Abstract
The safety and efficacy of spironolactone is uncertain in end-stage renal disease. We randomized 129 maintenance hemodialysis patients to placebo (n=51) or spironolactone 12.5 mg (n=27), 25 mg (n=26), or 50 mg (n=25) daily for 36 weeks in a double-blind, placebo-controlled, multiple dosage trial to assess safety, tolerability and feasibility and to explore cardiovascular efficacy. The primary safety endpoints were hyperkalemia (potassium > 6.5 mEq/L) and hypotension requiring emergency department visit or hospitalization. Diastolic function was assessed by Doppler echocardiography. 125 participants (97%) completed dose escalation, with no significant difference in permanent study drug discontinuation between the groups (27.5% in placebo versus 16.7% in the combined spironolactone groups and 28% in the 50 mg group). Hyperkalemia frequency was similar between spironolactone and placebo (0.49 versus 0.50 events per patient-year) but demonstrated a significant linear trend due primarily to an increased event rate at the 50 mg dose (0.89 events per patient-year). The primary hypotension outcome was infrequent and similar with spironolactone and placebo (0.11 versus 0 events per patient-year). Gynecomastia was rare and did not differ significantly between groups. Change in diastolic function was similar with spironolactone and placebo. Spironolactone appears safe in carefully monitored maintenance hemodialysis patients, but did not affect cardiovascular parameters in this small study. Hyperkalemia occurs more frequently as dosage increases to 50 mg daily.
Keywords: hemodialysis, multiple dosage, randomized controlled trial, mineralocorticoid blockade
Introduction
Cardiovascular disease underlies the majority of deaths in patients with dialysis-dependent end stage renal disease (ESRD).1 Standard cardiovascular therapies have rarely been tested in this population and with disappointing results. For example, three trials of HMG-CoA reductase inhibitors found that neither overall nor cardiovascular mortality was reduced in hemodialysis (HD) patients treated with statins compared to placebo.2-4 These and other findings highlight the need to evaluate therapies to reduce cardiovascular morbidity and mortality specifically in patients receiving maintenance dialysis.5
Several lines of evidence suggest that in ESRD, the heart undergoes progressive fibrosis and rarefaction of the microvasculature6-8 — structural changes that predispose to arrhythmias and contribute to heart failure by reducing myocardial perfusion and disrupting normal conduction and cardiac mechanical function. Aldosterone has been implicated as a key hormone underlying myocardial fibrosis and fluid retention in the setting of heart failure, and mineralocorticoid blockade with spironolactone (SPL) or eplerenone significantly reduces cardiovascular morbidity and mortality in patients with systolic heart failure not on dialysis.9-12 Whether mineralocorticoid blockade is beneficial in patients with ESRD is uncertain, and side effects including hypotension, hyperkalemia, and gynecomastia may be more common in the absence of kidney function.13 Furthermore, the optimal dosing regimen for patients receiving maintenance dialysis is uncertain.
We conducted the Spironolactone in Dialysis (SPin-D) trial to evaluate the safety and tolerability, and generate preliminary estimates of efficacy, of multiple dosages of SPL compared with placebo in patients receiving maintenance HD. SPin-D is one of the trials of the Hemodialysis Novel Therapies (HDNT) Consortium established by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to conduct early phase studies of interventions for patients receiving maintenance HD.
Results
Participants
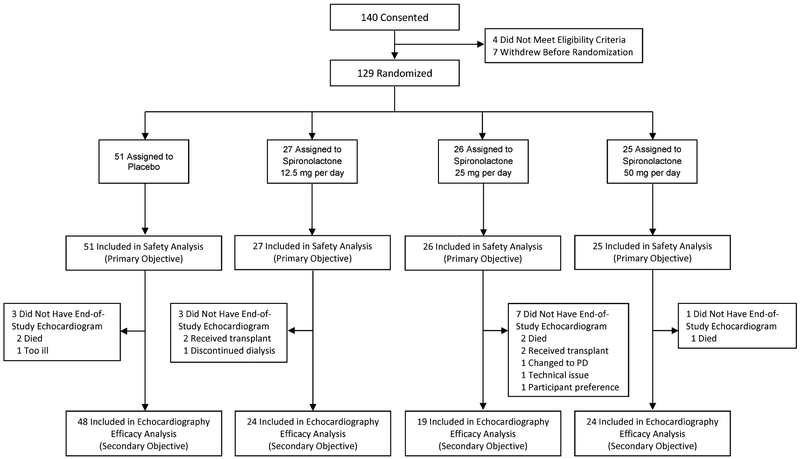
Between April 21, 2015 and September 1, 2016, 129 participants were randomized to placebo (N=51), SPL 12.5 mg daily (N=27), SPL 25 mg daily (N=26), or SPL 50 mg daily (N=25). Baseline characteristics were similar across groups (Table 1). The majority of participants (71%) were African American, 66% were male, and the mean age was 55.5 ± 12 years. The median duration of dialysis was 3.4 years (IQR 1.9 – 6.1). Nine participants withdrew because of kidney transplantation, dialysis discontinuation, change to peritoneal dialysis, or transfer to another dialysis unit, and five died during follow-up (Figure 1 and Table 2). Follow-up ended on June 8, 2017. All randomized participants were included in the primary safety analysis. For the secondary analyses exploring efficacy, 16 participants (12%) were excluded because they did not have end-of-treatment studies.
Table 1.
