Abstract
Transcription factors that coordinate migration, differentiation or proliferation of enteric nervous system (ENS) precursors are not well defined. To identify novel transcriptional regulators of ENS development, we performed microarray analysis at embryonic day (E) 17.5 and identified many genes that were enriched in the ENS compared to other bowel cells. We decided to investigate the T-box transcription factor Tbx3, which is prominently expressed in developing and mature ENS. Haploinsufficiency for TBX3 causes ulnar-mammary syndrome (UMS) in humans, a multi-organ system disorder. TBX3 also regulates several genes known to be important for ENS development. To test the hypothesis that Tbx3 is important for ENS development or function, we inactivated Tbx3 in all neural crest derivatives, including ENS progenitors using Wnt1-Cre and a floxed Tbx3 allele. Tbx3 fl/fl; Wnt1-Cre conditional mutant mice die shortly after birth with cleft palate and difficulty feeding. The ENS of mutants was well-organized with a normal density of enteric neurons and nerve fiber bundles, but small bowel glial cell density was reduced. Despite this, bowel motility appeared normal. Furthermore, although Tbx3 is expressed cardiac neural crest, Tbx3 fl/fl; Wnt1-Cre mice had structurally normal hearts. Thus, loss of Tbx3 within neural crest has selective effects on Tbx3-expressing neural crest derivatives.
Keywords: T-box, enteric nervous system, cleft palate, heart, Tbx3, Ulnar Mammary Syndrome
Introduction
TBX3 is a transcription and splicing factor that belongs to the TBX2 subfamily of T-box transcription factors (Kumar et al., 2014; Rowley et al., 2004). Despite sharing an evolutionarily conserved DNA binding domain of approximately 200 amino acids known as the T-box, all 20 known T-box transcription factors regulate different genes. Furthermore, TBX3-mediated regulation of target genes is context-specific, suggesting that association of TBX3 with different cofactors drives target specificity (Kumar et al., 2014; Kumar P et al., 2014; Tada and Smith, 2001). In humans, point mutations within and outside of the conserved T-box domain of TBX3 cause ulnar-mammary syndrome (UMS). UMS is an autosomal dominant disorder that affects limb, tooth, hair, apocrine gland and genital development (Bamshad et al., 1997). TBX3 is also known to be abundantly expressed in the ENS (Heanue and Pachnis, 2006; Memic et al., 2017a; Vohra et al., 2006), but roles for TBX3 in the ENS have not been previously defined.
The ENS is a complex nervous system in the bowel wall that controls motility, blood flow, and epithelial function. ENS defects can cause life-threatening medical problems like Hirschsprung disease or chronic intestinal pseudoobstruction, and our understanding of genes that regulate enteric nervous system (ENS) development remains incomplete (Amiel et al., 2008; Bergeron et al., 2013; Goldstein et al., 2013; Gui et al., 2017; Kapoor et al., 2015; Lake and Heuckeroth, 2013; Luzón-Toro et al., 2015; Mckeown et al., 2013; Obermayr et al., 2012). Several studies have shown ENS expression of Tbx3 at E11-12, E14.5, E15-16, and E18-19 (Heanue and Pachnis, 2006; Memic et al., 2017b; Vohra et al., 2006). Consistent with this, we profiled the transcriptome of the developing ENS at E17.5 by microarray and found abundant Tbx3 transcripts. In other developmental contexts, TBX3 regulates several genes known to impact ENS development (Zhu et al., 2016; Burgucu et al., 2012; Davenport, 2003; Emechebe et al., 2016; Osterwalder et al., 2014; Rallis, Del Buono, & Logan, 2005; Tümpel et al., 2002) increasing our interest in studying the role of TBX3 in the ENS.
The ENS is a neural crest derivative formed primarily from vagal neural-derived crest cells (NCCs) that migrate through fetal bowel, proliferate and then differentiate into diverse neuron and glia subtypes. Proliferation of progenitor cells, differentiation of mature neuronal phenotypes, and formation of functional neuronal circuits continues after birth and into adulthood (Sasselli et al., 2012). In vivo studies of ENS function in Tbx3 mutant mice were not previously possible because homozygous Tbx3 −/− mice have heart malformations that cause prenatal death (Bakker et al., 2008; Frank et al., 2013, 2012).
To further understand the role of Tbx3 in ENS development, we generated conditional Tbx3 fl/fl; Wnt1-Cre mutant mice. In this mouse line, Wnt1-Cre induces DNA recombination to inactivate Tbx3 in essentially all enteric nervous system precursors and their mature progeny (collectively called enteric neural crest-derived cells (ENCDC)) (Frank et al., 2013; Lake et al., 2016). Wnt1-Cre also inactivates Tbx3 in other neural crest-derived tissue including great vessels of the heart and craniofacial bones (Jiang et al., 2002; Lee et al., 2004). We found that Tbx3 fl/fl; Wnt1-Cre mice die within 24 hours of birth, but have normal enteric neuron density, bowel motility, and cardiac septation at birth. Interestingly, enteric glia density was reduced in small bowel of mutant mice. We also found that Tbx3 fl/fl; Wnt1-Cre mice have highly penetrant cleft palate and reduced levels of Osr2 in the developing palate. OSR2 is a transcription factor that regulates many genes involved in palate mesenchyme development (Fu et al., 2017; Lan, 2004), suggesting that reduced OSR2 may contribute to the cleft palate phenotype observed in Tbx3 fl/fl; Wnt1-Cre mutant mice.
Materials and Methods
Animals and genoiyping
Animal experiments were approved by the Institutional Animal Use and Care Committee at The Children’s Hospital of Philadelphia Research Institute, the University of Utah, and by the Washington University School of Medicine Animal Studies Committee. All mice were maintained on mixed genetic backgrounds (Wnt1-Cre (C57BL/6J x CBA/J)F1), Tbx3 floxed (Tbx3 fl/fl) (B16/SV129). The Tbx3 floxed (Tbx3 fl/fl) conditional allele was generated as described (Frank et al., 2013). Tbx3 fl/fl; Wnt1-Cre and EYFP; Wnt1-Cre reporter mice animals were generated by breeding Tbx3 flox/flox mice or Rosa26EYFP reporter mice (Gt(ROSA)26Sortm1 (EYFP) (Srinivas et al., 2001; RRID:IMSR_JAX:006148) to Tg(Wnt1cre)11Rth (RRID:IMSR_JAX:003829) (Danielian et al.,1998) mice (referred to as Wnt1-Cre). Tbx3 fl/fl and Tbx3 fl/wt; Wnt1-Cre males and females were then intercrossed to obtain Tbx3 fl/wt, Tbx3 fl/wt; Wnt1-Cre, or Tbx3 fl/fl (control genotypes) and the conditional knockout genotype, Tbx3 fl/fl; Wnt1-Cre. tdTomato reporter mice (Gt/ROSA)26Sortm9(CAG-tdTomato)Hze, stoc #007909), were bred into Tbx3;Wnt1-Cre mice. The vaginal plug day was counted as embryonic day 0.5. Newborn P0 mice were euthanized by decapitation. Genotyping reactions for Cre recombinase-containing transgenes and Rosa26EYFP used previously described primers (Lake et al., 2013). The Tbx3 wild-type and Tbx3 floxed alleles were genotyped using primer pair Tbx3-F: 5’ GTG TGA GAC AGA GAA ATC AGT GG 3’ and Tbx3–R: 5’ CCA ACT GGT ATC TTG ATA AAC CTC 3’, producing a 320 bp band from the wild-type allele and a 480 bp band from the conditional allele (Frank et al., 2013).
Bone and cartilage staining
Simultaneous staining with alizarin red S and alcian blue was performed with minor modifications to a published protocol (McLeod, 1980). P0 newborn mice were fixed in 95% ethanol for two hours, after which skin and organs were removed and fixation was continued in 95% ethanol for one week. Samples stored in 4% paraformaldehyde for more than 72 hours were rinsed in milliQ water overnight before evisceration and post fixation in 95% ethanol. Samples were moved to acetone for two days and then stained for 3 days at 37 °C in 0.015% alcian blue (Sigma #A5268), 0.005% alizarin red S (Sigma #5533), 5% glacial acetic acid, and 70% ethanol. Samples were washed with water and rocked in a 1% KOH solution at room temperature until skeletons became visible after 48 h. Samples were then passed through a graded series of 20%/1% KOH, 50%/1% KOH, and 80% glycerol/1% KOH baths over the course of several weeks or until tissues cleared. Skeletal preparations were stored and photographed in 80% glycerol/1% KOH.
Whole-mount immunofluorescent staining
P0 bowel was harvested, flushed with PBS, pinned flat and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 minutes at room temperature, and then incubated in blocking solution (5% normal donkey serum (Jackson ImmunoResearch #017-000-121) in 0.5% Triton-X 100 (PBS-T)) for 1 h at 25 °C. Samples were then incubated overnight at 4 °C in primary antibody diluted in 5% normal donkey serum in 0.5% PBS-T. Samples were washed 3 times with PBS for 5 minutes and incubated with secondary antibody at 25 °C for 20 minutes in PBS. Samples were mounted in 50% Glycerol in PBS.
Antibodies
Primary antibodies: ANNA–1 antiserum (a gift from Vanda Lennon, Mayo Clinic, RRID:AB_2313944, 1:2000), SOX10 (goat, RRID:AB_2195374, Santa Cruz Cat# SC-17342, 1:400), TuJ1 (rabbit, RRID :AB_10063850, Covance Research Products Inc Cat# PRB-435P-100,1:10,000), TBX3 (goat, RRID: AB_2240328, R&D Systems Cat #AF4509-SP, 1:100), and SI00β (rabbit, RRID: AB_882426, Abcam Cat#AB52642, 1:200).
Secondary antibodies: donkey anti rabbit, goat anti-human, donkey anti-goat Alexa fluor-488, - 594, or -647 (Invitrogen, 1:400).
