Abstract
Background and Aims:
Population-based studies often use plasma fatty acids (FAs) as objective indicators of FA intake, especially for n-3 FA and linoleic acid (LA). The relation between dietary and circulating FA in cardiometabolic patients is largely unknown. We examined whether dietary n-3 FA and LA were reflected in plasma lipid pools in post-myocardial infarction (MI) patients.
Methods and Results:
Patients in Alpha Omega Cohort filled out a 203-item food-frequency questionnaire from which eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), alpha-linolenic acid (ALA), and LA intake were calculated. Circulating individual FA (% total FA) were assessed in cholesteryl esters (CE; n=4,066), phospholipids (PL; n=838), and additionally in total plasma for DHA and LA (n=739). Spearman correlation coefficients (rs) were calculated for dietary vs. circulating FA. Circulating FA were also compared across dietary FA quintiles, overall and in subgroups by sex, obesity, diabetes, statin use, and high alcohol intake.
Patients were on average 69 years old and 79% was male. Moderate correlations between dietary and circulating levels were observed for EPA (rs~0.4 in CE and PL) and DHA (rs ~0.5 in CE and PL, ~0.4 in total plasma), but not for ALA (rs ~0.0). Weak correlations were observed for LA (rs 0.1 to 0.2). Plasma LA was significantly lower in statin users and in patients with a high alcohol intake.
Conclusions:
In post-MI patients, dietary EPA and DHA were well reflected in circulating levels. This was not the case for LA, which may partly be influenced by alcohol use and statins.
Keywords: dietary fatty acids, food frequency questionnaire, circulating fatty acids, plasma fatty acids, n-3 fatty acids, linoleic acid, biomarkers, cardiac patients, Alpha Omega Cohort
INTRODUCTION
There is continuing interest in the use of circulating fatty acids (FA) as biomarkers of dietary FA intake in epidemiological studies [1, 2], often in relation to cardiometabolic outcomes [3, 4]. Dietary FA that are frequently studied include n-3 FA, i.e. eicosapentaenoic acid (EPA, 20:5 n-3), docosahexaenoic acid (DHA, 22:6 n-3), alpha-linolenic acid (ALA, 18:3 n-3), and linoleic acid (LA, 18:2 n-6). LA intakes in Western diet generally range from 4 to 6 energy percent (en%), whereas intake of n-3 FA is much lower (mean intake of <500 mg/d for EPA+DHA and 1.0-1.5 g/d for ALA) [5, 6].
In cohorts of mainly healthy participants, correlations between dietary intake and plasma circulating levels are mostly moderate for EPA, DHA (0.4-0.6), and LA (0.2-0.3), and generally weak for ALA (<0.1) [1]. However, circulating levels of FA, which are expressed as proportions of total FA, are not only affected by dietary intake but also by fatty acid metabolism. For example, the endogenous synthesis of saturated and monounsaturated fatty acids may affect the proportions of other FA [1, 7]. Furthermore, metabolic impairments such as abdominal obesity, type 2 diabetes and/or impaired liver function may influence desaturase activity [8, 9], and hence circulating levels of n-3 and n-6 FA. To what extent lipid-modifying agents (e.g. statins) and lifestyle factors, such as alcohol use, affect circulating FA is not yet clear.
In our cohort of drug-treated post-myocardial infarction (MI) patients, we examined whether dietary LA and n-3 FA intake were reflected in circulating FA, using various plasma lipid pools (plasma cholesteryl esters, CE; plasma phospholipids, PL; total plasma). We also investigated whether these relations were modified by cardiometabolic risk factors, alcohol intake and statin use, which could have implications for using these biomarkers of intake in patient populations.
METHODS
Study design and population
The Alpha Omega Cohort (AOC) is a prospective cohort study and a continuation of the Alpha Omega Trial, which has been described in detail elsewhere [10, 11]. Briefly, 4,837 Dutch men and women aged 60-80 y who had an MI up to 10 years before study enrollment participated in a 3-year intervention study of n-3 fatty acid supplementation, which had no effect on recurrent cardiovascular events [11]. The study was approved by the medical ethics committee at the Haga Hospital (The Hague, The Netherlands) and all patients provided written informed consent before enrollment.
The present study is a cross-sectional analysis of baseline data of the AOC (2002-2006) (Supplemental Figure S1). Patients with missing food frequency questionnaire (FFQ) data (n=453) and patients with unreliable dietary data (n=238) were excluded, as previously described [12]. Patients with missing data on plasma CE (n=75) or >5% unknown FA in CE (n=5) were also excluded. In total, 4,066 patients with complete data of dietary intake and measurements of plasma FA in CE were analyzed. Circulating FAs were additionally measured in PL in 838 patients (21% of the cohort) and in total plasma in 739 patients (18% of the cohort).
