Abstract
Nicotine and alcohol addiction are leading causes of preventable death worldwide and continue to constitute a huge socio-economic burden. Both nicotine and alcohol perturb the brain’s mesocorticolimbic system. Dopamine (DA) neurons projecting from the ventral tegmental area (VTA) to multiple downstream structures, including the nucleus accumbens (NAc), prefrontal cortex (PFC) and the amygdala (AMG), are highly involved in the maintenance of healthy brain function. VTA DA neurons play a crucial role in associative learning and reinforcement. Nicotine and alcohol usurp these functions, promoting reinforcement of drug-taking behaviors. In this review, we will first describe how nicotine and alcohol individually affect VTA DA neurons by examining how drug exposure alters the heterogeneous VTA microcircuit and network-wide projections. We will also examine how coadministration or previous exposure to nicotine or alcohol may augment the reinforcing effects of the other. Additionally, this review briefly summarizes the role of VTA DA neurons in nicotine, alcohol and their synergistic effects in reinforcement and also addresses the remaining questions related to the circuit-function specificity of the dopaminergic system in mediating nicotine/alcohol reinforcement and comorbidity.
Keywords: VTA, dopamine, nicotine, alcohol, reinforcement, electrophysiology, heterogeneity, circuit
Graphical Abstract

Ventral Tegmental Area (VTA) dopamine (DA) neurons project to multiple brain areas, including the prefrontal cortex (PFC), amygdala (AMG) and nucleus accumbens (NAc). Nicotine and alcohol induce heterogeneous reinforcing effects across the VTA. Anatomical location of DA neurons within the VTA dictates sensitivity and response to nicotine (+/−) or to alcohol (+/+) across the anterior/posterior and medial/lateral axes.
Introduction
Nicotine and alcohol use disorders (AUD) are both leading causes of preventable death in developed countries; causing 9 million deaths worldwide. World Health Reports (2013 and 2014) state an economic burden estimated over 4% of the European GDP (EC report) and a cost of over $300 billion in the United States for tobacco addiction and $250 billion for AUD (Sacks et al., 2015). Multiple surveys and studies depict a strong association between nicotine and alcohol abuse; consumption of one promotes the other (Bobo & Husten, 2000; Grant et al., 2004). Social aspects, genetic vulnerabilities, and neurobiological factors all contribute to nicotine and alcohol consumption.
Numerous theories and models have been generated to determine how substance abuse can induce maladaptive behaviors, drug seeking, and addiction (Everitt & Robbins, 2005; Koob & Volkow, 2016). Nonetheless, the neuronal substrates and primary mechanisms leading the transition from recreational to abusive drug use and relapse have yet to be solidified. A common hypothesis states that several steps are involved during the transition from drug use to dependence, including initial primary reinforcing effects (Koob & Volkow, 2010; Piazza & Deroche-Gamonet, 2013). Reinforcement is a process promoting a reiterative behavior during a particular context or in response to cue presentation. Drugs of abuse, including nicotine and alcohol, are suggested to hijack this function and consequently increase the repetition of behavior associated with drug intake.
Numerous brain areas are involved in the reinforcement processes. The ventral tegmental area (VTA) is a brain region known to encode reward, novelty, and aversion (Schultz, 1997; Brischoux et al., 2009; Bromberg-Martin et al., 2010)). VTA dopamine (DA) neurons target multiple brain areas, including the nucleus accumbens (NAc), medial prefrontal cortex (mPFC), and amygdala (AMG). A large majority of drugs, including nicotine and alcohol, activate this mesolimbic system and increase DA concentration in the NAc (Di Chiara & Imperato 1985; Imperato et al., 1986; Di Chiara & Imperato 1988), a region known for encoding drug salience. In line with DA’s role in natural reinforcement, learning and motivational processes, electrical and optogenetic stimulation (Tye & Deisseroth, 2012) of VTA DA neurons mimics and/or promotes drug intake behaviors (Juarez et al., 2017).
In this comprehensive review, we will describe the endogenous functionality of VTA DA neurons, their heterogeneous responses to nicotine and alcohol exposure, and how VTA DA neurons encode their reinforcing properties. Finally, we will discuss the contribution of the VTA DA neurons in the comorbid use of nicotine and alcohol.
VTA dopamine neurons
DA neurons are relatively few in number, with a total of around ~600,000 in the human brain and ~45,000 in rats (German & Manaye, 1993). They are located primarily in the ventral part of the mesencephalon (Chinta & Andersen, 2005), which includes the nigrostriatal, mesolimbic, and mesocortical pathways. The VTA is functionally and anatomically heterogeneous, mainly composed of DA, GABA and glutamate neurons (Lammel et al., 2014). Inhibitory γ-aminobutyric acid (GABA) neurons account for 20–40% (Carr et al., 2000; Nair-Roberts et al., 2008; Margolis et al., 2012), glutamate neurons account for 2–3% and DA neurons account for the rest of the VTA cellular population ~60%. This phenotypic diversity can be compartmentalized into sub regions: rostral linear nucleus of the raphe, interfascicular nucleus, caudal linear nucleus, paranigral nucleus, and parabrachial pigmented nucleus (PBP) (Oades & Halliday, 1987). Based on responsivity to natural and drug stimuli, VTA subregion classification has been simplified to medial/lateral (m/l), anterior (aVTA)/posterior (pVTA), and dorsal/ventral (Mrejeru et al., 2015; Morales & Margolis 2017).
Historically, DA neurons have been characterized by broad action potentials (AP), the presence of a hyperpolarization-activated cyclic nucleotide-gated (HCN) channel-mediated Ih current, low-frequency (0.5–5hz) pacemaker activity, autoinhibition by DA-activated D2 receptors or G protein-coupled inwardly-rectifying potassium (GIRK) channels, and the presence of small-conductance activated (SK) channels (Grace & Bunney, 1984; Grace & Onn, 1989; Kitai et al., 1999; Margolis et al., 2006; Liss & Roeper, 2008). While VTA DA neurons are identified by the presence of tyrosine hydroxylase (TH), a rate-limiting enzyme in the production of DA (Javoy-Agid et al., 1981; Berger et al., 1982), recent data challenges this classification. Single-cell labeling studies have also shown the presence of Ih may be small or nonexistent in some VTA DA neurons (Jones & Kauer, 1999; Ford et al., 2006; Zhang et al., 2010; Witten et al., 2011), and others do not respond to direct application of DA (Bannon & Roth, 1983; Lammel et al., 2008). The heterogeneous response of VTA DA neurons to drugs will be expanded upon in later sections.
DA neurons display two distinct modes of firing in vivo: tonic, single-spike activity (0.5–5 hz) or phasic bursts (10–30 hz), comprising of 2–10 APs (Grace & Bunney, 1984; Hyland et al., 2002; Mameli-Engvall et al., 2006). VTA DA neurons exclusively display tonic activity in vitro, as acute slice preparation or dissociated neurons removes the influence of active synaptic inputs (Grace & Onn, 1989; Puopolo et al., 2007). The VTA DA neuron’s pattern of activity converges from intrinsic and extrinsic electrophysiological mechanisms. VTA DA neurons express a number of channels and receptors; anatomical location and heterogeneity dictates activation. Based on a compilation of recent studies (Morales & Margolis 2017) and in the scope of this review, VTA DA neurons can express a variety of potassium channels, including GIRK2 and GIRK3 (Cruz et al., 2004; Lüscher & Slesinger, 2010; Rifkin et al., 2017), but also GABABRs (Labouèbe et al., 2007; Margolis et al., 2012), D2 receptors (Lammel et al., 2008; Margolis et al., 2008b), μ-opioid and κ-opioid receptors (Margolis et al., 2008a; Kotecki et al., 2015), N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and acetylcholine (ACh) receptors (Klink et al., 2001), to name a few. In vivo, VTA DA neuron’s activity is under the control of multiple brain areas. Early studies mapped VTA circuitry, showing reciprocal connections to the PFC (Thierry et al., 1976; Divac et al., 1978), NAc, bed nucleus of the stria terminalis (BNST), basolateral amygdala (BLA), lateral hypothalamus (Lh), lateral habenula (LHb), locus coeruleus (LC), ventral pallidum (VP) and other sets of smaller nuclei (Phillipson, 1979). Inhibition of pallidal afferents selectively increases VTA DA population activity, while activation of the pedunculopontine (PPTg) and the laterodorsal tegmental nucleus (LDTg) increases firing activity (Clements & Grant, 1990; Oakman et al., 1995; Floresco et al., 2003; Lodge & Grace, 2006). Additionally, stimulation of the PFC induces patterns of activity in the VTA which resemble natural burst events (Tong et al., 1996). Further, the VP can regulate motivation and reinforcement by providing inhibitory control over VTA DA neurons (Hjelmstad et al., 2013).
For the past few decades VTA DA neurons have been hypothesized to encode rewards, reward-associated cues, and mediate the incentive signal, which promotes reward-seeking behavior. In this context, the transition from tonic to phasic/bursting activity plays a crucial role. This transition leads to sharp DA transients within VTA brain targets, which mediates the DA role in the reinforcement learning processes (Starkweather et al., 2017; Babayan et al., 2018) and encode incentive salience signal. According to the reward prediction error (RPE) hypothesis (Schultz et al., 1997, 1998, 2002), this fast DA transient encodes the reward value and its unpredictability; the larger the value, the stronger the DA signal. Over time, the cue predicting the reward induces DA neuron activity, while the reward itself no longer activates the dopaminergic system; the reward-associated cue becomes the reinforcer. The RPE hypothesis thus stipulates that if the cue predicts the reward value, no further activation would be observed when receiving the reward (RPE = 0), while if the reward is better than expected, VTA DA neuron’s activity increases (RPE > 0). Oppositely, the absence of an expected reward or presence of a negative event is considered less beneficial than the expected reward, inducing a decrease in DA neuron activity (RPE < 0). VTA DA neurons encode the difference between past experienced behavioral outcomes and present received outcomes, thus providing informative action value and shaping incentive salience and reiteration of the reward associated behavior. In line with this hypothesis, optogenetic phasic activation of VTA DA neurons induces a strong condition place preference (Tsai et al., 2009). Further, the presence of this RPE signal in the VTA has been tested during fMRI scans in humans when monetary gains and losses were used, where an increased blood oxygen level-dependent (BOLD) response was detected during a positive reward prediction error (D’Ardenne et al., 2008). More specifically, DA neurons in the VTA show phasic excitation after the presentation of reward-predicting odors, and effect that can be mimicked through optogenetic manipulation of single-cells in mice (Cohen et al., 2012).
Recent advances in cell- and circuit- probing techniques reveal VTA DA circuit-function heterogeneity, which challenges the simplified reward-encoding function proposed for DA neurons. Indeed, recent studies have described VTA DA neuron’s role in encoding salient and aversive events (Ungless et al., 2004; Brischoux et al., 2009; Bromberg-Martin et al., 2010, Chaudhury et al., 2013; Pignatelli & Bonci, 2015; Juarez & Han, 2016). Interestingly cell- and circuit- mapping studies have described anatomical function specificity (Lammel et al., 2012; Chaudhury et al., 2013; Beier et al., 2015). In particular, while dorsal VTA DA neurons are inhibited by electrical shock – in agreement with the RPE hypothesis, ventral VTA DA neurons are activated (Brischoux et al., 2009). Chronic models of stress and depression in rodents also show long-term changes in VTA DA activity (Anstrom & Woodward, 2005; Valenti et al., 2011). Specifically, chronic social stress exposure generates hyperactivity of VTA DA neurons projecting to the NAc, whereas the mPFC pathway exhibited hypoactivity (Chaudhury et al., 2013). Additionally, recent studies also show differential L/M cellular expression of VMAT2, DAT and D2R within the VTA, and functional response to D2/D3 receptor agonist quinpirole: medial VTA being insensitive to quinpirole, when compared to the lateral VTA (Li et al., 2013). These findings illustrate the importance of investigating projection specific pathway alterations from the VTA in the context of stress and aversion.
Primary exposure to nicotine and ethanol usurps the DA system by directly increasing the DA concentration in the synaptic cleft, particularly in the NAc, inducing their reinforcing properties. As a consequence, drug-associated cues become reinforcers while nicotine and alcohol directly activate the DA system and bypasses the initially established RPE. This direct activation has been suggested to encode hedonic and/or rewarding drug value, but also creates an aberrant incentive salience for drug-associated stimuli. Multiple theories, from the opponent processes, hedonic processes, incentive salience, to the habits/flexibility unbalance have been hypothesized to model the transition from recreative to compulsive drug taking behavior. These hypotheses are not mutually exclusive and overall agree on the primary drug reinforcing properties. Here, we will focus primarily on reinforcement and the VTA DA heterogeneous response of the two most commonly abused drugs, alcohol and nicotine.
Reinforcing properties of nicotine and subsequent neural alterations
While nicotine constitutes more than 40% of the tobacco alkaloids (Wonnacott et al., 2005), other molecules contribute to the reinforcing effect of tobacco. Tobacco smoke contains multiple toxic molecules and addictive or pro-addictive molecules. Amongst them, tobacco smoke contains nicotine and its derivate –i.e. nornicotine and anabasine, β-carbolines - harmane and norharmane, and other inhibitors of monoamine oxidase (IMAO) A and B (Rommelspacher et al., 1994). Harmanes stimulate LC neurons (Ruiz-Durántez et al., 2001), increase 5-hydroxytryptamine (5-HT) and DA levels in the NAc and potentiate the activation of DA neurons (Ergene & Schoener, 1993; Baum et al., 1995, 1996; Arib et al., 2010). Also, IMAOs inhibit the degradation of serotonin and catecholamines (Villégier et al., 2006). All together, they potentiate the reinforcing properties of nicotine. While it is of importance to note that tobacco smoke reinforcement results in the concomitant action of multiple molecules, we will focus on nicotine specifically.
Nicotine, the main addictive component of tobacco (Wonnacott et al., 2005; Marti et al., 2011) hijacks the endogenous acetylcholine (ACh) system and mediates its reinforcing properties by acting through nicotinic receptors (nAChRs). Activation of nAChRs leads to the entrance of cations and consecutive intracellular cascades. These receptors are ubiquitous and highly evolutionary conserved across species (Le Novère et al., 2002). The nAChRs are members of the cysteine-loop receptor family, and allosteric transmembrane proteins forming pentameric ligand-gated channels (Galzi & Changeux, 1995; Le Novère & Changeux, 1999; Lukas et al., 1999). Twelve nAChRs subunits have been cloned from neuronal tissues: 9 alpha-subunits (α2- α10) containing a cys-cys pair required for the agonist binding site (excluded α5nAChRs), and 3 beta-subunits (β2-β4) (Changeux, 2010). The remaining α1, β1, δ, γ, and ε subunits are referred to as muscular nAChRs subunits. Each subunit contains an extracellular N- and C-terminus and 4 transmembrane (TM) domains. Five nAChRs subunits constitute a functional receptor. The α subunit N-terminus forms the agonist-binding site in combination with the adjacent subunit. The association of the TM2 of each subunit forms a central pore with ion selectivity depending on the subunit combination (Miyazawa et al., 2003; Unwin, 2005; Albuquerque et al., 2009).
Agonist binding induces an allosteric conformational transition that leads to channel opening. This mechanism was initially described for hemoglobin by the Monod-Wyman-Changeux model (Monod et al., 1965) and was then generalized to nAChRs (Edelstein et al., 1996). Persistent binding desensitizes the receptor, which is then followed by closing of the channel. Agonist affinity, permeability selectivity, and activation/desensitization kinetics depend on the nAChR subunits forming the pentamere.
The nAChR subunits form various homopentameres or heteropentameres, creating a large portfolio of pharmacological and electrophysiologically distinct nAChRs throughout the brain. Of interest, the heteropentamere α4β2*nAChRs (* including non exclusively α4 and β2 nAChRs subunits) is highly sensitive to agonists –ACh and nicotine- while the homopentamere α7nAChRs has a low affinity for these agonists, a strong Ca2+ permeability, and fast kinetics of desensitization (Changeux, 2010). The α4β2*nAChRs and α7nAChRs are largely expressed throughout the brain, whereas other nicotinic subunits are present in specific brain areas (Gotti et al., 2009), including the retina (Moretti et al., 2004), the thalamus (Pauly & Collins, 1993; Heath et al., 2010), the area postrema (Funahashi et al., 2004), the habenula (Salas et al., 2009; Fowler et al., 2011; Frahm et al., 2011), the interpeduncular nuclei (IPn) (Salas et al., 2009; Harrington et al., 2016) and the DA nuclei (Le Novère et al., 1996; Klink et al., 2001; Azam et al., 2002; Champtiaux et al., 2003).
