Abstract
OBJECTIVES:
To characterize bone scan use, and potential overuse, after radical prostatectomy using a large, national integrated delivery system. Overuse of imaging is well documented in the setting of newly diagnosed prostate cancer, but whether overuse persists following radical prostatectomy remains unknown.
MATERIALS AND METHODS:
We identified 12,269 prostate cancer patients treated with radical prostatectomy between 2005-2008 using the Veterans Administration Central Cancer Registry. We used administrative and laboratory data to examine rates of bone scan use, including preceding PSA levels, and receipt of adjuvant or salvage therapy. We then performed multivariable logistic regression to identify factors associated with post-prostatectomy bone scan use.
RESULTS:
At a median follow up of 6.8 years, one in five men (22%) underwent a postoperative bone scan at a median PSA of 0.2 ng/mL. Half of bone scans (48%) were obtained in men who did not receive further treatment with androgen deprivation (ADT) or radiation therapy. After adjustment, post-prostatectomy bone scan was associated with a prior bone scan (adjusted Odds Ratio (aOR) 1.55, 95% Confidence Interval (CI) 1.32 – 1.84), positive surgical margin (aOR 1.68, 95% CI 1.40 – 2.01), preoperative PSA (aOR 1.02, 95% CI 1.01 – 1.03) as well as Hispanic ethnicity, black race, and increasing D’Amico risk category, but not with age or comorbidity.
CONCLUSION:
We found a substantial rate of bone scan utilization after radical prostatectomy. The majority was performed for PSA <1ng/mL where the likelihood of a positive test is low. More judicious use of imaging appears warranted in the post-prostatectomy setting.
Keywords: prostatic neoplasms, prostatectomy, radionuclide imaging, diagnostic imaging, neoplasm metastasis
INTRODUCTION
Many men diagnosed with prostate cancer will undergo diagnostic imaging at some point, either as part of initial staging or to investigate rising prostate-specific antigen (PSA) levels after treatment. Guidelines recommend radionuclide bone scan use for newly diagnosed men at high risk of metastasis or with symptoms concerning for metastatic disease, and after treatment in the setting of persistent or rising PSA.[1,2]
While bone scan use among the majority of newly diagnosed men is unlikely to yield useful clinical information (i.e., change treatment options), it remains common.[3–10] In fact, recent regional and national quality improvement initiatives target bone scan overuse among newly diagnosed men.[11,12] Whether similar initiatives are warranted to promote high-value imaging use after radical prostatectomy remains unknown. On the one hand, persistent (e.g., >0.2 ng/mL) or rising PSA levels after surgery define biochemical recurrence prompting imaging recommendations. On the other hand, although men with metastatic disease may present with low PSA values, most patients at these PSA levels are asymptomatic and the likelihood of bone scans finding metastatic disease among a cohort of post-prostatectomy men before PSA levels exceed 10 ng/mL is well below 10%, except in cases with extremely brisk PSA doubling times.[13–18] While patients with local recurrence may be candidates for salvage therapy, a PSA threshold of 10 ng/mL remains too high to inform clinical decision-making in many men with recurrence but low PSA values.
In this context, we examined bone scan use after radical prostatectomy in a national integrated delivery system. We characterized adjuvant and salvage therapy rates, investigated PSA levels at the time of bone scan, and identified predictors associated with post-prostatectomy bone scan use. A better understanding of bone scan use after prostate cancer treatment will inform high-value use of current imaging resources, and identify considerations for emerging, expensive next-generation imaging techniques.[19,20]
METHODS
Study population
We used data from the Veterans Administration (VA) Central Cancer Registry to identify men with pathologically confirmed incident diagnoses of prostate cancer between the years 2005 and 2008 and available follow up through 2013. These records were linked with administrative files to obtain clinical data. We excluded men with less than two years of follow up, a history of other malignancy, those enrolled in hospice within 30 days of diagnosis or who died within 6 months of diagnosis, and those who were diagnosed at autopsy. Our sample was then restricted to men who underwent radical prostatectomy as their primary therapy per the VA Central Cancer Registry, yielding a cohort of 12,269 patients.