Baseline Characteristics
| All (N=129) | Placebo (N=51) | SPL 12.5 mg (N=27) | SPL 25 mg (N=26) | SPL 50 mg (N=25) | |
|---|---|---|---|---|---|
| Male, No. (%) | 85 (65.9) | 32 (62.7) | 15 (55.6) | 19 (73.1) | 19 (76.0) |
| Age, yr; mean (SD) | 55.5 (12.0) | 56.8 (11.5) | 55.1 (13.6) | 53.3 (13.5) | 55.5 (9.8) |
| Black, No. (%) | 92 (71.3) | 39 (76.5) | 18 (66.7) | 18 (69.2) | 17 (68.0) |
| White, No. (%) | 27 (20.9) | 7 (13.7) | 8 (29.6) | 7 (26.9) | 5 (20.0) |
| Asian, No. (%) | 5 (3.9) | 2 (3.9) | 0 (0.00) | 1 (3.8) | 2 (8.0) |
| Hispanic/Latino, No. (%) | 11 (8.5) | 4 (7.8) | 3 (11.1) | 2 (7.7) | 2 (8.0) |
| BMI; mean (SD) | 31.4 (7.4) | 28.8 (6.9) | 33.4 (8.2) | 32.5 (7.4) | 33.6 (5.9) |
| Systolic BP, mm Hg; mean (SD) | 139.7 (22.2) | 140.0 (18.9) | 135.6 (22.7) | 141.1 (19.4) | 140.9 (25.0) |
| Diastolic BP, mm Hg; mean (SD) | 77.3 (12.1) | 78.2 (11.6) | 75.0 (8.3) | 81.1 (15.3) | 76.1 (12.1) |
| Hypertension, No. (%) | 120 (93.0) | 50 (98.0) | 24 (88.9) | 23 (88.5) | 23 (92.0) |
| Diabetes mellitus, No. (%) | 66 (51.2) | 24 (47.1) | 13 (48.1) | 14 (53.8) | 15 (60.0) |
| Coronary artery disease, # (%) | 28 (21.7) | 12 (23.5) | 1 (3.7) | 8 (30.8) | 7 (28.0) |
| Congestive heart failure, No. (%) | 21 (16.3) | 10 (19.6) | 2 (7.4) | 4 (15.4) | 5 (20.0) |
| Atrial fibrillation, No. (%) | 10 (7.8) | 7 (13.7) | 1 (3.7) | 2 (7.7) | 0 (0.0) |
| Stroke, No. (%) | 22 (17.1) | 14 (27.5) | 4 (14.8) | 1 (3.8) | 3 (12.0) |
| Peripheral arterial disease, No. (%) | 16 (12.4) | 6 (11.8) | 2 (7.4) | 4 (15.4) | 4 (16.0) |
| Hyperlipidemia, No. (%) | 49 (38.0) | 20 (39.2) | 9 (33.3) | 10 (38.5) | 10 (40.0) |
| Current tobacco use, No. (%) | 18 (14.0) | 8 (15.7) | 5 (18.5) | 1 (3.8) | 4 (16.0) |
| AV graft, No. (%) | 14 (10.9) | 6 (11.8) | 5 (18.5) | 1 (3.8) | 2 (8.0) |
| AV fistula, No. (%) | 109 (84.5) | 42 (82.4) | 21 (77.8) | 24 (92.3) | 22 (88.0) |
| Tunneled CVC No. (%) | 2 (1.6) | 1 (2.0) | 0 (0.00) | 0 (0.00) | 1 (4.0) |
| Other | 4 (3.1) | 2 (3.9) | 1 (3.7) | 1 (3.8) | 0 (0.00) |
| Dialysis vintage, yr; median (IQR) | 3.4 (1.9 - 6.1) | 3.4 (2.2 - 7.0) | 3.5 (1.5 - 5.7) | 3.3 (1.6 - 5.0) | 3.8 (1.8 - 6.1) |
| Dialysis <1 year, No. (%) | 13 (10.1) | 4 (7.8) | 4 (14.8) | 3 (11.5) | 2 (8.0) |
| Dialysis ≥1 year, No. (%) | 116 (89.9) | 47 (92.2) | 23 (85.2) | 23 (88.5) | 23 (92.0) |
| ACEI or ARB use, No. (%) | 39 (30.2) | 15 (29.4) | 9 (33.3) | 8 (30.8) | 7 (28.0) |
| Beta blocker use, No. (%) | 61 (47.3) | 22 (43.1) | 12 (44.4) | 12 (46.2) | 15 (60.0) |
| Statin use, No. (%) | 54 (41.9) | 21 (41.2) | 11 (40.7) | 13 (50.0) | 9 (36.0) |
| Anti-platelet agents use, No. (%) | 44 (34.1) | 17 (33.3) | 9 (33.3) | 10 (38.5) | 8 (32.0) |
| Potassium, mEq/L; mean (SD) | 4.8 (0.6) | 4.8 (0.6) | 4.7 (0.5) | 4.8 (0.6) | 4.8 (0.7) |
| Albumin, g/dL; mean (SD) | 3.6 (0.8) | 3.6 (0.8) | 3.5 (0.8) | 3.4 (0.8) | 3.6 (0.9) |
| Single pool Kt/V | 1.5 (0.3) | 1.6 (0.2) | 1.5 (0.2) | 1.5 (0.3) | 1.6 (0.5) |
| 24-hr urine volume, ml; median (IQR)a | 0.0 (0.0 - 240) | 0.0 (0.0 - 142) | 0.0 (0.0 - 289) | 99.7 (0.0 - 464) | 3.9 (0.0 - 219) |
Values determined for 118 of the 129 participants.
Abbreviations: SPL, spironolactone; BP, blood pressure, AV, arteriovenous; CVC, central venous catheter; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 1.
Participant enrollment and follow-up.
Table 2.
Feasibility Outcomes and Study Drug Adherence
| p-Values | ||||||
|---|---|---|---|---|---|---|
| Placebo (N=51) |
SPL 12.5 mg (N=27) |
SPL 25 mg (N=26) |
SPL 50 mg (N=25) |
Trend | Combinedy SPL vs Placebo |
|
| Withdrawal from trial, No. (%) | 4 (7.8) | 4 (14.8) | 5 (19.2) | 1 (4.0) | 0.6 | 0.5 |
| Kidney transplantation | 2 (3.9) | 2 (7.4) | 2 (7.7) | 0 (0.0) | ||
| Modality change to peritoneal dialysis | 0 (0.0) | 0 (0.0) | 1 (3.8) | 0 (0.0) | ||
| Transfer to non-participating dialysis unit | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0 (0.0) | ||
| Dialysis discontinuation | 0 (0.0) | 1 (3.7) | 0 (0.0) | 0 (0.0) | ||
| Death | 2 (3.9) | 0 (0.0) | 2 (7.7) | 1 (4.0) | ||
| Study drug permanent discontinuation, No. (%) | 14 (27.5) | 4 (14.8) | 2 (7.7) | 7 (28.0) | 0.8 | 0.3 |
| Hyperkalemia (K≥7.0 mEq/L) | 8 (15.6) | 1 (3.7) | 0 (0.0) | 3 (12.0) | ||
| Hypotension | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Gastrointestinal symptoms | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | ||
| Facial swelling | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Transfer to nursing home/unable to monitor | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Participant preference | 2 (3.9) | 1 (3.7) | 2 (7.7) | 2 (8.0) | ||
| Study drug permanent discontinuation or dose reduction, No. (%) | 16 (31.4) | 5 (18.5) | 6 (23.1) | 8 (32.0) | 0.9 | 0.6 |
| % Study drug adherence, mean (SD)a | 86.9 (19.4) | 82.7 (21.7) | 80.8 (26.6) | 84.2 (20.6) | 0.65 | 0.40 |
Determined from counts of returned pills: [(# dispensed - # returned) / # expected to have taken] X 100%. If the study drug was discontinued, pills that would have been expected to be taken after the date of discontinuation were not included in the denominator.