Endogenous contribution of the nicotinic modulation of VTA DA neurons
Within the VTA, nAChRs are expressed at the somatic, perisomatic and presynaptic levels of DA, GABA and glutamate neurons (Fig. 1). The precise expression and related role of VTA nAChRs is still unclear. Nonetheless, some cellular specificity has been described. The α4β2*nAChRs is the most expressed nAChR in the VTA. The majority of VTA DA neurons express α4, α5, α6, β2 and β4 subunits and half of them express α3 and α7nAChRs subunits (Fig. 1). GABA and glutamatergic neurons preferentially express β4, α7, β2 and α4 subunits (Le Novère et al., 1996; Klink et al., 2001; Azam et al., 2002, 2007; Champtiaux et al., 2003). Finally, glutamate afferents strongly express α7nAChRs, where they have been described to contribute to NMDA-dependent long-term synaptic potentiation (Mansvelder & McGehee, 2000; Mansvelder et al., 2002; Gao et al., 2010). The VTA receives endogenous cholinergic modulation from the PPTg and the LDTg (Mena-Segovia et al., 2008). Cholinergic modulation, in addition to GABAergic and glutamatergic transmission, play a crucial role in the spontaneous activity of VTA DA neurons (Faure et al., 2014). In particular, lesions of the LDTg and PPTg decrease the firing rate and the bursting activity of VTA DA neurons (Blaha et al., 1996). In anesthetized mice, deletion of the β2nAChRs subunit gene - β2KO mice (Picciotto et al., 1998) - induces a loss of VTA DA bursting activity, associated with an alteration of exploratory behavior and uncertainty processing (Mameli-Engvall et al., 2006; Avale et al., 2008; Maubourguet et al., 2008; Naudé et al., 2016). The viral re-expression of β2 nAChRs in both VTA DA and GABA neurons are required to restore spontaneous VTA DA neuron bursting activity (Maskos et al., 2005; Tolu et al., 2012). Similar approaches show the contribution of the α4 subunit in DA burst structure (i.e. Interspike Intervals, ISI) (Tolu et al., 2010). Further characterization of transgenic mouse lines has not revealed profound alteration of the spontaneous VTA DA firing activity in mice lacking α7, α5* and β4* nAChRs (Mameli-Engvall et al., 2006; Morel et al., 2014; Harrington et al., 2016), but has defined the contribution of these subunits in VTA DA neuron’s response to chronic nicotine and stress exposure.
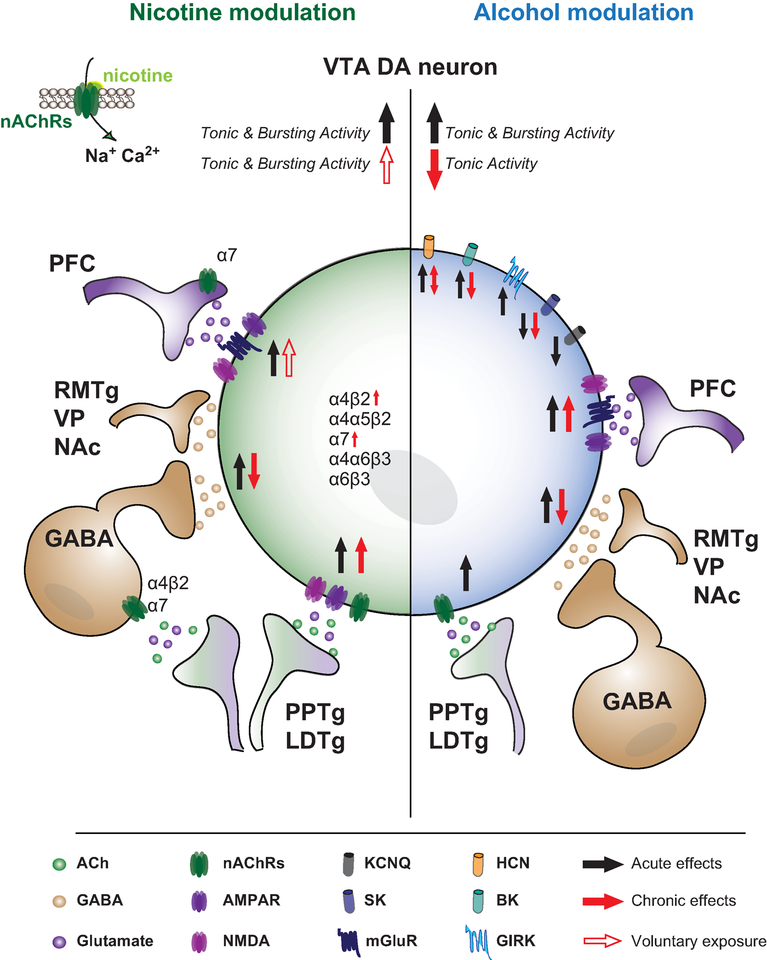
Figure 1: Schematic of the acute and chronic action of nicotine (left, green) and alcohol (right, blue) on VTA DA neurons.
VTA DA neurons receive glutamatergic inputs from: PFC, LHb, PPTg and LDTg, ACh inputs from PPTg and LDTg, and GABA inputs from local VTA GABA neurons, VP, NAc and RMTg. VTA neurons expresses a large portfolio of nAChRs (α4β2, α4α5β2, α7, α6β3, α6β4). Acute nicotine acts directly on VTA DA and GABA neurons, and potentiates the glutamatergic and GABA synaptic inputs, and overall increases the tonic and bursting activity of VTA DA neurons. Chronic nicotine exposure decreases the GABA synaptic inputs onto VTA DA neurons, and increases the expression and function of α4β2nAChRs and α7nAChRs, respectively. Voluntary chronic exposure to nicotine increases VTA DA neuron activity. Acute alcohol potentiates GABA and Glycine receptors, inhibits VTA GABA neurons, and has differing effects on K+ and HCN channels. Specifically, alcohol increases the function of HCN and BK channels, whereas SK channels are inhibited. SK channel inhibition persists after chronic exposure, and is thought to underlie increased bursting activity of VTA DA neurons in response to ethanol over time. Chronic alcohol exposure increases excitatory transmission onto VTA DA neurons, although this may be due to withdrawal effect.
Abbreviations: DA, dopamine neurons; nAChRs, nicotinic receptors; ACh, Acetylcholine; LDT, laterodorsal tegmentum, PPTg, pedonculopontine tegmentum; PFC, prefrontal cortex; Lateral habenula, LHb; RMTg, rostromedial tegmental nucleus; VP, ventral pallidum; NAc, nucleus accumbens; Big potassium channels, BK; Small-conductance activated channels; SK; Hyperpolarization-activated cyclic nucleotide-gated channels, HCN.
Primary nicotinic modulation of VTA DA neurons
Like natural rewards, nicotine increases DA release in VTA-projecting brain areas including the NAc (Imperato et al., 1986; Ferrari et al., 2002). DA release in the NAc induced by acute nicotine exposure is due to the transition of VTA DA neurons activity from tonic to bursting and pre-synaptic nicotine-gated DA release in the NAc, which is thought to mediate the nicotine reinforcing properties.
In vitro slice electrophysiological recordings have strongly contributed to the understanding of nicotine’s mode of action onto VTA DA neurons. In voltage-clamp mode, which allows for the characterization of agonist-induced currents, ACh “puff” application induces a brief inward current. In current-clamp mode, which allows for the monitoring of APs, ACh “puff” application induces a small amount of spiking activity transiently increased and then decreased by nicotine (Pidoplichko et al., 1997). Nicotine also acts on VTA GABA neurons and inputs (Fig. 1). In voltage-clamp, while recording VTA DA neurons, nicotine induces IPSCs that are abolished by bath application of DHBE (Dihydro-B-Erythroidine hydrobromide), a competitive nicotinic ACh receptor antagonist, suggesting a β2*nAChR dependent mechanism (Mansvelder et al., 2002). The bath application of nicotine also induces excitatory postsynaptic currents (EPSCs) that are abolished in the presence of methylcaconitine (MLA), an inhibitor of α7nAChRs, but not by mecamylamine, a non-selective, non-competitive antagonist of the nicotinic acetylcholine receptors. These studies suggest that nicotine binds onto glutamatergic presynaptic inputs and potentiates glutamatergic transmission via α7nAChRs (Fig. 1) (Mansvelder & McGehee, 2000).
The resulting in vivo effect of nicotine observed in electrophysiological recordings is a dose-dependent increase in both firing frequency and bursting activity, which persists over several minutes (Mameli-Engvall et al., 2006; Tolu et al., 2012; Morel et al., 2014). Nicotine also synchronizes VTA DA firing activity (Li et al., 2011). Pharmacological approaches (George et al., 2011), transgenic mice, and viral strategies confirm the necessary role of the β2*nAChRs in the reinforcing properties of nicotine. Indeed, the full deletion of β2*nAChRs, i.e. in β2KO mice, results in a loss of nicotine-induced VTA firing activity, and in failure to self-administer nicotine. These phenotypes are fully restored when β2nAChRs are re-expressed in the VTA (Picciotto et al., 1998; Maskos et al., 2005; Tolu et al., 2012).
VTA DA neurons have heterogeneous pharmacological and electrophysiological properties, and different modes of VTA DA neuron firing activity are observed within the same animal. In line with this heterogeneity, nicotine acts differently amongst VTA DA neurons (Fig. 1). In anesthetized mice, high bursting or firing neurons respond strongly to nicotine, with an increased tonic or bursting activity, respectively. Oppositely, low firing VTA DA neurons have a low response to the same amount of nicotine (Mameli-Engvall et al., 2006). Like many other drugs, Ach agonist intracranial self-administration (ICSA) efficiency varies across the anterior/posterior (a/p) axis (Fig. 2). Rats establish carbachol ICSA in the pVTA but not in the surrounding areas (Ikemoto & Wise, 2002). Supporting these results, the pVTA highly expresses α4, α6 and β4 nAChRs, which leads to a stronger nicotine-elicited activation of VTA DA neurons compared to aVTA DA neurons (Li et al., 2011; Zhao-Shea et al., 2011).
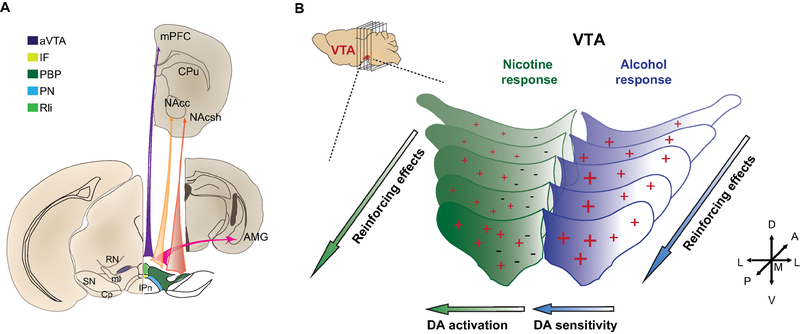
Figure 2: Dopaminergic circuit heterogeneous reinforcing effect of nicotine and alcohol.
(A) Schematic of VTA DA subnuclei and preferential projections. Recent studies using cell-specific tracing techniques established topographic preferential projection: from a medial to lateral axis, VTA DA neurons preferentially project to the mPFC, then the NAc, then the AMG and finally the NAcsh. (B) Schematic of VTA DA heterogenous neural and the consecutive behavioral response to nicotine (left, green) and alcohol (right, blue). Both nicotine and alcohol establish strong reinforcing effects when infused in the pVTA compared to the aVTA. VTA DA neurons display distinct activity in response to nicotine. Lateral VTA DA neurons are activated (+) by i.v. nicotine injection, medial VTA DA neurons are inhibited (−). VTA DA neurons have an increased sensitivity to alcohol across the medial/lateral axis (+, +).
Abbreviations: AMG, Amygdala; aVTA, anterior ventral tegmental area; BNST, bed nucleus of the stria terminalis; CPu, caudate putamen; CP, cerebral peduncle; IF, interfascicular nucleus; IL, infralimbic cortex; IPn, interpeduncular nucleus; LHb, lateral habenula; mHb, medial habenula; ml, medial lemniscus; mPFC, medial prefrontal cortex; NAcc, nucleus accumbens core; NAcsh, nucleus accumbens shell; PBP, parabrachial pigmented nucleus; PN, paranigral nucleus; pVTA, posterior ventral tegmental area; RN, red nucleus; RLi, rostral linear nucleus; SN, substantia nigra.
In anesthetized mice, while the majority of VTA DA neurons, preferentially recorded in the PBP, are activated in response to nicotine injection, a subpopulation is inhibited. In vivo juxtacellular neurobiotin labeling techniques allow for discrete cellular mapping and confirmed the TH+ expression profile of these two subpopulations. Nicotine-activated neurons are preferentially located in the lateral part of the PBP while nicotine-inhibited VTA DA neurons are preferentially located in the medial PBP (Fig. 2). Further investigations define a D2-receptor dependent inhibition, opening the question on the role of these neurons in nicotine reinforcement (Eddine et al., 2015).
While nicotinic action onto VTA DA neurons has been well established, the primary nicotinic action onto VTA GABA neurons remains unclear. Multiple lines of evidence suggest a disinhibition of VTA GABA neurotransmission onto VTA DA neurons, thus contributing to nicotine-induced VTA DA activity (Fig. 1) (Grieder et al., 2014; Buczynski et al., 2016). This disinhibition is attributed to the desensitization properties of VTA β2*nAChRs, while in parallel, nicotine activates the α7nAChRs expressed on glutamatergic inputs, strengthening glutamate neurotransmission that leads to VTA DA activation (Mansvelder & McGehee 2000; Mansvelder et al., 2002). The latest studies on β2KO mice show that the co-activation of both VTA GABA and DA neurons by nicotine is required to induce VTA DA bursting activity in response to nicotine exposure (Tolu et al., 2012). Indeed, the sole re-expression of β2nAChRs in VTA DA neurons of β2KO mice is sufficient to nicotine-induced tonic firing of VTA DA neurons, but is not sufficient to observe bursting and the associated maintenance of nicotine self-administration. Conversely, the specific re-expression of β2nAChRs exclusively on VTA GABA neurons leads to an expected inhibitory response of VTA DA neurons to nicotine and absence of nicotine self-administration. Interestingly, dual re-expression on both VTA GABA and DA neurons is necessary to observe firing frequency and bursting activity in response to nicotine, and prolonged nicotine self-administration (Tolu et al., 2012). These results highlight the crucial role of β2nAChRs, VTA DA bursting activity in mediating the reinforcing properties of nicotine, but also the complex modulation performed by VTA GABA neurons on these processes.
Hypersensitive α4nAChRs subunit overexpression increases VTA DA neuron’s nicotine sensitivity and promotes nicotine preference (Tapper et al., 2004). Conversely, α4KO mice recapitulate the phenotype of DA cell-type specific β2 re-expression in β2KO mice: loss of nicotine induced bursting activity and nicotine self-administration (Pons et al., 2008; Exley et al., 2011). Such a profile could be due to compensatory α6β2*nAChRs functions and an imbalance between VTA DA and GABA. The precise contribution of α6*nAChRs is unclear. Rats treated with an α6*nAChRs antagonist (Gotti et al., 2010) and α6KO mice both fail to self-administer nicotine during intravenous self-administration (IVSA) (Pons et al., 2008); whereas VTA ICSA is maintained (Exley et al., 2011). Fast-scan cyclic voltammetry (FSCV) studies have established the crucial role of α6β2*nAChRs in NAc pre-synaptic terminals, where the α6β2nAChRs gate VTA DA signals (Exley & Cragg 2007; Exley et al. 2011; Siciliano et al., 2017). In line with these results, hypersensitive α6nAChRs expression increases DA release (Wang et al., 2014) and VTA response to nicotine (Drenan et al., 2008).
Recent interests emerged for the gene locus 15q25, which encodes for the α3, α5 and β4 nAChRs. In particular, the single-nucleotide polymorphism (SNP) rs16969968 leads to a point mutation in the coding sequence of the α5*nAChRs subunits, D398N, which decreases α4α5β2nAChRs function (Bierut et al., 2008; Kuryatov et al., 2010). 398N-homozygote smokers are twice as susceptible to develop heavy tobacco addiction compared to 398D-homozygote smokers (Saccone et al., 2006). The α5*nAChRs is strongly expressed by 80% of VTA DA neurons (Klink et al., 2001) and their terminals, where this subunit preferentially forms an α4α5β2*nAChRs. The α5KO mice self-administer a higher concentration of nicotine compared to wild-type (WT) mice and have a decreased sensitivity to nicotine (Salas et al., 2003; Fowler et al. 2011; Morel et al. 2014). The VTA expression of the α5-N subunit, mimicking the human 398N mutation, is associated with VTA DA decreased sensitivity to nicotine compared to the α5-D subunit, which is causally related to higher doses of nicotine to induce acute nicotine self-administration (Morel et al., 2014).
Animal models of nicotine reinforcement
Numerous paradigms have been established to study the physiological consequences of nicotine exposure. While human pharmacokinetics or social aspects are absent, animal models offer the ability to control multiple aspects difficult to calibrate in human subjects, including: age of onset, gender, genetic profile, etc. Nicotine can be passively administered (i.e. injection, infusion, transdermal delivery, or vapor) or actively self-administered by the animal (Matta et al., 2007; Kallupi & George, 2017).
Nicotine self-administration protocols offer the ability to study the reinforcing properties of nicotine from primary reinforcing properties, to withdrawal and relapse. In these protocols, the animals perform an action associated with nicotine delivery, which mimics voluntary human drug intake. Nicotine self-administration has been established in various species including non-human primates, rats and mice (Goldberg et al., 1981; William A. Corrigall & Coen 1989; Le Foll & Goldberg 2009). Similar to opioid self-administration, nicotine self-administration dose-response curve follows an inverted U-shape. This profile may result from positive nicotine reinforcing effect –the ascending part of the curve, and the negative/aversive nicotine effects –descending part of the curve for higher dose of nicotine. Nicotine self-administration is often difficult to establish, thus, numerous paradigms include a training phase using food rewards or other drug pre-exposure and food restriction prior to nicotine self-administration (Caggiula et al., 2002; Fowler & Kenny 2011; Caille et al., 2012). This pre-exposure can induce some difficulty in fully understanding the initial mechanism of nicotine reinforcing effects, since the action leading to the nicotine intake (lever press, nose-poke, etc.) has already been positively reinforced during the training phase.