FITC dextran intestinal transit study
Bowel transit was determined by assessing the distribution of a FITC-conjugated dextran marker (70-kDa FITC-dextran; Sigma, Cat# 46945) in the bowel of control and Tbx3 fl/fl; Wnt1-Cre mice after feeding the non-absorbed fluorescent marker FITC-dextran as previously described (Butchbach et al., 2007). Pregnant dams were euthanized and mice were delivered at E19.5 by caesarian section to avoid having fed control and unfed mutant mice. All mice were fasted and kept on a warming pad for 1 h. Mice were then fed by mouth 7μL of a solution containing 50 mg FITC Dextran (70 kDa) per mL of 2% methylcellulose in water. Six hours later, mice were euthanized, and the bowel was divided into 8 segments (esophagus, stomach, small intestine 1-3, colon 1-3). Each bowel segment was opened and suspended in 100 μL of 1X phosphate buffered saline solution. Samples were vortexed for 15 seconds and centrifuged at 4000 rpm for 10 min, and fluorescent activity of the supernatant was measured using a fluorimeter (excitation 485 nm, emission at 525 nm). Bowel transit was analyzed using the intestinal “geometric center” of the distribution of dextran throughout the bowel and was calculated as described (Miller et al., 1981). For the geometric center calculation we included all bowel segments.
Fluorescence activated cell sorting (FACS) of cells from prenatal bowel
Prenatal bowel was dissected from reporter mouse strain (Tbx3; Wnt-1Cre; TdTomato or Wnt1Cre-EYFP) at E17.5 and digested in 0.5 mg/mL collagenase (Sigma Cat. #C-6885) and 0.5 mg/mL dispase (ThermoFisher Scientific, Cat. #17105-041) in PBS at 37 °C for 30 minutes. Digested samples were triturated using a P1000 pipette and filtered through a 40 μm cell strainer (Fisher #352340). Dissociated cells were re-suspended in FACS buffer (10mM HEPES, 1mg/mL BSA, 1% penicillin and streptomycin in HBSS). Samples were sorted into DMEM on a Beckman Coulter MoFlo (Siteman Flow Cytometry Core at Washington University in St. Louis School of Medicin) or a MoFlo Astrios EQ (Children’s Hospital of Philadelphia Flow Cytometry Core Laboratory) then re-suspended in Buffer RLT (RNEasy Micro Kit Qiagen #74004 or RNeasy Mini Kit (Qiagen #74104) to proceed immediately with RNA isolation as per the manufacturer’s instructions.
Microarray
Microarrays were performed using Affymetrix gene chips that contain probes for 28,000 mouse genes (GeneChip Mouse Gene 1.0 ST Array, performed at the Genome Technology Access Center, Washington University School of Medicine). N=3 chips for EYFP-positive samples, N=4 chips for EYFP-negative samples. Gene expression data was analyzed using the Partek Genomics Suite. Data across multiple arrays was normalized using Robust Multi-array Average (RMA) and ANOVA with correction for multiple comparisons was used to identify genes with > 2-fold higher expression in the EYFP-positive fraction (ENCDC) versus the EYFP-negative fraction (non-ENCDC) at a false discovery rate of 0.05. Genes were displayed using Bioconductor software and the Oligo package in R (Carvalho and Irizarry, 2010; Gentleman et al., 2004).
Histological analysis
Heads and hearts were fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin prior to sectioning at 6 μm (heads) or 10 μm (hearts). Sections were stained with hematoxylin and eosin using standard protocols.
EDU and TUNNEL staining
Four consecutive 6 μm sections from anterior and from posterior palate were taken from three embryos of each genotype at E13.5. Proliferation was measured using Click-iT® EdU Alexa Fluor® 488 Imaging Kit (Thermo Fisher Scientific #C10337). Percent of proliferative (EdU+) positive cells was defined as the number of EdU positive nuclei to total number of nuclei as detected by Hoechst counterstain in the mesenchyme of the palatal shelf tip region (150μm from the tip of the palate).
qRT-PCR
Total RNA was isolated from FACS sorted bowel cells or microdissected palatal shelves at E13.5 using RNeasy Mini Kit (Qiagen #74104) or RNEasy Micro Kit Qiagen #74004 and reverse-transcribed using Superscript II Reverse Transcriptase for cDNA synthesis (Invitrogen #18064014). qRT-PCR was performed on at least three biological replicates with three technical replicates per run using SsoFast™ EvaGreen® Supermix with Low ROX (Bio-Rad #1725211) and Bio-Rad Cycler CFX96. Primers are listed in Table 1.
Table 1.
Primers for qRT-PCR
| Gene Symbol | Unigene Title | Sequences a |
|---|---|---|
| Bmp2 | Bone Morphogenetic | f GCTTCTTAGACGGACTGCGG |
| Protein 4 | r GCAACACTAGAAGACAGCGGGT | |
| Bmp4 | Bone Morphogenetic | f AGCCCGCTTCTGCAGGA |
| Protein 4 | r AAAGGCTCAGAGAAGCTGCG | |
| Ccnd1 | Cyclin-D1 | f CTGGCCATGAACTACCTGGA |
| r GTCACACTTGATCACTCTGG | ||
| Dll1 | Delta like canonical | f CAACAAGAAGGCGGACTTTC |
| Notch ligand 1 | r CACTTGGTGTCACGTTTGCT | |
| Foxf2 | forkhead box F2 | f AGCATGTCTTCCTACTCGTTG |
| r TCTTTCCTGTCGCACACT | ||
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | f AACTTTGGCATTGTGGAAGG |
| r GTCTTCTGGGTGGCAGTGAT | ||
| Gli3 | f GCTCTTCAGCAAGTGGTTCC | |
| r TTGCTGTCGGCTTAGGATCT | ||
| Hes1 | Hes family BHLH transcription factor | f ACACCGGACAAACCAAAGAC |
| r ATGCCGGGAGCTATCTTTCT | ||
| Msx1 | Msh homeobox 1 | f AGTTCTCCAGCTCGCTCAGC |
| r GGAACCATATCTTCACCTGCGT | ||
| Osr1 | odd-skipped-related 1 | f TGTAGCGTCTTGTGGACAGC |
| r GCGACCTTACACCTGTGACAT | ||
| Osr2 | odd-skipped-related 1 | f TTGCTCATTCAGCAGAGGAC |
| r TCCCACACTCCTGACATTTG | ||
| Ptch1 | Protein patched homolog 1 | f GGCAGGAGGAGTTGATTGTGG |
| r CATAGTCGTAGCCCCTGAAGTG | ||
| Shh | Sonic hedgehog | f AAAGCTGACCCCTTTAGCCTA |
| r TTCGGAGTTTCTTGTGATCTTCC | ||
| Tbx2 | T-box 2 | f TCCTGCTAATGGACATCGTG |
| r AGACATAGGTGCGGAAGGTG | ||
| Tbx3 Exon 1/2 | T-box 3 | f TGAGGCCTCTGAAGACCATG |
| r TCAGCAGCTATAATGTCCATC | ||
| Tbx3 Exon 5/6 | T-box 3 | f GGGACATCCAACCTCAAAGA |
| r CCGTAGTGGTGGAAATCTTG |
5’ to 3’sequences; f = forward primer; r = reverse-strand primer
Microscopy
Photographs of whole fetuses, skeletal preparations, and hearts were acquired on an Olympus SZ40 stereomicroscope. Images of fluorescent whole-mount bowel were acquired as multiple optical sections using a Zeiss LSM 710 confocal (Zen software). FIJI (NIH ImageJ) software was used to process images including only cropping, stitching, rotating, centering, and uniform adjustments of brightness, contrast and saturation. Confocal images show flattened Z-stacks.
Statistics
GraphPad Prism (version 7.03 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com) was used for statistical analysis via student’s t-test. All studies include at least three biological replicates, unless otherwise noted in the text. Data are plotted with mean and positive standard error of the mean. A p-value < 0.05 was considered significant.
Results
Tbx3 is enriched in enteric neural crest-derived cells (ENCDC) compared to other cells of E17.5 bowel
To identify novel genes that might regulate development of the ENS during late embryonic development, we used a microarray to compare gene expression in ENS and non-ENS cells of the E17.5 bowel. For this study, Wnt1-Cre; Rosa26EYFP reporter mice were employed for fluorescence-activated cell sorting to separate EYFP expressing ENCDC from other cells of the bowel wall. This is a commonly used mouse line that expresses EYFP in essentially all ENCDC (>99.9%), but not in other cells of the bowel wall (Lake et al., 2016). As expected, we identified many differentially expressed genes encoding transcription factors, as well as other proteins (Figure 1). Differential expression of many of these genes have been described and validated previously (Heanue and Pachnis, 2006; Vohra et al., 2006), providing a high level of confidence in our results (Data will be uploaded to the NIH GEO database when the paper is in press).
Figure 1. Differentially expressed genes in ENS vs. non-ENS cells at E17.5 (A-F).
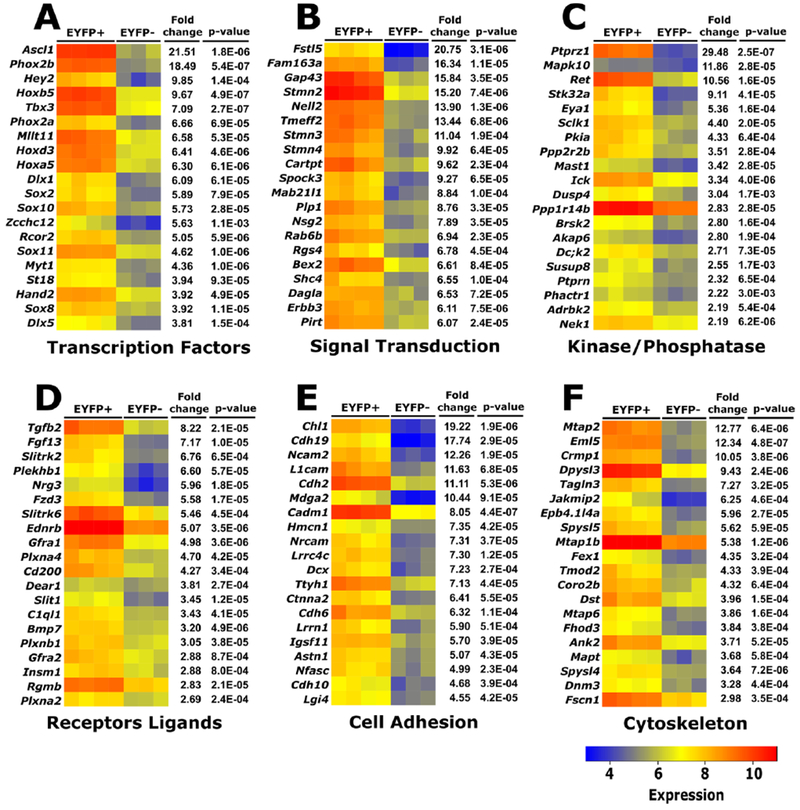
Genes differentially expressed in EYFP+ (ENS) vs. EYFP-(non-ENS) cells isolated from Wnt1-Cre; Rosa26EYFP reporter mice at E17.5. A full list of differentially expressed genes can be found in Supplementary Table 1.