Abbreviations: SPL, spironolactone
Feasibility and Tolerability
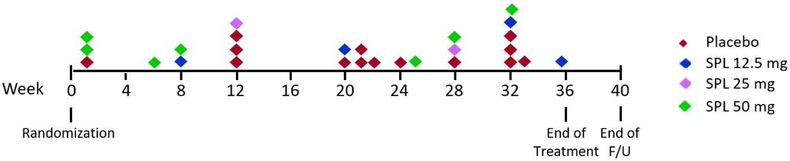
Participant retention and study drug adherence are shown in Table 2. Of the 129 participants, 125 (97%) completed the dose escalation in accordance with the protocol. The four participants who did not complete escalation were in the placebo (N=1), 25 mg (N=1), and 50 mg (N=2) dose groups. Twenty-seven participants (20.9%) permanently discontinued study drug prior to the end of follow-up. The most common reason for discontinuation was hyperkalemia (44%). Timing of permanent study drug discontinuations is shown in Figure 2. Mean adherence based on pill counts ranged from 80.8% to 86.9% across the randomized groups (Table 2).
Figure 2.
Timing of permanent discontinuations of study drug by treatment group.
Safety
Safety outcomes (listed in Supplementary Table 1) are reported for the intention-to-treat and as-treated analyses (Table 3 and Supplemental Table 2, respectively). For the primary safety outcomes of potassium concentration >6.5 mEq/L and hypotension requiring hospitalization or emergency room visit, there was not an overall difference between SPL and placebo groups but an effect of dosage on event rates was evident for both outcomes. Hyperkalemia frequency was similar with SPL and placebo (0.49 vs 0.50 events per patient-year; P=0.9) but demonstrated a significant linear trend due primarily to an increased event rate at 50 mg [0.50, 0.32, 0.23, and 0.89 events/patient-year (Ptrend=0.04)] in 9 (17.6%), 4 (14.8%), 4 (15.4%), and 8 (32%) patients in the placebo, 12.5, 25, and 50 mg groups. The primary hypotension outcome was infrequent and similar with SPL and placebo (0.11 vs 0 events per patient-year; P=0.1), with 0.0, 0.16, 0.0, and 0.16 events/patient-year (Ptrend=0.01) in 0, 2 (7.4%), 0, and 3 (12%) patients in the placebo, 12.5, 25, and 50 mg groups. The pattern was similar in as-treated analyses (Supplemental Table 2). Recurrent intra-dialytic hypotension, one of the most frequent safety outcome events, occurred in 76% of patients in the 50 mg group compared with 45%, 44% and 50% of patients in the placebo, 12.5, and 25 mg dosage groups, respectively. The event rate was 3.53 per patient-year in the 50 mg group and 2.40, 2.01, and 2.39 per patient-year in the placebo, 12.5 mg and 25 mg dosage groups, respectively; however, the linear trend was not significant. Similarly, for several other secondary hyperkalemia and hypotension outcomes, the proportion of patients and event rates were nominally higher for patients randomized to SPL 50 mg per day compared with lower dosages in both the intention-to-treat and as-treated analyses, but the linear trends across dosage groups were not significant. Per-patient mean serum potassium, systolic blood pressure, and diastolic blood pressure over follow-up were each similar across the four randomized groups.
Table 3.
Safety Outcomes: Intention-to-Treat Analysis
| Placebo (N=51) | SPL 12.5 mg (N=27) | SPL 25 mg (N=26) | SPL 50 mg (N=25) | p-Values for Event Rates |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pts w/event No. (%) |
Events per pt-year |
Pts w/event No. (%) |
Events per pt-year |
Pts w/event No. (%) |
Events per pt-year |
Pts w/event No. (%) |
Events per pt-year |
Trend | Combined SPL vs Placebo |
|
| Primary Safety Outcomes | ||||||||||
| Serum [K+] >6.5 mEq/L | 9 (17.6) | 0.50 | 4 (14.8) | 0.32 | 4 (15.4) | 0.23 | 8 (32.0) | 0.89 | 0.04 | 0.9 |
| Hypotension requiring hospitalization or ER visit | 0 (0.0) | 0.00 | 2 (7.4) | 0.16 | 0 (0.0) | 0.00 | 3 (12.0) | 0.16 | 0.01 | 0.1 |
| Secondary Safety Outcomes | ||||||||||
| Hyperkalemia requiring unscheduled dialysis, resin therapy, or hospitalization | 6 (11.8) | 0.16 | 2 (7.4) | 0.11 | 0 (0.0) | 0.00 | 7 (28.0) | 0.79 | <0.001 | 0.1 |