Distinct nicotine self-administration protocols illuminate different aspects of the nicotine reinforcement processes. Of interest, ICSA consists of a local nicotine infusion in discrete brain areas. ICSA studies provided the foundation for how VTA nAChRs established nicotine reinforcement (Corrigall et al., 1994). The acute IVSA paradigm performed on naïve animals offers the opportunity to detect the primary dose required to increase the propensity of an action associated with the drug delivery. For example, the paradigm used in Pons et al., the loss of nicotine reinforcing effect in β2KO, α4KO, α6KO, is described by the lack of increased nose-poke activity for the nicotine dose tested compared to WT mice (Pons et al., 2008). Nonetheless, other aspects critical to characterizing nicotine dependence require classic IVSA protocols, which assess acquisition, motivation, compulsion, and relapse (Caggiula et al., 2002; Kenny & Markou 2006; Fowler et al. 2011; Fowler & Kenny 2011; Orejarena et al. 2012).
Chronic nicotine modulation of VTA DA neurons
Long-term chronic exposure to nicotine affects multiple peripheral and cognitive functions: metabolism, immune response, memory and attention. Even though nicotine acts on the same nAChRs as endogenous ACh, chronic exposure to nicotine cannot be perceived as “hypercholinergy”. Nicotine is degraded by liver enzymes and has a longer systemic half-life (about 10–15min for rodents), whereas ACh esterase hydrolyses ACh in the synaptic cleft in milliseconds. Nicotine, compared to ACh, can thus desensitize nAChRs and be internalized by the surrounding cells. Chronic nicotine exposure induces the desensization (Pidoplichko et al., 1997; Grady et al., 2012) and the upregulation of nAChRs (Fig. 1) (Buisson & Bertrand 2001; Staley et al., 2006; Fasoli et al., 2016). These two phenomena are thought to contribute to withdrawal processes and overall nicotine addiction mechanisms. While nAChRs desensization is associated with impaired nAChRs functions and decoupling with the endogenous ACh tone (Pidoplichko et al., 1997), the nAChRs upregulation, suggested to compensate for nAChR hypofunction, increases receptor trafficking, turn over and alters endogenous nAChRs composition in the VTA (Nashmi et al., 2007; Baker et al., 2013).
The consequences of chronic nicotine exposure on VTA DA activity vary depending on the protocol used. Voluntary nicotine self-administration increases the spontaneous firing activity of VTA DA neurons both in tonic and bursting activities, whereas passive exposure via osmotic minipumps does not induce detectable effects (Besson et al. 2007; Caillé et al. 2009). Chronic nicotine exposure leads to the reorganization of VTA nAChRs and modifies their properties, uncovering the role of α7nAChRs. Indeed, in β2KO mice chronically exposed to nicotine, α7nAChRs contribute to the recovered VTA DA neuron’s firing activity (Besson et al., 2007), and restores to WT-like nicotine intake behaviors. Conversely, chronic exposure to nicotine induces a decrease in nicotine consumption in α7KO mice (Levin et al., 2009).
Taken together, VTA nAChRs mediate the reinforcing effects of nicotine differently on VTA DA, GABA and glutamate neurotransmission. The high affinity β2*nAChRs is the limiting factor, while α7nAChRs tune the system via glutamate neurotransmission. Finally, other subunits and their related polymorphisms, like α5nAChRs D398N, modulate the sensitivity of VTA DA neurons to nicotine and define the nicotine dose required to encode its reinforcing effects.
Network contributions
Nicotine acts not only on the VTA, but also on numerous brain areas contributing to the regulation of DA activity. Recent advances in circuit-probing techniques contribute to network contributions in nicotine reinforcement. The Infralimbic cortex (IL) – BNST circuit projecting on VTA (Kaufling et al. 2017; Kaufling et al. 2009), contributes to the increased spontaneous activity of VTA DA neurons (Fig. 3) following voluntary but not passive (Fig. 1), nicotine self-administration (Caillé et al., 2009). These results highlight network contributions in shaping VTA alterations during nicotine reinforcement.
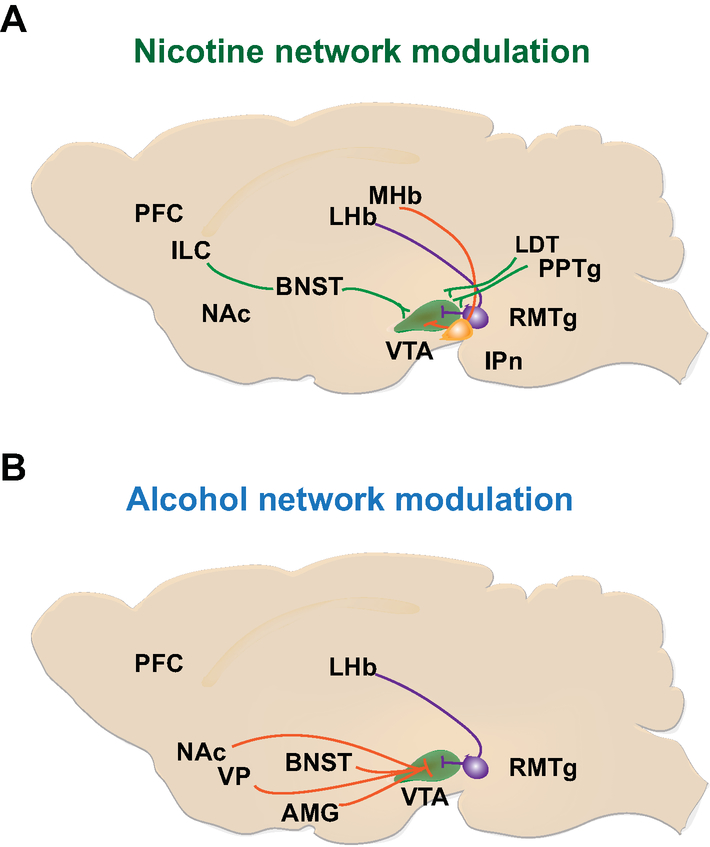
Figure 3: Network nicotine and alcohol modulation of VTA DA neurons.
(A) Network modulation onto VTA DA neurons in response to nicotine. The IL-BNST-VTA circuit (green) increases the spontaneous VTA DA activities following voluntary nicotine consumption. mHb-IPn circuit decreases the reinforcing properties of high concentration of nicotine by encoding aversive signal. The precise modulation of this pathway onto VTA DA neurons remains poorly understood. LHb-RMTg-VTA circuit requires further investigation to fully characterize its function in nicotine reinforcement. While LHb-RMTg activation inhibits VTA DA neurons, nicotine activation of RMTg neurons does not affect VTA DA neural response to nicotine. (B) Network modulation onto VTA DA neurons in response to alcohol. Activation of DA receptors in the NAcsh, VP, and PFC are involved in mediating the reinforcing effects of ethanol and inhibitory projections from the VP to the VTA may control alcohol seeking and reinstatement. The LHb-RMTg-VTA circuit has been hypothesized to contribute to aversive conditioning of ethanol response and may account for individual differences in drinking behaviors. Stress and withdrawal affects of chronic alcohol exposure are highly controlled by the connections between VTA and AMG.
Abbreviations: AMG, Amygdala; BNST, bed nucleus of the stria terminalis; CPu, caudate putamen; IL, infralimbic cortex; IPn, interpeduncular nucleus; LHb, lateral habenula; mHb, medial habenula; mPFC, medial prefrontal cortex; NAcc, nucleus accumbens core; NAcsh, nucleus accumbens shell.
Of recent interest, the habenulo-interpeduncular (mHb-IPn) pathway has been determined as a strong modulator of nicotine reinforcing properties (Fowler et al. 2011; Ables et al. 2017). IPn neurons project to the dorsal tegmentum and the raphe nucleus (Lecourtier & Kelly, 2007; Hsu et al., 2013; Antolin-Fontes et al., 2015). The mHb sends a glutamatergic projection to the IPn and encodes negative/aversive signals. Numerous nAChRs subtypes are highly expressed in the mHb-IPn pathway (Grady et al., 2009). The α5*nAChRs in the mHb-IPn circuit, similarly to the VTA, is a sensor of nicotine concentration. Fowler and collaborators defined that α5KO mice and knockdown rats self-administered for higher doses of nicotine than WT animals. This shift to a higher dose of nicotine is not only due to a decrease in nicotine’s reinforcing effects, but also to a decrease in the nicotinic aversive signal encoded by the mHb-IPn pathway (Fig. 3). In line with these results, functional alteration of β4*nAChRs in the mHb-IPn circuit modulate nicotine self-administration and NAc DA release and VTA DA neuron’s sensitivity to nicotine effects (Harrington et al., 2016). This phenotype is recovered by selective re-expression of β4nAChRs subunits in the IPn. In parallel, over-expression of β4nAChrs in the mHb-IPn pathway increases nicotine-induced aversive responses (Frahm et al., 2011).
The recent characterization of the tail of the VTA, the rostromedial tegmental nucleus (RMTg) (Sanchez-Catalan et al., 2014), contributes to the description of the LHb-RMTg-VTA circuit, also encoding for aversive signals (Perrotti et al., 2005). RMTg GABA neurons project to VTA DA neurons (Jhou et al., 2009; Kaufling et al., 2009; Hong et al., 2011) and LHb-RMTg-VTA circuit activation inhibits VTA DA neurons (Ji & Shepard 2007; P. L. Brown et al., 2017). Morphine and WIN decrease RMTg activity and thus promotes VTA DA neuron firing (Fig. 3). Unexpectedly, nicotine activation of RMTg does not elevate GABA inhibitory control over VTA DA neurons, and does not affect VTA DA neuronal activation by nicotine (Lecca et al., 2010; Lecca et al., 2012). Further investigations will be required to fully understand the contribution of the LHb-RMTg-VTA pathway in nicotine and drug reinforcement.
Alteration of non-drug functions of VTA DA neurons
Human smokers show impulsive behaviors, altered cognitive flexibility, and present a higher propensity to develop mood disorders than non-smokers. Smoking and impulsivity form a bidirectional relationship; increased impulsivity is associated with increased smoking vulnerability and increased nicotine dependence (Kale et al., 2018). A recent study described the role of VTA β2*nAChRs in uncertainty seeking behaviors (Naudé et al., 2016). Since nicotine exposure alters the endogenous function of β2*nAChRs in the VTA, it is expected that nicotine would affect uncertainty seeking processes and overall decision making (Naudé et al., 2015). Nicotine enhances the reinforcement of non-drug reinforcers both in humans (Perkins & Karelitz 2013; Perkins et al., 2018) and animals (Donny et al., 2003; Constantin & Clarke 2017). Nicotine’s ability to strengthen the reinforcing properties of non-drug reinforcers, in particular smoking-associated cues, is hypothesized to enhance primary reinforcing properties and promote nicotine intake (Caggiula et al., 2002; Donny et al., 2003; Palmatier et al., 2007; Farquhar et al., 2012).
Smoking dependence and mood disorders are highly comorbid in humans (Mathew et al., 2017). Smokers, although abstinent for several years, are threefold more likely to develop mood disorders (Breslau & Johnson, 2000); smoking as a coping strategy is highly prevalent in patients suffering from mood disorders. The role of nAChRs in mood disorders has been investigated in humans (Saricicek et al., 2012) and confirmed in animal models (Mineur et al., 2016, 2017). VTA DA neurons do not only encode positive reinforcement and reward, but also for negative and salient events, promoting adaptive behaviors. Thus, VTA DA neurons are highly involved in the maintenance of healthy brain functions and behaviors (Russo & Nestler 2013). In line with this role, alteration of VTA DA function is causally associated with depressive and anxiety-like phenotypes (Cao et al. 2010; Chaudhury et al. 2013; Barik et al. 2013; Friedman et al., 2014; Friedman et al., 2016). The stress-induced model of depression, chronic social defeat stress (CSDS), increases the spontaneous activity of VTA DA neurons projecting to the NAc, which leads to social avoidance behavior and anhedonia (Chaudhury et al., 2013) – two characteristic symptoms of depression. CSDS decreases VTA DA neuron’s nicotine-induced firing activity (Morel et al., 2017), which may decrease nicotine-reinforcing properties. This result coincides with human subjects who report an increased nicotine consumption following stressful events. Blocking β2*nAChRs in depressed mice exacerbates social avoidance behaviors, while blocking α7nAChRs rescues social interaction behaviors (Morel et al., 2017). Conversely, nicotine pre-exposure via α7nAChRs primes VTA DA maladaptations and the development of depressive phenotypes, following sub-threshold stress exposure (Morel et al., 2017). The opposite action of β2*nAChRs and α7nAChRs blockade in depressed mice requires the development of selective α7nAChRs inhibitors for further therapeutic approaches.
Reinforcing properties of ethanol and subsequent neural alterations
Ethanol is a two-carbon molecule that interacts with other biomolecules through weak hydrophobic interactions or hydrogen bonding. In addition to this, ethanol also has a low binding affinity to proteins, severely limiting its potency. The legal limit of 0.08% v/v is approximately 17mM in the human body, whereas the anesthetic concentration is 190 mM (Alifimoff et al., 1989). Upon ingestion, ethanol is ubiquitously distributed throughout total body water; the blood concentration approximates ethanol concentration in all organ and cellular compartments. Ethanol easily passes through the blood brain barrier and is known to have direct and indirect targets throughout the brain. Unlike other drugs of abuse, even low to intermediate concentrations of ethanol disrupt the normal functioning of neurotransmitter receptors, ion channels, intracellular signaling proteins (Yoshimura et al., 2006; Pany & Das, 2015; Ron & Barak, 2016; Mohamed et al., 2018), and also perturbs membrane lipids (Cui & Koob, 2017).
VTA DA neurons are a critical neural substrate for reward and ethanol reinforcement (Pfeffer & Samson, 1988; Samson et al., 1988; Nestler, 2005; Koob & Volkow, 2010). Acute exposure to ethanol facilitates VTA DA neuron’s firing both in vitro and in vivo, increases DA within the VTA (Fig. 1), as well as many downstream targets (Di Chiara and Imperato 1985; Brodie et al., 1990; Appel et al., 2003). Ethanol reinforcement was increased by microinjections of DA agonists in the NAc during a free choice operant task using 10% ethanol in rats (Hodge et al., 1992). Microdialysis studies augmented this finding by showing an increased concentration of DA in the NAc after self-administration of 10% ethanol but not saccharin (Weiss et al., 1993). Ethanol directly modulates the function of DA channels and receptors, while also manipulating extrinsic synaptic inputs onto DA neurons from within and outside of the VTA. Rats establish strong ethanol ICSA (Rodd-Henricks et al., 2000), and many behavioral paradigms show escalated intake of ethanol over time (Rhodes et al., 2005; Sharpe et al., 2005; Crabbe et al., 2011), despite ethanol’s aversive taste.
The complex nature of ethanol actions in the VTA is the further compounded by age, sex, and species differences. Different murine strains exhibit different drinking preferences and responses to acute and chronic ethanol (Cunningham et al., 1992; Belknap et al., 1993; Wanat et al., 2009). This is likely due to genetic or more recently epigenetic differences underlying reward and aversion processing (You et al., 2018). Age also effects the production and turnover of DA in the VTA (Woods & Druse, 1996), which may explain varying sensitivity of VTA DA neurons to ethanol across studies. While sex differences are not covered in this review, recent evidence clearly shows differences in ethanol consumption, response to reward, and aversion between males and females and need to be studied further (You et al., 2018). Within the scope of this review, the acute and chronic effects of ethanol on DA neurons are summarized below, starting with direct modulation of intrinsic excitability and moving onto extrinsic and circuit modulations.
In this review we will focus on ethanol’s reinforcing effects on VTA DA neurons specifically and subsequent microcircuit and network alterations. For more extensive molecular and anatomical reviews on ethanol throughout the brain, please see references (Harris et al., 2008; Trudell et al., 2014; Abrahao et al., 2017).
Primary ethanol modulation of VTA DA neurons
Acute application of ethanol increases the spontaneous firing frequency of VTA DA neurons in vivo, in vitro slice preparation and dissociated cell culture in ethanol-naïve animals (Gessa et al., 1985; Brodie et al., 1990, 1999; Brodie & Appel, 1998). Ethanol excitation of VTA DA neurons occurs despite being dissociated from synaptic and glial connections (Brodie et al., 1999), or by the presence of GABA, glutamate, or ACh antagonists (Nimitvilai et al., 2016). Modulation of both direct and indirect ethanol targets all contribute to the firing and functions of VTA DA neurons.
The HCN channel-mediated Ih current is known to contribute to the pacemaker firing of DA neurons and are direct ethanol targets (Harris & Constanti, 1995; Neuhoff et al., 2002). Treatment with ZD7288, an Ih blocker, irreversibly depresses basal firing frequency of VTA DA neurons (Fig. 1) and significantly attenuates the stimulatory effect of ethanol on firing (Okamoto, 2006). This excitatory Ih current is enhanced partly by ethanol’s ability to augment cyclic adenosine monophosphate (cAMP)-dependent facilitation of voltage-gating (Okamoto, 2006). However, this effect has been shown only in mice, not in rats (Appel et al., 2003; McDaid et al., 2008). Ethanol has also been shown to directly increase barium-sensitive inhibitory potassium currents in VTA DA neurons (McDaid et al., 2008), but this effect can only decrease firing if Ih is blocked.