Imaging use, biochemical recurrence, salvage and adjuvant therapy
We identified receipt of imaging using the Healthcare Common Procedure Coding System (HCPCS) (codes 78300, 78305, 78315, 78320). We defined pre-treatment imaging use as any bone scan ordered from the 6 months prior to diagnosis until the date of surgery. All bone scans ordered after the date of surgery were categorized as post-operative. We considered the last PSA value obtained prior to the treatment date from the laboratory data as the pre-treatment PSA.[21] We also assessed the post-treatment PSA nadir, as well as the PSA value at the time of bone scan.
To better understand post-prostatectomy treatment patterns influencing bone scan use, we defined biochemical recurrence as PSA ≥0.2 ng/mL in accordance with national guidelines.[1] We used claims and pharmacy data to classify any subsequent treatments as androgen deprivation therapy (ADT) or radiation therapy (XRT). Next, we characterized XRT according to timing after surgery to better understand whether it was intended as adjuvant or salvage. We defined adjuvant therapy as occurring within one year of surgery, and salvage therapy as occurring more than one year after prostatectomy. Lastly, we identified PSA values at the time of post-prostatectomy ADT or XRT use.
Statistical analysis
We used descriptive statistics to characterize our cohort according to post-treatment bone scan use. We examined a range of demographic and clinical covariates, including age, race (black, white, other), ethnicity (Hispanic, non-Hispanic, unknown), marital status (married, divorced, single/never married, widowed, unknown), cancer registry Gleason score, D’Amico risk group, surgical margin status (positive, negative, unknown), and Charlson comorbidity score. Next, we examined rates of, and time to, adjuvant and salvage therapy, as well as the corresponding PSA values at the time of each therapy. Finally, we used multivariable logistic regression to assess factors associated with receipt of bone scan after surgery. We selected variables a priori including: age, race, ethnicity, marital status, D’Amico risk group, Charlson comorbidity score, pretreatment PSA value, history of prior bone scan, and surgical margin status.
All analyses were performed in SAS, version 9.4 (SAS Institute, Cary, NC). Statistical significance was evaluated using a significance level of 0.05. This study was approved by the VA Ann Arbor Healthcare System Institutional Review Board.
RESULTS
Demographic characteristics of the 12,269 men treated with radical prostatectomy are displayed in Table 1. Mean age in this cohort was 62, and most men were diagnosed with low or intermediate risk disease at a median PSA of 5.6 ng/mL. The median follow up for the entire cohort was 81.4 months, and all patients were followed for at least 5 years.
Table 1.
Demographic characteristics of 12,269 men treated with radical prostatectomy for incident diagnoses of prostate cancer stratified by receipt of postoperative bone scan.