For this study, we decided to focus on Tbx3, as its transcripts are 7-fold more abundant in ENCDC compared to non-ENS cells of the bowel (p= 2.7E-7) (Figure 1). TBX3 protein was recently demonstrated to be expressed at high levels in most enteric neuron types at E18.5 and we previously demonstrated Tbx3 mRNA in the region of the ENS at E14 (Memic et al., 2017b; Vohra et al., 2006). Our primary hypothesis was that Tbx3 would be essential for some aspect of ENS development or function.
Deletion of Tbx3 in neural crest cells results in early postnatal death
To determine how loss of Tbx3 impacts the ENS, we generated conditional mutant mice using the Wnt1-Cre mouse line to recombine Tbx3 alleles in Tbx3 fl/fl mice. To ensure that CRE-mediated DNA recombination occurred as expected in the ENS, we confirmed loss of TBX3 protein and Tbx3 mRNA by immunohistochemistry and qRT-PCR (Supplemental Figure 1). Tbx3 fl/fl; Wnt1Cre mice were born in normal Mendelian ratios at E19.5 and appeared healthy at birth with normal weight and no exterior anatomic defects (Figure 2 A-B). Initially, Tbx3 fl/fl; Wnt1Cre mice were indistinguishable from littermates without cyanosis, respiratory distress or abnormal movements. However, by weaning at 21 days after birth (P21), there were no surviving Tbx3 fl/fl; Wnt1Cre mice (Chi2-test, p=0.000895, N=66) and we never found Tbx3 fl/fl; Wnt1-Cre mice that survived longer than 24 hours. Death during the neonatal period can result from cardiac defects, renal failure, or because of nutritional deficiency due to inadequate intake or intestinal dysfunction (Turgeon and Meloche, 2009).
Figure 2. Early deletion of Tbx3 in neural crest derived cells via Wnt1-Cre results in postnatal lethality.

(A) Tbx3 fl/fl; Wnt1-Cre mice were found in normal Mendelian ratios at P0 (Chi2-test, p>0.05, n=79) and appear normal. Mutants can be distinguished from wild type littermates by the absence of a milk spot in the stomach (black arrowheads). (B) Average weight is normal after birth (p-value<0.05, N= 37 control, N=11 mutant). Error bar = SEM. (C,F) External anatomy of the great vessels and outflow tract visualized using a Tdtomato reporter in control and Tbx3 fl/fl; Wnt1-Cre newborn mice appears normal. A=aorta, p=pulmonary artery, b=brachiocephalic trunk, lc=left common carotid artery. Scale bar= 200μm. N= 3 of each genotype. (D-E,G-H) Histological sections through the region of the ventricular outlets of control (D-E) and Tbx3 fl/fl;Wnt1-Cre (G-H) E18.5 hearts showing normal connection of the pulmonary trunk with the right ventricle (D,G) and ascending aorta with the left ventricle (E, H). a=aorta, pt=pulmonary trunk, pa=pulmonary artery rv=right ventricle, lv=left ventricle, ra, right atrium, la, left atrium, ivs, interventricular septum. Scale bar= 200μm. Control N= 2, Tbx3 fl/fl;Wnt1-Cre N=6.
Heart structure is normal in Tbx3 fl/fl; Wnt1 Cre mice
We evaluated heart anatomy because Tbx3 is required for normal cardiac outflow tract and conduction system development (Mesbah 2008, Bakker2008, Frank 2012 and 2013) and because Wnt1-Cre is active in cardiac neural crest cells required for outflow tract septation and proper smooth muscle cell investment of the great arteries. Furthermore, Tbx3 is expressed in a subset of the Wnt1-Cre+ neural crest-derived cells. We analyzed the outflow tract and major arteries of the heart using a tdTomato; Wnt1-Cre reporter and did not detect gross morphologic differences in the outflow tract or great vessels of Tbx3 fl/fl; Wnt1Cre mice (Figure 2 C, F). Histological analysis of mutant fetal hearts revealed normal ventriculo-arterial connections and no ventricular septal defects (N=6; Figure 2D-E, G-H). This ruled out structural heart disease as a cause of neonatal mortality.
Feeding problems in Tbx3 fl/fl; Wnt1-Cre newborn mice are not due to ENS defects
Mutant mice could be distinguished from their wild-type littermates by the absence of milk spots, consistent with empty stomachs and an inability to feed normally (Figure 2 A). Serious ENS defects in conjunction with functional bowel obstruction can cause poor neonatal feeding in children. Since TBX3 is abundant in enteric neurons, we pursued analysis of ENS structure and function. The ENS forms primarily from vagal neural crest-derived cells (ENCDC) that colonize the developing bowel in a rostrocaudal direction from E9.5 to E13.5 (Lake et al., 2013). To determine if Tbx3 is necessary for proper migration of ENCDC through the bowel, we stained E13.5 bowel with TuJ1 antibody, which binds neuron-specific class III β-tubulin. TuJ1 staining correlates well with the position of the most distal ENCDC in fetal bowel because neurons are generated as ENCDC colonize the bowel (Bergner et al., 2014; Lake et al., 2013). We found that the TuJ1 -staining pattern was similar in Tbx3 fl/fl; Wnt1Cre embryos and control littermates. Specifically, patterning of ENCDC and the extent of bowel colonization by TuJ1+ cells was equivalent (Figure 3 A-C). Colon length was also comparable in Tbx3 fl/fl; Wnt1Cre and control littermates (Figure 3 D). Because E13.5 is the stage at which ENCDC colonization of the bowel is nearly complete, these data provide convincing evidence that Tbx3 expression within ENCDC is not needed for bowel colonization or for major aspects of ENS organization during morphogenesis.
Figure 3. Enteric neural crest-derived precursors colonize the bowel normally in Tbx3 fl/fl; Wnt1-Cre mice at E13.5.
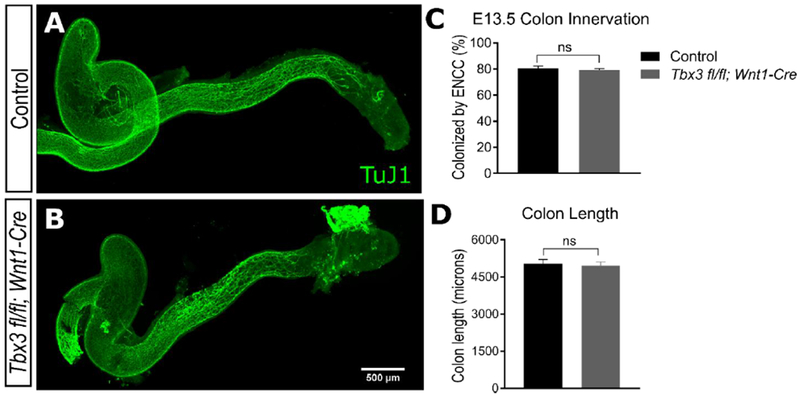
(A-B) At E 13.5 the colon is colonized by TuJ1+ ENCDC that have nearly completed their rostro-caudal migration from the vagal neural tube down to the distal colon in both Tbx3 fl/fl; Wnt1-Cre mice and control littermates. (C) Measurements of the percent of colon that is colonized by TuJ1+ fibers that accompany migrating ENCDC. (D) Mean absolute length of colon in Tbx3 fl/fl; Wnt1-Cre mice and control littermate is comparable. (Student’s t-test, p> 0.05, N=7 control, N=4 mutant). Error bar = SEM. Scale bar = 500 μm. Scale bar in B also applies to A.
To examine ENS structure at later stages, we stained newborn (P0) mouse bowel using ANNA-1 (anti-HuC/HuD) antibody to identify enteric neurons. We found a normal density of enteric neurons (Figure 4 A-F, M, S-U) in the small bowel and colon of Tbx3 fl/fl; Wnt1-Cre mice. We also found a normal density of SOX10+ cells in the proximal small intestine and colon of Tbx3 fl/fl; Wnt1-Cre mice (Figure 4 A, C, D, F, M, V, X), but there was a statistically significant reduction (22%, p=0.032) in SOX10+ cell density in distal small intestine of unclear physiologic or functional significance (Figure 4 B, E, W). SOX10 is expressed in all ENS precursors during early development, but then becomes restricted to enteric glia. To assess more mature glia, we stained P0 bowel with antibodies to S100β and SOX10 (Figure 4N) and found significantly reduced glial cell density in proximal (24% reduction, p < 0.05; Figure 4G, J, Y) and distal (48% reduction, p < 0.01; Figure 4H, K, Z) small bowel. Mean S100β+ cell density was also reduced in colon of Tbx3 fl/fl; Wnt1-Cre mice, but this was not statistically significant (28% reduction, p = 0.111 ; Figure 4I, L, A’). These data indicate that TBX3 is dispensable for differentiation of ENCDC into enteric neurons, but may be important for gliogenesis in the ENS. To determine which cells express TBX3 protein during development, we labeled normal P0 distal small bowel using an antibody against TBX3. Given the glial phenotype we were surprised that TBX3 protein was in HuC/D+ neurons (Figure 4O), but not in SOX10+ cells (Figure 4P) or in S100β+ glia (Figure 4Q). Collectively these data suggest that TBX3 is required for maturation of enteric glia, but that the effect is likely to be non-cell autonomous.