Ethanol also targets the M-current (Im) (Hansen et al., 2008), a sustained K + current that is activated at the subthreshold range of membrane potential, regulating AP generation (Brown & Adams, 1980; Aiken et al., 1995). In contrast to Ih, ethanol decreases Im conductance, inadvertently decreasing inhibition and increasing the spontaneous firing frequency of VTA DA neurons in vitro (Koyama et al., 2007). Kv7 channels are the principal molecular components of the slow voltage-gated M-channel (Brown & Passmore, 2009). Of recent interest, Kv7.4 channels have been shown to regulate ethanol intake and dependence (McGuier et al., 2018).
VTA DA neurons also express small conductance calcium-activated potassium (SK) and Big potassium (BK) channels (Fig. 1) that are also modulated by acute ethanol (Brodie et al., 2007), and express specific subtypes in the VTA (Stocker & Pedarzani, 2000). During an AP, membrane depolarization and Ca2+ influx activate BK and SK channels, which both help to terminate the AP and produce fast after-hyperpolarization (AHP) (Faber & Sah, 2007; Lee & Cui, 2010). Interestingly, acute ethanol seems to decrease SK channel function in a heterologous expression system (Dreixler et al., 2000), while potentiating BK channel function by increasing channel open probability (Mulholland et al., 2009; Dopico et al., 2014). The amount of the SK current present may dictate the sensitivity of VTA DA neurons to ethanol excitation (Brodie et al., 1999b), but inhibition of this channel with apamin, a peptide neurotoxin form bee venom that specifically blocks SK channels, failed to strongly reduce ethanol-induced VTA DA neuron excitation. Further, preliminary studies have shown that specific subunits of the BK channels can modulate ethanol sensitivity and can also influence drinking behavior (Bettinger et al., 2014) although further studies are needed on the role of both of these channels in VTA DA mediated ethanol reinforcement.
Ethanol modulates DA neuron’s excitability via the GIRK inwardly rectifying K + channels (Fig. 1). GIRK channels contribute to the resting membrane potential of VTA DA neurons and contributes to tonic/bursting switch of pattern activity (Lalive et al., 2014). Ethanol can directly module GIRK channels or indirectly through a G-coupled protein (Kobayashi et al., 1999; Lewohl et al., 1999; Blednov et al. 2001; Beckstead et al., 2004; Labouèbe et al., 2007; Aryal et al., 2009). For instance, ethanol has been shown to potentiate GABAB receptor-induced inhibition of VTA DA neurons through the downstream activation of GIRK channels (Federici et al., 2009). GIRK3 specifically has been shown to regulate ethanol sensitivity (Herman et al., 2015), although GIRK antagonists fail to fully inhibit ethanol excitation of DA neurons in other studies (Appel et al,. 2003; McDaid et al., 2008). This may, in part, be due to the differential distribution of GIRK subunits throughout the VTA (del Burgo et al., 2008; Rifkin et al., 2017). Accordingly, acute ethanol both directly and indirectly increases the function of GIRK channels on VTA DA neurons, potentially mediating inhibitory effects known to stabilize VTA DA firing.
Ethanol (30 to 100 mM) acts also on ligand-gated ion channels (LGICs) (Dildy-Mayfield et al., 1996; Mascia et al., 1996). For example, ethanol can potentiate the function of LGICs such as glycine and GABAA receptors and inhibit excitatory NMDA and AMPA glutamate receptors in brain region- and concentration-dependent manner (Lovinger & Roberto, 2010; Söderpalm et al., 2017). Canonical activation of ionotropic glutamate receptors (NMDA and AMPA) on VTA DA neurons increases in vivo firing and burst activity (Fig. 1) (Johnson et al., 1992; Chergui et al., 1993; Zweifel et al., 2009). Surprisingly, increased AMPAR-mediated excitatory synaptic transmission was determined in in vitro slice electrophysiology 24h after intraperitoneal (i.p.) injection (20mg/kg) in C57BL/6J mice (Saal et al., 2003), although this may be due to the effect of acute withdrawal. In contrast, a single ethanol exposure was shown to reduce AMPA/NMDA receptor function on VTA DA neurons 24h after intraperitoneal (i.p.) administration (2g/kg) in DBA/2J but not C57BL/6J mice (Wanat et al., 2009), revealing strain-specific ethanol sensitivity. The conflict in studies may be due to concentration of ethanol used. Acute ethanol can also act presynaptically by enhancing glutamatergic transmission onto VTA DA neurons and increasing the frequency but not the amplitude of spontaneous EPSCs (Deng et al., 2009; Xiao et al., 2009). While it is well known that ethanol can directly inhibit excitatory NMDA-mediated currents in a variety of other neurons/preparations (Valenzuela et al., 1998; Ronald et al., 2001), it remains unclear how acute ethanol directly modulates AMPA/NMDA receptors on VTA DA neurons and how this action may or may not contribute to ethanol reinforcement.
Acute ethanol potentiates the firing of DA neurons, but has differing effects on GABA release at DA synapses and inhibitory transmission within the VTA (Fig. 1) (Burkhardt & Adermark, 2014; Abrahao et al., 2017). Approximately 20–40% of VTA neurons are GABAergic and regulate VTA DA neuron activity (Carr et al., 2000; Margolis et al., 2012). VTA GABA neuron firing recorded directly in vivo (0.2–2.0 g/kg, i.p.) has been shown to be inhibited by acute ethanol administration (Steffensen et al., 2000; Stobbs et al., 2004). D2R and μ-opioid receptors are both expressed on VTA GABA neurons. They are targets of ethanol and affect VTA GABA neuron firing- activation decreases firing and increases DA release within the VTA (Xiao et al., 2007; Ludlow et al., 2009). However, a single, acute exposure to ethanol either i.p. (2g/kg) or through bath application (50 mM) in slice showed increased GABAergic transmission and GABA release onto VTA DA neurons (Melis et al., 2002; Theile et al., 2008; Theile et al., 2011). These inhibitory effects are most likely due to long-range projections coming from outside of the VTA, whereas the aforementioned studies focus on VTA GABA neurons directly. In contrast, low dose ethanol administered i.v. (0.01–0.1 g/kg) in the rat seems to stimulate VTA GABA neuron firing (Steffensen et al., 2009). The current hypothesis states that ethanol-induced increase in DA firing activity is partially due to “disinhibiting” DA neurons by blocking inhibitory effects (Xiao et al., 2007). Further investigation is needed to clarify whether these ethanol-induced synaptic effects are coming directly from VTA GABA interneurons or unique afferent projections from other brain structures.
Lastly, ethanol potently modulates nAChRs at many concentrations (100 μM–10 mM), but is not a direct agonist (Liu et al., 2013). The specific effects of this interaction are dependent upon the subunit composition of the receptor, and will be touched upon towards the end of this review when discussing interactions between nicotine and alcohol.
While a wide range of studies has been published on the effect of ethanol on DA neurons in the VTA (see additional review by Morikawa and Morrisett, 2010), its precise effect is still obscure. Numerous discrepancies arise from differences in species, behavioral model, and concentration of ethanol administered, but also from the VTA DA subpopulation being investigated (Lammel et al., 2008, 2014; Poulin et al., 2014). The majority of VTA DA neurons send specific projections to non-overlapping targets, further solidifying the importance of cell-type and projection specific research (Fallon, 1981; Rodd-Henricks et al., 2000; Lammel et al., 2008). Circuit-probing techniques highlight the different contribution of VTA DA projections in the reinforcing properties of ethanol (Fig. 2) (Juarez et al., 2017). Interestingly, medial VTA DA neurons seem more responsive to ethanol (Fig. 2) (Mrejeru et al., 2015; Avegno et al., 2016). Additionally, acute exposure to ethanol in the pVTA increases VTA DA neuron activity and decreases activity in the aVTA in conjunction with decreased and increased IPSCs (Guan et al., 2012). It also seems that the pVTA is more sensitive to the excitatory effects of ethanol; rats will self-administer via ICSA in the pVTA when compared to aVTA (Fig. 2) (Rodd-Henricks et al., 2000).
Network contributions to ethanol reinforcement
Changes in the brain induced by chronic ethanol intake are long lasting and recruit an extensive network of neural circuits. The magnitude of ethanol’s impact within specific neural circuits and cells is dictated by concentration, route of administration, and exposure frequency (i.e. acute v. chronic). Therefore, it is imperative to take into account network modulations occurring throughout the brain during chronic use (Seo & Sinha, 2014; Abrahao et al., 2017). Optogenetics and novel tracing viral strategies have now made it possible to assess behaviorally relevant inputs and anatomical connectivity between brain structures and how the function and sensitivity of these circuits change in the context of drug addiction (Osakada et al. 2011; Tye & Deisseroth, 2012).
Activation of DA receptors in the NAc shell, VP, and PFC are involved in mediating the reinforcing effects of ethanol in the pVTA (Ding et al., 2015). Indeed, one study found that self-administration of ethanol continued until NAc DA concentrations were normalized after chronic use (Weiss et al., 1996). Furthermore, systemic administration of ethanol in rats leads to an increase in the frequency of phasic DA transients in the NAc detected with FSCV, supporting the idea that ethanol promotes the occurrence of DA neuron bursts (Cheer et al., 2007; Robinson et al., 2009; Morikawa & Morrisett, 2010). Systemic injection of naltrexone can inhibit ethanol-induced NAc DA release associated with self-administration (Gonzales & Weiss, 1998). Neurotoxin lesioning of DA terminals in the NAc suppresses ethanol-taking behaviors as measured by two-bottle choice (2BC) or self-administration paradigms (Rassnick et al., 1993; Ikemoto et al., 1997). While both the AMG and PFC are also involved in drug seeking and decision-making, their role is less pronounced than the NAc in ethanol reinforcement (Meinhardt et al., 2013).
External projections onto VTA DA neurons have also been shown to influence ethanol related behaviors and reinforcement (Fig. 3). The ventral pallidum (VP) is a well-established structure for ethanol reinforcement and reinstatement of drug seeking and whose GABAergic neurons project directly to the VTA. Chemogenetic inactivation of the VP-VTA pathway successfully reduced alcohol seeking and reinstatement in rats (Prasad & McNally, 2016). The lateral habenula (LHb), comprised of mostly glutamatergic neurons, is also a key brain structure in modulating VTA DA neurons, specifically in the context of aversion. In rats given home cage access to 20% ethanol in an intermittent access 2BC paradigm, LHb lesioned animals escalated their voluntary ethanol consumption more rapidly than controls (Haack et al., 2014). The LHb projects directly to the VTA, but also influences VTA DA activity through the LHB-RMTg-VTA circuit. Stimulation of the LHb inhibits VTA DA neurons, potentially through the influence of RMTg inhibition and low doses of ethanol (0.25 g/kg i.p.) in rats have been shown to activate neurons in the LHb, potentially accounting for aversive conditioning and individual differences in drinking behaviors between and within animal strains (Fu et al., 2017). Many other brain areas project onto the VTA, including the PFC, NAc, BNST, and LDTg; however, their exact role in regulating VTA DA response to ethanol reinforcement needs to be studied further with cell-type and projection-specific tools.
Chronic ethanol modulation of VTA DA neurons
Over the last 50 years, researchers have used animal models of chronic ethanol exposure to determine the impact of repeated ethanol exposure on specific cells and neural circuits and more closely mimic human alcohol consumption and abuse (Becker, 2008; Leeman et al., 2010; Koob, 2014). Alcohol addiction can be conceptualized as a multi-stage cycle that involves escalating drug use and neuroplastic changes in the brain’s natural reward system over time (Koob & Volkow, 2016). Preclinical studies of chronic alcohol use elucidate the long-lasting effects of ethanol on the brain by revealing compensatory mechanisms, long-term plasticity changes and aberrant learning (Weiss & Porrino, 2002; Hyland et al., 2002). Various animal models of chronic alcohol exposure include: intermittent or 24 h access 2BC, drinking in the dark (DID), vapor chamber, chronic intermittent access (CIE), and repetitive i.p. injections (Crabbe et al., 2010). Many models of chronic ethanol exposure seek to model later phases of addiction, including drug seeking and relapse, intoxication, binge drinking and withdrawal. However, in this review we will focus on experimental studies that assess how the reinforcing properties of ethanol on VTA DA neurons change through repeated ethanol exposure.
Chronic ethanol has been shown to sensitize VTA DA neuron’s response to bath application of ethanol after chronic in vivo administration (3.5mg/kg 2× daily i.p., 21 days), with no change seen in baseline pacemaker activity (Brodie, 2002). Another group found a 25% reduction in Ih density and a subsequent reduction in the magnitude of ethanol stimulation of firing in mice in vitro (2 g/kg 1× daily i.p., 5 days) (Okamoto, 2006). This discrepancy in ethanol sensitization of VTA DA neurons may arise from length of exposure. Longer periods of alcohol exposure may induce homeostatic neuroadaptations not seen at short time intervals, but also the VTA DA neuron subpopulations investigated. Using a 10% ethanol 2BC paradigm of unlimited alcohol access, one group found that VTA-NAc DA neurons had significantly increased Ih current in low alcohol drinking mice and a significantly decreased Ih current in high alcohol drinking mice when compared to controls (Juarez et al., 2017). In contrast, in vitro recordings made from VTA DA neurons 1–6 days after cessation of chronic ethanol consumption in mice (liquid diet, ~23 days) revealed decreases in baseline firing (Bailey et al., 2001). The amount of ethanol consumed by the mice (25–32 g/kg/24 h) in this study may explain the attenuation of baseline VTA DA excitability, but also reveals profound differences in the physiological affects of ethanol on VTA DA activity based on route of administration and potential affects of withdrawal. A further complication is the disparity between passive (i.p.) and active self-administration of ethanol, although passive ethanol still can facilitate ethanol drinking in C57BL/6J mice (Lessov et al., 2001). In accordance with this, sensitization and tolerance of the DA system have been reported after different ethanol treatment protocols (Zapata et al., 2006; Ding et al., 2009).
Many different mechanisms may affect VTA DA neurons response to chronic ethanol and subsequent shifts in reinforcement. Microinjections of the dopamine D2/D3 agonist quinpirole in the VTA of Long-Evans rats reduced lever pressing for 10% ethanol, supporting the early hypothesis that DA activity in the VTA is involved in ethanol reinforcement (Hodge et al., 1993). The effects of D2R autoinhibition and subsequent activation of GIRK channels was studied after chronic administration of ethanol (2mg/kg i.p. 3× for 7 days). Patch-clamp recordings revealed increased activity of D(2)/GIRK-mediated inhibition whereas GABAB/GIRK mediated inhibition was not affected (Perra et al., 2011). SK channel function has also been shown to be reduced 7 days after rats are repeatedly exposed to ethanol (2g/kg i.p. 2× for 5 days) (Hopf et al., 2007). Because of this reduction in SK channel function, it has been hypothesized that this and several other alterations ultimately increase the ability of VTA neurons to produce burst firing and thus might increase ethanol reinforcement and contribute to addiction-related behaviors.
As discussed previously, there are multiple extrinsic inputs onto VTA DA neurons that regulate synaptic transmission and firing patterns- driving a form of aberrant learning and reward (Stuber et al., 2010). Chronic ethanol exposure can cause increased long-term potentiation (LTP) of NMDAR-mediated transmission in VTA DA neurons (Bernier et al., 2011), but only at very high doses of ethanol (6 g/kg/day). Chronic administration of ethanol (2–3.5 g/kg, i.p. 1–2×/day for 5–21 days) from two different groups shows no change in NMDA receptor function (Brodie, 2002; Hopf et al., 2007). Longer studies in rats, spanning up to 50 days of volitional intake, show an increase in AMPA-mediated synaptic transmission onto VTA DA neurons (Stuber et al., 2008). Lastly, it was found that only long-term exposure to ethanol increased levels of immunoreactivity of the NMDAR1 subunit, an obligatory component of NMDA receptors, and of the GluR1 subunit, a component of many AMPA receptors (Ortiz et al., 1995). As a result, the reinforcing properties of ethanol may also be partly driven by enhanced learning of both cue and reward through LTP.
Acute ethanol decreases the activity of VTA GABA neurons (Gallegos et al., 1999; Davies, 2003) and in addition, high doses of ethanol (3.5 g/kg i.p., daily for 21 days), seem to reduce the sensitivity of GABA-induced inhibition of VTA DA neuron firing (Brodie, 2002; Stobbs et al., 2004; Adermark et al., 2014; Burkhardt & Adermark, 2014). Tolerance to local ethanol inhibition by VTA GABA neurons was produced by 2 weeks of CIE/ethanol vapors (Gallegos et al., 1999), although this is less involved with reinforcement due to passive inhalation. Chronic ethanol exposure (5% liquid diet, ~2–3 weeks), has also been shown to down-regulate D2 receptors on VTA GABA neurons, potentially resulting from the persistent release of DA onto GABA neurons (Ludlow et al., 2009). It has also been reported that intra-VTA injection of a delta-opioid receptor (DOR) agonist decreases ethanol consumption in a 2BC paradigm, via presynaptic inhibition of GABA release onto VTA DA neurons (Margolis et al., 2008a). These results indicate selective adaptations after chronic ethanol exposure; excitatory transmission is perturbed to enforce the learning of reward, whereas inhibitory transmission is muted or diminished.