| Demographics | Bone scan (N = 2652) | No bone scan (N = 9617) | p-value |
|---|---|---|---|
| Mean age, y. (standard deviation) | 62.4 (8.0) | 62.1 (7.2) | 0.049 |
| Race, % | <.001 | ||
| Black | 26 | 22 | |
| White | 71 | 75 | |
| Other/unknown | 3 | 3 | |
| Ethnicity, % | <.001 | ||
| Hispanic | 7 | 4 | |
| Non-Hispanic | 92 | 95 | |
| Other/unknown | 1 | 1 | |
| Marital status, % | 0.033 | ||
| Married | 54 | 57 | |
| Divorced/separated | 31 | 29 | |
| Single/never | 8 | 8 | |
| Widowed | 7 | 6 | |
| Other | <1 | <1 | |
| Employment status, % | <.001 | ||
| Full-time | 13 | 16 | |
| Part-time | 5 | 4 | |
| Retired | 38 | 41 | |
| Self-employed | 3 | 4 | |
| Unemployed | 40 | 34 | |
| Active military | <.1 | 0 | |
| Unknown | 1 | 1 | |
| Charlson comorbidity index, % | <0.01 | ||
| 0 | 54 | 51 | |
| 1 | 25 | 24 | |
| 2+ | 21 | 25 | |
| Median PSA at diagnosis, ng/mL (Range) | 6.3 (0.1 – 96.4) | 5.5 (0.1 – 97.2) | <.001 |
| Gleason Score, % | <.001 | ||
| 6 | 30 | 44 | |
| 7 | 47 | 47 | |
| 8-10 | 23 | 9 | |
| D’Amico Risk Group, % | <.001 | ||
| Low | 22 | 35 | |
| Intermediate | 40 | 42 | |
| High | 38 | 23 | |
| Positive surgical margins, % | 31 | 19 | <.001 |
| Median follow up, mo. (IQR) | 83.3 (71.5 – 95.0) | 80.9 (69.5 – 93.5) | <.001 |
While one third of men in this cohort received a preoperative bone scan (33%), one in five (22%) received at least one bone scan (median 1, range 1 to 13) after radical prostatectomy, and 30% of men who received a bone scan underwent more than one. As illustrated in Figure 1, most patients undergoing a post-prostatectomy bone scan had low (22%) and intermediate (40%) risk prostate cancer. Moreover, the median PSA at time of post-prostatectomy bone scan was 0.2 ng/mL, with 78% of bone scans performed in men with PSA values below 1 ng/mL. While nearly half of patients (48%) undergoing a bone scan received no subsequent treatment, men with low risk disease were less likely, and those with high risk disease more likely, to have subsequent treatment with either ADT or radiation therapy (p<0.001).
Figure 1.
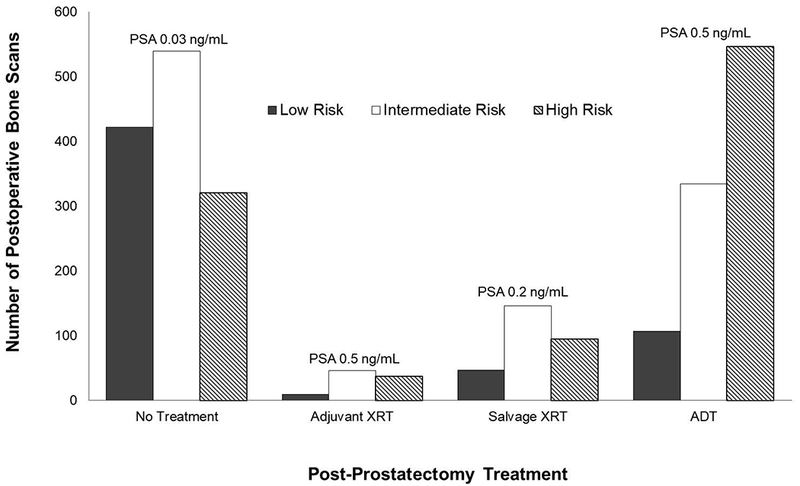
Distribution of postoperative bone scans across categories of adjuvant or salvage treatment and D’Amico risk group with median PSA values at the time of bone scan. Most bone scans were obtained in men who did not receive postoperative therapy, and most scans were in men with PSA values below 1ng/mL.
As shown in Table 2, both adjuvant and salvage radiation were more common in men who received bone scans (p<0.001), though time to radiation did not differ based on receipt of bone scan. Men who received bone scans were also more likely to receive ADT and do so later after surgery. The receipt of bone scan was associated with significantly higher PSA values at treatment for both salvage and adjuvant radiation, but not for ADT. After multivariable adjustment, factors significantly associated with bone scan were positive surgical margins, preoperative PSA, Hispanic ethnicity, black race, increasing D’Amico risk group, and history of a prior bone scan (Table 3). After adjustment, we found no differences in post-prostatectomy bone scan use according to patient comorbidity or age. Rates of preoperative and postoperative bone scan varied widely across facilities, and did not appear to be correlated within individual facilities (Figure 2).
Table 2.
