the intact skins. To examine the impact of CaSR ablation on wound repair, we compared the progression of wound healing in EpidCasr−/− mice and their Casrfl/fl control littermates by measuring the wound size. Wound closure in EpidCasr−/− mice was delayed as reflected by their significantly bigger wound sizes versus controls (Figure 1c and 1d). Measurements of the span of the neo-epithelia in wounds three days after injury showed that the distance of epidermal keratinocytes traveled across the wound beds was reduced by 37% in EpidCasr−/− mice (Figure 1e and 1f), suggesting that CaSR ablation delayed wound closure by effecting on re-epithelialization.
Figure 1. Delayed wound closure in the EpidCasr−/− mice.
Four-mm full thickness skin wounds were made on the back of (a, b) C57Bl/6J and (c-f) EpidCasr−/− mice and their control littermates. (a) Sections of intact and wounded skin were stained with an antibody against CaSR. Red arrow and black racket indicate the wound margin and nascent epithelial tongue, respectively. CaSR(+) basal keratinocytes (arrowheads) are shown in insets. (b) QPCR assessment of CaSR message level in wounded and intact skins. Data were presented as mean+/−SE (n=6), * P<0.01. (c) Representative images and (d) closure rate of skin wounds in EpidCasr−/− (KO) and control (cont) mice. Areas of the wounds were measured at specified time points after injury and normalized to the wound area at day 0. (e) H & E staining of wounds three days after injury. Epithelial tongues are outlined with dotted blue lines. Scale bar = 50 (e), 100 (a), or 800 μm (c). (f) The distances traveled by migratory keratinocytes are defined as the span between the wound margins and the tip of the epithelial tongues. Mean+/−SE (n=16–20). * P<0.01.
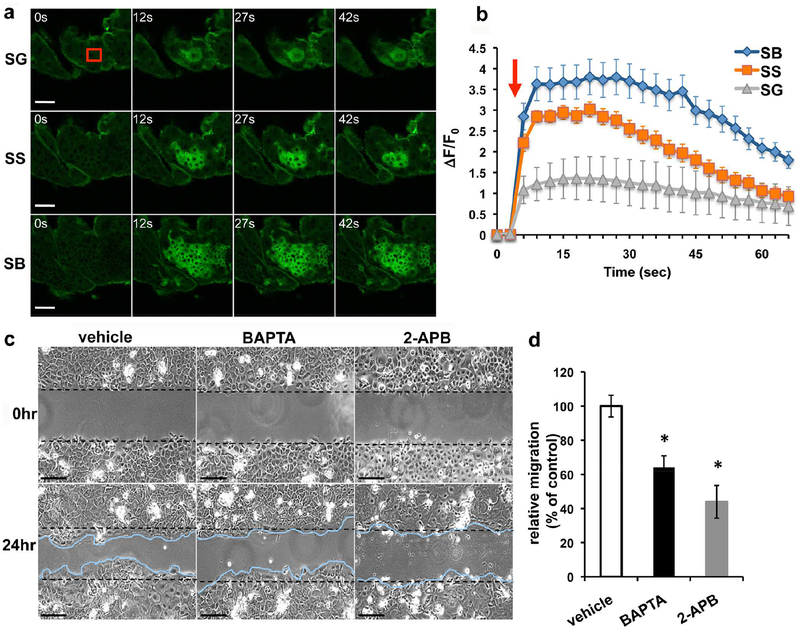
To determine whether injury alters the Ca2+i levels, we compared epidermal Ca2+i dynamics in response to wounding in transgenic mice expressing the fluorescent Ca2+ sensor protein GCaMP3 (Tian et al., 2009) under the control of keratin14 promoter. We performed focal laser wounding on mouse dorsal skins and imaged GCaMP signals in different epidermal cell layers using a confocal microscopy. Laser irradiation in the upper stratum granulosum (SG) immediately triggered a surge of Ca2+i, as indicated by the intensified GCaMP fluorescence, which radiated from wound site to the neighboring cell layers (Figure 2a). The Ca2+i signals were spread crosswise in SG and downward through the stratum spinosum (SS) to the stratum basale (SB) where Ca2+i propagated with a peak intensity >3 fold stronger than the SG (Figure 2b). These Ca2+i propagation events typically persisted for several minutes before dissipated. We subsequently employed an in vitro scratch wound model to test whether the transient Ca2+i surges are required for wound restitution. Scratching confluent keratinocyte sheets immediately induced Ca2+i waves spreading from the wound site to distant undamaged areas (Supplementary Figure S1a and S1b). We treated keratinocytes with BAPTA, an intracellular Ca2+ chelator, or 2-APB, an IP3R and TRP channel blocker, for 15 min prior to wounding to prevent Ca2+i propagation (Supplementary Figure S1c) and examined its impact on wound closure 24 hours later. Pretreatment with BAPTA or 2-APB markedly inhibited the ability of keratinocyte to migrate collectively to close wound (Figure 2c and 2d), demonstrating the prerequisite of Ca2+i signals in re-epithelialization.
Figure 2. Epidermal Ca2+i propagation is required for wound closure.
(a, b) Dorsal skins excised from EpidCasr+/+//GCaMP+/+ mice were subjected to focal laser irradiation in a 20×20 μm2 area (red box) in the upper stratum granulosum (SG). (a) Time-lapse GCaMP fluorescent images in SG, stratum spinosum (SS), and stratum basale (SB) before and after laser wounding. (b) Temporal changes in the intensity of GCaMP signal (ΔF/F0). Red arrow indicates the laser irradiation. Mean+/−SE (n=20–65 cells), P<0.01. The results are representative of 6 separate experiments. (c, d) Confluent keratinocyte cultures were treated with 0.1% DMSO (vehicle), 10μM BAPTA-AM, or 75μM 2-APB for 15 min prior to scratch wounding. (c) Representative micrographs of keratinocyte sheets at 0 and 24hr after scratch wounding. Scale bar = 50μm. Dashed black lines mark the boundaries of scratched areas. Migratory fronts of keratinocytes are outlined in blue. (d) Quantitation of scratched areas re-occupied by migrating keratinocytes were made 24hr after wounding and normalized to vehicle control. Mean+/−SE (n=12), * P<0.01.