| Hyperkalemia requiring adjustment in dialysate [K+] or study drug discontinuation | 13 (25.5) | 0.47 | 2 (7.4) | 0.11 | 5 (19.2) | 0.80 | 7 (28.0) | 1.11 | <0.001 | 0.2 |
| Serum [K+] ≥6.0 mEq/L | 23 (45.1) | 2.08 | 13 (48.1) | 1.27 | 10 (38.5) | 1.48 | 10 (40.0) | 2.26 | 0.5 | 0.5 |
| Recurrent intra-dialytic hypotensiona | 23 (45.1) | 2.40 | 12 (44.4) | 2.01 | 13 (50.0) | 2.39 | 19 (76.0) | 3.53 | 0.1 | 0.7 |
| Recurrent intra-dialytic blood pressure <80 mm Hgb | 8 (15.7) | 0.82 | 3 (11.1) | 0.58 | 2 (7.7) | 0.28 | 9 (36.0) | 1.42 | 0.7 | 0.9 |
| Inter-dialytic hypotensionc | 6 (11.8) | 0.45 | 4 (14.8) | 0.26 | 6 (23.1) | 0.46 | 5 (20.0) | 0.47 | 0.7 | 0.8 |
| Myocardial infarction | 0 (0.0) | 0.00 | 0 (0.0) | 0.00 | 3 (11.5) | 0.17 | 1 (4.0) | 0.05 | 0.02 | 0.2 |
| Stroke | 0 (0.0) | 0.00 | 0 (0.0) | 0.00 | 1 (3.8) | 0.06 | 0 (0.0) | 0.00 | 0.2 | 0.9 |
| Cardiovascular deathd | 1 (2.0) | 0.03 | 0 (0.0) | 0.00 | 2 (7.7) | 0.11 | 1 (4.0) | 0.05 | 0.3 | 0.6 |
| Death – all cause | 2 (3.9) | 0.05 | 0 (0.0) | 0.00 | 2 (7.7) | 0.11 | 1 (4.0) | 0.05 | 0.5 | 0.9 |
| Secondary Safety Outcomes, continuous measures | ||||||||||
|
Placebo (N=51) Mean (SD) |
SPL 12.5 mg (N=27) Mean (SD) |
SPL 25 mg (N=26) Mean (SD) |
SPL 50 mg (N=25) Mean (SD) |
p-Values | ||||||
| Trend |
Combined SPL vs Placebo |
|||||||||
| Per-patient mean serum [K+], mEq/L | 4.82 (0.48) | 4.84 (0.44) | 4.76 (0.43) | 4.93 (0.41) | 0.2 | 0.8 | ||||
| Per-patient mean systolic blood pressure, mm Hg | 146.1 (14.8) | 147.1 (16.1) | 146.1 (16.4) | 148.1 (20.5) | 0.6 | 0.4 | ||||
| Per-patient mean diastolic blood pressure, mm Hg | 79.3 (10.1) | 81.2 (11.2) | 82.8 (9.9) | 82.4 (13.7) | 0.2 | 0.03 | ||||
Systolic blood pressure <80 mm Hg during ≥3 dialysis sessions per 30-day period or treatment for either hypotension or symptoms of hypotension during ≥3 dialysis sessions per 30-day period
Systolic blood pressure <80 mm Hg during ≥3 dialysis sessions per 30-day period
Inter-dialytic systolic blood pressure <90 mm Hg or inter-dialytic hypotension requiring adjustment in anti-hypertensive medications or treatment in a hospital or emergency room.
Cardiovascular death was defined as death due to myocardial infarction, congestive heart failure, cardiac valvular disease, arrhythmia, sudden death, stroke, or peripheral arterial disease
Abbreviations: SPL, spironolactone; pt, patient; [K+], potassium concentration; ER, emergency room
Study drug was discontinued or dose-reduced in 16 (31.4%), 5 (18.5%), 6 (23.1%) and (32.0%) of placebo and SPL 12.5, 25 and 50 mg/day groups (Table 2, Ptrend=0.9, PSPL vs. placebo=0.6). Hyperkalemia requiring unscheduled dialysis, resin therapy or hospitalization, occurred at rates of 0.16, 0.11, 0.00 and 0.79 events/patient year in the placebo 12.5, 25 and 50 mg/day groups (Ptrend<0.001, PSPL vs. placebo=0.10) whereas hyperkalemia requiring adjustment in dialysate potassium or study drug discontinuation occurred at rates of 0.47, 0.11, 0.80 and 1.11 events/patient year (Ptrend<0.001, PSPL vs. placebo=0.2) (Table 3).
Five participants died during the trial. Causes of death were sepsis (placebo), sudden death (placebo and 25 mg), myocardial infarction (25 mg), and peripheral vascular disease with gangrene (50 mg). None of the deaths was thought to be related to study drug. Myocardial infarction (fatal or non-fatal) occurred in 3 participants in the SPL 25 mg group and 1 participant in the 50 mg group, and stroke (non-fatal) occurred in 1 participant in the 25 mg group.
The prespecified adverse events of interest are shown in Table 4. Rash, nausea, and constipation were more common in the combined SPL groups. No participants discontinued study drug because of adverse events of interest including breast enlargement or tenderness. A full listing of adverse events is provided in Supplemental Table 3.
Table 4.