Treatment type and PSA levels among men who received additional therapy following radical prostatectomy stratified by receipt of postoperative bone scan.
| Characteristic | Bone scan (n=2652) | No bone scan (n=9617) | p-value |
|---|---|---|---|
| Any therapy (%) | 51.6% | 11.8% | <.001 |
| Radiation therapy (%) | 14.3% | 3.6% | <.001 |
| Adjuvant | 3.5% | 1.1% | <.001 |
| Salvage | 10.8% | 2.5% | <.001 |
| Time to radiation therapy, months (median, IQR) | 23.8 (12.5 - 46.4) | 21.0 (10.6 - 43.0) | 0.06 |
| Adjuvant | 8.1 (5.8- 9.9) | 7.7 (5.4 - 9.8) | 0.6 |
| Salvage | 33.4 (20.2 - 52.7) | 33.8 (18.9 - 49.6) | 0.3 |
| PSA at radiation therapy, ng/mL (median, range) | 0.3 (0.0 - 0.9) | 0.2 (0.0 - 3.7) | <.001 |
| Adjuvant | 0.5 (0.0 - 9.1) | 0.1 (0.0 - 3.7) | <.001 |
| Salvage | 0.3 (0.0 - 9.4) | 0.2 (0.0 - 3.3) | <.001 |
| ADT (%) | 37.3% | 8.2% | <.001 |
| PSA at ADT, ng/mL (median, range) | 0.9 (0 - 2513) | 0.2 (0 - 598) | 0.1 |
| Time to ADT, months (median, IQR) | 12.2 (2.9 - 38.6) | 8.3 (1.8 - 36.6) | 0.05 |
Table 3.
Multivariable logistic regression results modeling the receipt of bone scan following radical prostatectomy.
| Covariate | Adjusted odds ratio (95% CI) |
|---|---|
| Age | 0.99 (0.98 – 1.01) |
| Race | |
| White | Referent |
| Black | 1.32 (1.10 – 1.59) |
| Other | 0.81 (0.51 – 1.30) |
| Ethnicity | |
| Non-Hispanic | Referent |
| Hispanic | 1.58 (1.22 – 2.05) |
| Marital status | |
| Married | Referent |
| Divorced/separated | 0.95 (0.79 – 1.13) |
| Never married | 0.79 (0.58 – 1.07) |
| Widowed | 0.97 (0.68 – 1.38) |
| D’Amico risk group | |
| Low | Referent |
| Intermediate | 1.44 (1.16 – 1.77) |
| High | 1.88 (1.49 – 2.36) |
| Charlson comorbidity score | |
| 0 | Referent |
| 1 | 0.85 (0.71 – 1.03) |
| 2+ | 1.12 (0.91 – 1.38) |
| Positive surgical margin | 1.68 (1.40 – 2.01) |
| Preoperative PSA | 1.02 (1.01 – 1.03) |
| Prior bone scan | 1.55 (1.32 – 1.84) |
Figure 2.
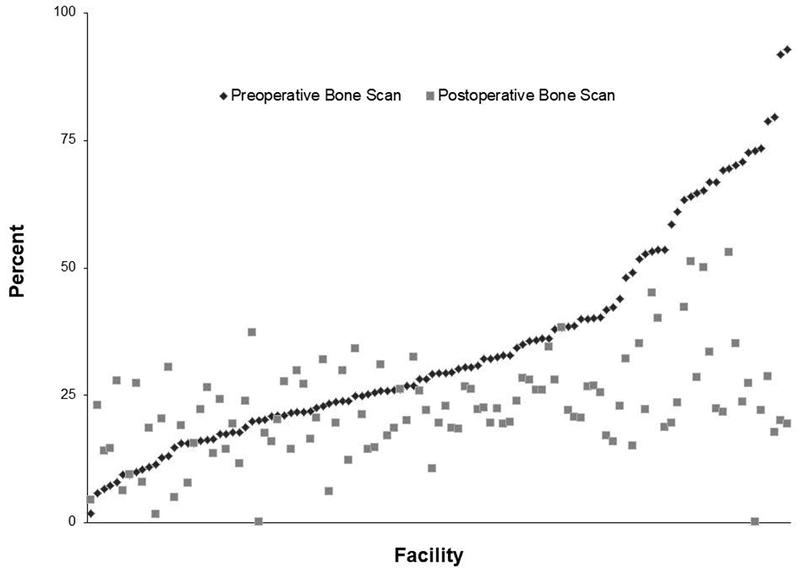
Preoperative and postoperative bone scan rates across VHA facilities. The range of preoperative bone scan rates is large, with little apparent correlation between the facility level use of preoperative and postoperative bone scan.