Figure 4. Normal density of neurons and glia are present in Tbx3 fl/fl; Wnt1-Cre mice at P0.

(A-F) ANNA–1 (anti-HuC/HuD) staining (red) of enteric neurons and SOX10 staining of glia and precursor cells (green) reveals that Tbx3 fl/fl; Wnt1-Cre and their wild-type littermates have a comparably dense network of enteric neurons but fewer SOX10+ cells in distal small bowel at P0. Scale bar =100 μm. Scale bar in F applies to A-E. (G-L) S100β (red) and SOX10 (green) staining reveals decreased numbers of SOX10+ S100β+ glia in Tbx3 fl/fl; Wnt1-Cre P0 mice compared to their control littermates. Scale bar =100 μm. Scale bar in L applies to G-L. (M) Expanded view of B. White arrowhead indicates a HuC/D+ neuron; white arrow indicates a SOX10+ cell. (N) Expanded view of H. White arrowheads indicate SOX10+S100β+ cells (counted); white arrows indicate SOX10+ S100β- cells. (O-Q) TBX3 antibody (green) labels HuC/D+ neurons, but not S100β+ glia or SOX10+ cells in P0 myenteric plexus. (R) TBX3 antibody (green) does not label Tbx3 fl/fl; Wnt1-Cre myenteric plexus. cKO = conditional knockout. Scale bar =10 μm. Scale bar in R applies to M-R. (S-U) Quantification of neuronal cell bodies (ANNA-1+) reveals normal numbers in the proximal small intestine, distal small intestine and the colon of Tbx3 fl/fl; Wnt1-Cre mice. (Student’s t-test, p> 0.05). (V-W) Quantification of SOX10+ cells reveals normal numbers in the proximal small intestine and colon of Tbx3 fl/fl; Wnt1-Cre mice (Student’s t-test, p> 0.05). A 22% reduction in SOX10+ cell density was statistically significant in the distal small intestine of Tbx3 fl/fl; Wnt1-Cre mice. (Student’s t-test, p = 0.0316). (Y-A’) Quantification of glia (SOX10+, S100β+ cells) reveals reduced glial density in proximal (24% reduction, Student’s t-test, p = 0.020) and distal (48% reduction, Student’s t-test, p = 0.007) small intestine of Tbx3 fl/fl; Wnt1-Cre mice. Glial density was not significantly different from control in colon of Tbx3 fl/fl; Wnt1-Cre mice (Student’s t-test, p = 0.111). * p ≤ 0.05, ** p ≤ 0.01. n = at least 3 for each genotype. Error bar = SEM.
Little is known about mechanisms that control enteric gliogenesis, but in many regions of the nervous system including the ENS, Notch signaling is be critical. In particular, Hedgehog pathway activation was reported to robustly induce the Notch ligand Dll1 and the Notch signaling molecule Hes1 to promote ENCDC proliferation and glial differentiation (Charrier and Pilon, 2017; Ngan et al., 2011). To determine if Tbx3 mutants have reduced induction of Dll1 and Hes1 as a function of altered Hedgehog or Notch signaling, we performed quantitative PCR. ENCDC were FACS-isolated from E17.5 control and Tbx3 fl/fl; Wnt1-Cre; TdTomato distal small bowel. Levels of Dll1 and Hes1 mRNA were normal in ENCDC of Tbx3 mutant bowel (Figure 5A-B). Together, these results suggest that gliogenesis defects in Tbx3 bowel are mediated by a neuron-dependent, but Dll1 and Hes1-independent pathway.
Figure 5: Notch pathway members Dll1 and Hes1 are expressed at normal levels in Tbx3fl/fl; Wnt1-Cre distal small intestine, (A-B).

Quantitative PCR was performed on ENCDC from E17.5 distal small intestine. Levels of Notch pathway members Dll1 (Mann-Whitney Rank Sum Test, p = 1; n=5 control, 3 mutant) or Hes1 (Student’s t-test, p = 0.803; n=5 control, 3 mutant) were normal in the ENS of Tbx3 fl/fl; Wnt1-Cre; Tdtomato mice. Expression levels are relative to mean expression in Tbx3 controls. Error bar = SEM.
Functional bowel motility appears normal in Tbx3 fl/fl; Wnt1-Cre newborn mice
Bowel dysmotility can be profound even without striking anatomic defects in the ENS (Gianino, 2003). We therefore tested the hypothesis that ENS dysfunction and bowel dysmotility might underlie perinatal death in Tbx3 fl/fl; Wnt1-Cre+ mice because TBX3 was absent from ENCDC. In support of this hypothesis, some Tbx3 fl/fl; Wnt1-Cre mice accumulated large amounts of air in the small bowel, a problem that can occur with serious bowel dysmotility (Figure 6 A). Although Tbx3 fl/fl; Wnt1-Cre mice were not spontaneously feeding (evidenced by no milk in their stomachs as compared to littermates), we used a previously established protocol (Butchbach et al., 2007; Miller et al., 1981) to administer Fluorescein isothiocyanate (FITC) dextran to newborn mice by mouth. FITC dextran is poorly absorbed, so intraluminal FITC abundance is a good measure of transit through the bowel and should be abnormal if bowel motility is impaired. Unlike adult mice, where bowel motility moves FITC dextran through the small bowel and into the colon within 1-2 hours, bowel transit is much slower in P0 mice. To quantify transit through the bowel, we measured FITC dextran levels in sequential bowel regions six hours after oral administration. FITC dextran distribution was indistinguishable between Tbx3 fl/fl; Wnt1-Cre mice and control littermates (Figure 6 B-C). These results indicate that the connectivity and function of the ENS at P0 is sufficient to coordinate normal bowel motility in the small intestines of Tbx3 fl/fl; Wnt1-Cre newborns. Further analysis of colon motility was not practical because of slow bowel transit in neonatal mice and < 24 hour lifespan of the Tbx3 fl/fl; Wnt1-Cre animals. Nonetheless, these data provide clear evidence that Tbx3 fl/fl; Wnt1-Cre mice can swallow (since FITC-dextran was given orally) and that bowel motility or esophageal motility defects are unlikely to account for neonatal death or the absence of milk within the stomach. Observation of the neonates also did not reveal problems with motor function or coordination that should affect feeding.
Figure 6. Fluorescein isothiocyanate (FITC) dextran transit after 6 hours is normal in Tbx3 fl/fl; Wnt1-Cre mice delivered by caesarian section.

(A) P0 Tbx3 fl/fl; Wnt1-Cre mice have air in the stomach (St) and throughout the proximal small intestine (SI). (B) One hour after delivery by caesarian section, neonatal mice were fed FITC-dextran and the distribution of FITC was assessed along 8 segments of bowel. FITC dextran was concentrated in the proximal small intestine in both Tbx3 fl/fl; Wnt1-Cre mice and control littermates. (Student’s t-test, p> 0.05). N=23 control, N=14 mutant. Error bar = SEM. (C) The geometric center was calculated for each replicate and revealed no significant difference in FITC-dextran transit after 6 hours in Tbx3 fl/fl; Wnt1-Cre mice. (Student’s t-test, p> 0.05). N=23 control, N=14 mutant. Error bar = SEM.
TBX3 is required for palate development
Since defects in swallowing and bowel motility could not account for the abnormal accumulation of air and absence of milk spots in stomachs of Tbx3 fl/fl; Wnt1-Cre mice, we examined the palate of the mutants. Cleft palate causes serious feeding problems because the secondary palate is needed to generate negative pressure for suckling (Turgeon and Meloche, 2009). We found that 96.5% (55/57) of Tbx3 fl/fl; Wnt1-Cre mice had a cleft secondary palate (Figure 7). Bone and cartilage staining with Alcian Blue and Alizarin Red confirmed and defined the secondary palate defect in Tbx3 fl/fl; Wnt1-Cre mice (Figure 7 A-O). Two of the Tbx3 fl/fl; Wnt1-Cre mice analyzed in this study had what appeared to be a normally fused palate (Figure 7 K-O), but this was early in the process of interbreeding Tbx3 fl/fl to Wnt1-Cre mice, suggesting strain background effects on penetrance. Abnormalities in other craniofacial structures were not observed in any Tbx3 fl/fl; Wnt1-Cre mice, suggesting that the failure of palatal shelf fusion is an intrinsic defect of the palatal shelf. Consistent with this hypothesis, Wnt1 regulatory elements drive Cre expression in premigratory cranial neural crest cells that contribute to the palatal mesenchyme (Ito, 2003).
Figure 7. Cleft of the secondary palate in newborn Tbx3 fl/fl; Wnt1-Cre mice.

(A-E) Whole-mount and Alizarin Red (bone) and Alcian Blue (cartilage) simultaneous staining of newborn mice heads show that wild-type littermates have normal fused palates. Cranial and lateral views show normal skull anatomy and no other major craniofacial defects. Note that in (E) the vomer (Vo) and presphenoid (PSp) bones cannot be seen because they are underneath the maxilla (Max) and palatine (PL). N=10. (F-J) Most Tbx3 fl/fl; Wnt1-Cre mice have overt cleft palatal defects. Note that in (J) the vomer (Vo) and presphenoid (PSp) bones are exposed. N=7. (K-O) Two mutant animals were identified and revealed a normally fused palate. N=2. Abbreviations: BO, basioccipital; BS, basisphenoid; Pt, pterygoid; LO, lamina obturans; PMx, premaxilla
To better define this developmental defect, we compared palate development in Tbx3 fl/fl; Wnt1-Cre mice versus control littermates. Palatal shelf morphogenesis occurs by coordinated survival, proliferation, migration and differentiation of mesenchymal cells and their epithelial lining (Bush and Jiang, 2012). We visualized palate development in paraffin sections from anterior and posterior palate between E12.5 and E15.5 (Figure 8) in control and Tbx3 fl/fl; Wnt1-Cre mice. Beginning at E11.5 bilateral palatal shelves arise from the oral surface of the maxillary processes. Then in control mice (Figure 8), proliferation of epithelial and mesenchymal cells causes vertical downward growth of palatal shelves between E12 and E14 (Figure 8 A, E). By E14.5, the palatal shelves normally undergo elevation, a process defined by asynchronous reorientation into the horizontal position (Figure 8 I). Palatal shelves continue to grow horizontally above the tongue until they meet and initiate fusion at a transient multilayered epithelium called the midline epithelial seam (MES) at E15 (Figure 8 I). By E15.5 the MES has disappeared and the palate has a single continuous mesenchymal layer lined by epithelial cells (Figure 8 M). Histological analysis at key developmental time points demonstrated a failure of palatal shelf elevation in Tbx3 fl/fl; Wnt1Cre mice (Figure 8 J, L, N, P). These results suggest that TBX3 is required in the palatal mesenchyme for normal development of the secondary palate.