Wound-induced Epidermal Ca2+i Responses and Keratinocyte Migration Require CaSR and E-cadherin
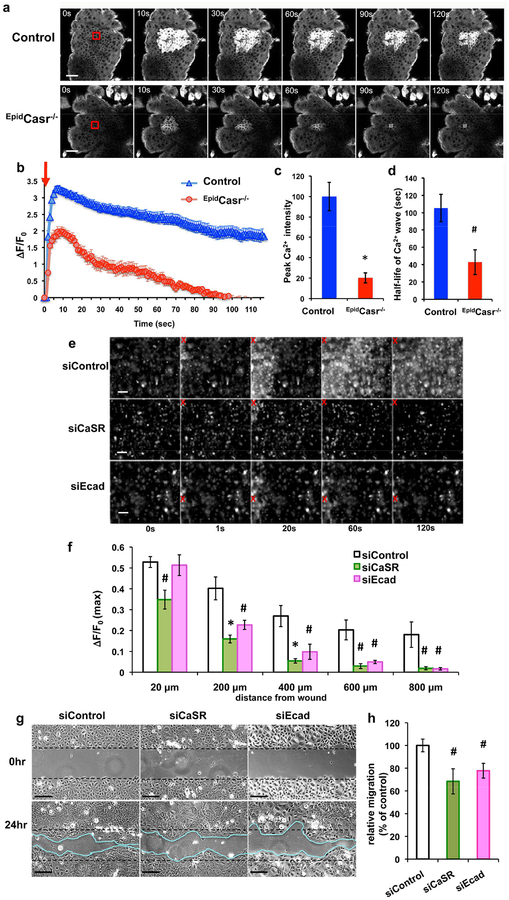
The robust Ca2+i propagation in SB keratinocytes (Figure 2b) coincided with the predominant CaSR expression, suggesting a role for CaSR in controlling Ca2+i mobilization responding to wounding. Indeed, as shown in Figure 3a, the epidermal Ca2+i wave triggered by laser wounding in the basal cell layer in the skins of CaSR knockout (EpidCasr−/−//GCaMP+/+) mice was reduced to 20–40% of the Ca2+i response in the control (EpidCasr+/+//GCaMP+/+) skins (Figure 3b, 3c, and 3d).
Figure 3. Blocking CaSR or E-cadherin expression diminished wound-induced Ca2+i propagation and collective keratinocyte migration.
(a-d) Dorsal skins excised from EpidCasr−/−//GCaMP+/+ (EpidCasr−/−) and EpidCasr+/+//GCaMP+/+ (control) mice were subjected to laser irradiation in the stratum basale (SB). (a) Time-lapse GCaMP fluorescent images of SB and the quantitative measurements of (b) magnitude, (c) peak intensity, and (d) duration of the Ca2+i propagation following wounding. Mean+/−SE (n=35–60 cells), * P<0.01, # P<0.05. (e-h) Human keratinocytes were transfected with scrambled siRNA (siControl) or specific siRNA targeting CaSR (siCaSR) or E-cadherin (siEcad) prior to scratch wounding. (e, f) Confluent cultures were loaded with calcium green before imaging. (e) Time-lapse fluorescent images and (f) quantitation of temporal changes in the intensity (peak ΔF/F0) of Ca2+i propagations at various distances from wound site. Red X indicates wounded sites. Data were presented as mean+/−SE of 6 separate experiments. *P<0.01, #P<0.05. (g) Representative micrographs of keratinocyte sheets at 0 and 24hr after scratch wounding. Bar = 50 (a, g) or 100 μm (e). (h) Scratched areas re-occupied by migrating keratinocytes were quantified 24hr after wounding and normalized to siControl, (n=14), # P<0.05.
Our previous studies unveiled that E-cadherin is a critical downstream effector for CaSR in regulating keratinocyte survival, adhesion, and differentiation (Tu et al., 2008; Tu et al., 2011). Immunohistochemistry of 3-day-old wounds showed that CaSR and E-cadherin were co-expressed in all keratinocyte layers in the nascent epithelia including the migratory front (Supplementary Figure S2a, S2a’, S2b, and S2b’) where the expression of desmoglein1, a desmosome component, was excluded (Supplementary Figure S2c and S2c’). The intense presence of CaSR and E-cadherin in the cell-cell membrane between migratory keratinocytes located at the tip of the epithelial tongues (Supplementary Figure S2a’’ and S2b’’) suggested their involvement in mediating collective keratinocyte migration during re-epithelialization. To test this idea, we examined the effects of gene silencing of CaSR and E-cadherin on wound restitution. Blocking CaSR expression inhibited the translocation of E-cadherin from cytoplasmic compartment to cell membrane (Supplementary Figure S3a). Consistent with the role of E-cadherin-dependent AJ in the CaSR-mediated Ca2+i accrual (Tu et al., 2011; Xie et al., 2009), inhibiting the expression of either CaSR or E-cadherin with CaSR- (siCaSR) or E-cadherin-targeting siRNA (siEcad) (Supplementary Figure S3a and S3b) profoundly diminished the wound-induced Ca2+i propagation (Figure 3e and 3f) and, the collective directional keratinocyte migration after wounding (Figure 3g and 3h). These data indicate that CaSR and E-cadherin modulate the epithelial sheet migration, at least partly, via Ca2+i signals.