DISCUSSION
One in five men in this large national integrated delivery system underwent at least one postoperative bone scan following radical prostatectomy. Nearly half of these scans were performed in men who did not receive any additional treatment. The median PSA among men who received bone scans was 0.2 ng/mL, which suggests that half of patients who underwent a bone scan after prostatectomy did so before their PSA had reached the level of a biochemical recurrence. Even after adjustment for patient and disease characteristics, receipt of preoperative bone scan was a significant predictor of postoperative imaging, which suggests that there may be non-clinical variables (e.g., provider preference for “baseline” studies) influencing the decision to pursue bone scan. The wide range of usage across facilities further suggests an opportunity for increased standardization. Taken together, these results suggest that there is opportunity for more judicious use of postoperative bone scan, just as in the pretreatment setting.
These findings suggest a higher rate of post-prostatectomy bone scan use than found in prior studies, and are reminiscent of imaging overuse in the pretreatment setting.[19] While imaging overuse in the workup of newly diagnosed prostate cancer has been extensively studied, there is a paucity of literature investigating post-treatment use. Interestingly, our findings are from an integrated delivery system which lacks incentives for imaging, potentially underestimating bone scan use in fee-for-service systems, and justifying post-treatment efforts to decrease low value imaging. Our results regarding ethnicity are somewhat surprising given that studies from other settings have found that minority populations generally receive a lower intensity of follow up, and we did observe differences based on race/ethnicity with African-American/Hispanic men more likely to undergo a bone scan.[22] However, these findings may be reflective of more aggressive disease in these men possibly incompletely controlled for in our model.[23]
For this population-based study, we were able to use PSA values prompting bone scan use among men treated with radical prostatectomy. Unfortunately, our finding of low PSA values at the time of imaging indicates the results were unlikely to be clinically useful. In other words, a negative imaging test at low PSA levels is unable to differentiate local versus distant metastatic disease, and therefore adds little value to clinical decision-making for men with biochemical recurrence as currently defined. In fact, guidelines recommend consideration of bone scan in the setting of biochemical recurrence, however indicate the likelihood of a positive result in the absence of symptoms and with PSA values below 10 ng/mL is low.[1] For example, one study found that men with PSA values below 10 ng/mL had positive bone scan rates ranging from 0 to 11%, depending on if PSA doubling time was greater or less than 6 months, and another estimated the probability of a positive bone scan below 5% until PSA values exceeded 40 ng/mL.[14,17] However, this situation is made more complicated by results suggesting that up to one in four men with bone metastases after prostatectomy present with PSA levels below 10 ng/mL.[13] Post-prostatectomy bone scan is likely warranted in men with rapidly rising PSA values or values closer to 10 ng/mL, however that still excludes the many men in our cohort who received bone scans at far lower values. Interestingly, studies suggest that implementation of salvage radiotherapy earlier in the postoperative period and at lower PSA values may confer benefits in the form of lower rates of additional recurrence and metastasis.[24] In light of this, eliminating bone scan altogether and proceeding directly to salvage therapy for men with biochemical recurrence but low PSA values could be an approach to lower the use of uninformative imaging while improving clinical outcomes.