Adverse Events of Interest and Serious Adverse Events
| Placebo (N=51) | SPL 12.5 mg (N=27) | SPL 25 mg (N=26) | SPL 50 mg (N=25) | p-Values for Event Rates |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pts w/event No. (%) |
Events per pt-year |
Pts w/event No. (%) |
Events per pt-year |
Pts w/event No. (%) |
Events per pt-year |
Pts w/event No. (%) |
Events per pt-year |
Trend | Combined SPL vs Placebo |
|
| Breast enlargement or tenderness | 2 (3.9) | 0.05 | 0 (0.0) | 0.00 | 1 (3.8) | 0.06 | 2 (8.0) | 0.11 | 0.7 | 0.9 |
| Rash | 2 (3.9) | 0.05 | 1 (3.7) | 0.05 | 1 (3.8) | 0.06 | 2 (8.0) | 0.11 | 0.3 | <0.001 |
| Nausea | 6 (11.8) | 0.16 | 8 (29.6) | 0.48 | 4 (15.4) | 0.23 | 1 (4.0) | 0.05 | 0.2 | 0.01 |
| Vomiting | 5 (9.8) | 0.13 | 7 (25.9) | 0.42 | 2 (7.7) | 0.40 | 1 (4.0) | 0.11 | 0.8 | 0.1 |
| Diarrhea | 5 (9.8) | 0.24 | 4 (14.8) | 0.21 | 5 (19.2) | 0.40 | 3 (12.0) | 0.21 | 0.7 | 0.8 |
| Anorexia | 0 (0.0) | 0.00 | 0 (0.0) | 0.00 | 1 (3.8) | 0.06 | 0 (0.0) | 0.00 | 0.2 | 0.9 |
| Constipation | 2 (3.9) | 0.05 | 1 (3.7) | 0.05 | 1 (3.8) | 0.28 | 0 (0.0) | 0.00 | 0.03 | <0.001 |
| Any gastrointestinal symptoms | 11 (21.6) | 0.45 | 11 (40.7) | 0.79 | 8 (30.8) | 1.14 | 4 (16.0) | 0.32 | 0.6 | 0.1 |
| Serious adverse eventa | 27 (52.9) | 1.79 | 13 (48.1) | 1.43 | 13 (50.0) | 2.68 | 12 (48.0) | 2.26 | 0.2 | 0.5 |
Serious adverse events include any adverse event that: is fatal or result in death, is life-threatening, requires or prolongs hospitalization, results in persistent or significant disability or incapacity, results in congenital anomalies or birth defects or is considered an important medical event Abbreviation: SPL, spironolactone; pt, patient
Efficacy
Efficacy outcomes were considered exploratory with a goal of detecting signals rather than clearly demonstrating efficacy. For mitral annular E’ velocity, the primary efficacy outcome, the increase from baseline to week 36 was highest in the SPL 50 mg group but there was not a statistically significant trend across groups (Table 5). Similarly, for E/E’, where a reduction over time reflects improvement, only the 50 mg group showed a decrease. Left ventricular mass index, left ventricular ejection fraction, and left ventricular global longitudinal strain did not improve.
Table 5.
Efficacy Outcomes
| Primary Efficacy Outcome - Echocardiography | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Placebo (N=40) |
SPL 12.5 mg (N=20) |
SPL 25 mg (N=15) |
SPL 50 mg (N=22) |
p-Valuesa | ||||||||||
| Baseline | 36-Week | Change | Baseline | 36-Week | Change | Baseline | 36-Week | Change | Baseline | 36-Week | Change | Trend |
Combined SPL vs Placebo |
|
| MA E’, cm/sec |
7.4 (1.7) | 7.5 (1.9) | 0.1 (1.1) |
7.6 (1.8) | 7.4 (1.9) | −0.2 (1.0) |
7.8 (1.9) | 7.7 (1.4) | −0.1 (1.2) |
7.0 (1.9) | 7.3 (1.9) | 0.3 (1.6) |
0.5 | 0.8 |
| Secondary Efficacy Outcomes - Echocardiography | ||||||||||||||
|
Placebo (N=48) |
SPL 12.5 mg (N=24) |
SPL 25 mg (N=19) |
SPL 50 mg (N=24) |
p-Valuesa | ||||||||||
| Baseline | 36-Week | Change | Baseline | 36-Week | Change | Baseline | 36-Week | Change | Baseline | 36-Week | Change | Trend |
Combined SPL vs Placebo |
|
| LVMI | 105.2 (25.2) |
94.8 (22.4) |
−10.4 (11.9) |
115.5 (26.7) |
104.6 (27.0) |
−10.9 (18.1) |
116.4 (26.9) |
109.1 (30.5) |
−7.3 (21.0) |
106.3 (29.2) |
96.5 (25.7) |
−9.8 (9.3) |
0.6 | 0.3 |
| LVEF, % | 68.9 (4.0) |
70.7 (3.1) |
1.8 (4.1) |
65.9 (8.5) |
66.9 (9.9) |
1.0 (4.7) |
66.0 (11.4) |
65.3 (13.9) |
−0.7 (7.3) |
68.2 (5.8) |
69.5 (6.3) |
1.3 (3.7) |
0.03 | 0.03 |
| E/E’ | 10.7 (5.8) |
11.5 (7.0) |
0.9 (3.6) |
11.8 (6.2) |
12.2 (8.0) |
0.4 (4.4) |
9.2 (5.0) |
10.6 (3.9) |
1.4 (2.2) |
12.5 (5.6) |
11.9 (5.5) |
−0.6 (4.0) |
0.1 | 0.1 |
| LVGLS | −17.2 (2.8) |
−18.1 (2.8) |
−0.8 (2.3) |
−16.7 (3.3) |
−17.0 (3.3) |
−0.3 (3.3) |
−17.2 (4.2) |
−17.0 (3.9) |
0.2 (2.7) |
−17.4 (3.0) |
−18.2 (3.0) |
−0.7 (2.9) |
0.9 | 0.4 |
Adjusted for baseline values
Values are mean (SD) unless otherwise specified
Abbreviations: SPL, spironolactone; MA E’, early diastolic mitral annular velocity; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; E/E’, ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E’); LVGLS, left ventricular global longitudinal strain.
The cardiac PET sub-study enrolled 28 participants and was not powered to detect statistically significant effects. Global left ventricular coronary flow reserve decreased between baseline and week 36 in the placebo group whereas for the higher dosages of SPL (25 mg and 50 mg) there were modest increases (Supplemental Table 4). Among the sub-group with heart rate variability measured at baseline and end of the treatment, there was not a clear signal for a spironolactone effect. There was no evidence for effects of SPL on the circulating markers of inflammation, fibrosis, or cardiac function.