Ethanol has also been shown to block long-term potentiation of GABAergic synapses via μ-opioid receptors (Guan & Ye, 2010). Naltrexone, a non-selective opioid antagonist, decreases the rewarding and euphoric effects of alcohol and is one of the only current therapies for recovering alcoholics. While administration of naltrexone did not block the acute release of dopamine in the VTA of ethanol-naïve rats, it did prevent this release from being prolonged (Valenta et al., 2013), a mechanism which may modulate the rewarding effects of ethanol but only in chronic users. VTA neurons express a wide variety of opioid receptors, but specifically targeting them in the VTA to increase therapeutic efficacy has not yet been achieved. Stimulation of another ethanol target, the κ-opioid receptor, also suppresses ethanol consumption and preference in rats (Nestby et al., 1999; Lindholm et al., 2001; Logrip et al., 2009)
Effects of ethanol withdrawal and stress on VTA DA neurons
Repeated exposure to ethanol and withdrawal creates an imbalance between excitatory and inhibitory mechanisms controlling VTA DA neurons, and also increases the frequency of negative affect and stress- two main drivers to relapse. The main effects of alcohol withdrawal on synaptic physiology and behavior have been extensively studied in other brain regions such as the AMG and PFC (Roberto & Varodayan, 2017; Varodayan et al., 2018), including withdrawal affects on the excitability and synaptic transmission of DA neurons and impact on reinforcement.
Initially, primary exposure to ethanol increases DA release in the VTA and NAc,. Ethanol withdrawal has been shown to decrease tonic and phasic DA firing in rats (Diana et al., 1993, 1995), returning to control levels after 2 months (Bailey et al., 2001). This hypodopaminergic state is said to drive drug seeking and relapse, shifting the sensitivity of the mesolimbic DA system overall. In humans imaging studies, AUD are correlated with decreased D2 receptor availability and DA release in the striatum of alcoholics (Martinez et al., 2005; Volkow et al., 2002, 2007). Many studies have also shown a decrease in tonic DA levels in the NAc in rodents withdrawn from chronic ethanol exposure (Rossetti et al., 1992; Diana et al., 1993; Weiss et al., 1996). Contrastingly, protracted abstinence in rodents who have undergone chronic vapor exposure exhibit a hyperdopaminergic state (Hirth et al., 2016). The convergence of hypodopaminergic states during withdrawal and hyperdopaminergia at later time points may, in fact, drive the neuroadaptations seen in late-stage alcoholics. This may be due to a shift in brain stimulation reward, which has been modeled using ICSA in rats (Schulteis et al., 1995) and can be seen after exposure to many drugs of abuse.
Ethanol also stimulates the hypothalamic-pituitary-adrenal (HPA) axis by acting on corticotropin-releasing factor (CRF), increasing levels of this peptide throughout the brain and affecting alcohol and anxiety-related behaviors, specifically during withdrawal states (Allen et al., 2011). Recently, it has been shown that GABAA inhibition of VTA DA neurons is regulated by presynaptic actions of CRF in mice following 3 days of withdrawal from four weeks of CIE (Harlan et al., 2018). The VTA receives CRF from projecting terminals of the BNST, the central amygdala (CeA), and even from local CRF synthesizing neurons in the VTA (Korotkova et al., 2006; Rodaros et al., 2007; Grieder et al., 2014). The CeA, for example, is mostly composed of GABA neurons (Roberto et al., 2012), receives a dense VTA DA projection, and is integral to mediating fear and anxiety response (Ressler, 2010). Together, the CeA and HPA axis constitute a major player in the withdrawal phase, or negative affect state experienced by many alcoholics. CRF enhances GABAergic transmission in the CeA, potentially affecting the processing of aversive stimuli or negative emotional salience (Bajo et al., 2008; Roberto et al., 2010; Cruz et al., 2012; Koob, 2015). Projections between the AMG and VTA may regulate response to ethanol and should be further characterized and considered, especially when assessing function and sensitivity of VTA DA neurons during acute or protracted ethanol withdrawal.
Nicotine and ethanol interactions on VTA DA neurons
Tobacco and alcohol comorbidity is highly associated (World Health Reports 2013, 2014). While nicotine dependence is prevalent amongst AUD patients and is shown to promote alcohol consumption, a history of AUD increases vulnerability to develop nicotine addiction (Bobo & Husten 2000; Grant et al., 2004; Hughes et al., 2000). Multiple socio-economical and genetic factors (True et al., 1999) contribute to the development of this polyaddiction. This is also largely driven by the legality of both of these drugs. In this section we will focus on the synergistic effects of nicotine and ethanol on VTA DA function in reinforcement.
Concomitant acute injection of nicotine and ethanol increases VTA DA neurons firing both in vitro (Clark & Little, 2004) and in vivo (Tolu et al., 2017), followed by a subsequent release of DA in the NAc (Tizabi et al., 2002, 2007). Nicotine and ethanol exhibit an additive effect for “low” doses, which is blunted at higher doses. This suggests an initially synergistic but later opposing effect on common brain circuits. In line with this hypothesis, rats establish ICSA in the pVTA using a sub-reinforcing dose of nicotine and ethanol. However, systemic interactive effects diverge depending on exposure and the addiction stage observed, suggesting distinct cross-sensitization mechanisms.
Several studies have shown that pre-exposure to nicotine increases alcohol consumption (Smith et al., 1999; Tolu et al., 2017) and the acquisition of self-administration (Clark et al., 2001; Doyon et al., 2013). As previously mentioned, nicotine exposure directly alters innate VTA DA neuron functionality due to its similarity to ACh mechanism of action, but later promotes the reinforcing properties of other natural reinforces. Thus, nicotine may prime VTA DA neurons to encode the reinforcing properties of ethanol more strongly. Consequently, nicotine’s priming effect on ethanol consumption is occluded by prior exposure to nAChRs antagonists, involving the nAChRs as a substrate of nicotine-ethanol interactive effects. In line with these studies, nAChRs inhibition or genetic manipulation of nAChRs also alters ethanol consumption (Ericson et al., 1998; Blomqvist et al., 1993) and VTA DA neuron’s activity in response to alcohol (Liu et al., 2013; Tolu et al. 2017).
In addition, nicotine potentiates glutamatergic transmission (Mansvelder & McGehee 2000; Saal et al., 2003) and alters GABA modulation onto VTA DA neurons (Mansvelder et al., 2002), contributing to potential altered ethanol effects. Doyon and collaborators have established that nicotine pre-exposure blunts VTA DA neurons activation in response to alcohol, due to increased inhibitory synaptic transmission (Doyon et al., 2013). A recent longitudinal study showed that nicotine exposure during adolescence increased GABAergic inhibition of DA neurons in the lateral VTA in response to ethanol exposure and led to a long-lasting self-enhancement of alcohol self-administration in rodents (Thomas et al., 2018). Such effects are regulated by the neuroendocrine system, in particular by the stress hormone corticosterone. These results highlight the broad impact of nicotine throughout the brain, particularly since its targets are much more discrete than ethanol’s. Nicotine modulates not only VTA microcircuit function but also crucial afferents form distal brain regions, including circuits encoding aversion (i.e. LHb and mHb circuits) and stress responses (i.e. HPA axis).
The precise impact of ethanol pre-exposure on nicotine reinforcement driven by VTA DA neurons is not yet clear. Ethanol exposure alters nAChRs expression profile in the brain (Booker & Collins, 1997; Tarren et al., 2017). Ethanol also alters the endogenous function of choline acetyltransferase, suggested to lead to ACh hypofunctions (Pelham et al., 1980; Floyd et al., 1997). Ethanol pre-exposure may decrease (Korkosz et al., 2006) nicotine-induced withdrawals, but also exacerbates (Meck, 2007) nicotine effects. Rats selectively bred to develop high sensitivity to ethanol have also high sensitivity to nicotine (de Fiebre et al., 1991). Further investigation should be performed to define how previous exposure to ethanol primes or blunts nicotine’s reinforcing properties. Due to the multi-dimensional impact of ethanol, it is reasonable to hypothesize a large impact of ethanol pre-exposure on VTA DA neuron’s function in encoding nicotine-reinforcing properties; in particular, the negative/aversive effects of high nicotine doses on the VTA microcircuit and other important afferent projections.
Future directions and conclusions
In the current review, the role of VTA DA neurons in nicotine and alcohol reinforcement was discussed. Both nicotine and ethanol cause a transient increase in VTA DA neuron tonic and burst firing, and over time, alter the endogenous functions of the VTA, resulting in an imbalance between excitatory and inhibitory transmission, microcircuit, and overall brain function. Further, other phases of the addiction cycle- intoxication and withdrawal- are equally important in explaining how drug use can lead to pathological states. Social aspects, other drug co-exposure and mood disorders, are not covered in this review but are also well known risk factors. Other factors known to contribute to nicotine and ethanol addiction include: sex hormones (Lenz et al., 2012), immune function (Warden et al., 2016; Roberto et al., 2017), species differences (Juarez & Han, 2016), age of exposure and development (Burke & Miczek, 2014; Smith et al., 2015), glial function (Lacagnina et al., 2017) genetics, (Walters, 2002; Liu et al., 2004; Vink et al., 2005), and most recently, epigenetics (Nestler, 2013). Sex differences are being investigated, as males and females respond differentially to most drugs of abuse (Calipari et al., 2017; Riley et al., 2018). The contribution of sex hormones to the reinforcing, and other stages, of the addiction cycle, are also currently under investigation. In parallel, the immune system shows involvement with drug-taking behaviors and withdrawal. Equal in complexity to the nervous system, the immune system and relevant immunomodulatory molecules are becoming new therapeutic targets for addiction (Blednov et al., 2017; Harris et al., 2017).
The heterogeneity of the VTA increases the complexity and findings of drug reinforcement studies, with differences in responses on all axes (anterior/posterior; medial/lateral). Advent of new technologies, advances in optics, transgenic animal models, and cell targeting, will allow investigation of cell-type and projection-specific function in drug reinforcement. Lastly, in vivo longitudinal monitoring of distinct neural populations, such as VTA DA neurons, using calcium imaging (Cai et al.,. 2016; Chen et al., 2013; Gunaydin et al., 2014) and electrophysiological recordings will provide vital information from the primary drug reinforcing effect to the development of pathological behaviors.
Acknowledgements:
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01 AA022445, MHH). We thank Michal Bajo for his helpful guidance and review of theses topics.
Abbreviations
- 5-HT
5-hydroxytryptamine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ACh
Acetylcholine (ACh)
- AP
Action Potentials
- AHP
After-hyperpolarization
- AUD
Alcohol Use Disorders
- AMG
Amygdala
- a/p
Anterior/posterior axis
- aVTA
Anterior VTA
- BLA
Basolateral amygdala
- BNST
Bed nucleus of the stria terminalis
- BK
Big potassium channels
- Ca2+
Calcium
- CeA
Central amygdala
- CIE
Chronic intermittent access
- CSDS
Chronic social defeat stress
- CRF
Corticotropin-releasing factor
- cAMP
Cyclic adenosine monophosphate
- DHBE
Dihydro-B-Erythroidine hydrobromide
- DA
Dopaminergic or dopamine
- DAT
Dopamine transporter
- DOR
delta-opioid receptor
- DID
Drinking in the dark
- EPSCs
Excitatory postsynaptic currents
- FSCV
Fast-scan cyclic voltammetry
- GABA
γ-aminobutyric acid
- GIRK
G protein-coupled inwardly-rectifying potassium channel
- mHb-IPn
Habenulo-interpeduncular nucleus
- HCN
Hyperpolarization-activated cyclic nucleotide-gated channels
- HPA
Hypothalamic-pituitary-adrenal
- IL
Infralimbic cortex
- IMAO
Inhibitors of monoamine oxidase
- IPn
Interpeduncular nuclei
- IVSA
Intravenous self-administration
- ICSA
Intracranial self-administration
- ISI
Interspike intervals
- Lh
Lateral hypothalamus
- LHb
Lateral habenula
- LDTg
Laterodorsal tegmental nucleus
- LGICs
Ligand-gated ion channels
- LC
Locus coeruleus
- LTP
Long-term potentiation
- Im
M-current
- mHb
Medial habenular
- m/l
Medial/lateral
- MLA
Methylcaconitine
- mPFC
Medial prefrontal cortex
- nAChRs
Nicotinic acetylcholine receptors
- NMDA
N-methyl-D-aspartate
- NAc
Nucleus accumbens
- PBP
Parabrachial pigmented nucleus
- PN
paranigral nucleus
- PPTg
Pedunculopontine Tegmentum
- K +
Potassium
- pVTA
posterior VTA
- PFC
Prefrontal cortex
- PKC
Protein kinase C
- RPE
Reward prediction error
- RMTg
Rostromedial tegmental nucleus
- SK
Small-conductance activated channels
- TM
Transmembrane
- 2BC
Two-bottle choice
- TH
Tyrosine hydroxylase
- VP
Ventral pallidum
- VTA
Ventral tegmental area
- VMAT
Vesicular monoamine transporter
- WT
Wild-type
Footnotes
The authors declare no conflict of interest.