Our findings are also relevant to emerging diagnostic techniques posited to improve post-treatment prostate cancer surveillance, namely, positron emission tomography (PET) imaging. Early findings suggest PET-based approaches may improve staging of lymph nodes and distant metastases (e.g., bone) for men with newly diagnosed prostate cancer.[25,26] Relevant to our work, PET imaging is also increasingly used to evaluate biochemical recurrence. The inability of current bone scan imaging techniques to adequately assess the source of low but detectable PSA levels after surgery may be frustrating for clinicians, spurring the promise and use of PET imaging.[27,28] PET imaging utilizing prostate-specific membrane antigen (PSMA) in particular appears to hold promise in the evaluation of biochemical recurrence, especially at lower PSA values where traditional bone scan has been of limited use. Recent data from Gadzinski et al. suggests that nearly half of post-prostatectomy men with a PSA of 0.2 ng/mL may have detectable lesions using PSMA-PET, with the detection rate improving to 100% among included men with PSA values greater than 6 ng/mL.[29] Continuing work should help to clarify the most effective ways to apply this new technology and identify those men most likely to benefit. Next-generation PET imaging could be harnessed to improve the value of care by identifying patients with metastases who would not benefit from local therapies such as salvage radiation. However, the ability of advanced imaging techniques to improve decision-making and clinical outcomes for these patients remains to be fully explored.
While significant efforts are directed towards decreasing imaging overuse in prostate cancer staging, we found similar issues post-treatment that are not being addressed. From a clinical perspective, a negative postoperative bone scan may alleviate patient and clinician concerns about rising, though low, PSA values. It could also lead to salvage treatment with radiation therapy, as many scans in these data may have been ordered to verify the absence of disseminated disease prior to localized radiotherapy. However, using negative bone scans as reassurance and to differentiate local versus distant disease when it is unlikely to yield accurate results could mislead patients and clinicians resulting in misuse and overuse of treatment. These risks must be weighed against the benefit of possible discovery of some metastatic disease. In addition, we found a prior bone scan was associated with post-prostatectomy bone scan use suggesting that some may be ordered based on non-clinical factors such provider perception or preference. Lastly, consideration should be given to the natural history of PSA progression after radical prostatectomy and to the life expectancy of patients experiencing biochemical recurrence years after their surgery as we found age was not a factor in our adjusted analyses.[30] Better understanding competing risks for individual patients, and how best to approach de-implementation of low value imaging in light of these risks appears warranted.
There are limitations to this study. This study did not include an assessment of PSA kinetics found to be at least as important as PSA levels in predicting bone scan positivity after biochemical recurrence. A subset of the patients in this study likely had rapid doubling times prompting imaging in spite of low PSA values. However, given the median PSA at bone scan of 0.2 ng/mL, our overall conclusions regarding the extent to which imaging is potentially low value is unchanged. The data used in this study lack information regarding the indication for which imaging was obtained. It is possible that a number of scans were obtained to work up common benign conditions such as persistent low back pain to rule out malignancy as an etiologic factor. It is unlikely that many men in this study would have suffered from symptoms from metastasis-related back pain due to the low PSA values at the time of bone scan. Additionally, prior studies examining bone scan positivity after biochemical recurrence did not observe an association between common symptoms such as fatigue or back pain and bone scan results.[15] Next, whether recent advances in the management of locally recurrent and metastatic prostate cancer might have impacted imaging use among prostate cancer survivors in our study remains unknown. However, use of advanced therapies would theoretically only increase bone scan use to monitor treatment effects. We also did not exclude men participating in clinical trials. Lastly, we did not have results of the bone scan studies. However since many men were not treated after the bone scan it was unlikely that these scans were positive for disease. As ADT was the most commonly used salvage treatment, it remained unclear whether this was used to target biochemical recurrence, an increasingly scrutinized practice[31], or metastatic disease to the bone which would be unusual for most PSA levels prompting bone scans in this study.