Discussion
In this double-blind, placebo-controlled trial, we randomized 129 individuals on maintenance HD to 36 weeks of placebo or spironolactone at daily dose of 12.5, 25 or 50 mg. Compared with placebo, SPL was well tolerated without higher event rates for most of the prespecified adverse events of interest, and increases in constipation and rash that were minimal and generally not dose-limiting. Both the primary hyperkalemia and hypotension safety endpoints and secondary safety endpoints using alternative definitions of hyperkalemia, inter-dialytic and intradialytic hypotension occurred with similar frequency in placebo and SPL-treated patients. Although SPL appeared to have a good safety profile, we did not detect definitive signals of a beneficial impact on cardiovascular function or structure. Lastly, while the overall SPL to placebo comparisons suggest that the drug can be used safely in the HD population, the frequency of hyperkalemia and hypotension appeared to be dosage-related with increased rates primarily in the 50 mg daily group. An increase in hyperkalemia events was also observed in a recent placebo-controlled study in hemodialysis of the aldosterone antagonist, eplerenone.14
Landmark trials have established that mineralocorticoid blockade improves survival and reduces cardiovascular morbidity in patients with heart failure with reduced ejection fraction.10, 12 This suggests that spironolactone could be particularly beneficial in end stage renal disease — a condition characterized by abnormalities in left ventricular structure and function, and an extraordinarily high incidence of cardiovascular morbidity and mortality.1, 6, 7, 15
Several recent trials provide support for a beneficial effect of SPL on pump structure and function in patients treated with maintenance HD or peritoneal dialysis16-18. In addition, in an open label trial of 309 oligo-anuric Japanese HD patients SPL reduced the composite of cardiovascular hospitalization or death by 60%, and all-cause mortality by 65%.19 Results were similar in a 2nd, placebo-controlled, trial of 250 HD and peritoneal dialysis patients in China17 and a meta-analysis including 721 randomized patients from a variety of trial designs and dosing strategies.13
Three of the studies tested a dosage of 25 mg daily17-19 while one used a comparable weekly dose of 50 mg administered 3 times per week.16 Thus, whether 25 mg daily is the optimal dosage in terms of safety and efficacy remains a critical question to address before investing the substantial resources required for definitive trials powered to evaluate effects on hard clinical outcomes. SPin-D differs from previous trials by evaluating multiple dosages of SPL. We did not identify significant effects of drug dosage on tolerability or the need for dosage reduction or discontinuation. However, hypotension and hyperkalemia rates increased. While the trial did not provide clear cardiovascular efficacy signals, changes in measures of diastolic function (E’ and E/E’) were greatest in the 50 mg group. Similarly, the myocardial perfusion sub-study demonstrated numerical, but non-significant, trends suggestive of dose-related improvements in coronary flow reserve.
How should our findings be interpreted in the context of previous studies demonstrating improvement in cardiovascular outcomes in dialysis patients treated with SPL? It should be emphasized that we designed the trial with the objective of evaluating safety rather than efficacy. Study duration was short compared with some previous trials (9 months versus ≥2 years)16-19 and might not have been sufficient to observe measurable changes in cardiac structure and function. Further measures of diastolic function and left ventricular mass were well-preserved at baseline leaving little room for improvement. Additionally, SPin-D enrolled a high proportion of black and Hispanic patients and differences in dialysis practices or the genetic background of patients in the United States and elsewhere could account for differences in observed efficacy between trials. Given the promising signals in other studies and confirmed efficacy in systolic heart failure, our findings should not be interpreted as a rationale for abandoning SPL but rather as an indication of remaining equipoise and the need for adequately powered studies reflecting the diversity of hemodialysis patient populations and dialysis practices.
Our data suggest that for maintenance HD patients SPL is safer at a dosage of 25 mg/day than 50 mg/day. If a single, fixed dose is used, 25 mg daily is likely to be the best choice. This is comparable to the average dose achieved in a landmark systolic heart failure trial.10 However, whether this is the correct dose to use in large outcome trials is open to interpretation. The same dosage did not improve mortality in a recent trial of heart failure patients with preserved ejection fraction20 raising the possibility that a higher dosage could be required in populations not primarily selected for systolic dysfunction. In SPin-D the incidence of the primary hyperkalemia and hypotension safety endpoints in the 50 mg group were only 0.89 and 0.16 events per patient-year, respectively. Severe hyperkalemia to ≥7.0 mEq/L occurred with a similar incidence in the 50 mg and placebo groups, and measures of intra-dialytic hypotension and the need for drug discontinuation or dosage reduction were not significantly different across groups. Given the low absolute rates of safety events, and their manageable nature without requirement for dosage reduction or discontinuation in most cases, a trial design in which each participant’s maximal-tolerated dosage up to 50 mg/day is defined during a period of close observation might maximize the potential for significant efficacy while still providing an adequate safety margin.
Strengths of this study include recruitment from multiple centers, assessment of multiple dosages of SPL, use of a central, core facility for assessment of echocardiographic findings, rigorous ascertainment of safety outcomes using standardized criteria, and employment of a range of techniques to assess effects on cardiovascular physiology including echocardiography, PET, rhythm monitoring, and measurement of circulating markers. Our results should also be interpreted within the context of several limitations. Despite randomization, there were small imbalances in some baseline characteristics. Follow-up was relatively short and the sample size was modest, both of which may have reduced the power to detect small, but clinically relevant changes in outcomes. Enrolled patients were younger than the overall HD population in the United States and, on average, measurements of cardiovascular function and structure at baseline were normal or near normal, both factors that might account for the low overall mortality and lack of an effect on cardiovascular efficacy parameters.
In conclusion, in this trial of multiple dosages of SPL administered for 36 weeks to individuals receiving maintenance HD, SPL was well tolerated compared with placebo. The rates of safety events were low overall but there was evidence of increasing hyperkalemia and hypotension with higher dosages, and there were no convincing improvements in cardiac structure or function. Our data suggest that, with appropriate monitoring, SPL can be used safely in HD patients particularly at dosages ≤25 mg daily. The findings also indicate clinical equipoise regarding efficacy despite dramatic improvements in cardiovascular health and survival found in some prior trials, and emphasize the need for adequately-powered, well-designed, definitive outcomes trials that are informed by the results of SPin-D.
Methods
Design
SPin-D (NCT02285920) was a parallel group, double-blind, randomized, placebo-controlled, multiple dosage trial of SPL. The full study protocol is available in the Supplemental Materials.