References
- Ables JL, Görlich A, Antolin-Fontes B, Wang C, Lipford SM, Riad MH, Ren J, Hu F, Luo M, Kenny PJ, Heintz N, & Ibañez-Tallon I (2017) Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl. Acad. Sci, 114, 13012–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahao KP, Salinas AG, & Lovinger DM (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Söderpalm B, & Burkhardt JM (2014) Brain region specific modulation of ethanol-induced depression of GABAergic neurons in the brain reward system by the nicotine receptor antagonist mecamylamine. Alcohol, 48, 455–461. [DOI] [PubMed] [Google Scholar]
- Aiken SP, Lampe BJ, Murphy PA, & Brown BS (1995) Reduction of spike frequency adaptation and blockade of M-current in rat CA1 pyramidal neurones by linopirdine (DuP 996), a neurotransmitter release enhancer. Br. J. Pharmacol, 115, 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, & Rogers SW (2009) Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev, 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alifimoff JK, Firestone LL, & Miller KW (1989) Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br. J. Pharmacol, 96, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, & Rivier C (2011) Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic–pituitary–adrenal axis in adult and adolescent rats. Brain. Behav. Immun, 25, S50–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK & Woodward DJ (2005) Restraint Increases Dopaminergic Burst Firing in Awake Rats. Neuropsychopharmacology, 30, 1832–1840. [DOI] [PubMed] [Google Scholar]
- Antolin-Fontes B, Ables JL, Görlich A, & Ibañez-Tallon I (2015) The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology, 96, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SB, Liu Z, McElvain MA, & Brodie MS (2003) Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J. Pharmacol. Exp. Ther, 306, 437–446. [DOI] [PubMed] [Google Scholar]
- Arib O, Rat P, Molimard R, Chait A, Faure P, & de Beaurepaire R (2010) Electrophysiological characterization of harmane-induced activation of mesolimbic dopamine neurons. Eur. J. Pharmacol, 629, 47–52. [DOI] [PubMed] [Google Scholar]
- Aryal P, Dvir H, Choe S, & Slesinger PA (2009) A discrete alcohol pocket involved in GIRK channel activation. Nat. Neurosci, 12, 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, Faure P, Pons S, Robledo P, Deltheil T, David DJ, Gardier AM, Maldonado R, Granon S, Changeux J-P, & Maskos U (2008) Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc. Natl. Acad. Sci. U. S. A, 105, 15991–15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avegno EM, Salling MC, Borgkvist A, Mrejeru A, Whitebirch AC, Margolis EB, Sulzer D, & Harrison NL (2016) Voluntary adolescent drinking enhances excitation by low levels of alcohol in a subset of dopaminergic neurons in the ventral tegmental area. Neuropharmacology, 110, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y, & Leslie FM (2007) Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience, 144, 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, & Leslie FM (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol, 444, 260–274. [DOI] [PubMed] [Google Scholar]
- Babayan BM, Uchida N, Gershman SJ (2018) Belief state representation in the dopamine system. Nat Commun, 9, 1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, & Little HJ (2001) Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology, 41, 989–999. [DOI] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, & Roberto M (2008) Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc. Natl. Acad. Sci, 105, 8410–8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LK, Mao D, Chi H, Govind AP, Vallejo YF, Iacoviello M, Herrera S, Cortright JJ, Green WN, Mcgehee DS, & Vezina P (2013) Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitises locomotor responding to the drug. Eur. J. Neurosci, 37, 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ & Roth RH (1983) Pharmacology of mesocortical dopamine neurons. Pharmacol. Rev, 35, 53–68. [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, & Tronche F (2013) Chronic Stress Triggers Social Aversion via Glucocorticoid Receptor in Dopaminoceptive Neurons. Science, 339, 332–335. [DOI] [PubMed] [Google Scholar]
- Baum SS, Hill R, & Rommelspacher H (1995) Norharman-induced changes of extracellular concentrations of dopamine in the nucleus accumbens of rats. Life Sci, 56, 1715–1720. [DOI] [PubMed] [Google Scholar]
- Baum SS, Hill R, & Rommelspacher H (1996) Harman-induced changes of extracellular concentrations of neurotransmitters in the nucleus accumbens of rats. Eur. J. Pharmacol, 314, 75–82. [DOI] [PubMed] [Google Scholar]
- Becker HC (2008) Alcohol dependence, withdrawal, and relapse. Alcohol Res. Health, 31, 348–361. [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, & Williams JT (2004) Vesicular Dopamine Release Elicits an Inhibitory Postsynaptic Current in Midbrain Dopamine Neurons. Neuron, 42, 939–946. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, & Luo L (2015) Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell, 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, & Young ER (1993) Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl)., 112, 503–510. [DOI] [PubMed] [Google Scholar]
- Berger B, Di Porzio U, Daguet MC, Gay M, Vigny A, Glowinski J, & Prochiantz A (1982) Long-term development of mesencephalic dopaminergic neurons of mouse embryos in dissociated primary cultures: morphological and histochemical characteristics. Neuroscience, 7, 193–205. [DOI] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, & Morikawa H (2011) Previous Ethanol Experience Enhances Synaptic Plasticity of NMDA Receptors in the Ventral Tegmental Area. J. Neurosci, 31, 5205–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Granon S, Mameli-Engvall M, Cloëz-Tayarani I, Maubourguet N, Cormier A, Cazala P, David V, Changeux J-P, & Faure P (2007) Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc. Natl. Acad. Sci, 104, 8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger JC, Davies AG, (2014). The role of the BK channels in ethanol response behaviors: evidence from model organism and human studies. Front. Physiol 9 5:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PAF, Mayo K, Nurnberger J, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, & Goate AM (2008) Variants in Nicotinic Receptors and Risk for Nicotine Dependence. Am. J. Psychiatry, 165, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, & Winn P (1996) Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J. Neurosci, 16, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Black M, Chernis J, Da Costa A, Mayfield J, & Harris RA (2017) Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol. Clin. Exp. Res, 41, 516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. (2001) Potassium channels as targets for ethanol: Studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther 298(2): 521–530. [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, & Söderpalm B (1993) The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur. J. Pharmacol, 249, 207–213. [DOI] [PubMed] [Google Scholar]
- Bobo JK & Husten C (2000) Sociocultural influences on smoking and drinking. Alcohol Res. Health, 24, 225–232. [PMC free article] [PubMed] [Google Scholar]
- Booker TK & Collins a C. (1997) Long-term ethanol treatment elicits changes in nicotinic receptor binding in only a few brain regions. Alcohol, 14, 131–140. [DOI] [PubMed] [Google Scholar]
- Breslau N & Johnson EO (2000) Predicting smoking cessation and major depression in nicotine-dependent smokers. Am. J. Public Health, 90, 1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, & Ungless MA (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci, 106, 4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, & Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res, 508, 65–69. [DOI] [PubMed] [Google Scholar]
- Brodie MS & Appel SB (1998) The Effects of Ethanol on Dopaminergic Neurons of the Ventral Tegmental Area Studied with Intracellular Recording in Brain Slices. Alcohol. Clin. Exp. Res, 22, 236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, & Appel SB (1999a) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol. Clin. Exp. Res, 23, 1848–1852. [PubMed] [Google Scholar]
- Brodie MS, McElavin MA, Bunney EB, Appel SB (1999b) Pharmacological reduction of small conductance calcium-activated potassium current (SK) potentiates the excitatory effect of ethanol on ventral tegmental area dopamine neurons. J. Pharmacol Exp. Ther, 1:325–33 [PubMed] [Google Scholar]
- Brodie MS (2002) Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol. Clin. Exp. Res, 26, 1024–1030. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Scholz A, Weiger TM, & Dopico AM (2007) Ethanol Interactions With Calcium-Dependent Potassium Channels. Alcohol. Clin. Exp. Res, 31, 1625–1632. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, & Hikosaka O (2010) Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron, 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA & Adams PR (1980) Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone [18]. Nature. [DOI] [PubMed] [Google Scholar]
- Brown DA & Passmore GM (2009) Neural KCNQ (Kv7) channels. Br. J. Pharmacol,.156, 1185–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Palacorolla H, Brady D, Riegger K, Elmer GI, & Shepard PD (2017) Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus. J. Neurosci, 37, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Herman MA, Hsu KL, Natividad LA, Irimia C, Polis IY, Pugh H, Chang JW, Niphakis MJ, Cravatt BF, Roberto M, Parsons LH (2016) Diacylglycerol lipase disinhibits VTA dopamine neurons during chronic nicotine exposure. Proc. Natl. Acad. Sci, 113, 1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B & Bertrand D (2001) Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J. Neurosci, 21, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR & Miczek KA (2014) Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl)., 231, 1557–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JM & Adermark L (2014) Locus of onset and subpopulation specificity of in vivo ethanol effect in the reciprocal ventral tegmental area-nucleus accumbens circuit. Neurochem. Int, 76, 122–130. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, & Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl)., 163, 230–237. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, Flores SE, Kim I, Sano Y, Zhou M, Baumgaertel K, Lavi A, Kamata M, Tuszynski M, Mayford M, Golshani P, & Silva AJ (2016) A shared neural ensemble links distinct contextual memories encoded close in time. Nature, 534, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, & Cador M (2012) Modeling nicotine addiction in rats. Methods Mol. Biol, 243–256 [DOI] [PubMed] [Google Scholar]
- Caillé S, Guillem K, Cador M, Manzoni O, & Georges F (2009) Voluntary Nicotine Consumption Triggers In Vivo Potentiation of Cortical Excitatory Drives to Midbrain Dopaminergic Neurons. J. Neurosci, 29, 10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han M-H, & Nestler EJ (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun, 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, & Han M-H (2010) Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci, 30, 16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, & Wong C (2000) Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci, 20, 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, & Changeux J-P (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci, 23, 7820–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci, 11, 389–401. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai H-C, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, & Han M-H (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature, 493, 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, Aragona BJ, Phillips PEM, & Wightman RM (2007) Phasic Dopamine Release Evoked by Abused Substances Requires Cannabinoid Receptor Activation. J. Neurosci, 27, 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, & Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature, 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Charléty PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, & Chouvet G (1993) Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur. J. Neurosci, 5, 137–144. [DOI] [PubMed] [Google Scholar]
- Chinta SJ & Andersen JK (2005) Dopaminergic neurons. Int. J. Biochem. Cell Biol, 37, 942–946. [DOI] [PubMed] [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, & Little HJ (2001) Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology, 41, 108–117. [DOI] [PubMed] [Google Scholar]
- Clark A & Little HJ (2004) Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend, 75, 199–206. [DOI] [PubMed] [Google Scholar]
- Clements JR & Grant S (1990) Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci. Lett, 120, 70–73. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, & Uchida N (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature, 482, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin A & Clarke PBS (2017) Reinforcement enhancement by nicotine in adult rats: behavioral selectivity and relation to mode of delivery and blood nicotine levels. Psychopharmacology (Berl)., 1–10. [DOI] [PubMed] [Google Scholar]
- Corrigall WA & Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl)., 99, 473–478. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, & Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res, 653, 278–284. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, & Belknap JK (2010) The complexity of alcohol drinking: Studies in rodent genetic models. Behav. Genet, 40, 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, & Koob GF (2011) Preclinical studies of alcohol binge drinking. Ann. N. Y. Acad. Sci, 1216, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn M-L, Stoffel M, Slesinger PA, & Lüscher C (2004) Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat. Neurosci, 7, 153–159. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, & Roberto M (2012) Nociceptin/Orphanin FQ Blockade of Corticotropin-Releasing Factor-Induced Gamma-Aminobutyric Acid Release in Central Amygdala Is Enhanced After Chronic Ethanol Exposure. Biol. Psychiatry, 71, 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C & Koob GF (2017) Titrating Tipsy Targets: The Neurobiology of Low-Dose Alcohol. Trends Pharmacol. Sci, 38, 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, & Prather LK (1992) Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl)., 107, 385–393. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, & Cohen JD (2008) BOLD Responses Reflecting Dopaminergic Signals in the Human Ventral Tegmental Area. Science. 319, 1264–1267. [DOI] [PubMed] [Google Scholar]
- Davies M (2003) The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci, 28, 263–274. [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, Romm E, Collins JT, Draski LJ, Deitrich RA, & Collins AC (1991) Responses to Cholinergic Agonists of Rats Selectively Bred for Differential Sensitivity to Ethanol. Alcohol. Clin. Exp. Res, 15, 270–276. [DOI] [PubMed] [Google Scholar]
- del Burgo LS, Cortes R, Mengod G, Zarate J, Echevarria E, & Salles J (2008) Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J. Comp. Neurol, 510, 581–606. [DOI] [PubMed] [Google Scholar]
- Deng C, Li KY, Zhou C, & Ye JH (2009) Ethanol enhances glutamate transmission by retrograde dopamine signaling in a postsynaptic neuron/synaptic bouton preparation from the ventral tegmental area. Neuropsychopharmacology, 34, 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G & Imperato A (1985) Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur. J. Pharmacol, 115, 131–132. [DOI] [PubMed] [Google Scholar]
- Di Chiara G & Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A, 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, & Rossetti ZL (1993) Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc. Natl. Acad. Sci. U. S. A, 90, 7966–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni AL, & Gessa GL (1995) Ethanol withdrawal does not induce a reduction in the number of spontaneously active dopaminergic neurons in the mesolimbic system. Brain Res, 682, 29–34. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Mihic SJ, Liu Y, Deitrich RA, & Harris RA (1996) Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br. J. Pharmacol, 118, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z-M, Ingraham CM, Rodd ZA, & McBride WJ (2015) The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain cortico-limbic systems. Addict. Biol, 20, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, & McBride WJ (2009) Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol. Clin. Exp. Res, 33, 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I, Björklund A, Lindvall O, & Passingham RE (1978) Converging projections from the mediodorsal thalamic nucleus and mesencephalic dopaminergic neurons to the neocortex in three species. J. Comp. Neurol, 180, 59–71. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, & Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl)., 169, 68–76. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Bukiya AN, & Martin GE (2014) Ethanol modulation of mammalian BK channels in excitable tissues: molecular targets and their possible contribution to alcohol-induced altered behavior. Front. Physiol, 5, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, & Dani JA (2013) Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron, 79, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler JC, Jenkins A, Cao YJ, Roizen JD, & Houamed KM (2000) Patch-clamp analysis of anesthetic interactions with recombinant SK2 subtype neuronal calcium-activated potassium channels. Anesth. Analg, 90, 727–732. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, & Lester HA (2008) In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha6* nicotinic acetylcholine receptors. Neuron, 60, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddine R, Valverde S, Tolu S, Dautan D, Hay A, Morel C, Cui Y, Lambolez B, Venance L, Marti F, & Faure P (2015) A concurrent excitation and inhibition of dopaminergic subpopulations in response to nicotine. Sci. Rep, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein SJ, Schaad O, Henry E, Bertrand D, & Changeux JP (1996) A kinetic mechanism for nicotinic acetylcholine receptors based on multiple allosteric transitions. Biol. Cybern, 75, 361–379. [DOI] [PubMed] [Google Scholar]
- Ergene E & Schoener EP (1993) Effects of harmane (1-methyl-beta-carboline) on neurons in the nucleus accumbens of the rat. Pharmacol. Biochem. Behav, 44, 951–957. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, & Söderpalm B (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur. J. Pharmacol, 358, 189–196. [DOI] [PubMed] [Google Scholar]
- Everitt BJ & Robbins TW (2005) Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci, 8, 1481–1489. [DOI] [PubMed] [Google Scholar]
- Exley R & Cragg (2007) Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br. J. Pharmacol, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux J-P, Maskos U, Cragg SJ, & Faure P (2011) Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc. Natl. Acad. Sci. U. S. A, 108, 7577–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber EL & Sah P (2007) Functions of SK channels in central neurons Clin. Exp. Pharmacol. Physiol, 34, 1077–1083. [DOI] [PubMed] [Google Scholar]
- Fallon JH (1981) Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J. Neurosci, 1, 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MJ, Latimer MP, & Winn P (2012) Nicotine self-administered directly into the VTA by rats is weakly reinforcing but has strong reinforcement enhancing properties. Psychopharmacology (Berl)., 220, 43–54. [DOI] [PubMed] [Google Scholar]
- Fasoli F, Moretti M, Zoli M, Pistillo F, Crespi A, Clementi F, Mc Clure-Begley T, Marks MJ, & Gotti C (2016) In vivo chronic nicotine exposure differentially and reversibly affects upregulation and stoichiometry of α4β2 nicotinic receptors in cortex and thalamus. Neuropharmacology, 108, 324–331. [DOI] [PubMed] [Google Scholar]
- Faure P, Tolu S, Valverde S, & Naudé J (2014) Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience, 282, 86–100. [DOI] [PubMed] [Google Scholar]
- Federici M, Nisticò R, Giustizieri M, Bernardi G, & Mercuri NB (2009) Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur. J. Neurosci, 29, 1369–1377. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novère N, Picciotto MR, Changeux JP, & Zoli M (2002) Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur. J. Neurosci, 15, 1810–1818. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, & Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci, 6, 968–973. [DOI] [PubMed] [Google Scholar]
- Floyd EA, Young-Seigler AC, Ford BD, Reasor JD, Moore EL, Townsel JG, & Rucker HK (1997) Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol, 14, 93–98. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, & Diana M (2004) Acetaldehyde Increases Dopaminergic Neuronal Activity in the VTA. Neuropsychopharmacology, 29, 530–536. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, & Williams JT (2006) Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci, 26, 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD & Kenny PJ (2011) Intravenous nicotine self-administration and cue-induced reinstatement in mice: Effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology, 61, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, & Kenny PJ (2011) Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature, 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Ślimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine J-F, Tsetlin V, Maskos U, & Ibañez-Tallon I (2011) Aversion to Nicotine Is Regulated by the Balanced Activity of β4 and α5 Nicotinic Receptor Subunits in the Medial Habenula. Neuron, 70, 522–535. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, Chaudhury D, Zhang S, Hawkins A, Dietz DM, Murrough JW, Ribadeneira M, Wong EH, Neve RL, & Han MH (2016) KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun, 7, 11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, & Han MH (2014) Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 344, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Mei Q, Zuo W, Li J, Gregor D, Bekker A, & Ye J (2017) Low-dose ethanol excites lateral habenula neurons projecting to VTA, RMTg, and raphe. Int. J. Physiol. Pathophysiol. Pharmacol, 9, 217–230. [PMC free article] [PubMed] [Google Scholar]
- Funahashi M, Mitoh Y, & Matsuo R (2004) Nicotinic modulation of area postrema neuronal excitability in rat brain slices. Brain Res, 1017, 227–233. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Lee R-S, Criado JR, Henriksen SJ, & Steffensen SC (1999) Adaptive Responses of γ-Aminobutyric Acid Neurons in the Ventral Tegmental Area to Chronic Ethanol. J. Pharmacol. Exp. Ther, 291. [PubMed] [Google Scholar]