Figure 8. Tbx3 fl/fl; Wnt1-Cre mice present defects in palatal shelf elevation.

(A-P) Histological staining with hematoxylin and eosin in the anterior and posterior region of the secondary palate at key developmental stages show a delay in palatal shelf elevation at E14.5. N=3 of each genotype for each time point. Abbreviations: Ps, palatal shelf, T: tongue, MES: midline epithelial seam
The role of Tbx3 in palatal shelf elevation
Although the prevailing model of palatal shelf elevation suggests that this process is independent of palatal shelf-growth (Bush and Jiang, 2012; Walker and Fraser, 1956), some mouse models with delayed palatal shelf elevation have disturbed cell proliferation and apoptosis of mesenchymal cells in the developing palate (Goudy et al., 2010; Lan, 2004). To determine whether the defect of palatal shelf elevation observed in Tbx3 fl/fl; Wnt1Cre mice was caused by changes in cell proliferation and apoptosis, we analyzed EdU incorporation into dividing palate mesenchymal cells and stained for active caspase-3 in E13.5 embryos, a time just prior to palatal shelf elevation. We found that Tbx3 fl/fl; Wnt1Cre mice had normal levels EdU incorporation in both the anterior and posterior region of palatal shelf mesenchyme (Figure 9 A-F) and very low levels of active capsase-3 in both control and mutant animals (Figure 9 G-L). These data suggest that defects in elevation of the palatal shelves of Tbx3 fl/fl; Wnt1Cre mice are not a consequence of altered cell proliferation or apoptosis at E13.5.
Figure 9. Molecular mechanisms of palatal shelf elevation.

(A-F) EdU incoorporation assay was used to identify proliferative cells at E13.5 in the palatal mesenchyme four hours after EdU injection. The ratio of EdU+ cells to total cells (Hoechst+) does not differ significantly in the anterior (A-C) or posterior (D-F) region of the palatal shelf in Tbx3 fl/fl; Wnt1-Cre mice. (Student’s t-test, p> 0.05, N=3 of each genotype). (G-L) Immunostaining for cleaved caspase 3 was used to identify apoptotic cells at E13.5 in the palatal mesenchyme. The ratio of cleaved caspase3+ cells to total cells does not differ significantly in the anterior (G-I) or posterior (J-L) region of the palatal shelf in Tbx3 fl/fl; Wnt1-Cre mice. (Student’s t-test, p> 0.05, N=3 of each genotype). (M) Quantitative RT-PCR analysis was performed to determine expression of genes known to regulate palate development. Osr2 mRNA levels were markedly reduced in Tbx3- deficient mice at E13.5 (p = 0.0079). Expression levels are relative to mean expression in Tbx3 controls. Error bar = SEM. (N=5 of each genotype). Scale bar =100 μm.
Molecular mechanisms of TBX3 in palatal shelf elevation
To understand the molecular mechanisms through which TBX3 regulates palatal shelf elevation, we analyzed gene expression in the palate of Tbx3 fl/fl; Wnt1-Cre and control mice at E13.5. Genes selected for analysis by qRT-PCR have known roles in palate morphogenesis or are known to interact with TBX3 in other contexts. For example, sonic hedgehog (SHH) binds the receptor patched (PTC) to activate smoothened (SMO) and signals downstream via several of the GLI family transcription factors (Bush and Jiang, 2012). Mice with inactivation of Shh, Smo, Gli2 or Gli3 have cleft palate defects (Huang et al., 2008; Lan and Jiang, 2009; Mo et al., 1997) and TBX3 influences expression of SHH related genes during limb development (Emechebe et al., 2016). Similarly, TBX3 and BMP4 act in an autoregulatory loop to control mesenchymal proliferation in palatal shelves (Lee et al., 2007) and MSX1 is a target of BMP2 and BMP4 signaling critical for palate development (Zhang et al., 2002). We therefore examined expression of each of these genes in E13.5 palatal shelves from Tbx3 fl/fl; Wnt1-Cre and control mice, but gene expression levels were equivalent (Figure 9M). We next evaluated Tbx2 and cyclin D1 (Ccndl) mRNA levels because of reported partial functional redundancy between Tbx2 and Tbx3 in the palate (Zirzow et al., 2009); these mRNA were also of normal abundance in Tbx3 fl/fl; Wnt1-Cre mice. Finally, Osr2−/− mutant mice also have cleft palate and disturbed palatal shelf elevation (Lan, 2004). We found that Osr2 mRNA levels were reduced by 55% (p = 0.0079) in E13.5 palatal mesenchyme (Figure 9 M) suggesting palate defects in Tbx3 fl/fl; Wnt1-Cre mice might result at least in part from reduced OSR2 in mutant mice.
Discussion
The ENS forms from neural crest-derived precursors that colonize the bowel from E9.5 to E13.5 in mice. These cells differentiate into a wide array of neurons and glia that control most aspects of bowel function. Over the past two decades, many genes that control these early phases of ENS development have been identified, but much less is known about molecular and cellular mechanisms that guide differentiation of specific enteric neuron types, that support axon guidance and synaptogenesis, or that influence enteric glial development. In an attempt to identify genes that control these later developmental processes, we performed microarray analysis at E17.5 and identified numerous genes that are differentially expressed in the ENS versus other cells of the bowel wall. Many of these genes were previously identified and validated by our group and others at E14 and E15.5 (Heanue and Pachnis, 2006; Memic et al., 2017b; Vohra et al., 2006). Furthermore, our cell selection strategy robustly separates ENS from non-ENS cells at E17.5. Thus, many of the genes in Figure 1 and Supplementary Table 1 may be worthy of investigation as we strive to define mechanisms of ENS morphogenesis.
For this study, we investigated the role of Tbx3, a transcription factor that is highly expressed in developing ENS relative to other cells of the bowel. We demonstrated that Wnt1-Cre efficiently depleted TBX3 from ENCDC, as expected since prior studies show Wnt1-Cre induces almost complete recombination in the ENS lineage (Lake et al., 2016). We found that Tbx3 is not required within ENCDCs for prenatal bowel colonization by ENS precursors, that Tbx3 fl/fl; Wnt1-Cre mice had a normal density of enteric neurons at P0, and that transit of FITC-dextran though the upper gastrointestinal tract occurred at a normal rate despite a reduced number of enteric glia. Nonetheless, Tbx3 fl/fl; Wnt1-Cre mice die within 24 hours after birth with no milk in their stomachs. We attribute neonatal death to the highly penetrant cleft palate that occurred in >95% of Tbx3 fl/fl; Wnt1-Cre mice. The mechanistic link between the loss of Tbx3 and cleft palate is unclear at this point: we found normal levels of many candidate genes in the palate of E13.5 Tbx3 mutant mice. There was a significant reduction in Osr2 mRNA levels and since Osr2 null mutants have cleft palate (Lan, 2004), TBX3 may directly or indirectly regulate Osr2 mRNA levels and contribute cleft palate in Tbx3 fl/fl; Wnt1-Cre mice.
TBX3 in prenatal ENS and cardiac development
Our work and prior studies show that Tbx3 mRNA and TBX3 protein are abundant in the developing ENS from E11 to E19 in mice and that TBX3 is present in the human ENS at week 10 of gestation, shortly after colonization of fetal bowel by ENCDC (Heanue and Pachnis, 2006; Memic et al., 2017a; Vohra et al., 2006). Prior studies also show that TBX3 is co-expressed with many enteric neuron subtype markers including tyrosine hydroxylase, calcitonin gene related peptide, calbindin, neuronal nitric oxide synthase, neuropeptide Y, serotonin and vasoactive intestinal peptide (Memic et al., 2017). Furthermore, in other cellular contexts TBX3 regulates many genes that are critical for ENS development, including Gli3, Bmp4, Shh, Hand2, and PTEN (Burgucu et al., 2012; Chalazonitis, 2004; D’Autréaux et al., 2011; Emechebe et al., 2016; Fu et al., 2006; Goldstein et al., 2005; Hendershot et al., 2007; Lee et al., 2007; Osterwalder et al., 2014; Puig et al., 2009; Rallis et al., 2005; Suzuki et al., 2004; Yang et al., 1997; Zhu et al., 2016; Zirzow et al., 2009). Therefore, we hypothesized that loss of TBX3 within ENCDC would impact ENS development or function. Instead, we found that TBX3 is not required for efficient colonization of the bowel by ENCDC, that Tbx3 mutant mice have normal neuron numbers, and that Tbx3 loss within the ENS does not affect transit of FITC-dextran through neonatal bowel even though TBX3 is prominently expressed in enteric neurons at P0. Specifically, our functional studies suggest that P0 Tbx3 fl/fl; Wnt1-Cre mice can swallow liquids (although inefficiently with cleft palate), have normal gastric emptying and normal transit of luminal contents though the small bowel, but that they also have excess air swallowing due to cleft palate. Unfortunately, the Tbx3 fl/fl; Wnt1-Cre mice die as neonates, so we could not test colon motility or later aspects of ENS development or function. It remains possible that TBX3 has important roles after birth or that the closely related gene Tbx2 compensates for the loss of Tbx3 as has been shown in other contexts (Singh et al., 2012; Weidgang et al., 2013).