Dietary assessment
Patients filled out a 203-item FFQ, which was an extended and updated version of a previously biomarker-validated FFQ to estimate the intake of different types of fat and cholesterol. The FFQ has shown high reproducibility for assessing daily intake of food groups including edible fats and oils, with Spearman r up to 0.9. Intakes of total energy, total fat and saturated fat estimated by FFQ correlated well with estimates from the dietary history method (Pearson r ~0.8). Double data entry was performed and returned questionnaires were checked. In case of missing data on relevant parts of the FFQ, patients were called by dietitians for additional data collection. The FFQ included specific questions on types and brands of fats and oils used in food preparation. Food items were linked to the 2006 Dutch food composition table (NEVO) [13] to calculate total energy (MJ/d) and nutrient intakes, including EPA, DHA, ALA and LA. Dietary intakes of EPA and DHA were expressed in mg/d, ALA in g/d and LA as % of total energy intake (en%). Additionally, individual n-3 FA were reported as en% and LA as g/d. Patients used no fish oil supplements, which was an exclusion criterion for the Alpha Omega Trial [10]. The FFQ included detailed questions on alcoholic beverages from which total ethanol intake (g/d) was computed. Alcohol use was categorized as no intake (0 g/d), low intake (>0 to 10 g/d), moderate intake (>10 to 20 g/d for women and >10 to 30 g/d for men), or high intake (>20 g/d for women and >30 g/d for men).
Risk factor assessment
Demographic data and information on lifestyle factors, medical history and medication use were obtained by questionnaires. Physical activity was assessed with the validated Physical Activity Scale for the Elderly (PASE) and classified as low (no activity or only light activity, defined as ≤ 3 metabolic equivalents (METs)), medium (>0 to <5 days per week of moderate or vigorous activity, >3 METs), or high (≥5 days per week of moderate or vigorous activity, >3 METs) [14]. Medication was coded according to the Anatomical Therapeutic Chemical (ATC) Classification system, with codes C10AA and C10B for statins [15]. Patients underwent a physical examination at home or in the hospital by trained research nurses. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Obesity was defined as BMI≥30 kg/m2. A total of 30 mL of venous blood was drawn from each patient, of which 10 mL was collected in EDTA tubes for FA composition analysis. If patients had their last meal ≥8 hours before blood sampling, they were considered fasted. Samples for plasma FA composition analysis were stored at −80°C. Serum lipids and plasma glucose were measured using standard kits as described previously [10]. Diabetes mellitus was considered present in case of a self-reported physician’s diagnosis, use of anti-diabetes medication, or plasma glucose ≥7.0 mmol/L (fasting) or ≥11.1 mmol/L (non-fasting) [16].
Measurement of FA in plasma CE and PL
Circulating FA in CE and PL pools were analyzed at the Division of Human Nutrition and Health, Wageningen University, the Netherlands. Briefly, extracted total lipids from plasma samples were separated into CE and PL by solid phase extraction silica columns. FA were transesterified into fatty acid methyl esters and analyzed by gas chromatography. FA were identified by comparing retention times with FA standards, and expressed as weight percentage relative to total FA (% total FA). Laboratory analyses of FA in CE took place in different years. We observed stable FA over 6-9 years of storage at −80°C, shown by high intraclass correlation coefficients (r>0.90) for EPA, DHA, ALA and LA. Others have also observed stable FA in CE, PL and triglycerides over a time span of 10-12 years when stored at −80°C [17, 18]. Detailed laboratory and quality control methods are described in Supplemental Methods and Supplemental Table 1.
Measurement of FA in total plasma
Stored plasma EDTA samples (300 μl) were sent in 2014 to Nightingale Health, Ltd (Helsinki, Finland; formerly Brainshake, Ltd) for profiling of metabolic markers. A high-throughput proton nuclear magnetic resonance (1H-NMR) metabolomics platform was used to quantify 231 metabolic measures, including selected FA (linoleic acid and DHA) as reported in detail elsewhere [19]. Linoleic acid and DHA were expressed as relative to the total FA content in total plasma (% of total FA) and in absolute concentrations (in mmol/L). To monitor quality of the measurements, two control samples, of which one sample represented human plasma, were placed in each box of 96 samples. The measurement CV, as reported by the Nightingale Health, was calculated for a control sample over 10 boxes and was <5% for both LA and DHA [20].
Statistical analysis
Normality of distribution of values for all variables was checked visually using histograms and Q-Q plots. Patient characteristics were computed as means ± standard deviation (SD) for normally distributed variables, medians (interquartile range) for non-normally distributed variables, and percentages for categorical variables. Dietary and circulating FA were expressed as medians (interquartile range) because of skewed distributions, and as mean ± SD (linoleic acid only).
Dietary FA were related to circulating levels in different plasma lipid pools by means of Spearman rank correlation coefficients (rs) and partial rs, adjusting for age (y), sex, and total energy intake. Fisher’s z transformation was used to obtain 95% confidence interval (95% CI) for correlation coefficients. Circulating FA were also reported in quintiles of dietary FA to obtain insight in potential dose-response relationships. Least-squares means and 95% CI of circulating FA in quintiles of dietary FA were obtained by multivariable linear regression, adjusting for age, sex, total energy intake, obesity (absent vs. present), physical activity (low, medium, or high), smoking status (current, former, or never), fasting status at blood collection (yes or no), total serum cholesterol (mmol/L), measurement year (for CE), high alcohol intake, and prevalent diabetes. No adjustment was made for statin use to avoid multicollinearity with serum cholesterol, which was already in the model. Missing data on adjustment variables were 5.9% of total sample. All analyses were done using complete cases. The median values of intake in quintiles of dietary FA were used as explanatory variables to calculate p-for-trend.