CaSR Ablation Impairs Keratinocyte Proliferation and Differentiation During Wound Reepithelialization
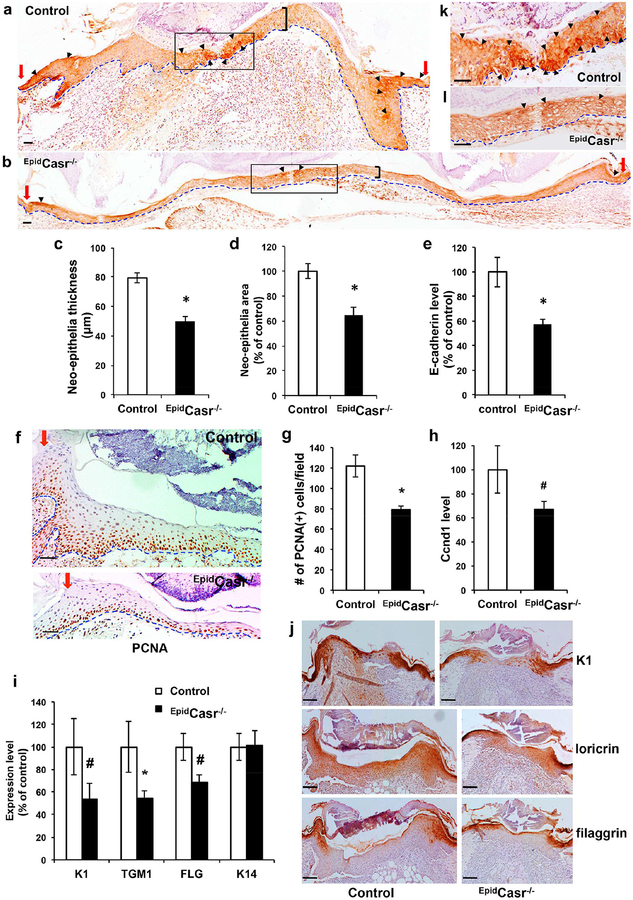
To ascertain that wound re-epithelialization was impacted by CaSR ablation, we compared the morphology and gene expression of neo-epithelia in 5-day-old skin wounds from EpidCasr−/− mice and control littermates. The neo-epithelia in EpidCasr−/− mice (Figure 4b) appeared thinner than those in the controls (Figure 4a and 4c) with reduced numbers of cell layers comprised of flattened nucleated keratinocytes. Measurement of neo-epithelial areas showed a 35% decrease in EpidCasr−/− mice (Figure 4d). Immunohistochemical staining of wound sections for proliferating cell nuclear antigen (PCNA) showed decreased numbers of proliferating keratinocytes in the neo-epithelia in EpidCasr−/− mice (Figure 4f and 4g), correlated with a declined cyclin D1 (Ccnd1) expression (Figure 4h).
Figure 4. CaSR ablation reduced E-cadherin expression, keratinocyte proliferation, and differentiation in the neo-epithelia.
Skin wounds were excised from EpidCasr−/− mice and control littermates (a-h, k, l) four or (i-j) five days after injury, sectioned, and stained with antibodies against (a, b) E-cadherin, (f) PCNA, or (j) differentiation markers. Basement membranes are outlined with dotted blue lines. Red arrows denote the wound margins and black brackets mark the spans of neo-epithelia. Arrowheads indicate areas with the strongest staining of E-cadherin in the cell membrane. Boxed areas in a and b are enlarged and shown in panel k and l, respectively. Bar = 50 (a, b, f, k, l) or 100 m (j). The (c) thickness and (d) tissue size of neo-epithelia and (g) the number of PCNA(+) cells in representative fields were quantified and presented as mean+/−SE (n=16). Expression of E-cadherin (e), cyclin D1 (h) and differentiation markers (i) in wounds were determined by qPCR and normalized to the levels in control mice (n=6–8); * P<0.01, # P<0.05.
Immunohistochemistry detected strong E-cadherin localizations in the cell-cell membrane in the migratory keratinocytes at the tip of the epithelial tongues and in the suprabasal keratinocytes adjacent to the wound margins in control mice (Figure 4a and 4k), while the E-cadherin expression was significantly diminished in the neo-epithelia of EpidCasr−/− mice (Figure 4b and 4l). QPCR assays confirmed a 45% reduction in E-cadherin expression in wounds from EpidCasr−/− mice (Figure 4e) versus controls. Notably, epidermal differentiation in the neo-epithelia started in the suprabasal cell layers near the wound margins (Figure 4j), where intense cell-cell membrane localizations of E-cadherin were first noticed (Figure 4a and Supplementary Figure S2b), corroborating a role for E-cadherin-mediated cell-cell adhesion in promoting keratinocyte differentiation. Corresponding to a reduction of membrane-localized E-cadherin, qPCR analyses (Figure 4i) and immunohistochemical staining (Figure 4j) showed that the levels of keratinocyte differentiation markers keratin (K) 1, transglutaminase (TGM) 1, loricrin, and filaggrin (FLG) in the neo-epithelia of EpidCasr−/− mice were reduced by 32–47%.