Despite these limitations, our study has important implications for current and future practice. While the American Urological Association has partnered with the American Board of Internal Medicine’s Choosing Wisely campaign to help reduce the routine use of bone scans in the staging of men with low risk prostate cancer, it appears that the potential for imaging overuse in this population may persist in the postoperative phase. As a point of comparison from another osteophilic malignancy, historic data recommended obtaining serial post-treatment bone scans in women following treatment for breast cancer. Subsequent evidence has led to guidelines recommending against the use of routine follow up imaging in otherwise asymptomatic women.[32,33] Focusing increased attention on this aspect of prostate cancer survivorship care will help decrease the burdens of unnecessary testing and procedures. As imaging paradigms in prostate cancer evolve, it will be critically important to leverage these insights in guidelines and best practices. The application of carefully considered testing thresholds will help to minimize low yield evaluations among prostate cancer survivors, even as technology enables improved diagnostic efficiency at lower PSA values. De-implementation of unnecessary imaging in the post-prostatectomy setting will spare asymptomatic prostate cancer survivors inconvenience and cost, likely without compromising quality and quantity of life.
We found relatively high rates of bone scan use following radical prostatectomy, with many performed at PSA levels below the threshold for biochemical recurrence. Our results emphasize the need to optimize post-prostatectomy imaging practices, much like those among newly diagnosed men.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Ryan Blake for assistance with data collection.
Funding: Ruth L. Kirschstein National Research Service Award 4TL1TR000435-10 (PSK), National Cancer Institute T32-CA180984 (TB), National Institutes of Health Claude Pepper Center AG-024824 (DAS), Agency for Healthcare Research and Quality R01-HS-025707 (BKH), National Cancer Institute R01-CA-222885-01 (TAS)
Footnotes
Conflicts of Interest: None
REFERENCES
- [1].National Comprehensive Cancer Network. Practice Guidelines in Oncology: Prostate Cancer v2. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed Jun. 2017.
- [2].American Urological Association. PSA Testing for the Pretreatment Staging and Posttreatment Management of Prostate Cancer: 2013 Revision of 2009 Best Practice Statement. https://auanet.org/documents//education/clinical-guidance/Prostate-Specific-Antigen.pdf. Accessed Jun 2017.
- [3].Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol 2004; 171: 2122–7. [DOI] [PubMed] [Google Scholar]
- [4].Choi WW, Williams SB, Gu X, Lipsitz SR, Nguyen PL, Hu JC. Overuse of imaging for staging low risk prostate cancer. J Urol 2011; 185: 1645–9. [DOI] [PubMed] [Google Scholar]
- [5].Falchook AD, Hendrix LH, Chen RC. Guideline-discordant use of imaging during work-up of newly diagnosed prostate cancer. J Oncol Pract 2015; 11: e239–46. [DOI] [PubMed] [Google Scholar]
- [6].Lavery HJ, Brajtbord JS, Levinson AW, Nabizada-Pace F, Pollard ME, Samadi DB. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology 2011; 77: 274–8. [DOI] [PubMed] [Google Scholar]
- [7].Makarov D V, Hu EYC, Walter D, Braithwaite RS, Sherman S, Gold HT, et al. Appropriateness of Prostate Cancer Imaging among Veterans in a Delivery System without Incentives for Overutilization. Health Serv Res 2016; 51: 1021–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palvolgyi R, Daskivich TJ, Chamie K, Kwan L, Litwin MS. Bone scan overuse in staging of prostate cancer: an analysis of a Veterans Affairs cohort. Urology 2011; 77: 1330–6. [DOI] [PubMed] [Google Scholar]
- [9].Porten SP, Smith A, Odisho AY, Litwin MS, Saigal CS, Carroll PR, et al. Updated trends in imaging use in men diagnosed with prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prasad SM, Gu X, Lipsitz SR, Nguyen PL, Hu JC. Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer 2012; 118: 1260–7. [DOI] [PubMed] [Google Scholar]