For plasma FA in CE (n=4,066), analyses in quintiles of dietary FA intake were repeated in strata of sex, obesity (absent vs. present), prevalent diabetes (absent vs. present), statin use (no vs. yes), and high alcohol intake (>30 vs. ≤30 g/d in men; >20 vs. ≤20 g/d in women), while holding all other variables at the observed population mean. Partial Spearman correlations (rs) between dietary and circulating FA were also obtained in these subgroups. Analyses were carried out with SAS 9.4 (Cary, NC, USA). A two-sided p-value <0.05 was considered statistically significant.
RESULTS
The cohort with CE data was 69±6 years old and mostly male (79%). Most patients were treated with cardiovascular drugs, including statins (86%). Twenty percent had diabetes, 23% had obesity, and 16% had a high alcohol intake (Table 1). Patient characteristics in subcohorts of other lipid pools were roughly similar, except for use of statins, use of antihypertensive drugs, serum lipids and fasting status, which were slightly different in patients with PL data.
Table 1.
Characteristics of patients of the Alpha Omega Cohort by availability of FA in different plasma lipid poolsa
| Cholesteryl esters (n=4,066) | Phospholipids (n=838) | Total plasma (n=739) | |
|---|---|---|---|
| Age (y) | 69.0 ± 5.6 | 69.1 ± 5.7 | 70.0 ± 5.8 |
| Men, n (%) | 3,226 (79.3) | 652 (77.8) | 578 (78.2) |
| BMI (kg/m2)b | 27.7 ± 3.8 | 27.9 ± 4.0 | 27.6 ± 3.8 |
| Obesity, n (%)b,c | 947 (23.3) | 201 (24.0) | 166 (22.5) |
| Total daily energy intake (MJ)d | 8.0 ± 2.0 | 8.0 ± 2.0 | 8.0 ± 2.1 |
| Alcohol intake, n (%)e | |||
| No | 198 (4.9) | 55 (6.6) | 40 (5.4) |
| Low | 2,157 (53.1) | 443 (52.9) | 379 (51.3) |
| Moderate | 1,065 (26.2) | 215 (25.7) | 202 (27.3) |
| High | 646 (15.9) | 125 (14.9) | 118 (16.0) |
| Smoking status, n (%)b | |||
| Never | 660 (16.2) | 142 (17.0) | 117 (15.8) |
| Former | 2,740 (67.4) | 571 (68.2) | 496 (67.1) |
| Current | 665 (16.4) | 124 (14.8) | 126 (17.1) |
| Physical activity, n (%)b,f | |||
| Low | 1,646 (40.7) | 310 (37.2) | 342 (46.5) |
| Medium | 1,531 (37.8) | 345 (41.4) | 253 (34.4) |
| High | 868 (21.5) | 178 (21.4) | 140 (19.1) |
| Medication use, n (%) | |||
| Statins | 3,494 (85.9) | 750 (89.5) | 630 (85.3) |
| Anti-hypertensive drugs | 3,644 (89.6) | 789 (94.2) | 668 (90.4) |
| Anti-diabetes drugs | 593 (14.6) | 126 (15.0) | 116 (15.7) |
| Prevalent diabetes, n (%)g | 811 (20.0) | 182 (21.7) | 164 (22.2) |
| Plasma glucose (mmol/L)b | 5.60 (5.06-6.59) | 5.98 (5.38-6.97) | 5.64 (5.10-6.67) |
| Serum lipids (mmol/L)h | |||
| Total cholesterol | 4.71 ± 0.96 | 4.45 ± 0.90 | 4.72 ± 0.98 |
| LDL cholesterol | 2.57 ± 0.82 | 2.26 ± 0.75 | 2.59 ± 0.83 |
| HDL cholesterol | 1.28 ± 0.34 | 1.37 ± 0.34 | 1.28 ± 0.35 |
| Triglycerides | 1.66 (1.21-2.31) | 1.59 (1.18-2.31) | 1.63 (1.18-2.30) |
| Fasting at blood collection, n (%)i | 1,390 (35.5) | 226 (28.2) | 249 (35.1) |
Values are shown as mean ± standard deviation, median (interquartile range) or n (%);
Missing values for <1% of patients;
Obesity defined as BMI ≥30 kg/m2;
To convert MJ to kcal, multiply by 238.8;
Categorized as “no: 0 g/d”, “low: >0 to 10 g/d”, “moderate: >10 to 20 g/d for women and >10 to 30 g/d for men”, and “high: >20 g/d for women and >30 g/d for men”;
Categorized as “low: no activity or only light activity (≤ 3 METs), “medium: >0 to <5 days per week of moderate or vigorous activity (>3 METs), and “high: ≥5 days per week of moderate or vigorous activity;
Defined as a self-reported physician’s diagnosis, use of anti-diabetes medication, or plasma glucose ≥7.0 mmol/L (fasting) or ≥11.1 mmol/L (non-fasting);
Missing values for 61 patients for total cholesterol, HDL-C and TG, and for 253 patients for LDL-C;
Defined as consumption of the last meal ≥8 hours before blood sampling; missing values for 155 patients.