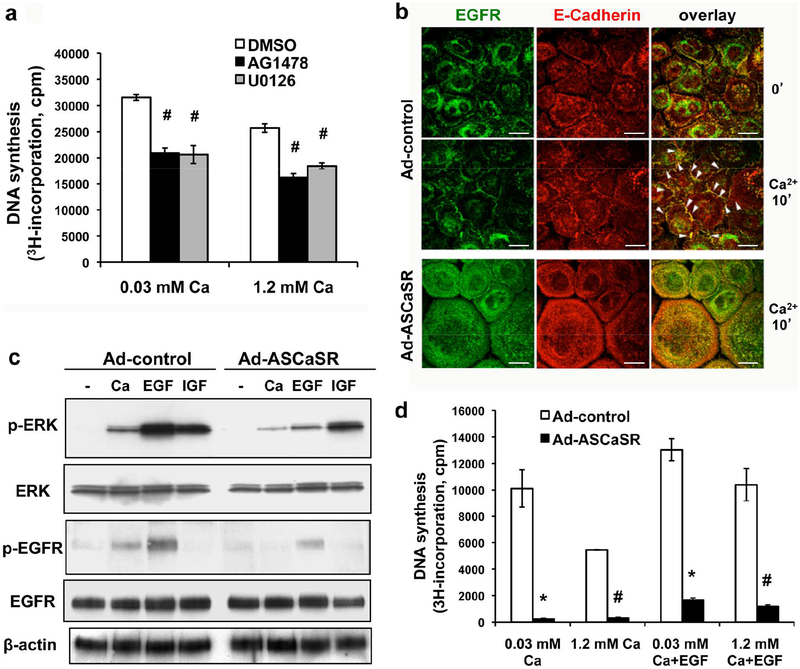
Cell proliferation during wound re-epithelialization is critically regulated by growth factors and their downstream signaling cascades, including the EGFR/MAPK signaling axis (Muller et al., 2012; Repertinger et al., 2004; Seeger and Paller, 2015). As shown in Figure 5a, blocking the activity of EGFR and MEK with specific inhibitors, AG1478 and U0126, respectively, inhibited cell proliferation in cultured keratinocytes. Growing evidence indicate that clustering membrane-localized E-cadherin is required to activate EGFR and stimulate MAPK in epithelial cells (Fedor-Chaiken et al., 2003; Pece and Gutkind, 2000). In epidermal keratinocytes, CaSR stabilizes AJ through recruitment and activation of Rho-family GTPase and induction of cytoskeletal reorganization (Tu et al., 2011; Tu and You, 2014). Fluorescence immunostaining (Supplementary Figure S4a, S4b, S4c), and co-immunoprecipitation (Supplementary Figure S4d) demonstrated that blocking CaSR expression with adenoviruses carrying a full-length CaSR antisense cDNA (Ad-ASCaSR; Supplementary Figure S3c) suppressed the Ca2+-induced trans-localization of RhoA and E-cadherin to the cell membrane, hence inhibited the formation of E-cadherin/β-catenin adhesion complex and the insertion of cytoskeletal actin into AJ at cell-cell contact. We then tested whether inhibition of CaSR expression affected the E-cadherin/EGFR interaction and MAPK activation. Fluorescence immunostaining (Figure 5b) demonstrated that inducing the formation of cell-cell contacts by raising Ca2+o concentration from 0.03 to 2 mM substantially increased the colocalization of EGFR with E-cadherin at the cell membrane in the control keratinocytes, whereas inhibiting CaSR expression by Ad-ASCaSR reduced the association of EGFR with E-cadherin (Figure 5b). The E-cadherin/EGFR interaction was verified by co-immunoprecipitation of plasma membrane protein using antibodies for either protein (Supplementary Figure S5a). Additionally, Ad-ASCaSR infection markedly suppressed the ability of Ca2+ and EGF to activate EGFR and ERK, reflected by reduced levels of phosphorylated EGFR and phosphorylated ERK1/2, respectively, (Figure 5c, Ad-ASCaSR vs. Ad-control). The impact of CaSR depletion on ERK activation was specific for EGFR/MAPK signaling, since Ad-ASCaSR infection had no effect on the IGF-induced ERK phosphorylation, which is mediated via PI3K pathway (Haase et al., 2003). Conversely, EGFR inhibitor AG1478 inhibited Ca2+-stimulated phosphorylation of EGFR and ERK (Supplementary Figure S5b), substantiating the crosstalk between CaSR and EGFR pathways. As a consequence of the diminished E-cadherin/EGFR/ERK signaling, CaSR depletion greatly inhibited keratinocyte proliferation (Figure 5d).
Figure 5. Blocking CaSR expression abolished interactions between E-cadherin and EGFR and suppressed EGFR-mediated ERK activation and keratinocyte proliferation.
(a) Keratinocytes were pre-treated with vehicle (0.1% DMSO), 5μM AG1478, or 5μM U0126 for 30 min and cell proliferation was assessed in EGF-free medium containing 0.03 or 1.2 mM CaCl2 for 24 hrs in the presence of [3H]thymidine. (b, c, d) Keratinocytes were infected with adenoviruses carrying antisense CaSR cDNA (Ad-ASCaR) or empty viral vector (Ad-control) in medium with 0.03 mM CaCl2 prior to calcium or growth factor treatments. (b) Cells were exposed to 2 mM CaCl2 for 15 min to induce formation of cell-cell junctions. Fluorescence immunostaining was performed using antibodies against E-cadherin (red) and EGFR (green). Overlapped staining sites were visualized as yellow. Arrowheads indicate the colocalization of EGFR with E-cadherin at the cell membrane. Bar = 20 μm. (c) Keratinocytes were treated with EGF (50 ng/ml) or IGF1 (50 ng/ml) for 5 min, or CaCl2 (2mM) for 15 min. Protein levels of ERK, phosphorylated ERK (p-ERK), EGFR, and phosphorylated EGFR (p-EGFR) were assessed by immunoblotting analyses of cell lysates. β-actin was used as a loading control. (d) Cell proliferation was assessed by [3H]thymidine incorporation in medium with or without supplementation of EGF (0.1 ng/ml) for 24 hrs.