Study Population
Participants were enrolled from dialysis units affiliated with four U.S. academic medical centers. The Institutional Review Boards affiliated with the clinical centers and with the data coordinating center approved the protocol and all participants provided informed consent. Inclusion criteria included age 18 to 85 years, maintenance HD for ≥6 months or for 3-6 months if there were no changes in target dry weight during the prior 2 weeks and no hospitalizations during the prior 6 weeks. Women of child-bearing potential were required to use birth control until 4 weeks after completing study drug. Major exclusion criteria included: a) serum potassium ≥ 6.5 mEq/L or unscheduled dialysis for hyperkalemia within 3 months; b) potassium ≥6.0 mEq/L within 2 weeks prior to baseline; c) pre-dialysis systolic blood pressure <100 mm Hg within 2 weeks prior to screening or at baseline; d) ≥2 dialysis sessions within the month prior to screening with blood pressure <80 mm Hg or treatment for cramping, light-headedness, nausea or hypotension; e) use of digoxin, spironolactone, eplerenone, or dual use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers; f) allergy to spironolactone; g) inability to maintain dialysis machine blood flow ≥300 mL/min during the 3 sessions before screening; and h) anticipated pregnancy, transplantation, modality transfer, or transfer to a non-participating dialysis unit within nine months. Individuals with a history of mitral valve surgery or severe mitral valve disease were excluded because of anticipated inability to determine mitral annular E’ velocity.
Randomization and Intervention
Participants were randomized in a 2:1:1:1 ratio to placebo or SPL 12.5, 25, or 50 mg daily for 36 weeks. Using a random number generator, the data coordinating center prepared permuted blocks of random sizes with stratification by center, use of ACE inhibitors or angiotensin receptor blockers, and dialysis duration (<1 versus ≥1 year). Web-based randomization was performed with participants and all research personnel masked to the treatment assignment.
Participants were evaluated either in person or by telephone weekly during the 6-week dose escalation phase and then monthly for a total follow-up of 40 weeks to assess hyperkalemia, hypotension, study drug tolerability, adverse events, and medication changes. Dialysis treatment records were reviewed and participants were questioned about breast enlargement or tenderness, gastrointestinal symptoms, and rash. Study drug dose was increased in a blinded manner every 2 weeks until the target dose was reached. Serum potassium was checked 3-5 days and 2 weeks after each dose increase and monthly thereafter. In addition to protocol-dictated measurements, all available clinical potassium values were reviewed. Potassium was re-measured within 1 week after any value >6.0 mEq/L. The dose of the study drug could be decreased in a blinded manner twice before discontinuation for adverse effects and was permanently discontinued if serum potassium was ≥7.0 mEq/L. Adherence was measured by performing pill counts.
Continuous heart rate and rhythm monitoring using a wearable patch (Medtronic SEEQ™ Mobile Cardiac Telemetry System) for 7 days prior to randomization, at week 6, and during weeks 32-36 was added as an optional procedure by protocol amendment on January 22, 2016 after 64 participants had enrolled. Participants who were enrolled before the amendment were eligible for week 6 and/or week 32-36 monitoring. Assessment of rest and adenosine-induced stress myocardial perfusion and coronary flow reserve using positron emission tomography (PET) at baseline and week 36 was performed as an optional study at one center.
Outcomes
The complete list of primary and secondary outcomes with definitions is provided in Supplemental Table 1. The principal objective was to evaluate the safety of SPL in the HD population, and thus the primary endpoints were a) the incidence of serum potassium >6.5 mEq/L; and b) the incidence of serious hypotension defined as hypotension requiring hospitalization or treatment in an emergency room and not attributable to an obvious cardiovascular or infectious cause. Tolerability was assessed by the need for study drug dose reduction or discontinuation, and feasibility was based on recruitment, retention, and loss-to-follow-up. Change in diastolic function, as measured by mitral annular E’ velocity (average of lateral and septal early diastolic myocardial velocity), was pre-specified as the primary efficacy signal.
Key secondary outcomes included: a) hyperkalemia requiring hospitalization, emergency dialysis, or resin therapy; b) mean potassium during follow-up; c) symptomatic or recurrent intradialytic hypotension; d) inter-dialytic hypotension; e) change in left ventricular mass index; f) heart rate variability; and g) change in coronary flow reserve in the subset undergoing PET.
Laboratory Measurements
Blood was collected pre-dialysis at baseline and 36 weeks. Serum and EDTA-treated plasma were stored at −80° C in 500 μl aliquots. Plasma concentrations of Interleukin-6, Interleukin-10, soluble ST2, galectin-3, and vascular cell adhesion molecule-1 (VCAM-1) were measured using a microfluidic fluorescent immunoassay run in triplicate (Protein Simple, San Jose, CA). High-sensitivity C-reactive protein was measured using a chemiluminescent immunometric assay (Siemens Health Care). Serum aldosterone was measured in duplicate using a competitive enzyme-linked immunoassay (ALPCO Diagnostics, Salem, NH). Serum albumin concentrations were determined using a colorimetric assay (Ortho Clinical, Raritan, NJ).
Study Oversight
An external Data and Safety Monitoring Board (DSMB) appointed by the NIDDK approved the protocol and reviewed study progress, data quality, and safety.
Sample Size Determination and Statistical Analysis
Because the primary objective of this pilot study was to evaluate safety and feasibility rather than efficacy, we utilized conventional power analyses as a guide for selecting the sample size recognizing that we were not planning to conduct definitive hypothesis testing. The sample size of 125 participants with 50 in the placebo group and 25 in each SPL group was expected to provide 80% power to detect an incidence of hyperkalemia of 22% across the SPL dose groups assuming an incidence of 5% in the placebo group. This would also provide 80% power to detect a 0.1 mEq/L difference in time-averaged serum potassium concentration between the placebo and SPL groups. We estimated that mitral annular E’ would be 5.8 ±1.8 cm/sec at baseline.21-24 Assuming a correlation of ≥0.2 between baseline and end-of-treatment E’ and a withdrawal rate of 10%, our sample size would provide 80% power to detect a difference in the change in E’ of 0.7 standard deviations, or 1.3 cm/sec. The trend test, in particular, borrows strength across dose levels with increased power to detect trends if event rates are low.
The primary analyses were based on incidence rate in intention-to-treat populations with all participants analyzed based on randomized group assignment. For safety outcomes, as-treated analyses were also performed as sensitivity analyses. Study drug adherence was determined from returned pill counts.
Outcomes are presented as mean (standard deviation) frequency (%), or incidence rate. P values for linear trends using equally spaced scores and for comparisons between the combined SPL dose groups and placebo were determined using generalized estimating equations with independent correlation structure accounting for clustering effect of centers: specifically, Poisson for events that can occur repeatedly, binomial for binary and incidence measures, and Gaussian distribution for continuous measures. All analyses were performed using SAS version 9.4 (SAS Institute Inc.) and geepack packages in R version 3.4.3 (https://www.r-project.org). Given the pilot nature of the study with a focus on safety, no corrections were made for multiple comparisons.