Relation between intake and circulating n-3 FA
In our study population, median dietary intake of EPA+DHA was 108 mg/d (0.05 energy%) and intake of ALA was 0.45 energy%. Circulating EPA was comparable in CE (median: 1.06% of total FA) and PL (1.13%). DHA was higher in PL (4.49%) than in CE (0.67%) and total plasma (1.24%). Median circulating ALA was 0.49% in CE and 0.14% in PL (Table 2). Individual circulating n-3 FA were strongly positively correlated across different plasma lipid pools (all rs ≥0.80; Supplemental Table 2).
Table 2.
Circulating and dietary n-3 fatty acids and linoleic acid in different plasma lipid pools, and correlations between circulating and dietary fatty acids in the Alpha Omega Cohort
| Plasma lipid pool | Circulating levels of fatty acid | Dietary intake of fatty acid | Spearman rank correlation (rs)a |
|||||
|---|---|---|---|---|---|---|---|---|
| Crude | P | Partial | P | |||||
| Cholesteryl esters (n=4,066) | EPA (%) | 1.06 (0.79-1.52) | EPA (mg/d) | 41.3 (12.6-72.2) | 0.39 (0.36, 0.41) | <0.001 | 0.39 (0.37, 0.42) | <0.001 |
| DHA (%) | 0.67 (0.53-0.84) | DHA(mg/d) | 67.4 (29.7-114.9) | 0.44 (0.41, 0.46) | <0.001 | 0.45 (0.43, 0.48) | <0.001 | |
| ALA (%) | 0.49 (0.41-0.59) | ALA (g/d) | 0.93 (0.68-1.33) | −0.01 (−0.04, 0.02) | 0.56 | −0.02 (−0.05, 0.01) | 0.25 | |
| LA (%) | 49.9±5.0 | LA (en%) | 5.7±2.2 | 0.16 (0.13, 0.19) | <0.001 | 0.15 (0.12, 0.18) | <0.001 | |
| Phospholipids (n=838) | EPA (%) | 1.13 (0.85-1.56) | EPA (mg/d) | 45.0 (12.8-74.1) | 0.35 (0.29, 0.41) | <0.001 | 0.37 (0.31, 0.43) | <0.001 |
| DHA (%) | 4.49 (3.68-5.39) | DHA(mg/d) | 72.3 (30.9-120.2) | 0.48 (0.42, 0.53) | <0.001 | 0.50 (0.45, 0.55) | <0.001 | |
| ALA (%) | 0.14 (0.11-0.17) | ALA (g/d) | 0.92 (0.67-1.30) | −0.00 (−0.07, 0.06) | 0.89 | −0.01 (−0.07, 0.06) | 0.85 | |
| LA (%) | 18.7±3.0 | LA (en%) | 5.8±2.2 | 0.11 (0.05, 0.18) | 0.001 | 0.10 (0.03, 0.17) | 0.003 | |
| Total plasmab (n=739) | DHA (%) | 1.24 (1.05-1.55) | DHA (mg/d) | 63.5 (25.8-109.5) | 0.41 (0.35, 0.47) | <0.001 | 0.43 (0.37, 0.49) | <0.001 |
| LA (%) | 26.3±3.6 | LA (en%) | 5.7±2.3 | 0.17 (0.10, 0.24) | <0.001 | 0.16 (0.08, 0.23) | <0.001 | |
| DHA (mmol/L) | 0.14 (0.12-0.18) | DHA (mg/d) | 63.5 (25.8-109.5) | 0.34 (0.27, 0.40) | <0.001 | 0.37 (0.31, 0.43) | <0.001 | |
| LA (mmol/L) | 3.05±0.72 | LA (en%) | 5.7±2.3 | 0.12 (0.04, 0.19) | 0.002 | 0.13 (0.06, 0.20) | <0.001 | |
Values are shown as mean ± standard deviation, median (interquartile range);
rs (95% CI), with P-values, for association between dietary and circulating fatty acids in corresponding plasma pools. Partial correlations were adjusted for age, sex, and total energy intake;
EPA and ALA were not measured in total plasma;
ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
Moderate correlations between dietary and circulating EPA and DHA were observed in crude analysis and in analysis with adjustment for age, sex and total energy intake (Table 2). Partial correlations were 0.37-0.39 for EPA and 0.43-0.50 for DHA (Table 2). Partial correlations between dietary and circulating ALA were weak (rs −0.02 in CE; Table 2), as was the correlation between dietary ALA and circulating EPA (rs −0.06 in CE; data not in Table). Partial correlations obtained in a subsample that had fasted >12 hours showed similar results (0.35-0.38 for EPA, 0.38-0.42 for DHA, and ~0.01 for ALA in CE; data not in Table). Figure 1 shows results from multivariable linear regression analysis, with additional adjustment for obesity, physical activity, smoking status, high alcohol intake and prevalent diabetes. A positive dose-response relation between dietary and circulating levels (in CE) was observed for EPA and DHA, whereas for ALA no association was found (Figure 1). Similar associations were observed in PL and total plasma (Supplemental Figures 2-3).
Figure 1.