Activating Endogenous CaSR Facilitates Wound Healing
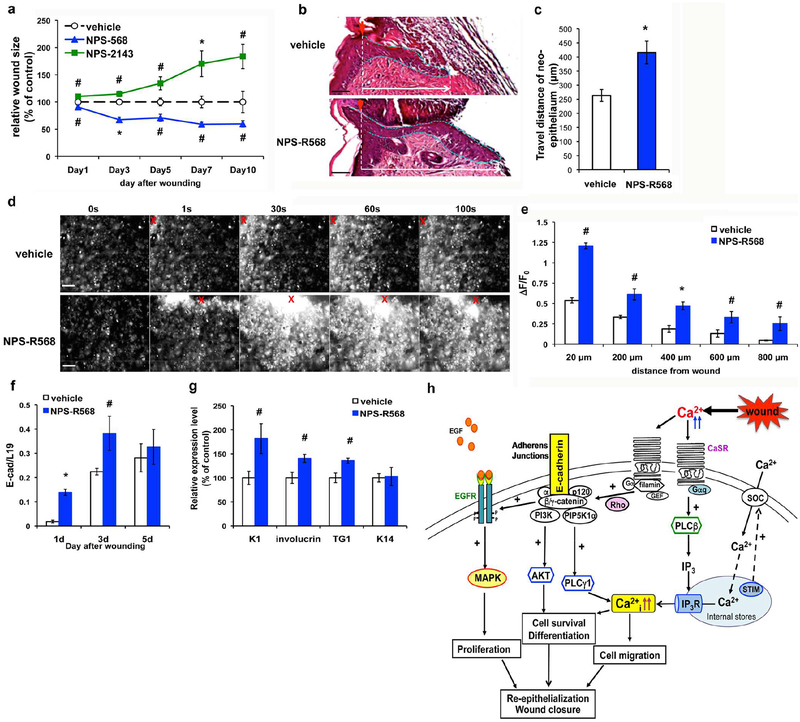
To test whether manipulating endogenous CaSR activity affects wound repair, we topically treated wounds on the dorsal skins of wild-type C57Bl/6J mice with allosteric CaSR agonist (calcimimetic) or antagonist (calcilytic) and examined their effects on wound closure. As shown in Figure 6a, the calcimimetic NPS-R568 accelerated, whereas the calcilytic NPS-2143 delayed, wound closure. Measurements of the length of the neo-epithelia in 3-day-old wounds demonstrated that NPS-R568 increased wound re-epithelialization by 56% (Figure 6b and 6c), correlated with the ability of NPS-R568 to augment the intensity and expanse of the wound-induced Ca2+i waves (Figure 6d and 6e). QPCR analyses of wounded skins showed that topical NPS-R568 treatment up-regulated E-cadherin expression (Figure 6f) and increased the levels (30–73%) of differentiation markers in the neo-epithelia (Figure 6g). Also, as shown in Supplementary Figure S6, pre-treating keratinocyte sheets with NPS-R568 increased the total and cell membrane-localized E-cadherin after wounding. These data demonstrated that activating endogenous epidermal CaSR effectively facilitated epidermal wound repair by enhancing the Ca2+i and E-cadherin signaling.
Figure 6. Activating endogenous CaSR augmented wound-induced Ca2+i propagation and re-epithelialization.
(a) Four-mm full thickness skin wounds on the back of C57Bl/6J mice were topically treated with vehicle (0.05% DMSO), 0.1 nmole NPS-R568, or 0.1nmole NPS-2143 daily. Sizes of the wounds were measured at times indicated and normalized to the control wounds treated with vehicle. (b) H & E staining of wounds treated with NPS-R568 or vehicle for three days. Epithelial tongues are outlined with dotted blue lines. (c) The distances traveled by migratory keratinocytes in neo-epithelium were measured and presented as mean+/−SE (n=8), *P<0.01. (d, e) Cultured keratinocyte sheets were loaded with calcium green and pretreated with 5μM NPS-R568 or vehicle for 15 minutes prior to scratch wounding. (d) Time-lapse fluorescent images. Red Xs indicate wounded sites. Bar = 50 (b) or 100 μm. (e) Quantitation of peak ΔF/F0 at various distances from wound site. Mean+/−SE of 6 separate experiments. *P<0.01, #P<0.05. Expressions of (f) E-cadherin and (g) epidermal differentiation markers in wounds treated with NPS-R568 or vehicle were assessed by qPCR. Mean+/−SE (n=8); * P<0.01, # P<0.05. (h) A model depicting CaSR-mediated regulation of wound re-epithelialization. Details were described in Discussion.
CaSR Ablation in Keratinocytes Impacts on Acute immune Response and Dermal Repair
To examine whether deleting CaSR from keratinocytes influences other wound repair processes, we evaluated inflammatory cell infiltration and the distribution of the myofibroblasts in the dermal granulation tissue. While fluorescence immunostaining of 1-day-old skin wounds using CD45 antibody (Supplementary Figure S7a) showed that EpidCasr−/− mice and control mice exhibited comparable leukocytes infiltration near the wound margins; however, qPCR analyses of skin wounds (Supplementary Figure S7b) revealed a 39–58% reduction in the expressions of pro-inflammatory cytokines (IL-1β, IL-6, and TNFα) and regulators for immune response (cathelicidin, TLR2, MCP1, MIP1α, and Cox2) in EpidCasr−/−. Furthermore, as the message levels of various dermal extracellular matrix (ECM) were equivalent in EpidCasr−/− and control wounds five days after injury (Supplementary Figure S7d), the number of α-smooth muscle actin (αSMA)-positive myofibroblasts (Supplementary Figure S7c) and the expression of several ECM-remodeling enzymes (MMP3, 9, and 10) were decreased by 33–53% in EpidCasr−/− (Supplementary Figure S7d). It was conceivable that these changes in acute immune responses and dermal remodeling also contributed to the delayed wound healing in EpidCasr−/− mice.