Supplementary Material
Acknowledgments
Project officers from the National Institute of Diabetes and Digestive and Kidney Diseases worked collaboratively with the investigators in designing the study, monitoring the study performance, interpreting data, and preparing the manuscript. Medtronic, Inc. had no involvement in designing or conducting the study, analyzing or interpreting the data, or preparing the article. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers. The authors would like to thank the participating patients, dialysis unit personnel, and dialysis provider organizations for their important contributions to this work.
Funding: This trial was funded by the following cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases: U01 DK096189, U01 DK099923, U01 DK099914, and U01 DK099919. Additional support was provided by the Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. A. Hung. was supported by I01CX000982 CSR&D. The SEEQ Mobile Cardiac Telemetry (MCl) Systems used for heart rate and rhythm monitoring were donated by Medtronic, Inc.
Footnotes
Disclosures: David M. Charytan received research support from Medtronic Inc. and consulting fees from Medtronic Inc. and Zoll Inc. Amanda H. Anderson received an honorarium and travel support from Kyowa Hakko Kirin. Daniel E. Weiner receives salary support paid to his institution by Dialysis Clinic, Inc. Marcelo Di Carli received a research grant from SpectrumDynamics, and consulting honoraria from Sanofi and General Electric. Portions of these results were presented at the 2017 American Society of Nephrology Kidney Week in New Orleans, LA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David M. Charytan, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Jonathan Himmelfarb, Kidney Research Institute, Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA.
T. Alp Ikizler, Division of Nephrology and Hypertension, Department of Medicine, and Vanderbilt Center for Kidney Disease, Vanderbilt University Medical Center, Nashville, TN.
Dominic S. Raj, Division of Renal Diseases and Hypertension, George Washington University School of Medicine, Washington, DC.
Jesse Y. Hsu, Department of Biostatistics, Epidemiology and Informatics, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
J. Richard Landis, Department of Biostatistics, Epidemiology and Informatics, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, Philadelphia PA.
Amanda H. Anderson, Department of Biostatistics, Epidemiology and Informatics, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Adriana M. Hung, Division of Nephrology and Hypertension, Department of Medicine, and Vanderbilt Center for Kidney Disease, Vanderbilt University Medical Center and VA Tennessee Valley Healthcare System, Nashville, TN.
Rajnish Mehrotra, Kidney Research Institute and Harborview Medical Center, Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA.
Shailendra Sharma, Division of Renal Diseases and Hypertension, George Washington University School of Medicine, Washington D.C..
Daniel E. Weiner, Division of Nephrology, Tufts Medical Center, Boston, MA.
Mark Williams, Renal Division, Beth Israel Deaconess Medical Center, Boston, MA.
Marcelo DiCarli, Departments of Radiology and Medicine, Brigham and Women’s Hospital, Boston, MA.
Hicham Skali, Cardiovascular Division, Department of Medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA.
Paul L. Kimmel, National Institute of Diabetes Digestive and Kidney Diseases Bethesda, MD.
Alan S. Kliger, Department of Medicine, Yale School of Medicine and Yale New Haven Health System, New Haven, CT.
Laura M. Dember, Renal, Electrolyte and Hypertension Division, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
References
- 1.U.S. Renal Data System. USRDS 2016 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, 2016. [Google Scholar]
- 2.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377: 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353: 238–248. [DOI] [PubMed] [Google Scholar]
- 4.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–1407. [DOI] [PubMed] [Google Scholar]
- 5.Damman K, Perez AC, Anand IS, et al. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J Am Coll Cardiol 2014; 64: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 6.Amann K, Breitbach M, Ritz E, et al. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol 1998; 9: 1018–1022. [DOI] [PubMed] [Google Scholar]
- 7.Charytan DM, Padera R, Helfand AM, et al. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol 2014; 176: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amann K, Wiest G, Zimmer G, et al. Reduced capillary density in the myocardium of uremic rats--a stereological study. Kidney Int 1992; 42: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 9.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000; 102: 2700–2706. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 13.Quach K, Lyvtvyn L, Baigent C, et al. The safety and efficacy of mineralocorticoid recepotr antagonists in patients who require dialysis: a systematic review and meta-analysis. Am J Kidney Dis 2016; In Press. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M, Manns B, Garg AX, et al. The Safety of Eplerenone in Hemodialysis Patients: A Noninferiority Randomized Controlled Trial. Clin J Am Soc Nephrol 2015; 10: 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal N, Keane M, Delafontaine P, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol 2013; 8: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vukusich A, Kunstmann S, Varela C, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C, Zhang Q, Zhang H, et al. Long-Term Effects of Low-Dose Spironolactone on Chronic Dialysis Patients: A Randomized Placebo-Controlled Study. J Clin Hypertens (Greenwich) 2016; 18: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Mizuno M, Suzuki Y, et al. Long-Term Effects of Spironolactone in Peritoneal Dialysis Patients. J Am Soc Nephrol 2014; 25: 1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto Y, Mori Y, Kageyama S, et al. Spironolactone reduces cardio- and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 2014; 63: 528–536. [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 21.Edwards NC, Ferro CJ, Kirkwood H, et al. Effect of spironolactone on left ventricular systolic and diastolic function in patients with early stage chronic kidney disease. Am J Cardiol 2010; 106: 1505–1511. [DOI] [PubMed] [Google Scholar]
- 22.Wang AY, Wang M, Lam CW, et al. Left ventricular filling pressure by Doppler echocardiography in patients with end-stage renal disease. Hypertension 2008; 52: 107–114. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi SY, Rohani M, Lindholm B, et al. Left ventricular function in patients with chronic kidney disease evaluated by colour tissue Doppler velocity imaging. Nephrol Dial Transplant 2006; 21: 125–132. [DOI] [PubMed] [Google Scholar]
- 24.Mendes L, Ribeiras R, Adragao T, et al. Load-independent parameters of diastolic and systolic function by speckle tracking and tissue doppler in hemodialysis patients. Rev Port Cardiol 2008; 27: 1011–1025. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.