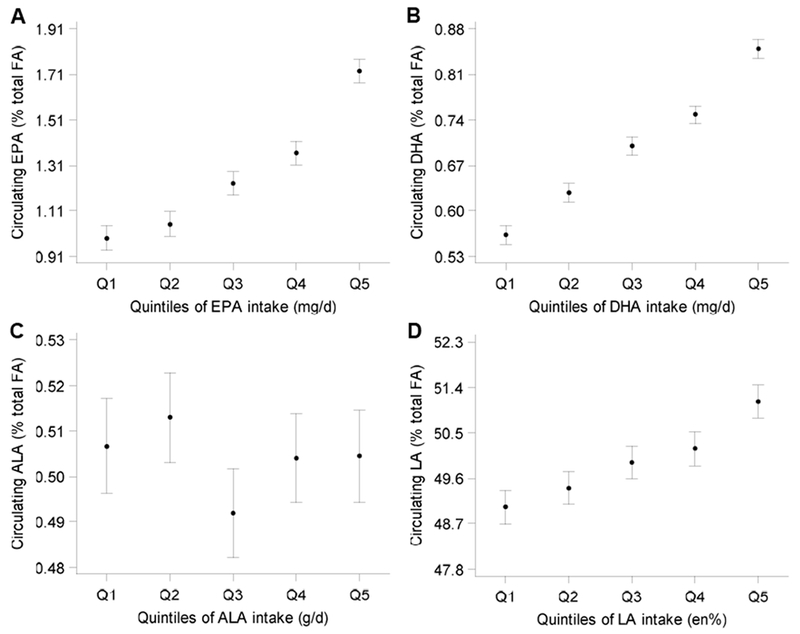
Relation between dietary and circulating (A) EPA, (B) DHA, (C) ALA, and (D) LA in plasma cholesteryl esters (n=4,066). Least-squares means and 95%CI were adjusted for age, sex, total energy intake, obesity, physical activity, smoking status, fasting status, total serum cholesterol, measurement year, alcohol intake, and prevalent diabetes.
Intake ranges: EPA (mg/d; Q1:0.0-8.3, Q3:28.7-50.9, Q5:85.6-692.0); DHA (mg/d; Q1:0.1-23.4, Q3:51.3-84.3, Q5:134.5-1061.6); ALA (g/d; Q1:0.19-0.63, Q3:0.83-1.07, Q5:1.46-3.89); LA (en%; Q1:1.2-3.8, Q3: 4.8-5.9, Q5:7.2-19.3).
P-for-trend <0.001 for EPA, DHA, and LA, and 0.80 for ALA.
ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acids; LA, linoleic acid.
Sex, obesity and diabetes did not affect overall circulating levels of n-3 FA (Supplemental Figures 4-8). Statin users, however, had higher circulating EPA and DHA and lower circulating ALA compared to non-statin users. Circulating EPA (but not DHA and ALA) was also higher in patients with a high alcohol intake. In all subgroups, there was a dose-response relation between dietary and circulating EPA and DHA (but not for ALA), comparable to the total cohort. Correlation coefficients between dietary and circulating n-3 FA were also comparable to the total cohort in most subgroups (Supplemental Table 3). In patients with a high alcohol intake, however, dietary ALA was more strongly correlated with circulating ALA in PL compared to the total cohort, but this may be a chance finding due to small sample size.
Relation between intake and circulating linoleic acid
Dietary linoleic acid intake was 5.7 ±2.2 en% (12.1±5.5 g/d) and levels of circulating linoleic acid were 49.9 ±5.0 % in CE, 18.7 ±3.0 % in PL, and 26.3 ±3.6 % in total plasma. Correlations for linoleic acid across different plasma lipid pools ranged from 0.71 to 0.85, with the strongest correlation between CE and total plasma (rs= 0.85). Partial correlations between dietary and circulating linoleic acid (Table 2) were <0.2 for all plasma lipid pools, with some evidence for a dose-response relationship (Figure 1D). Partial correlations in subsample that had fasted >12 hours showed similar results (rs=0.06-0.16; data not in Table). When dietary linoleic acid was expressed as % of total fat, the partial correlation with linoleic acid in CE was 0.13, and when expressed in g/d, it was 0.15 (data not shown in Table).
Subgroup analyses for dietary and circulating linoleic acid (in CE) are shown in Figure 2A-E. Men had somewhat higher circulating linoleic acid than women. Circulating linoleic acid did not strongly differ between obese and non-obese or diabetic and non-diabetic patients. However, circulating linoleic acid was substantially lower in statin users (Figure 2D) and in patients with a high alcohol intake (Figure 2E). In all subgroups, there was a dose-response relation between dietary and circulating linoleic acid in CE, comparable to the total cohort (Figure 2A-E). The partial correlations between dietary and circulating linoleic acid within subgroups were also comparable to the total cohort, although some fluctuations were seen in PL and total plasma, resulting from smaller sample sizes (Supplemental Table 4).
Figure 2.