Notably, we found that Tbx3 fl/fl; Wnt1-Cre mice have a marked reduction in S100β+ enteric glia in small bowel without a comparable loss of SOX10+ cells. Since SOX10 is expressed in uncommitted ENS precursors and in enteric glia, but S100β is only expressed in the more mature glial lineage, this finding suggests that TBX3 enhances enteric glial cell differentiation. Remarkably, we did not detect TBX3 protein in enteric glia, but only in enteric neurons. Collectively, these data suggest that TBX3 promotes maturation enteric glia in a non-cell autonomous manner. A potential mechanism for this finding would be altered Notch signaling, since Notch enhances enteric gliogenesis (Ngan et al., 2011), but we did not detect changes in expression of the Notch ligand Dll1 or the transcriptional effector Hes1 that were previously implicated in ENS gliogenesis. Reduced glial numbers did not alter small bowel transit, but this was expected since even more complete glial loss does not affect this parameter (Rao et al., 2017).
Our studies of Tbx3 fl/fl; Wnt1-Cre mouse heart anatomy revealed no defects in cardiac outflow tract alignment or in the great vessels of the heart. This is in contrast to results from constitutive Tbx3 deficient embryos which showed arterial pole alignment defects (Bakker et al., 2008; Frank et al., 2012; Mesbah et al., 2008), suggesting that Tbx3 acts primarily in non Wnt1-Cre lineage cells to regulate aspects of cardiac development.
Tbx3 is necessary for palatal shelf elevation
Development of the secondary palate from cranial ectoderm-derived epithelial cells and Wnt1 expressing cranial neural crest-derived mesenchymal cells is mediated by a network of growth factors and transcription factors (Chai et al., 2000; Chai and Maxson, 2006; Ferguson, 1988). Our observations show for the first time that palatal shelf elevation requires TBX3, but suggest that TBX3 effects on palatal shelf elevation are not due to changes in cell proliferation or apoptosis. These findings are consistent with the prevailing model of palatal shelf elevation as a process mediated by an internal mechanical force that drives the rapid and asynchronous reorientation of each palatal shelf independent of changes in cell number (Jin et al., 2008; Walker and Fraser, 1956).
Our work also complements previous studies of Tbx3 in palate development (Lee et al., 2007; Zirzow et al., 2009). For example, studies of wild-type palatal shelf explants isolated at E13.5 and cultured for 48 hours showed that BMP4 induces Tbx3, but TBX3 reduces Bmp4 levels in mesenchyme, creating a negative feedback loop that influenced cell proliferation (Lee et al., 2007). In contrast, our in vivo data show that loss of TBX3 in palatal shelf mesenchyme did not alter Bmp4 mRNA levels or mesenchymal proliferation at E13.5. It remains possible that interactions between TBX3 and BMP4 regulate cell proliferation at other stages in palate development, including during the initial vertical growth of palatal shelves or the horizontal growth that occurs after palatal shelf elevation. Alternatively, the apparently contradictory results between our work and prior studies of TBX3/BMP4 interaction may reflect differences in the timing of TBX3 manipulation or the presence of factors in vivo that were missing from the published in vitro studies.
Our results in Tbx3 fl/fl; Wnt1-Cre mice are surprising considering that prior in vivo studies suggest a less important role for Tbx3 in palate fusion than for the closely related gene Tbx2. Tbx2−/− mice have increased proliferation and apoptosis in palatal shelf mesenchyme and have partially penetrant cleft palate in two different mouse strains (Zirzow et al., 2009). Furthermore, Zirzow showed that while 38% of Tbx2+/− Tbx3+/− mice had a cleft secondary palate, the one Tbx3 null that survived to E15.5 mouse had a partially fused palate at E15.5 suggesting palatal shelf growth and fusion occur normally, but may be delayed. While it is possible that these differences reflect global Tbx3 loss versus Wnt1-Cre driven Tbx3 loss, the NMRI background strain of the Tbx3 −/− mouse in prior studies (Zirzow et al., 2009) may be more resilient to cleft palate relative to the B16/SV129/FVB mixed background used in our studies. In support of this hypothesis, two Tbx3 fl/fl; Wnt1-Cre mice with a fused palate at P0 were discovered during our initial breeding between Tbx3 fl/fl and Wnt1-Cre lines. As our studies progressed, we were unable to identify additional Tbx3 fl/fl; Wnt1-Cre mice with a fused palate, consistent with the hypothesis that strain background affects penetrance of the mutant Tbx3 cleft palate phenotype. Our finding that Tbx3 loss can cause cleft palate independent of altered Tbx2 gene expression argues against the fully redundant functional role of Tbx3 and Tbx2 in palatogenesis. Consistent with this hypothesis, Tbx3 and Tbx2 have previously been shown to have independent functions in heart (Aanhaanen et al., 2009; Bakker et al., 2008; Frank et al., 2013, 2012; Harrelson, 2004; Hoogaars et al., 2007; Mesbah et al., 2008; Zirzow et al., 2009), limb (Emechebe et al., 2016; Frank et al., 2013; Suzuki et al., 2004) and other contexts (Douglas and Papaioannou, 2013; Rowley et al., 2004).
Osr2 levels are reduced in the palate of Tbx3 fl/fl; Wnt1-Cre mice
Defining molecular mechanisms through which TBX3 impacts palate development is challenging given that TBX3 regulates the expression of many genes as well as mRNA splicing. Our approach was to evaluate mRNA levels for selected genes in microdissected E13.5 palate. We focused on genes that impact palate development and interact with TBX3. While our studies were not comprehensive, we did discover reduced levels of Osr2 mRNA in Tbx3 fl/fl; Wnt-Cre mouse palate compared to control littermates, but unchanged levels for many other genes. Similar to Tbx3 fl/fl; Wnt1-Cre mice, Osr2−/− mice have a delay or failure of palatal shelf elevation (Lan, 2004). In situ hybridization for Tbx3 and Osr2 suggests that they are co-expressed in palatal shelf mesenchyme (Zirzow et al., 2009) adding credence to the hypothesis that reduced Osr2 contributes to cleft palate in Tbx3 fl/fl; Wnt1-Cre mice. TBX3 could directly regulate the expression of Osr2 through DNA-binding interactions, or indirectly via RNA binding proteins and splicing factors that drive alternative splicing (Kumar et al., 2014). Using published ChIP-Seq data from adult mouse heart, we noted a peak for TBX3 binding near the promoter sequence of Osr2 (Van Den Boogaard et al., 2012) suggesting that TBX3 may directly regulate Osr2 gene expression. How changes in Tbx3 and Osr2 regulate the internal mechanical forces needed to drive the rapid reorientation of the palatal shelves remains to be defined. It also remains likely that other TBX3 regulated genes are the critical for palatal development since Osr2 heterozygous mice do not have cleft palate (Lan, 2004) and we found a roughly 50% reduction in Osr2 mRNA in the palate of our Tbx3 fl/fl; Wnt1-Cre mice. Nonetheless, our data clearly show a role for TBX3 in normal palate morphogenesis.
Summary:
Our E17.5 microarray studies identify a large number of transcription factors, axon guidance molecules, cell adhesion proteins, signaling molecules, receptors and ligands that are enriched in the ENS or surrounding cells at late gestation. Our studies show that TBX3 is not needed for bowel colonization by ENCDC or for small bowel motility, but is important for enteric glial differentiation. TBX is also required for palatal shelf reorientation and fusion, in a mechanism that is independent of altered cell proliferation.
Supplementary Material
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Human anti- ANNA-1 | a gift from Vanda Lennon, Mayo Clinic | RRID:AB_2313944 |
| Goat polyclonal anti-SOX10 | Santa Cruz | Cat# SC-17342; RRID:AB_2195374, |
| Rabbit polyclonal anti-TuJ1 | Covance Research Products Inc | Cat# PRB-435P-100;RRID:AB 10063850 |
| Goat polyclonal anti- TBX3 | R&D Systems | Cat #AF4509-SP; RRID: AB 2240328 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | ThermoFisher Scientific | Cat# A-31573 |
| Goat anti-Human IgG (H+L) Secondary Antibody, Alexa Fluor 594 | ThermoFisher Scientific | Cat# A-11014 |
| Donkey anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | ThermoFisher Scientific | Cat# A-21447 |
| Donkey anti-Goat IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | ThermoFisher Scientific | Cat# A-11055 |
| Normal Donkey Serum | Jackson ImmunoResearch | #017-000-121 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| alizarin red S | Sigma-Aldrich | Cat# 5533 |
| alcian blue | Sigma-Aldrich | Cat# A5268 |
| FITC dextran molecular weight 70,000 | Sigma-Aldrich | Cat# 46945 |
| Collagenase from Clostridium h | Sigma-Aldrich | Cat# C6885 |
| Dispase II | ThermoFisher Scientific | Cat. #17105-041 |
| SsoFast™ EvaGreen® Supermix with Low ROX | Bio-Rad | #1725211 |
| SuperScript II Reverse Transcriptase | Invitrogen | #18064014 |
| Critical Commercial Assays | ||
| RNeasy Mini Kit | Qiagen | #74104 |
| RNEasy Micro Kit | Qiagen | #74004 |
| Click-iT® EdU Alexa Fluor® 488 Imaging Kit | Thermo Fisher Scientific | #C10337 |
| Deposited Data | ||
| Raw and analyzed data | This paper | Will be uploaded to NIH GEO when accepted |
| Experimental Models: Organisms/Strains | ||
| Mouse: Tbx3 floxed (Tbx3 fl/fl), Strain: Bl6/SV129 | (Frank et al., 2013) | N/A |
| Mouse: Tg(Wnt1cre)11Rth; Strain: (C57BL/6J × CBA/J)F1) | The Jackson Laboratory | Cat# 003829; RRID:IMSR_JAX:003829 |
| Mouse: (Gt(ROSA)26Sortm1(EYFP); Strain: 129X1/SvJ and C57BL/6J | The Jackson Laboratory | Cat#:006148; RRID:IMSR JAX:006148 |
| Mouse: Gt(ROSA)26Sortm9(CAG-tdTomato)Hze; Strain: C57BL/6J | The Jackson Laboratory | Cat# JAX:007909; RRID:IMSR_JAX:007909 |
| Oligonucleotides | ||
| Primers for qPCR data, See Table 1 | This paper | N/A |