- Galzi JL & Changeux JP (1995) Neuronal nicotinic receptors: molecular organization and regulations. Neuropharmacology, 34, 563–582. [DOI] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, & Wu J (2010) Mechanisms Involved in Systemic Nicotine-Induced Glutamatergic Synaptic Plasticity on Dopamine Neurons in the Ventral Tegmental Area. J. Neurosci, 30, 13814–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, & Koob GF (2011) Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl)., 213, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC & Manaye KF (1993) Midbrain dopaminergic neurons (nuclei A8, A9, and A10): Three-dimensional reconstruction in the rat. J. Comp. Neurol, 331, 297–309. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, & Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res, 348, 201–203. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, & Goldberg DM (1981) Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science, 214, 573–575. [DOI] [PubMed] [Google Scholar]
- Gonzales RA & Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J. Neurosci, 18, 10663–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, & Zoli M (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol, 78, 703–711. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, & Zoli M (2010) Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J. Neurosci, 30, 5311–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA & Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci, 4, 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA & Onn SP (1989) Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J. Neurosci, 9, 3463–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, & Gotti C (2009) Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci, 29, 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Wageman CR, Patzlaff NE, & Marks MJ (2012) Low concentrations of nicotine differentially desensitize nicotinic acetylcholine receptors that include α5 or α6 subunits and that mediate synaptosomal neurotransmitter release. Neuropharmacology, 62, 1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, & Dawson DA (2004) Nicotine Dependence and Psychiatric Disorders in the United States: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry, 61, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, Chwalek M, Maal-Bared G, Freiling J, Schlosburg JE, Clarke L, Crawford E, Koebel P, Repunte-Canonigo V, Sanna PP, Tapper AR, Roberto M, Kieffer BL, Sawchenko PE, Koob GF, van der Kooy D, George O (2014) VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci, 17, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Xiao C, Krnjevic K, Xie G, Zuo W, & Ye J-H (2012) GABAergic Actions Mediate Opposite Ethanol Effects on Dopaminergic Neurons in the Anterior and Posterior Ventral Tegmental Area. J. Pharmacol. Exp. Ther, 341, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y & Ye J-H (2010) Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology, 35, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, & Deisseroth K (2014) Natural neural projection dynamics underlying social behavior. Cell, 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, & Taha SA (2014) Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One, 9, e92701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Waroux O, Seutin V, Jentsch TJ, Aznar S, & Mikkelsen JD (2008) Kv7 channels: Interaction with dopaminergic and serotonergic neurotransmission in the CNS. In Journal of Physiology. Wiley-Blackwell, pp. 1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan B, Becker H, Woodward J, & Riegel A (2018) Opposing actions of CRF-R1 and CB1 receptors on VTA-GABAergic plasticity following chronic exposure to ethanol. Neuropsychopharmacology, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, Viñals X, Herrera-Solís A, Flores A, Morel C, Tolu S, Faure P, Maldonado R, Maskos U, & Robledo P (2016) Role of β4* Nicotinic Acetylcholine Receptors in the Habenulo-Interpeduncular Pathway in Nicotine Reinforcement in Mice. Neuropsychopharmacology, 41, 1790–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC & Constanti A (1995) Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J. Neurophysiol, 74, 2366–2378. [DOI] [PubMed] [Google Scholar]
- Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM, de Guglielmo G, Lasek AW, Logrip ML, Vendruscolo LF, Roberts AJ, Roberts E, George O, Mayfield J, Billiar TR, Hackam DJ, Mayfield RD, Koob GF, Roberto M, & Homanics GE (2017) Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J. Neurosci, 37, 1139–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, & Mihic SJ (2008) Ethanol’s molecular targets. Sci. Signal,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, King SL, Gotti C, Marks MJ, & Picciotto MR (2010) Cortico-Thalamic Connectivity is Vulnerable to Nicotine Exposure During Early Postnatal Development through a4/b2/a5 Nicotinic Acetylcholine Receptors. Neuropsychopharmacology, 35, 2324–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Sidhu H, Stouffer DG, Kreifeldt M, Le D, Cates-Gatto C, Munoz MB, Roberts AJ, Parsons LH, Roberto M, Wickman K, Slesinger PA, & Contet C (2015) GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol. Proc. Natl. Acad. Sci. U. S. A, 112, 7091–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Broccoli L, Vengeliene V, Roßmanith M, Perreau-Lenz S, Köhr G, Sommer WH, Spanagel R, & Hansson AC (2016) Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc. Natl. Acad. Sci, 113, 3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Xia Y, Margolis EB, & Fields HL (2013) Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J. Neurosci, 33, 6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, & Samson HH (1993) Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol. Clin. Exp. Res, 17, 370–375. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, & Haraguchi M (1992) Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacol. Biochem. Behav, 43, 249–254. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, & Hikosaka O (2011) Negative Reward Signals from the Lateral Habenula to Dopamine Neurons Are Mediated by Rostromedial Tegmental Nucleus in Primates. J. Neurosci, 31, 11457–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, & Bonci A (2007) Withdrawal From Intermittent Ethanol Exposure Increases Probability of Burst Firing in VTA Neurons In Vitro. J. Neurophysiol, 98, 2297–2310. [DOI] [PubMed] [Google Scholar]
- Hsu Y-WA, Tempest L, Quina LA, Wei AD, Zeng H, & Turner EE (2013) Medial Habenula Output Circuit Mediated by 5 Nicotinic Receptor-Expressing GABAergic Neurons in the Interpeduncular Nucleus. J. Neurosci, 33, 18022–18035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Rose GL, & Callas PW (2000) Nicotine is more reinforcing in smokers with a past history of alcoholism than in smokers without this history. Alcohol. Clin. Exp. Res, 24, 1633–1638. [PubMed] [Google Scholar]
- Hyland BI, Reynolds JNJ, Hay J, Perk CG, & Miller R (2002) Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience, 114, 475–492. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, McBride WJ, Murphy JM, Lumeng L, & Li TK (1997) 6-OHDA-lesions of the nucleus accumbens disrupt the acquisition but not the maintenance of ethanol consumption in the alcohol-preferring P line of rats. Alcohol. Clin. Exp. Res, 21, 1042–1046. [PubMed] [Google Scholar]
- Ikemoto S & Wise RA (2002) Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J. Neurosci, 22, 9895–9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Mulas A, & Di Chiara G (1986) Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol, 132, 337–338. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F, Ploska A, & Agid Y (1981) Microtopography of tyrosine hydroxylase, glutamic acid decarboxylase, and choline acetyltransferase in the substantia nigra and ventral tegmental area of control and Parkinsonian brains. J. Neurochem, 37, 1218–1227. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, & Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron, 61, 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H & Shepard PD (2007) Lateral Habenula Stimulation Inhibits Rat Midbrain Dopamine Neurons through a GABAA Receptor-Mediated Mechanism. J. Neurosci, 27, 6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, & North RA (1992) Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science, 258, 665–667. [DOI] [PubMed] [Google Scholar]
- Jones S & Kauer JA (1999) Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J. Neurosci, 19, 9780–9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B & Han MH (2016) Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacology,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Morel C, Ku SM, Liu Y, Zhang H, Montgomery S, Gregoire H, Ribeiro E, Crumiller M, Roman-Ortiz C, Walsh JJ, Jackson K, Croote DE, Zhu Y, Zhang S, Vendruscolo LF, Edward S, Roberts A, Hodes GE, Lu Y, Calipari ES, Chaudhury D, Friedman AK, & Han MH (2017) Midbrain circuit regulation of individual alcohol drinking behaviors in mice. Nat. Commun, 8, 2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale D, Stautz K, & Cooper A (2018) Impulsivity related personality traits and cigarette smoking in adults: A meta-analysis using the UPPS-P model of impulsivity and reward sensitivity. Drug Alcohol Depend, 185, 149–167. [DOI] [PubMed] [Google Scholar]
- Kallupi M & George O (2017) Nicotine vapor method to induce nicotine dependence in rodents. Curr. Protoc. Neurosci, 2017, 8.41.1–8.41.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Girard D, Maitre M, Leste-Lasserre T, & Georges F (2017) Species-specific diversity in the anatomical and physiological organisation of the BNST-VTA pathway. Eur. J. Neurosci, 45, 1230–1240. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier M-J, & Barrot M (2009) Afferents to the GABAergic tail of the ventral tegmental area in the rat. J. Comp. Neurol, 513, 597–621. [DOI] [PubMed] [Google Scholar]
- Kenny PJ & Markou A (2006) Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology, 31, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, & Scroggs R (1999) Afferent modulation of dopamine neuron firing patterns. Curr. Opin. Neurobiol, 9, 690–697. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, & Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci, 21, 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, & Kumanishi T (1999) Ethanol opens G-protein-activated inwardly rectifying K+channels. Nat. Neurosci, 2, 1091–1097. [DOI] [PubMed] [Google Scholar]
- Koob GF (2014) Frameworks of Alcohol Addiction: Alcohol Addiction as a Reward Deficit and Stress Surfeit Disorder. In Neurobiology of Alcohol Dependence. Frontiers Media SA, pp. 3–28. [Google Scholar]
- Koob GF (2015) The dark side of emotion: The addiction perspective. Eur. J. Pharmacol, 753, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Rocio M, Carrera A, Gold LH, Heyser CJ, Maldonado-Irizarry C, Markou A, Parsons LH, Roberts AJ, Schulteis G, Stinus L, Walker JR, Weissenborn R, & Weiss F (1998) Substance dependence as a compulsive behavior. J. Psychopharmacol, 12, 39–48. [DOI] [PubMed] [Google Scholar]
- Koob GF & Volkow ND (2010) Neurocircuitry of Addiction. Neuropsychopharmacology, 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF & Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry, 8, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkosz A, Zatorski P, Taracha E, Plaznik A, Kostowski W, & Bienkowski P (2006) Ethanol blocks nicotine-induced seizures in mice: comparison with midazolam and baclofen. Alcohol, 40, 151–157. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, & Haas HL (2006) Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci, 23, 2677–2685. [DOI] [PubMed] [Google Scholar]
- Kotecki L, Hearing M, McCall NM, Marron Fernandez de Velasco E, Pravetoni M, Arora D, Victoria NC, Munoz MB, Xia Z, Slesinger PA, Weaver CD, & Wickman K (2015) GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner. J. Neurosci, 35, 7131–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, & Appel SB (2007) Ethanol Inhibition of M-Current and Ethanol-Induced Direct Excitation of Ventral Tegmental Area Dopamine Neurons. J. Neurophysiol, 97, 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, & Lindstrom J (2010) Acetylcholine Receptor (AChR) 5 Subunit Variant Associated with Risk for Nicotine Dependence and Lung Cancer Reduces (4 2)2 5 AChR Function. Mol. Pharmacol, 79, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, & Lüscher C (2007) RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci, 10, 1559–1568. [DOI] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, & Bilbo SD (2017) Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology, 42, 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive AL, Munoz MB, Bellone C, Slesinger PA, Luscher C, & Tan KR (2014) Firing Modes of Dopamine Neurons Drive Bidirectional GIRK Channel Plasticity. J. Neurosci, 34, 5107–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, & Roeper J (2008) Unique Properties of Mesoprefrontal Neurons within a Dual Mesocorticolimbic Dopamine System. Neuron, 57, 760–773. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, & Malenka RC (2014) Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology, 76, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, & Malenka RC (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature, 491, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B & Goldberg SR (2009) Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb. Exp. Pharmacol, 335–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère N, Corringer P-J, & Changeux J-P (2002) The diversity of subunit composition in nAChRs: Evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol, 53, 447–456. [DOI] [PubMed] [Google Scholar]
- Le Novère N & Changeux JP (1999) The Ligand Gated Ion Channel Database. Nucleic Acids Res, 27, 340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère N, Zoli M, & Changeux JP (1996) Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur. J. Neurosci, 8, 2428–2439. [DOI] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, & Pistis M (2010) Effects of Drugs of Abuse on Putative Rostromedial Tegmental Neurons, Inhibitory Afferents to Midbrain Dopamine Cells. Neuropsychopharmacology, 36, 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, & Pistis M (2012) Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology, 37, 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L & Kelly PH (2007) A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci. Biobehav. Rev, 31, 658–672. [DOI] [PubMed] [Google Scholar]
- Lee US & Cui J (2010) BK channel activation: structural and functional insights. Trends Neurosci, 33, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, & O’Malley SS (2010) Ethanol consumption: How should we measure it? Achieving consilience between human and animal phenotypes. Addict. Biol, 15, 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz B, Müller CP, Stoessel C, Sperling W, Biermann T, Hillemacher T, Bleich S, & Kornhuber J (2012) Sex hormone activity in alcohol addiction: Integrating organizational and activational effects. Prog. Neurobiol, 96, 136–163. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, & Phillips TJ (2001) Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berl)., 155, 91–99. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, & Rose JE (2009) Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav. Brain Res, 196, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, & Harris RA (1999) G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat. Neurosci, 2, 1084–1090. [DOI] [PubMed] [Google Scholar]
- Li W, Doyon WM, & Dani JA (2011) Acute in vivo nicotine administration enhances synchrony among dopamine neurons. In Biochemical Pharmacology. pp. 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang H-L, & Morales M (2013) Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct. Funct, 218, 1159–1176. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brené S, & Franck J (2001) The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav. Brain Res, 120, 137–146. [DOI] [PubMed] [Google Scholar]
- Liss B & Roeper J (2008) Individual dopamine midbrain neurons: Functional diversity and flexibility in health and disease. Brain Res. Rev, 58, 314–321. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Dodd PR, Randall PK, Harris RA, & Mayfield RD (2004) Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J. Neurochem, 90, 1050–1058. [DOI] [PubMed] [Google Scholar]
- Liu L, Hendrickson LM, Guildford MJ, Zhao-Shea R, Gardner PD, & Tapper AR (2013) Nicotinic Acetylcholine Receptors Containing the α4 Subunit Modulate Alcohol Reward. Biol. Psychiatry, 73, 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ & Grace AA (2006) The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc. Natl. Acad. Sci, 103, 5167–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, & Ron D (2009) Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol, 43, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM & Roberto M (2010) Synaptic Effects Induced by Alcohol. In Current Topics in Behavioral Neurosciences. pp. 31–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow KH, Bradley KD, Allison DW, Taylor SR, Yorgason JT, Hansen DM, Walton CH, Sudweeks SN, & Steffensen SC (2009) Acute and Chronic Ethanol Modulate Dopamine D2-Subtype Receptor Responses in Ventral Tegmental Area GABA Neurons. Alcohol. Clin. Exp. Res, 33, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, & Wonnacott S (1999) International Union of Pharmacology.Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol. Rev, 51, 397–401. [PubMed] [Google Scholar]
- Lüscher C & Slesinger PA (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci, 11, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux J-P, & Faure P (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron, 50, 911–921. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, & McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron, 33, 905–919. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD & McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron, 27, 349–357. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, & Fields HL (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol, 577, 907–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, & Mitchell JM (2008a) Opioid Receptor Expression in the Ventral Tegmental Area Protects Against Elevated Alcohol Consumption. J. Neurosci, 28, 12672–12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, & Fields HL (2008b) Midbrain Dopamine Neurons: Projection Target Determines Action Potential Duration and Dopamine D2 Receptor Inhibition. J. Neurosci, 28, 8908–8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, & Fields HL (2012) Identification of Rat Ventral Tegmental Area GABAergic Neurons. PLoS One, 7, e42365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti F, Arib O, Morel C, Dufresne V, Maskos U, Corringer P-J, de Beaurepaire R, & Faure P (2011) Smoke Extracts and Nicotine, but not Tobacco Extracts, Potentiate Firing and Burst Activity of Ventral Tegmental Area Dopaminergic Neurons in Mice. Neuropsychopharmacology, 36, 2244–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang D-R, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, & Abi-Dargham A (2005) Alcohol Dependence Is Associated with Blunted Dopamine Transmission in the Ventral Striatum. Biol. Psychiatry, 58, 779–786. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Machu TK, & Harris RA (1996) Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br. J. Pharmacol, 119, 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux J-P, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani I, Bemelmans A-P, Mallet L, Gardier AM, David V, Faure P, Granon S, & Changeux J-P (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature, 436, 103–107. [DOI] [PubMed] [Google Scholar]
- Mathew AR, Hogarth L, Leventhal AM, Cook JW, & Hitsman B (2017) Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction, 112, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, Mccallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, & Zirger JM (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacol, 190, 269–319. [DOI] [PubMed] [Google Scholar]
- Maubourguet N, Lesne A, Changeux J, Maskos U, & Faure P (2008) Behavioral Sequence Analysis Reveals a Novel Role for ss2* Nicotinic Receptors in Exploration. PLoS Comput. Biol, 4, e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, & Brodie MS (2008) Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J. Neurophysiol, 100, 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Rinker JA, Cannady R, Fulmer DB, Jones SR, Hoffman M, & Mulholland PJ (2018) Identification and validation of midbrain Kcnq4 regulation of heavy alcohol consumption in rodents. Neuropharmacology, 138, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH (2007) Acute ethanol potentiates the clock-speed enhancing effects of nicotine on timing and temporal memory. Alcohol. Clin. Exp. Res, 31, 2106–2113. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stählin O, Heilig M, Harper C, Drescher KU, Spanagel R, & Sommer WH (2013) Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J. Neurosci, 33, 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, & Bonci A (2002) Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci, 22, 2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spiga S, & Diana M (2005) The Dopamine Hypothesis of Drug Addiction: Hypodopaminergic State. Int. Rev. Neurobiol, 63, 101–154. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Winn P, & Bolam JP (2008) Cholinergic modulation of midbrain dopaminergic systems. Brain Res. Rev, 58, 265–271. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Fote GM, Blakeman S, Cahuzac ELM, Newbold SA, & Picciotto MR (2016) Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology, 41, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Mose TN, Blakeman S, & Picciotto MR (2017) Hippocampal α7 nicotinic ACh receptors contribute to modulation of depression-like behaviour in C57BL/6J mice. Br. J. Pharmacol, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, & Unwin N (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature, 423, 949–955. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, & Changeux JP (1965) On the nature of allosteric transitions: A plausible model. J. Mol. Biol, 12, 88–118. [DOI] [PubMed] [Google Scholar]
- Morales M & Margolis EB (2017) Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci, 18, 73–85. [DOI] [PubMed] [Google Scholar]
- Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, De Biasi M, Lathrop M, Fratta W, Maskos U, & Faure P (2014) Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry, 19, 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C, Fernandez SP, Pantouli F, Meye FJ, Marti F, Tolu S, Parnaudeau S, Marie H, Tronche F, Maskos U, Moretti M, Gotti C, Han M-H, Bailey A, Mameli M, Barik J, & Faure P (2017) Nicotinic receptors mediate stress-nicotine detrimental interplay via dopamine cells’ activity. Mol. Psychiatry, 1–9. [DOI] [PubMed] [Google Scholar]