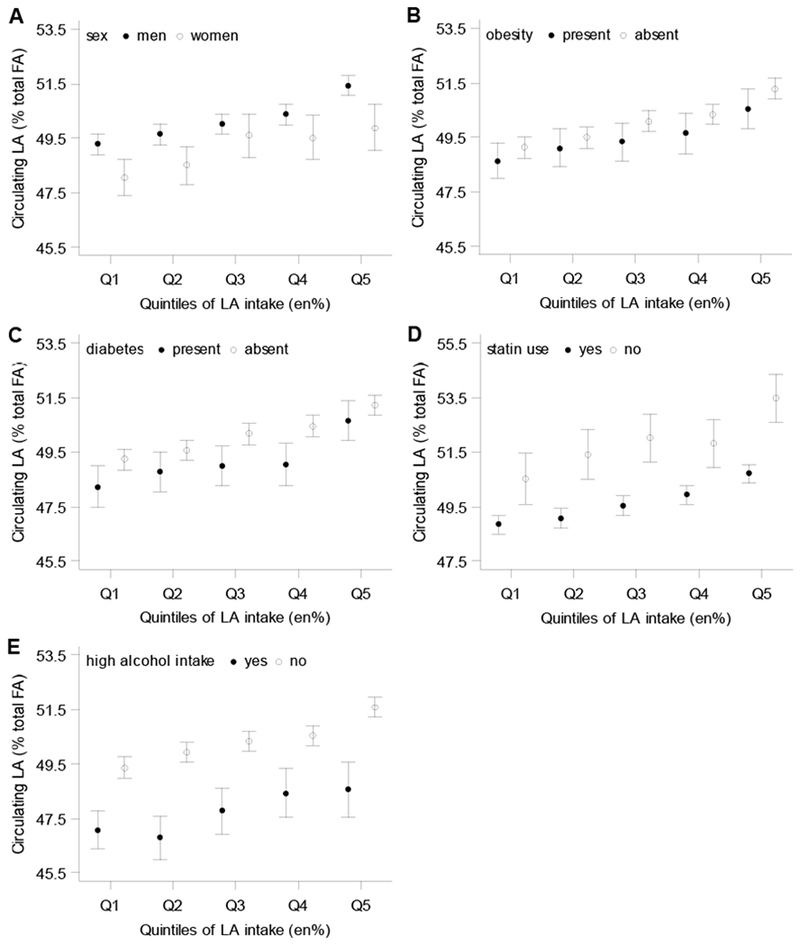
Relation between dietary and circulating linoleic acid (LA) in cholesteryl esters in strata of (A) sex, (B) obesity, (C) diabetes, (D) statin use, and (E) alcohol intake. Least-squares means and 95%CI were adjusted for age, sex, total energy intake, obesity, physical activity, smoking status, fasting status, total serum cholesterol, measurement year, alcohol intake, and prevalent diabetes. When stratified by statin use, total serum cholesterol was not included as adjustment variable in the model.
LA intake ranges (en%): Q1:1.2-3.8, Q3: 4.8-5.9, Q5:7.2-19.3.
P-for-trend <0.001 in all subgroups of sex, obesity, statin use, and prevalent diabetes, and in patients without high alcohol intake; P-for-trend=0.003 in patients with high alcohol intake.
P-values for interaction terms: sex, p=0.60; obesity, p=0.47; diabetes, p=0.79; statin use, p=0.18; alcohol intake, p=0.85.
DISCUSSION
In our cohort of post-MI patients, we observed moderate correlations between intake and circulating levels of EPA and DHA (0.4-0.5), and no correlations for ALA, which was consistent across various plasma lipid pools. Correlations between dietary and circulating linoleic acid were weak (<0.2). Circulating levels of LA appeared to be lower for statin users and patients with a high alcohol intake.
Circulating FA have repeatedly been used as biomarkers of FA intake in (relatively) healthy populations [1, 4]. For individual n-3 FA, the present study in post-MI patients showed similar correlations between dietary and circulating levels to those in population-based studies. In previous reviews [1, 21] and in the more recent Multi-Ethnic Study of Atherosclerosis (MESA) cohort [22], correlations varied between 0.2 and 0.5 for EPA and DHA. For ALA, weak correlations between dietary and circulating levels were also reported by others [21, 22]. Rapid oxidation of ingested ALA [23] and dietary measurement error may explain this finding. Conversion of ALA into EPA is not a likely explanation because the correlation between ALA intake and plasma EPA was only weak in our cohort.
We have shown dose-response relationships between dietary and circulating EPA and DHA in CE irrespective of cardiometabolic risk factors and statin use. In four longitudinal UK and Finnish cohorts, statin use was associated with only a small increase in circulating proportion of DHA in total plasma [24]. In Australian adults, associations between dietary and circulating EPA and DHA in PL varied by sex, but were not modified by history of MI (3% of total participants), presence of diabetes (4%), lipid-lowering medication use (3%), or alcohol intake [25].
Correlations between dietary and circulating linoleic acid in the Alpha Omega Cohort were considerably weaker than in other studies, which were mostly conducted in non-patient populations (r=0.20-0.34) [25–29]. The unit for linoleic acid intake may possibly affect the size of the correlation. However, in our study, expressing dietary linoleic acid as % total fat instead of energy% did not result in stronger correlation. Our findings are in line with the MESA study that showed a weak correlation (r=0.13) between dietary linoleic acid, expressed in g/d, and circulating linoleic acid measured in phospholipids [22]. Part of our cohort was not in fasting state during blood sampling. However, this cannot explain the absence of a correlation since we obtained similar results in a subsample of the cohort that had fasted >12 hours. This finding is in line with the study by Hodge et al in 4,439 Australian adults [25] who also found that fasting status did not modify the association between dietary and circulating unsaturated FA. The average level and range of linoleic acid intake in our cohort was comparable to that of the general older Dutch population [30] and to that of other populations with Western diets [25–29]. Therefore, we consider lack of contrast in linoleic acid intake not a likely explanation for the weak correlation with circulating linoleic acid.