DISCUSSION
Ca2+ is a key regulator of keratinocyte differentiation, but its actions and underlying mechanisms in wound healing remain unclear. In the present study, we discovered that epidermis responds to injury with a profound increase in CaSR expression and our results demonstrated pivotal roles played by the epidermal CaSR in initiating early Ca2+i responses and E-cadherin signaling to stimulate keratinocyte proliferation, migration, and differentiation for wound reepithelialization.
The rapid induction of the Ca2+i propagation at a wound site signifies Ca2+i as a transcription-and translation-independent damage signal to initiate epithelial healing (Cordeiro and Jacinto, 2013). Wound-elicited Ca2+i waves protect corneal endothelium from excess apoptosis (Justet et al., 2016). In Drosophila embryos epidermal Ca2+i surge instigates H2O2 release via activation of a NAPDH oxidase to recruit inflammatory cells at the wound site (Razzell et al., 2013). The epidermal Ca2+i signal acts upstream of Rho-GTPases to modulate actin polymerization and actin-myosin network constriction to close wound in nematodes and Xenopus embryos, respectively (Soto et al., 2013; Xu and Chisholm, 2011). In this study we showed that wound-induced Ca2+i propagation is required for effective epithelial sheet migration (Figure 2c). Interestingly, we uncovered differential Ca2+i responses to wounding in separate keratinocyte layers of mammalian stratified epidermis (Figure 2a), with the strongest response in the basal layer where CaSR was highly expressed and cells were activated to proliferate and migrate after wounding. In line with the obligatory role of CaSR in controlling Ca2+i mobilization in keratinocytes (Tu et al., 2007), inhibiting CaSR expression abolished wound-induced epidermal Ca2+i response, suppressed collective directional keratinocyte migration (Figure 3), and impeded wound restitution in vivo (Figure 1).
Despite conventional views linking the down-regulation of E-cadherin with increased cell motility, E-cadherin appears to promote epithelial migration under several circumstances (Kardash et al., 2010; Rodriguez et al., 2012). In Drosophila, E-cadherin functions as an integrator of mechanical signals and is necessary for collective directional migration of border cell clusters (Cai et al., 2014). E-cadherin-dependent traction forces of the leading-edge cells coordinate migration of renal epithelial sheets (Li et al., 2012). E-cadherin acts downstream to CaSR to mediate keratinocyte sheet migration likely through regulating intercellular adhesion and Ca2+i accrual (Xie et al., 2009). Membrane expression of E-cadherin was reduced in the leading edges of the shortened epithelial tongues in EpidCasr−/− wounds (Figure 4b), consistent with the decreased migration rate of CaSR- or E-cadherin-deficient keratinocytes in vitro (Figure 3g).
Members of the EGF family induce the rapid proliferation and migration of keratinocytes at the wound edge through the EGFR/MAPK signaling cascade to promote re-epithelialization (Haase et al., 2003; Loo et al., 2011; Repertinger et al., 2004; Shirakata et al., 2005). The engagement of E-cadherin in newly formed cell contacts is critical for recruitment and sensitization of EGFR to allow responses to low levels of EGF and activation of downstream MAPK and Rac1 signaling cascades (Betson et al., 2002; Fedor-Chaiken et al., 2003; Pece and Gutkind, 2000). The findings that depleting CaSR inhibited EGF-stimulated phosphorylation of EGFR and ERK (Figure 5c) while EGF inhibitor blocked Ca2+-induced activation of ERK (Supplementary Figure S5b) support a signaling scheme in which the E-cadherin/EGFR interface couples the CaSR to MAPK activation. Depleting CaSR reduced the interaction between E-cadherin and EGFR (Figure 5b and Supplementary Figure S5a) and diminished MAPK signaling (Figure 5c), leading to decreased cell proliferation in vitro (Figure 5d) and in vivo (Figure 4f). Furthermore, in the neo-epithelia of EpidCasr−/− mice the membrane-localized E-cadherin in suprabasal keratinocyte layers was markedly reduced (Figure 4b), similar to the lack of E-cadherin adhesion complex formation in CaSR-depleted keratinocytes in vitro (Supplementary Figure S4), rendering the delay of epidermal differentiation during wound repair (Figure 4j).
In contrast to the negative impacts of genetic knockdown or pharmacological inhibition of CaSR on re-epithelialization, stimulating endogenous CaSR with an allosteric activator, NPS-R568, amplified the magnitude and duration of injury-induced Ca2+i propagation and accelerated wound closure (Figure 6). During cytokine-guided intestinal restitution, elevated Rho signaling stimulates the membrane localizations of E-cadherin and F-actin to reduce the paracellular space and enhance the epithelium barrier integrity (Hwang et al., 2012). Likewise, NPS-R568 increased the localization of E-cadherin to the cell-cell junctions between keratinocytes during wound restitution (Supplementary Figure S6c). Our studies support that Ca2+/CaSR signal is essential for cutaneous wound repair and convey clinical implications for the use of calcimimetics in improving outcomes of wound healing.
In summary, our previous and current studies support a working model for the actions of the CaSR in wound re-epithelialization (Figure 6h). Injury increases CaSR expression in keratinocytes at the wound margins and raises the Ca2+ levels in the extracellular milieu of those cells, activating the CaSR and downstream pathways: (1) CaSR couples to the Gαq and activates PLC to generate IP3, which triggers calcium release from internal stores and subsequent Ca2+ influx through membrane channels, resulting in a transient increase in Ca2+i. The Ca2+i signals promote the reorganization of actin cytoskeleton and changes of cell adhesion likely through Rho-mediated signaling, enabling keratinocyte to migrate collectively. (2) CaSR forms a signaling complex with Rho and GEF to stabilize the E-cadherin/catenin adhesion complexes at the cell-cell contacts, which in turn activate EGFR and downstream MAPK pathway to support keratinocyte proliferation. (3) E-cadherin-mediated AJs engage PIP5K1α and PI3K and stimulate their effectors, PLCγ 1 and Akt, to promote Ca2+i accumulation, cell survival, and differentiation. CaSR integrates extracellular cues (wounding, Ca2+o) and intracellular signals (Ca2+i, E-cadherin, MAPK) to coordinate keratinocyte proliferation, migration, and differentiation to re-epithelialize the wounds.