- [11].Hurley P, Dhir A, Gao Y, Drabik B, Lim K, Curry J, et al. A Statewide Intervention Improves Appropriate Imaging in Localized Prostate Cancer. J Urol 2017; 197: 1222–8. [DOI] [PubMed] [Google Scholar]
- [12].Makarov D V, Loeb S, Ulmert D, Drevin L, Lambe M, Stattin P. Prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Natl Cancer Inst 2013; 105: 1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Loeb S, Makarov D V, Schaeffer EM, Humphreys EB, Walsh PC. Prostate specific antigen at the initial diagnosis of metastasis to bone in patients after radical prostatectomy. J Urol 2010; 184: 157–61. [DOI] [PubMed] [Google Scholar]
- [14].Cher ML, Bianco FJ, Lam JS, Davis LP, Grignon DJ, Sakr WA, et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol 1998; 160: 1387–91. [PubMed] [Google Scholar]
- [15].Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol 2008; 179: 906–10. [DOI] [PubMed] [Google Scholar]
- [16].Kane CJ, Amling CL, Johnstone PAS, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 2003; 61: 607–11. [DOI] [PubMed] [Google Scholar]
- [17].Okotie OT, Aronson WJ, Wieder JA, Liao Y, Dorey F, DeKERNION JB, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol 2004; 171: 2260–4. [DOI] [PubMed] [Google Scholar]
- [18].Dotan ZA, Bianco FJ, Rabbani F, Eastham JA, Fearn P, Scher HI, et al. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J Clin Oncol 2005; 23: 1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hussein AA, Punnen S, Zhao S, Cowan JE, Leapman M, Tran TC, et al. Current Use of Imaging after Primary Treatment of Prostate Cancer. J Urol 2015; 194: 98–104. [DOI] [PubMed] [Google Scholar]
- [20].Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol 2016; 13: 226–35. [DOI] [PubMed] [Google Scholar]
- [21].Mittakanti HR, Thomas I- C, Shelton JB, Makarov D V, Skolarus TA, Cooperberg MR, et al. Accuracy of Prostate-Specific Antigen Values in Prostate Cancer Registries. J Clin Oncol 2016; 34: 3586–7. [DOI] [PubMed] [Google Scholar]
- [22].Shavers VL, Brown M, Klabunde CN, Potosky AL, Davis W, Moul J, et al. Race/ethnicity and the intensity of medical monitoring under “watchful waiting” for prostate cancer. Med Care 2004; 42: 239–50. [DOI] [PubMed] [Google Scholar]
- [23].Chu DI, Moreira DM, Gerber L, Presti JC, Aronson WJ, Terris MK, et al. Effect of race and socioeconomic status on surgical margins and biochemical outcomes in an equal-access health care setting. Cancer 2012; 118: 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol 2016; 34: 3648–54. [DOI] [PubMed] [Google Scholar]
- [25].Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol 2016; 195: 1436–43. [DOI] [PubMed] [Google Scholar]
- [26].Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J, Aygun A, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun 2015; 36: 582–7. [DOI] [PubMed] [Google Scholar]
- [27].Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med 2015; 56: 668–74. [DOI] [PubMed] [Google Scholar]
- [29].Gadzinski AJ, Greene KL, Carroll P, Ryan CJ, Feng FY, Hope T. Detection of prostate cancer lesions using Gallium-68 PSMA-11 PET in men with biochemical recurrence following radical prostatectomy. J Clin Oncol 2018; 36: 236. [Google Scholar]
- [30].Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–9. [DOI] [PubMed] [Google Scholar]
- [31].Fu AZ, Tsai H- T, Haque R, Yood MU, Cassidy-Bushrow AE, Van Den Eeden SK, et al. Mortality and Androgen Deprivation Therapy as Salvage Treatment for Biochemical Recurrence after Primary Therapy for Clinically Localized Prostate Cancer. J Urol 2017; 197: 1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gerber FH, Goodreau JJ, Kirchner PT, Fouty WJ. Efficacy of preoperative and postoperative bone scanning in the management of breast carcinoma. N Engl J Med 1977; 297: 300–3. [DOI] [PubMed] [Google Scholar]
- [33].Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 2016; 34: 611–35. [DOI] [PubMed] [Google Scholar]