In our study, patients with high alcohol intake had lower mean circulating linoleic acid than patients who consumed less alcohol, despite having the same dietary linoleic acid intake. In several other studies, alcohol intake was inversely associated with circulating linoleic acid and directly with palmitic acid (16:0) [28, 31]. Palmitic acid and linoleic acid are two major FA in CE, PL and total plasma [1]. Circulating palmitic acid does not reflect only palmitic acid intake but also its endogenous synthesis. Changes in palmitic acid will indirectly affect the measured proportion of linoleic acid, since they are expressed as relative concentrations in lipid pools [32].
We observed generally lower circulating linoleic acid levels in statin users compared to nonusers, despite similar linoleic acid intake. A decrease in circulating linoleic acid levels associated with statin use was also observed in total serum of four population-based cohorts [24]. Hodge et al. [25], however, did not find that use of lipid-lowering drugs (3% of cohort) modified the association between dietary and circulating FA in 4,439 Australian adults. In vitro research in human white blood cells suggested that statins may enhance the activity of desaturase enzymes, which converts LA into arachidonic acid (20:4 n-6). However, the underlying mechanisms remain unclear [33].
Around 20% of our cohort suffered from diabetes. For a given linoleic acid intake, linoleic acid in CE tended to be lower in diabetic patients than in other patients. In a cross-sectional analysis of 70-year-old Swedish men, a lower linoleic acid in CE was associated with reduced insulin sensitivity [34], and another study observed a lower plasma CE linoleic acid in patients with non-alcoholic fatty liver disease compared to age- and sex-matched healthy individuals [9]. Diabetic patients may be at higher risk of liver dysfunction, which could possibly affect circulating linoleic acid and its correlation with dietary intake. However, we did not assess insulin resistance or liver dysfunction to confirm this hypothesis.
Correlations between dietary and circulating FA partly depend on the dietary assessment method. We used an FFQ that provided very detailed information on sources of fat and types and brands of fats and oils used for food preparation. Using this FFQ, we found a strong inverse association of dietary unsaturated FA (replacing saturated and trans FA) with cardiovascular mortality in the Alpha Omega Cohort [12]. In the current analysis, we also found expected correlations of dietary with circulating n-3 FA, further supporting the validity of this FFQ.
A limitation needs to be considered while interpreting our results. The proportion of patients in different subgroups was unbalanced (e.g. only 14% non-statin users). This complicated the comparison of correlation coefficients in different subgroups and formal testing of interaction. Our results in subgroups therefore need confirmation in other studies.
To conclude, circulating EPA and DHA in various plasma lipid pools are good indicators of dietary intake in a cohort of post-MI patients, whereas circulating ALA is not. Concerning linoleic acid, circulating levels may not well reflect dietary linoleic acid intake. This may partly be due to alcohol use and/or statins.
Supplementary Material
Highlights.
Population-based studies often use plasma fatty acids as objective indicator of fatty acids intake.
To what extent linoleic acid in blood reflects dietary intake in post-myocardial infarction patients is largely unknown.
Cardiometabolic risk factors and statin use could affect levels of plasma fatty acids.
Dietary marine n-3 fatty acids were well reflected in plasma levels, but linoleic acid was not.
Statin use and high alcohol intake were associated with lower plasma levels of linoleic acid; this could limit the utility of circulating linoleic acid as an indicator of dietary intake.
ACKNOWLEDGMENTS
This research received financial support from the Netherlands Organisation for Scientific Research (NWO) through its Graduate Programme on Food Structure, Digestion and Health. Funding for plasma fatty acid analyses was obtained from Unilever R&D and from the Dutch biobank collaboration BBMRI-NL. Data collection for the Alpha Omega Cohort was funded by the Dutch Heart Foundation (topdown program 200T401) and the National Institutes of Health (NIH/NHLBI grant no. R01HL076200).
We thank Eveline Waterham for management of Alpha Omega Cohort database and biobank and Dr. Paul Hulshof, Robert Hovenier and Marlies Diepeveen-de Bruin for analysis of plasma fatty acids.