Unexpectedly, our results indicate that the loss of keratinocyte CaSR had broader effects on wound repair besides re-epithelialization. It was uncertain whether the altered acute immune response and dermal matrix remodeling in EpidCasr−/− mice skin (Supplementary Figure S7) was a direct consequence of impaired functions of keratinocytes, which have the ability to produce pro-inflammatory factors and ECM modifying enzymes, or an indirect outcome due to the impacts on immune cells and fibroblasts. Further studies are needed in the future to address these questions and decipher the underlying mechanism.
MATERIALS AND METHODS
Mice and In Vivo Wounding
Generation of Casrfl/fl control and EpidCasr−/− mice, in which the entire transmembrane domain and intracellular portion of the CaSR is deleted in Keratin 14 (Krt14)-expressing keratinocytes, and the verification of gene ablation were described previously (Tu et al., 2012). EpidCasr−/− and K14-cre were bred with ROSA-GCaMP3 mice (Ai38; Jackson Laboratory, Sacramento, CA) to create EpidCasr−/−//GCaMP+/+ and EpidCasr+/+//GCaMP+/+, respectively. All mice were bred into the C57Bl/6J background and maintained on a normal calcium (1.3%) diet. Mice at 8 weeks of age were used in wound repair assessment. Four-mm full-thickness skin wounds were made on the backs of mice with a biopsy punch after hair depilation. To calculate the wound closure rate the wounds were photographed daily during a 7 to 10-day recovery period, and areas of the wounds were quantitated using ImageJ software (NIH). Wounds from at least 8 mice per genotype were evaluated. Wounds and their 2 mm-wide peripheral areas were excised for immunohistochemical or RNA extraction for gene expression analyses at the specified time points after wounding. The Institutional Animal Care and Use Committee at the San Francisco Veterans Affairs Medical Center approved all protocols.
Human Keratinocytes and Gene Silencing
Human neonatal foreskin keratinocytes (NHKs) were cultured in serum-free growth medium 154CF (Thermo Fisher Scientific) containing Human Keratinocyte Growth Supplement (Thermo Fisher Scientific) and 0.03 mM CaCl2. Pre-confluent keratinocytes were transfected with 20 nM siRNA targeting CASR and CDH1 (E-cadherin) (siGENOME SMART pool; Dharmacon Inc., Lafayette, CO) using PepMute siRNA transfection reagent (SignaGen Laboratories, Ijamsville, MD), or infected with the Ad-ASCaR adenovirus (60 pfu/cell) in growth medium containing 0.03 mM CaCl2 and cultured for 3 days prior to growth factor treatments or calcium (2 mM) exposure for AJ induction. The committee for Human Research at the San Francisco Veteran Affair Medical Center and the University of California San Francisco approved the use of human keratinocytes.
Keratinocyte Migration Assay (Scratch Wounding Model)
Confluent keratinocyte cultures were exposed to 7.5 μg/mL mitomycin for 4 hours to stop cell proliferation. The cultures were switched to medium containing 1.2 mM CaCl2 for 1h before scratched with a sterile 20 μL-pipette tip (Mettler Toledo Rainin, LLC, Oakland, CA). In some experiments, keratinocyte cultures were pre-treated with vehicle (0.1% DMSO), 1,2-bis(2-aminophenoxy)ethane-N ,N ,N’ ,N’-tetraacetic acid-AM (BAPTA-AM, 10μM) or 2-aminoethoxydiphenyl borate (2-APB, 75μM) (Sigma-Aldrich, St Louis, MO) for 15 min prior to wounding. Cell migration was performed in the presence of 0.6 mM CaCl2 and monitored by taking phase-contrast photographs on the same field before and after wounding. Migration efficiency was determined by quantitating the scratched areas that were re-occupied by migrating keratinocytes 24h after wounding using ImageJ. Statistical significance was evaluated using at least twelve different fields for four independent batches of keratinocytes.
Descriptions of other methods used in this study are available in the Supplementary Materials online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alicia Menendez and Rustin Zomorodi for their assistance in animal management and the Laboratory for Cell Analysis Core for providing access to two-photon excitation microscope. This work was supported by National Institutes of Health grants, RO1-AR056256 and RO1-AR067291, and Merit Review Awards IBX001066 and BX003453 from the Department of Veterans Affairs.