- Moretti MM, Vailati SS, Zoli MM, Lippi GG, Riganti LL, Longhi RR, Viegi AA, Clementi FF, & Gotti CC (2004) Nicotinic acetylcholine receptor subtypes expression during rat retina development and their regulation by visual experience. Mol. Pharmacol, 66, 85–96. [DOI] [PubMed] [Google Scholar]
- Morikawa H & Morrisett RA (2010) Ethanol Action on Dopaminergic Neurons in the Ventral Tegmental Area. In International Review of Neurobiology. 235–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrejeru A, Martí-Prats L, Avegno EM, Harrison NL, & Sulzer D (2015) A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience, 290, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Hopf FW, Bukiya AN, Martin GE, Liu J, Dopico AM, Bonci A, Treistman SN, & Chandler LJ (2009) Sizing up ethanol-induced plasticity: The role of small and large conductance calcium-activated potassium channels. Alcohol. Clin. Exp. Res, 33, 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, & Ungless MA (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience, 152, 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, & Lester HA (2007) Chronic nicotine cell specifically upregulates functional alpha4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J. Neurosci, 27, 8202–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudé J, Dongelmans M, & Faure P (2015) Nicotinic alteration of decision-making. Neuropharmacology, 96, 244–254. [DOI] [PubMed] [Google Scholar]
- Naudé J, Tolu S, Dongelmans M, Torquet N, Valverde S, Rodriguez G, Pons S, Maskos U, Mourot A, Marti F, & Faure P (2016) Nicotinic receptors in the ventral tegmental area promote uncertainty-seeking. Nat. Neurosci, 19, 471–478. [DOI] [PubMed] [Google Scholar]
- Nestby P, Schoffelmeer AN, Homberg JR, Wardeh G, De Vries TJ, Mulder AH, & Vanderschuren LJ (1999) Bremazocine reduces unrestricted free-choice ethanol self-administration in rats without affecting sucrose preference. Psychopharmacology (Berl)., 142, 309–317. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci, 2, 119–128. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat. Neurosci, 8, 1445–1449. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2013) Cellular basis of memory for addiction. Dialogues Clin. Neurosci, 15, 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, & Roeper J (2002) I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J. Neurosci, 22, 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, You C, Arora DS, McElvain MA, Vandegrift BJ, Brodie MS, & Woodward JJ (2016) Differential effects of toluene and ethanol on dopaminergic neurons of the ventral tegmental area. Front. Neurosci, 10, 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD & Halliday GM (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res, 434, 117–165. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, & Hartman BK (1995) Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J. Neurosci, 15, 5859–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T (2005) Hyperpolarization-Activated Cation Current (Ih) Is an Ethanol Target in Midbrain Dopamine Neurons of Mice. J. Neurophysiol, 95, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orejarena MJ, Herrera-Solís A, Pons S, Maskos U, Maldonado R, & Robledo P (2012) Selective re-expression of β2 nicotinic acetylcholine receptor subunits in the ventral tegmental area of the mouse restores intravenous nicotine self-administration. Neuropharmacology, 63, 235–241. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, & Nestler EJ (1995) Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse, 21, 289–298. [DOI] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, & Callaway EM (2011) New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron, 71, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakri Mohamed RM, Mokhtar MH, Yap E, Hanim A, Abdul Wahab N, Jaffar FHF, Kumar J (2018) Ethanol-Induced Changes in PKCε: From Cell to Behavior. Front Neurosci.12:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, & Sved AF (2007) The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend, 89, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pany S & Das J (2015) Alcohol binding in the C1 (C1A+C1B) domain of protein kinase C epsilon. Biochim. Biophys. Acta - Gen. Subj, 1850, 2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR & Collins AC (1993) An autoradiographic analysis of alterations in nicotinic cholinergic receptors following 1 week of corticosterone supplementation. Neuroendocrinology, 57, 262–271. [DOI] [PubMed] [Google Scholar]
- Pelham RW, Marquis JK, Kugelmann K, & Munsat TL (1980) Prolonged Ethanol Consumption Produces Persistent Alterations of Cholinergic Function in Rat Brain. Alcohol. Clin. Exp. Res, 4, 282–287. [DOI] [PubMed] [Google Scholar]
- Perkins KA & Karelitz JL (2013) Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl)., 228, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Boldry MC (2018) Reinforcement Enhancing Effects of Nicotine via Patch and Nasal Spray. Nicotine Tob. Res, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, & Morikawa H (2011) In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology, 36, 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Bolaños CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, & Barrot M (2005) ΔFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci, 21, 2817–2824. [DOI] [PubMed] [Google Scholar]
- Pfeffer AO & Samson HH (1988) Haloperidol and apomorphine effects on ethanol reinforcement in free feeding rats. Pharmacol. Biochem. Behav, 29, 343–350. [DOI] [PubMed] [Google Scholar]
- Phillipson OT (1979) Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: A horseradish peroxidase study in the rat. J. Comp. Neurol, 187, 117–143. [DOI] [PubMed] [Google Scholar]
- Piazza PV & Deroche-Gamonet V (2013) A multistep general theory of transition to addiction. Psychopharmacology (Berl)., 229, 387–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, & Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature, 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Wiliams JT, & Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature, 390, 401–404. [DOI] [PubMed] [Google Scholar]
- Pignatelli M & Bonci A (2015) Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron, 86, 1145–1157. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, & Fratta W (2008) Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci, 28, 12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J-F, Zou J, Drouin-Ouellet J, Kim K-YA, Cicchetti F, & Awatramani RB (2014) Defining Midbrain Dopaminergic Neuron Diversity by Single-Cell Gene Expression Profiling. Cell Rep, 9, 930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA & McNally GP (2016) Ventral Pallidum Output Pathways in Context-Induced Reinstatement of Alcohol Seeking. J. Neurosci, 36, 11716–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Raviola E, & Bean BP (2007) Roles of Subthreshold Calcium Current and Sodium Current in Spontaneous Firing of Mouse Midbrain Dopamine Neurons. J. Neurosci, 27, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, & Koob GF (1993) The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res, 623, 16–24. [DOI] [PubMed] [Google Scholar]
- Ressler KJ (2010) Amygdala Activity, Fear, and Anxiety: Modulation by Stress. Biol. Psychiatry, 67, 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav, 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Rifkin RA, Moss SJ, & Slesinger PA (2017) G Protein-Gated Potassium Channels: A Linkto Drug Addiction. Trends Pharmacol. Sci, 38, 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL, Hempel BJ, & Clasen MM (2018) Sex as a biological variable: Drug use and abuse. Physiol. Behav, 187, 79–96. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, & Parsons LH (2010) Corticotropin Releasing Factor-Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence. Biol. Psychiatry, 67, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, & Siggins GR (2012) The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb. Perspect. Med, 2, a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Patel RR, & Bajo M (2017) Ethanol and Cytokines in the Central Nervous System. In Handbook of Experimental Pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M & Varodayan FP (2017) Synaptic targets: Chronic alcohol actions. Neuropharmacology, 122, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, & Wightman RM (2009) Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol. Clin. Exp. Res, 33, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, & Stewart J (2007) Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience, 150, 8–13. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, & McBride WJ (2000) Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl)., 149, 217–224. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, May T, & Salewski B (1994) Harman (1-methyl-beta-carboline) is a natural inhibitor of monoamine oxidase type A in rats. Eur. J. Pharmacol, 252, 51–59. [DOI] [PubMed] [Google Scholar]
- Ron D & Barak S (2016) Molecular mechanisms underlying alcohol-drinking behaviours. Nat. Rev. Neurosci, 17, 576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, & Woodward JJ (2001) Ethanol Inhibition of N-Methyl-d-aspartate Receptors Is Reduced by Site-directed Mutagenesis of a Transmembrane Domain Phenylalanine Residue. J. Biol. Chem, 276, 44729–44735. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, & Gessa GL (1992) Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Ann. N. Y. Acad. Sci, 654, 513–516. [DOI] [PubMed] [Google Scholar]
- Ruiz-Durántez E, JA R-O, Pineda J, & Ugedo L (2001) Stimulatory effect of harmane and other beta-carbolines on locus coeruleus neurons in anaesthetized rats. Neurosci. Lett, 308, 197–200. [DOI] [PubMed] [Google Scholar]
- Russo SJ & Nestler EJ (2013a) The brain reward circuitry in mood disorders. Nat. Rev. Neurosci, 14, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, & Malenka RC (2003) Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron, 37, 577–582. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, & Bierut LJ (2006) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet, 16, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, & Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med, 49, 73–79. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, & De Biasi M (2003) The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol, 63, 1059–1066. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, & De Biasi M (2009) Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci, 29, 3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi K, & Haraguchi M (1988) Relation of ethanol self-administration to feeding and drinking in a nonrestricted access situation in rats initiated to self-administer ethanol using the sucrose-fading technique. Alcohol, 5, 375–385. [DOI] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, & Barrot M (2014) The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience, 282, 198–216. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, & Bhagwagar Z (2012) Persistent β2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am. J. Psychiatry, 169, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, & Koob GF (1995) Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. U. S. A, 92, 5880–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (1997) Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol, 7, 191–197. [DOI] [PubMed] [Google Scholar]
- Schultz W (1998) Predictive Reward Signal of Dopamine Neurons. J. Neurophysiol, 80, 1–27. [DOI] [PubMed] [Google Scholar]
- Schultz W (2002) Getting formal with dopamine and reward. Neuron, 36, 241–263. [DOI] [PubMed] [Google Scholar]
- Schultz W (2007) Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci, 30, 259–288. [DOI] [PubMed] [Google Scholar]
- Seo D & Sinha R (2014) The neurobiology of alcohol craving and relapse. In Handbook of Clinical Neurology. 355–368. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, & Ryabinin AE (2005) Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol. Clin. Exp. Res, 29, 1419–1426. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, McIntosh JM, Jones SR, & Ferris MJ (2017) Α6Β2 Subunit Containing Nicotinic Acetylcholine Receptors Exert Opposing Actions on Rapid Dopamine Signaling in the Nucleus Accumbens of Rats With High-Versus Low-Response To Novelty. Neuropharmacology, 126, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, & Amit Z (1999) Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl)., 142, 408–412. [DOI] [PubMed] [Google Scholar]
- Smith RF, McDonald CG, Bergstrom HC, Ehlinger DG, & Brielmaier JM (2015) Adolescent nicotine induces persisting changes in development of neural connectivity. Neurosci. Biobehav. Rev, 55, 432–443. [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Lidö HH, & Ericson M (2017) The Glycine Receptor-A Functionally Important Primary Brain Target of Ethanol. Alcohol. Clin. Exp. Res, 41, 1816–1830. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley SS, & van Dyck CH (2006) Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J. Neurosci, 26, 8707–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather CK, Babayan BM, Uchida N, Gershman SJ (2017) Dopamine reward prediction errors reflect hidden-state inference across time. Nature Neuroscience. 20, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Nie Z, Criado JR, & Siggins GR (2000) Ethanol Inhibition ofN-Methyl-d-aspartate Responses Involves Presynaptic γ-Aminobutyric AcidB Receptors. J. Pharmacol. Exp. Ther, 294. [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, & Criado JR (2009) Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol. Biochem. Behav, 92, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, & Steffensen SC (2004) Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther, 311, 282–289. [DOI] [PubMed] [Google Scholar]
- Stocker M & Pedarzani P (2000) Differential Distribution of Three Ca(2+)-Activated K(+) Channel Subunits, SK1, SK2, and SK3, in the Adult Rat Central Nervous System. Mol. Cell. Neurosci, 15, 476–493. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, & Bonci A (2008) Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol. Clin. Exp. Res, 32, 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, & Bonci A (2010) Neuroplastic Alterations in the Limbic System Following Cocaine or Alcohol Exposure. In Current Topics in Behavioral Neurosciences. pp. 3–27. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, & Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science, 306, 1029–1032. [DOI] [PubMed] [Google Scholar]
- Tarren JR, Lester HA, Belmer A, & Bartlett SE (2017) Acute Ethanol Administration Upregulates Synaptic α4-Subunit of Neuronal Nicotinic Acetylcholine Receptors within the Nucleus Accumbens and Amygdala. Front. Mol. Neurosci, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, & Morrisett RA (2008) Ethanol Enhances GABAergic Transmission Onto Dopamine Neurons in the Ventral Tegmental Area of the Rat. Alcohol. Clin. Exp. Res, 32, 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, & Morrisett RA (2011) GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience, 172, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, & Glowinski J (1976) Selective activation of the mesocortical DA system by stress. Nature, 263, 242–244. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, & Dani JA (2018) Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration. Cell Rep, 23, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, & Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol, 42, 413–416. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Louis V. a, & Taylor RE (2002) Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol. Clin. Exp. Res, 26, 394–399. [PubMed] [Google Scholar]
- Tolu S, Avale ME, Nakatani H, Pons S, Parnaudeau S, Tronche F, Vogt A, Monyer H, Vogel R, de Chaumont F, Olivo-Marin J-C, Changeux J-P, & Maskos U (2010) A versatile system for the neuronal subtype specific expression of lentiviral vectors. FASEB J, 24, 723–730. [DOI] [PubMed] [Google Scholar]
- Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, Baudonnat M, Husson M, Besson M, Reperant C, Zemdegs J, Pagès C, Hay YAH, Lambolez B, Caboche J, Gutkin B, Gardier AM, Changeux JP, Faure P, & Maskos U (2012) Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry, 18, 382–393. [DOI] [PubMed] [Google Scholar]
- Tolu S, Marti F, Morel C, Perrier C, Torquet N, Pons S, De Beaurepaire R, & Faure P (2017) Nicotine enhances alcohol intake and dopaminergic responses through β2* and β4* nicotinic acetylcholine receptors. Sci. Rep, 7, 45116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, & Clark D (1996) Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse, 22, 195–208. [DOI] [PubMed] [Google Scholar]
- Trudell JR, Messing RO, Mayfield J, & Harris RA (2014) Alcohol dependence: Molecular and behavioral evidence. Trends Pharmacol. Sci,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PAF, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, & Tsuang M (1999) Common genetic vulnerability for nicotine and alcohol dependence in men. Arch. Gen. Psychiatry, 56, 655–661. [DOI] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, & Deisseroth K (2009) Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science. 324, 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM & Deisseroth K (2012) Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci, 13, 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, & Bolam JP (2004) Uniform Inhibition of Dopamine Neurons in the Ventral Tegmental Area by Aversive Stimuli. Science, 303, 2040–2042. [DOI] [PubMed] [Google Scholar]
- Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol, 346, 967–989. [DOI] [PubMed] [Google Scholar]
- Valenta JP, Job MO, Mangieri RA, Schier CJ, Howard EC, & Gonzales RA (2013) μ-Opioid receptors in the stimulation of mesolimbic dopamine activity by ethanol and morphine in Long-Evans rats: a delayed effect of ethanol. Psychopharmacology (Berl)., 228, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, & Grace AAA (2011) Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J. Neurosci, 31, 4280–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Bhave S, Hoffman P, & Harris RA (1998) Acute effects of ethanol on pharmacologically isolated kainate receptors in cerebellar granule neurons: comparison with NMDA and AMPA receptors. J. Neurochem, 71, 1777–1780. [DOI] [PubMed] [Google Scholar]
- Varodayan FP, Sidhu H, Kreifeldt M, Roberto M, & Contet C (2018) Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology, 133, 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villégier A-S, Salomon L, Granon S, Changeux J-P, Belluzzi JD, Leslie FM, & Tassin J-P (2006) Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology, 31, 1704–1713. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, & Boomsma DI (2005) Heritability of Smoking Initiation and Nicotine Dependence. Behav. Genet, 35, 397–406. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, & Goldstein RZ (2002) Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn. Mem, 78, 610–624. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, & Wong C (2007) Profound Decreases in Dopamine Release in Striatum in Detoxified Alcoholics: Possible Orbitofrontal Involvement. J. Neurosci, 27, 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, & Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci, 18, 741–752. [DOI] [PubMed] [Google Scholar]
- Walters GD (2002) The heritability of alcohol abuse and dependence: a meta-analysis of behavior genetic research. Am. J. Drug Alcohol Abuse, 28, 557–584. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Sparta DR, Hopf FW, Bowers MS, Melis M, & Bonci A (2009) Strain Specific Synaptic Modifications on Ventral Tegmental Area Dopamine Neurons After Ethanol Exposure. Biol. Psychiatry, 65, 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lee JW, Oh G, Grady SR, McIntosh JM, Brunzell DH, Cannon JR, & Drenan RM (2014) Enhanced synthesis and release of dopamine in transgenic mice with gain-of-function α6* nAChRs. J. Neurochem, 129, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden A, Erickson E, Robinson G, Harris RA, & Mayfield RD (2016) The neuroimmune transcriptome and alcohol dependence: potential for targeted therapies. Pharmacogenomics, 17, 2081–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, & Koob GF (1993) Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J. Pharmacol. Exp. Ther, 267, 250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, & Koob GF (1996) Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci, 16, 3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F & Porrino LJ (2002) Behavioral neurobiology of alcohol addiction: recent advances and challenges. J. Neurosci, 22, 3332–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1996) Neurobiology of addiction. Curr. Opin. Neurobiol,. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, & Deisseroth K (2011) Recombinase-Driver Rat Lines: Tools, Techniques, and Optogenetic Application to Dopamine-Mediated Reinforcement. Neuron, 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, & Balfour DJK (2005) Nicotine: from molecular mechanisms to behaviour. Curr. Opin. Pharmacol, 5, 53–59. [DOI] [PubMed] [Google Scholar]
- Woods JM & Druse MJ (1996) Effects of chronic ethanol consumption and aging on dopamine, serotonin, and metabolites. J. Neurochem, 66, 2168–2178. [DOI] [PubMed] [Google Scholar]
- Xiao C, Shao XM, Olive MF, Griffin WC, Li KY, Krnjević K, Zhou C, & Ye JH (2009) Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology, 34, 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjević K, & Ye JH (2007) Effects of Ethanol on Midbrain Neurons: Role of Opioid Receptors. Alcohol. Clin. Exp. Res, 31, 1106–1113. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Pearson S, Kadota Y, & Gonzalez CE (2006) Identification of Ethanol Responsive Domains of Adenylyl Cyclase. Alcohol. Clin. Exp. Res, 30, 1824–1832. [DOI] [PubMed] [Google Scholar]
- You C, Vandegrift B, & Brodie MS (2018) Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl)., 235(6): 1711–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, & Shippenberg TS (2006) Repeated Ethanol Intoxication Induces Behavioral Sensitization in the Absence of a Sensitized Accumbens Dopamine Response in C57BL/6J and DBA/2J Mice. Neuropsychopharmacology, 31, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, & Dani JA (2010) In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology, 59, 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, & Tapper AR (2011) Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology, 36, 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PEM, & Palmiter RD (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci, 106, 7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]