Abbreviations:
- ALA
alpha-linolenic acid
- CE
cholesteryl esters
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acids
- MI
myocardial infarction
- PL
phospholipids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- [2].Wanders AJ, Alssema M, De Hoon SEM, Feskens EJM, van Woudenbergh GJ, van der Kallen CJ, et al. Circulating polyunsaturated fatty acids as biomarkers for dietary intake across subgroups: The CODAM and Hoorn Studies. Ann Nutr Metab 2018;72:117–25. [DOI] [PubMed] [Google Scholar]
- [3].Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39740 adults from 20 prospective cohort studies. Lancet Diabetes & Endocrinol 2017;5:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, et al. Ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: Pooling project of 19 cohort studies. JAMA Intern Med 2016;176:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harika RK, Eilander A, Alssema M, Osendarp SJM, Zock PL. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann Nutr Metab 2013;63:229–38. [DOI] [PubMed] [Google Scholar]
- [6].Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014;348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of fatty acids and insulin action. Ann N Y Acad Sci 2002;967:183–95. [DOI] [PubMed] [Google Scholar]
- [8].Alsharari ZD, Riserus U, Leander K, Sjogren P, Carlsson AC, Vikstrom M, et al. Serum fatty acids, desaturase activities and abdominal obesity – A population-based study of 60-year old men and women. PLoS One 2017;12:e0170684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Park H, Hasegawa G, Shima T, Fukui M, Nakamura N, Yamaguchi K, et al. The fatty acid composition of plasma cholesteryl esters and estimated desaturase activities in patients with nonalcoholic fatty liver disease and the effect of long-term ezetimibe therapy on these levels. Clin Chim Acta 2010;411:1735–40. [DOI] [PubMed] [Google Scholar]
- [10].Geleijnse JM, Giltay EJ, Schouten EG, de Goede J, Griep LMO, Teitsma-Jansen AM, et al. Effect of low doses of n-3 fatty acids on cardiovascular diseases in 4,837 post-myocardial infarction patients: Design and baseline characteristics of the Alpha Omega Trial. Am Heart J 2010;159:539–46.e2. [DOI] [PubMed] [Google Scholar]
- [11].Kromhout D, Giltay EJ, Geleijnse JM. n–3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26. [DOI] [PubMed] [Google Scholar]
- [12].Mölenberg FJ, de Goede J, Wanders AJ, Zock PL, Kromhout D, Geleijnse JM. Dietary fatty acid intake after myocardial infarction: a theoretical substitution analysis of the Alpha Omega Cohort. Am J Clin Nutr 2017;106:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Institute for Public Health and the Environment (RIVM). Nederlands Voedingsstoffenbestand (NEVO) (Dutch food composition database). Bilthoven: National Institute for Public Health and the Environment (RIVM); 2006. [Google Scholar]
- [14].Schuit AJ, Schouten EG, Westerterp KR, Saris WHM. Validity of the physical activity scale for the elderly (PASE): According to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol 1997;50:541–6. [DOI] [PubMed] [Google Scholar]
- [15].WHO Collaborating Centre for Drug Statistics Methodology Anatomical Therapeutic Chemical Classification System (ATC). Oslo: World Health Organization; 2009. [Google Scholar]
- [16].World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. Geneva: World Health Organization; 2006. [Google Scholar]
- [17].Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res 2010;51:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr 2000;54:367–72. [DOI] [PubMed] [Google Scholar]
- [19].Soininen P, Kangas AJ, Wurtz P, Tukiainen T, Tynkkynen T, Laatikainen R, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009;134:1781–5. [DOI] [PubMed] [Google Scholar]
- [20].Kettunen J, Demirkan A, Wurtz P, Draisma HHM, Haller T, Rawal R, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun 2016;7:11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Serra-Majem L, Nissensohn M, Øverby NC, Fekete K. Dietary methods and biomarkers of omega 3 fatty acids: a systematic review. Br J Nutr 2012;107:S64–S76. [DOI] [PubMed] [Google Scholar]
- [22].de Oliveira Otto MC, Wu JHY, Baylin A, Vaidya D, Rich SS, Tsai MY, et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 2005;45:581–97. [DOI] [PubMed] [Google Scholar]
- [24].Würtz P, Wang Q, Soininen P, Kangas AJ, Fatemifar G, Tynkkynen T, et al. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J Am Coll Cardiol 2016;67:1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O’Dea K, et al. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis 2007;17:415–26. [DOI] [PubMed] [Google Scholar]
- [26].Astorg P, Bertrais S, Laporte F, Arnault N, Estaquio C, Galan P, et al. Plasma n-6 and n-3 polyunsaturated fatty acids as biomarkers of their dietary intakes: a cross-sectional study within a cohort of middle-aged French men and women. Eur J Clin Nutr 2008;62:1155–61. [DOI] [PubMed] [Google Scholar]
- [27].Cabout M, Alssema M, Nijpels G, Stehouwer CDA, Zock PL, Brouwer IA, et al. Circulating linoleic acid and alpha-linolenic acid and glucose metabolism: The Hoorn study. Eur J Nutr 2016:2171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr 1995;62:564–71. [DOI] [PubMed] [Google Scholar]
- [29].Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81. [DOI] [PubMed] [Google Scholar]
- [30].Ocké MC, Buurma-Rethans E, de Boer E, Wilson-van den Hooven C, Etemad-Ghameslou Z, Drijvers J, et al. Diet of community-dwelling older adults : Dutch National Food Consumption Survey Older adults 2010-2012. Bilthoven: National Institute for Public Health and the Environment (RIVM); 2013. [Google Scholar]
- [31].Simon JA, Fong J, Bemert JJT, Browner WS. Relation of smoking and alcohol consumption to serum fatty acids. Am J Epidemiol 1996;144:325–34. [DOI] [PubMed] [Google Scholar]
- [32].Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol 2006;17:22–7. [DOI] [PubMed] [Google Scholar]
- [33].Risé P, Colombo C, Galli C. Effects of simvastatin on the metabolism of polyunsaturated fatty acids and on glycerolipid, cholesterol, and de novo lipid synthesis in THP-1 cells. J Lipid Res 1997;38:1299–307. [PubMed] [Google Scholar]
- [34].Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 1994;37:1044–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