Abbreviations:
- CaSR
Ca2+-sensing receptor
- Ca2+i
intracellular Ca2+
- Ca2+o
extracellular Ca2+
- PLC
phospholipase C
- AJ
adherens junctions
- PI3K
phosphatidylinositol 3-kinase
- PIP5K1α
phosphatidyl inositol 4-phosphate 5-kinase 1α
- siRNA
small interfering RNA
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal regulated kinase
- EGFR
epidermal growth factor receptor
- Vdr
vitamin D receptor
- α -SMA
smooth muscle actin-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Agle KA, Vongsa RA, Dwinell MB. Calcium mobilization triggered by the chemokine CXCL12 regulates migration in wounded intestinal epithelial monolayers. The Journal of biological chemistry 2010;285(21):16066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betson M, Lozano E, Zhang J, Braga VM. Rac activation upon cell-cell contact formation is dependent on signaling from the epidermal growth factor receptor. The Journal of biological chemistry 2002;277(40):36962–9. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, et al. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014;157(5):1146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. The Journal of biological chemistry 2005;280(38):32856–65. [DOI] [PubMed] [Google Scholar]
- Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nature reviews 2013;14(4):249–62. [DOI] [PubMed] [Google Scholar]
- Fedor-Chaiken M, Hein PW, Stewart JC, Brackenbury R, Kinch MS. E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes 2003;10(2):105–18. [PubMed] [Google Scholar]
- Grzesiak JJ, Pierschbacher MD. Shifts in the concentrations of magnesium and calcium in early porcine and rat wound fluids activate the cell migratory response. The Journal of clinical investigation 1995;95(1):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci 2003;116(Pt 15):3227–38. [DOI] [PubMed] [Google Scholar]
- Hwang S, Zimmerman NP, Agle KA, Turner JR, Kumar SN, Dwinell MB. E-cadherin is critical for collective sheet migration and is regulated by the chemokine CXCL12 protein during restitution. The Journal of biological chemistry 2012;287(26):22227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungman E, Pirot F, Maibach H. Ex vivo calcium percutaneous eggression in normal and tape-stripped human skin. Cutan Ocul Toxicol 2012;31(1):1–6. [DOI] [PubMed] [Google Scholar]
- Justet C, Hernandez JA, Torriglia A, Chifflet S. Fast calcium wave inhibits excessive apoptosis during epithelial wound healing. Cell Tissue Res 2016;365(2):343–56. [DOI] [PubMed] [Google Scholar]
- Kardash E, Reichman-Fried M, Maitre JL, Boldajipour B, Papusheva E, Messerschmidt EM, et al. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nature cell biology 2010;12(1):47–53; sup pp 1–11. [DOI] [PubMed] [Google Scholar]
- Kawai K, Larson BJ, Ishise H, Carre AL, Nishimoto S, Longaker M, et al. Calcium-based nanoparticles accelerate skin wound healing. PLoS One 2011;6(11):e27106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdown AB, Sampson B, Rowe A. Sequential changes in trace metal, metallothionein and calmodulin concentrations in healing skin wounds. J Anat 1999;195 (Pt 3):375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hartley R, Reiss B, Sun Y, Pu J, Wu D, et al. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell Mol Life Sci 2012;69(16):2779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo AE, Ho R, Halliwell B. Mechanism of hydrogen peroxide-induced keratinocyte migration in a scratch-wound model. Free Radic Biol Med 2011;51(4):884–92. [DOI] [PubMed] [Google Scholar]
- Muller AK, Meyer M, Werner S. The roles of receptor tyrosine kinases and their ligands in the wound repair process. Semin Cell Dev Biol 2012;23(9):963–70. [DOI] [PubMed] [Google Scholar]
- Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol 2016; 164:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Hu L, Nguyen T, Fong C, Tu CL, Bikle DD. Combined deletion of the vitamin D receptor and calcium-sensing receptor delays wound re-epithelialization. Endocrinology 2017; 158(6):1929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Hu L, Nguyen T, Fong C, Zhang J, Guo P, Bikle DD. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. The Journal of investigative dermatology 2018; pii: S0022–202X(18)31971–7. doi: 10.1016/j.jid.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JH, Kraemer A, Stehbens SJ, Frame MC, Yap AS. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signaling to E-cadherin function. The Journal of biological chemistry 2005;280(4):3043–50. [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. The Journal of biological chemistry 1999;274(27):19347–51. [DOI] [PubMed] [Google Scholar]
- Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. The Journal of biological chemistry 2000;275(52):41227–33. [DOI] [PubMed] [Google Scholar]
- Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 2013;23(5):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR enhances early healing after cutaneous incisional wounding. The Journal of investigative dermatology 2004;123(5):982–9. [DOI] [PubMed] [Google Scholar]
- Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin’s dark side: possible role in tumor progression. Biochim Biophys Acta 2012;1826(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger MA, Paller AS. The Roles of Growth Factors in Keratinocyte Migration. Adv Wound Care (New Rochelle) 2015;4(4):213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 2005;118(Pt 11):2363–70. [DOI] [PubMed] [Google Scholar]
- Soto X, Li J, Lea R, Dubaissi E, Papalopulu N, Amaya E. Inositol kinase and its product accelerate wound healing by modulating calcium levels, Rho GTPases, and F-actin assembly. Proceedings of the National Academy of Sciences of the United States of America 2013;110(27):11029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 2009;6(12):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Goto M, Denda M. Dynamics of intracellular calcium in cultured human keratinocytes after localized cell damage. Exp Dermatol 2013;22(5):367–9. [DOI] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. The Journal of investigative dermatology 2007;127(5):1074–83. [DOI] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. The calcium-sensing receptor-dependent regulation of cell-cell adhesion and keratinocyte differentiation requires Rho and filamin A. The Journal of investigative dermatology 2011;131(5):1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. The Journal of biological chemistry 2008;283(6):3519–28. [DOI] [PubMed] [Google Scholar]
- Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. The Journal of investigative dermatology 2012;132(10):2350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu CL, You M. Obligatory roles of filamin A in E-cadherin-mediated cell-cell adhesion in epidermal keratinocytes. J Dermatol Sci 2014;73(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W Wound healing: calcium flashes illuminate early events. Curr Biol 2012;22(1):R14–6. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. The Journal of biological chemistry 2007;282(12):8695–703. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Molecular biology of the cell 2009;20(6):1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chisholm AD. A Galphaq-Ca(2)(+) signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr Biol 2011;21(23):1960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Hsiao TI, Chisholm AD. The wounded worm: Using C. elegans to understand the molecular basis of skin wound healing. Worm 2012;1(2):134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. The Journal of cell biology 1989;109(3):1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.