| Software and Algorithms | ||
| GraphPad Prism version 7.03 | GraphPad Software | www.graphpad.com |
| RStudio (Bioconductor software, Oligo package) | Carvalho and Irizarry, 2010; Gentleman et al., 2004) | http://www.rstudio.com/. |
| ImageJ (1.51s) | Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2016 | http://imagej.nih.gov/ij |
Highlights:
Microarray identified many genes expressed in E17.5 mouse enteric nervous system
TBX3 loss in Wnt1-Cre+ neural crest lineage caused cleft palate and reduced Osr2
TBX3 loss reduced small bowel enteric glia, but TBX3 protein was in enteric neurons
FITC-dextran transit through small bowel was normal in TBX3 conditional mutant mice
Acknowledgments
We thank Dr. Meenakshi Rao for thoughtful comments and insight. We also thank Deepika Kothakapa and Beth Maguire for assistance with mouse colony management. We are grateful to Vanda Lennon for the human ANNA–1 serum. We also appreciate assistance and technical guidance of Florin Tuluc, Lily Wu, and Jennifer Murray at the Children’s Hospital of Philadelphia Flow Cytometry Core and Daniel Martinez, Socrates Agrio, and Joon Jung from the Pathology Core Laboratory. This work was supported by: the Irma and Norman Braman Endowment (ROH), the Suzi and Scott Lustgarten Center Endowment (ROH): The Children’s Hospital of Philadelphia Research Institute (ROH); Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (grant no. MD-II2013-269) (ROH); NIH grants RO1 DK087715 (ROH) and R01 HD046767 (AMM), March of Dimes 6-FY15-235 (ROH) and Basil O’Connor Award (AMM); the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (grant no. 1008525) (ROH); the NIH SPARC Program OT2OD023859. RK acknowledges support from the Fondation pour la Recherche Médicale (DEQ20150331717).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanhaanen WTJ, Brons JF, Domínguez JN, Rana MS, Norden J, Airik R, Wakker V, De Gier-De Vries C, Brown NA, Kispert A, Moorman AFM, Christoffels VM, 2009. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ. Res. 104, 1267–1274. 10.1161/CIRCRESAHA.108.192450 [DOI] [PubMed] [Google Scholar]
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, De Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KKY, Lyonnet S, Chakravarti A, Tam PKH, Ceccherini I, Hofstra RMW, Fernandez R, 2008. Hirschsprung disease, associated syndromes and genetics: A review. J. Med. Genet. 45, 1–14. https://doi.org/10.1136/jmg.2007.053959 [DOI] [PubMed] [Google Scholar]
- Bakker ML, Boukens BJ, Mommersteeg MTM, Brons JF, Wakker V, Moorman AFM, Christoffels VM, 2008. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ. Res. 102, 1340–1349. 10.1161/CIRCRESAHA.107.169565 [DOI] [PubMed] [Google Scholar]
- Bamshad M, Lin RC, Law DJ, Watkins WS, Krakowiak PA, Moore ME, Franceschini P, Lala R, Holmes LB, Gebuhr TC, Bruneau BG, Schinzel A, Seidman JG, Seidman CE, Jorde LB, 1997. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat. Genet. 16, 311–315. 10.1038/ng0797-311 [DOI] [PubMed] [Google Scholar]
- Bergeron K-F, Silversides D, Pilon N, 2013. The developmental genetics of Hirschsprung’s disease. Clin. Genet. 83, 15–22. 10.1111/cge.12032 [DOI] [PubMed] [Google Scholar]
- Bergner AJ, Stamp LA, Gonsalvez DG, Allison MB, Olson DP, Myers MG, Anderson CR, Young HM, 2014. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J. Comp. Neurol. 522, 514–527. 10.1002/cne.23423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgucu D, Guney K, Sahinturk D, Ozbudak IH, Ozel D, Ozbilim G, Yavuzer U, 2012. Tbx3 represses PTEN and is over-expressed in head and neck squamous cell carcinoma. BMC Cancer 12 10.1186/1471-2407-12-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Jiang R, 2012. Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development 139, 828–828. 10.1242/dev.079152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchbach MER, Edwards JD, Schussler KR, Burghes AHM, 2007. A novel method for oral delivery of drug compounds to the neonatal SMNDelta7 mouse model of spinal muscular atrophy. J. Neurosci. Methods. 10.1016/j.jneumeth.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho BS, Irizarry RA, 2010. A framework for oligonucleotide microarray preprocessing. Bioinformatics 26, 2363–2367. 10.1093/bioinformatics/btq431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, Soriano P, McMahon a P., Sucov HM, 2000. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE, 2006. Recent advances in craniofacial morphogenesis. Dev. Dyn. 235, 2353–2375. 10.1002/dvdy.20833 [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, 2004. Bone Morphogenetic Protein-2 and −4 Limit the Number of Enteric Neurons But Promote Development of a TrkC-Expressing Neurotrophin-3-Dependent Subset. J. Neurosci. 24, 4266–4282. 10.1523/JNEUROSCI.3688-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier B, Pilon N, 2017. Toward a better understanding of enteric gliogenesis. Neurogenesis. 10.1080/23262133.2017.1293958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Autréaux F, Margolis KG, Roberts J, Stevanovic K, Mawe G, Li Z, Karamooz N, Ahuja A, Morikawa Y, Cserjesi P, Setlick W, Gershon MD, 2011. Expression level of Hand2 affects specification of enteric neurons and gastrointestinal function in mice. Gastroenterology 141, 576–587. 10.1053/j.gastro.2011.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP, 1998. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–S2. 10.1016/S0960-9822(07)00562-3 [DOI] [PubMed] [Google Scholar]
- Douglas NC, Papaioannou VE, 2013. The T-box transcription factors TBX2 and TBX3 in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 18, 143–147. 10.1016/j.micinf.2011.07.011.Innate [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emechebe U, Pavan Kumar P, Rozenberg JM, Moore B, Firment A, Mirshahi T, Moon AM, 2016. T-box3 is a ciliary protein and regulates stability of the Gli3 transcription factor to control digit number. Elife 5, 1–28. 10.7554/eLife.07897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW, 1988. Palate development. Development 103 Suppl, 41–60. [DOI] [PubMed] [Google Scholar]
- Frank DU, Carter KL, Thomas KR, Burr RM, Bakker ML, Coetzee WA, Tristani-Firouzi M, Bamshad MJ, Christoffels VM, Moon AM, 2012. Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis. Proc. Natl. Acad. Sci. 109, E154–E163. 10.1073/pnas.1115165109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DU, Emechebe U, Thomas KR, Moon AM, 2013. Mouse Tbx3 Mutants Suggest Novel Molecular Mechanisms for Ulnar-Mammary Syndrome. PLoS One 8, 1–7. 10.1371/journal.pone.0067841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Vohra BPS, Wind D, Heuckeroth RO, 2006. BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncaml polysialic acid addition. Dev. Biol. 299, 137–150. 10.1016/j.ydbio.2006.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Xu J, Chaturvedi P, Liu EL, Jiang R, Lan Y, 2017. Identification of osr2 Transcriptional Target Genes in Palate Development. J. Dent. Res. 96, 1451–1458. 10.1177/0022034517719749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, others, 2004. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianino S, 2003. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development, 10.1242/dev.00433 [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Brewer KC, Doyle AM, Nagy N, Roberts DJ, 2005. BMP signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech. Dev. 122, 821–833. 10.1016/j.mod.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Hofstra RMW, Bums AJ, 2013. Building a brain in the gut: Development of the enteric nervous system. Clin. Genet. 83, 307–316. 10.1111/cge.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudy S, Law A, Sanchez G, Baldwin HS, Brown C, 2010. Tbxl is necessary for palatal elongation and elevation. Mech. Dev. 127, 292–300. 10.1016/j.mod.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui H, Schriemer D, Cheng WW, Chauhan RK, Antiňolo G, Berrios C, Bleda M, Brooks AS, Brouwer RWW, Bums AJ, Chemy SS, Dopazo J, Eggen BJL, Griseri P, Jalloh B, Le TL, Lui VCH, Luzón-Toro B, Matera I, Ngan ESW, Pelet A, Ruiz-Ferrer M, Sham PC, Shepherd IT, So MT, Sribudiani Y, Tang CSM, van den Hout MCGN, van der Linde HC, van Ham TJ, van IJcken WFJ, Verheij JBGM, Amiel J, Borrego S, Ceccherini I, Chakravarti A, Lyonnet S, Tam PKH, Garcia-Barceló MM, Hofstra RMW, 2017. Whole exome sequencing coupled with unbiased functional analysis reveals new Hirschsprung disease genes. Genome Biol. 18, 1–13. 10.1186/sl3059-017-1174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrelson Z, 2004. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development 131, 5041–5052. 10.1242/dev.01378 [DOI] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V, 2006. Expression profiling the developing mammalian enteric nervous system identifies marker and candidate Hirschsprung disease genes. Proc. Natl. Acad. Sci. U. S. A 103, 6919–24. 10.1073/pnas.0602152103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Sarkar AA, Giovannucci DR, Clouthier DE, Abe M, Howard MJ, 2007. Expression of Hand2 is sufficient for neurogenesis and cell type-specific gene expression in the enteric nervous system. Dev. Dyn. 236, 93–105. 10.1002/dvdy.20989 [DOI] [PubMed] [Google Scholar]
- Hoogaars WMH, Engel A, Brons JF, Verkerk AO, De Lange FJ, Wong LYE, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AFM, Verheijck EE, Christoffels VM, 2007. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 21, 1098–1112. 10.1101/gad.416007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Goudy SL, Ketova T, Litingtung Y, Chiang C, 2008. Gli3-deficient mice exhibit cleft palate associated with abnormal tongue development. Dev. Dyn. 237, 3079–3087. 10.1002/dvdy.21714 [DOI] [PubMed] [Google Scholar]
- Ito Y, 2003. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 130, 5269–5280. 10.1242/dev.00708 [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM, 2002. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 241, 106–116. 10.1006/dbio.2001.0487 [DOI] [PubMed] [Google Scholar]
- Jin JZ, Li Q, Higashi Y, Darling DS, Ding J, 2008. Analysis of Zfhxla mutant mice reveals palatal shelf contact-independent medial edge epithelial differentiation during palate fusion. Cell Tissue Res. 333, 29–38. 10.1007/s00441-008-0612-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Jiang Q, Chatterjee S, Chakraborty P, Sosa MX, Berrios C, Chakravarti A, 2015. Population variation in total genetic risk of Hirschsprung disease from common RET, SEMA3 and NRGl susceptibility polymorphisms. Hum. Mol. Genet. 24, 2997–3003. 10.1093/hmg/ddv051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Franklin S, Emechebe U, Hu H, Moore B, Lehman C, Yandell M, Moon AM, 2014. TBX3 Regulates Splicing In Vivo: A Novel Molecular Mechanism for Ulnar-Mammary Syndrome. PLoS Genet. 10 10.1371/journal.pgen.1004247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P P, Emechebe U, Smith R, Franklin S, Moore B, Yandell M, Lessnick SL, Moon AM, 2014. Coordinated control of senescence by IncRNA and a novel T-box3 co-repressor complex. Elife 3, 1–28. 10.7554/eLife.02805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, Avetisyan M, Zimmermann AG, Heuckeroth RO, 2016. Neural crest requires Impdh2 for development of the enteric nervous system, great vessels, and craniofacial skeleton. Dev. Biol. 409, 152–165. 10.1016/j.ydbio.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, Heuckeroth RO, 2013. Enteric nervous system development: migration, differentiation, and disease. AJP Gastrointest. Liver Physiol. 305, G1–G24. 10.1152/ajpgi.00452.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, Tusheva OA, Graham BL, Heuckeroth RO, 2013. Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J. Clin. Invest. 123, 4875–4887. 10.1172/JCI69781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, 2004. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 131, 3207–3216. 10.1242/dev.01175 [DOI] [PubMed] [Google Scholar]
- Lan Y, Jiang R, 2009. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development 136, 1387–1396. 10.1242/dev.028167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Kléber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L, 2004. Instructive Role of Wnt/β-Catenin in Sensory Fate Specification in Neural Crest Stem Cells. Science (80-. ). 10.1126/science.1091611 [DOI] [PubMed] [Google Scholar]
- Lee JM, Kim JY, Cho KW, Lee MJ, Cho SW, Zhang Y, Byun SK, Yi CK, Jung HS, 2007. Modulation of cell proliferation during palatogenesis by the interplay between Tbx3 and Bmp4. Cell Tissue Res. 327, 285–292. 10.1007/s00441-006-0271-8 [DOI] [PubMed] [Google Scholar]
- Luzón-Toro B, Gui H, Ruiz-Ferrer M, Sze-Man Tang C, Fernández RM, Sham PC, Torroglosa A, Kwong-Hang Tam P, Espino-Paisán L, Cherny SS, Bleda M, Enguix-Riego MDV, Dopazo J, Antiñolo G, García-Barceló MM, Borrego S, 2015. Exome sequencing reveals a high genetic heterogeneity on familial Hirschsprung disease. Sci. Rep. 5, 1–10. 10.1038/srep16473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckeown SJ, Stamp L, Hao MM, Young HM, 2013. Hirschsprung disease: A developmental disorder of the enteric nervous system. Wiley Interdiscip. Rev. Dev. Biol. 2, 113–129. 10.1002/wdev.57 [DOI] [PubMed] [Google Scholar]
- McLeod MJ, 1980. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299–301. 10.1002/tera.1420220306 [DOI] [PubMed] [Google Scholar]
- Memic F, Knoflach V, Morarach K, Sadler R, Laranjeira C, Hjerling-Leffler J, Sundström E, Pachnis V, Marklund U, 2017a. Transcription and Signaling Regulators in Developing Neuronal Subtypes of Mouse and Human Enteric Nervous System. Gastroenterology 1–13. 10.1053/j.gastro.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memic F, Knoflach V, Morarach K, Sadler R, Laranjeira C, Hjerling-Leffler J, Sundström E, Pachnis V, Marklund U, 2017b. Transcription and Signaling Regulators in Developing Neuronal Subtypes of Mouse and Human Enteric Nervous System. Gastroenterology 624–636. 10.1053/j.gastro.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Théveniau-Ruissy M, Papaioannou VE, Kelly RG, 2008. Tbx3 is required for outflow tract development. Circ. Res. 103, 743–750. 10.1161/CIRCRESAHA.108.172858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Galligan JJ, Burks TF, 1981. Accurate measurement of intestinal transit in the rat. J. Pharmacol. Methods 6, 211–217. 10.1016/0160-5402(81)90110-8 [DOI] [PubMed] [Google Scholar]
- Mo R, Freer a M., Zinyk DL, Crackower M. a, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner a L., Hui C, 1997. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 124, 113–123. [DOI] [PubMed] [Google Scholar]
- Ngan ESW, Garcia-Barceló MM, Yip BHK, Poon HC, Lau ST, Kwok CKM, Sat E, Sham MH, Wong KKY, Wainwright BJ, Cherny SS, Hui CC, Sham PC, Lui VCH, Tam PKH, 2011. Hedgehog/notch-induced premature gliogenesis represents a new disease mechanism for Hirschsprung disease in mice and humans. J. Clin. Invest. 10.1172/JCI43737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermayr F, Hotta R, Enomoto H, Young HM, 2012. Development and developmental disorders of the enteric nervous system. Nat. Rev. Gastroenterol. Hepatol. 10, 43–57. 10.1038/nrgastro.2012.234 [DOI] [PubMed] [Google Scholar]
- Osterwalder M, Speziale D, Shoukry M, Mohan R, Ivanek R, Kohler M, Beisel C, Wen X, Scales SJ, Christoffels VM, Visel A, Lopez-Rios J, Zeller R, 2014. HAND2 targets define a network of transcriptional regulators that compartmentalize the early limb bud mesenchyme. Dev. Cell 31, 345–357. 10.1016/j.devcel.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig I, Champeval D, De Santa Barbara P, Jaubert F, Lyonnet S, Larue L, 2009. Deletion of Pten in the mouse enteric nervous system induces ganglioneuromatosis and mimics intestinal pseudoobstruction. J. Clin. Invest. 119, 3586–3596. 10.1172/JCI39929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallis C, Del Buono J, Logan MPO, 2005. Tbx3 can alter limb position along the rostrocaudal axis of the developing embryo. Development 132, 1961–70. 10.1242/dev.01787 [DOI] [PubMed] [Google Scholar]
- Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G, 2017. Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice. Gastroenterology. 10.1053/j.gastro.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M, Grothey E, Couch FJ, 2004. The role of Tbx2 and Tbx3 in mammary development and tumorigenesis. J. Mammary Gland Biol. Neoplasia 9, 109–118. 10.1023/B:JOMG.0000037156.64331.3f [DOI] [PubMed] [Google Scholar]
- Sasselli V, Pachnis V, Bums AJ, 2012. The enteric nervous system. Dev. Biol. 366, 64–73. 10.1016/j.ydbio.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Singh R, Hoogaars WM, Barnett P, Grieskamp T, Sameer Rana M, Buermans H, Farm HF, Petry M, Heallen T, Martin JF, Moorman AFM, Hoen PAC, Kispert A, Christoffels VM, 2012. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cell. Mol. Life Sci. 69, 1377–1389. 10.1007/s00018-011-0884-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F, 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 1–8. 10.1186/1471-213X-l-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Takeuchi J, Koshiba-Takeuchi K, Ogura T, 2004. Tbx genes specify posterior digit identity through Shh and BMP signaling. Dev. Cell 6, 43–53. 10.1016/S1534-5807(03)00401-5 [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC, 2001. T-targets: Clues to understanding the functions of T-box proteins. Dev. Growth Differ. 43, 1–11. 10.1046/j.1440-169X.2001.00556.x [DOI] [PubMed] [Google Scholar]
- Turgeon B, Meloche S, 2009. Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol. Rev. 89, 1–26. 10.1152/physrev.00040.2007 [DOI] [PubMed] [Google Scholar]
- Van Den Boogaard M, Wong LYE, Tessadori F, Bakker ML, Dreizehnter LK, Wakker V, Bezzina CR, ‘T Hoen PAC, Bakkers J, Barnett P, Christoffels VM, 2012. Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J. Clin. Invest. 122, 2519–2530. 10.1172/JCI62613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BPS, Tsuji K, Nagashimada M, Uesaka T, Wind D, Fu M, Armon J, Enomoto H, Heuckeroth RO, 2006. Differential gene expression and functional analysis implicate novel mechanisms in enteric nervous system precursor migration and neuritogenesis. Dev. Biol. 298, 259–271. 10.1016/j.ydbio.2006.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BE, Fraser FC, 1956. Closure of the Secondary Palate in Three Strains of Mice. J. Embryol. Exp. Morphol. 4, 176–189. [Google Scholar]
- Weidgang CE, Russell R, Tata PR, Kühl SJ, Illing A, Müller M, Lin Q, Brunner C, Boeckers TM, Bauer K, Kartikasari AER, Guo Y, Radenz M, Bernemann C, Weiß M, Seufferlein T, Zenke M, Iacovino M, Kyba M, Schöler HR, Kühl M, Liebau S, Kleger A, 2013. TBX3 directs cell-fate decision toward mesendoderm. Stem Cell Reports 1, 248–265. 10.1016/j.stemcr.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Liu CZ, Villavicencio EH, Yoon JW, Walterhouse D, Iannaccone PM, 1997. Expression of human GLI in mice results in failure to thrive, early death, and patchy Hirschsprung-like gastrointestinal dilatation. Mol. Med. 3, 826–35. [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y, 2002. Rescue of cleft palate in Msxl-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129, 4135–4146. [DOI] [PubMed] [Google Scholar]
- Zhu B, Zhang M, Williams EM, Keller C, Mansoor A, Davie JK, 2016. TBX2 represses PTEN in rhabdomyosarcoma and skeletal muscle. Oncogene 35, 4212–4224. 10.1038/onc.2015.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirzow S, Lüdtke THW, Brons JF, Petry M, Christoffels VM, Kispert A, 2009. Expression and requirement of T-box transcription factors Tbx2 and Tbx3 during secondary palate development in the mouse. Dev. Biol. 336, 145–155. 10.1016/j.ydbio.2009.09.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


