Abstract
Frailty is an issue of paramount importance for cardiologists, because of the aging of patients admitted to hospital for acute coronary syndrome (ACS) and the straight relationship between aging and frailty. Several tools have been provided in this setting, in order to objectively assess frailty status, but important questions are still unsolved. There are conflicting data about a unique definition of frailty in subjects with cardiovascular diseases, the timing to perform a frailty evaluation in the context of an acute myocardial infarction, the mean to assess frailty in these patients and the usefulness of the information derived from the frailty assessment. Frailty results from the analysis of several items and a multidomain evaluation including laboratory values, clinical data and physical performance assessment is required for a comprehensive frailty assessment. However, regardless of the frailty tool, the prevalence of frailty in older ACS patients is high and it could add important information to the decision-making process about invasive strategy, the multivessel disease management, dual antiplatelet therapy and secondary prevention programs. The present overview tries to summarize the current knowledge about the definition and prevalence of frailty in older adults admitted to hospital for ACS, suggesting how frailty assessment may improve the management of older ACS patients.
Keywords: Acute coronary syndrome, Frailty, The elderly
1. Introduction
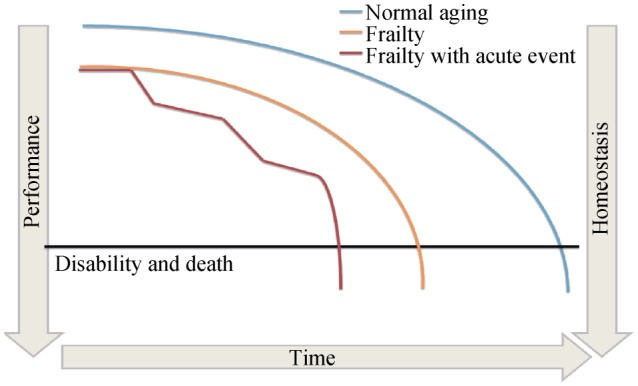
The aging of the population and the increasing of the mean age of patients admitted to hospital for acute coronary syndrome (ACS) represent a challenge for cardiologists. Compared to the last decade, cardiologists have to deal with a growing number of older adults with several comorbidities and a higher risk of complications. Thus, it is not surprising that the notion of frailty has become of interest, aiming for a better characterization of patients and for an optimization of treatments. Frailty is characterized by a loss of biological reserves, which leads to failure of homeostatic mechanisms following stressor events.[1] If not recognized and treated, frailty has several implications such as dependency, disability and death (Figure 1).[2],[3] Despite the highlighted importance of frailty, several aspects remain controversial in older patients admitted to hospital for ACS. Firstly, a universal definition of frailty for patients with cardiac disease is missing. Then, it is unclear which is the best tool and/or scale to discriminate frailty in patients with ACS, there is no agreement about the perfect timing to perform frailty evaluation and finally, there are not conclusive data on how the information derived from frailty assessment can be useful to optimize the management of ACS patients.
Figure 1. Fall to disability and death: comparison between normal aging, frailty and frailty associated with an acute event.
The current overview tries to summarize available knowledge about the definition and prevalence of frailty in older adults admitted to hospital for ACS and which are the main tools and scales for frailty assessment. Finally, we speculate on how frailty assessment may improve the management of older ACS patients.
2. How to assess frailty
Frailty represents a complex clinical syndrome of increased vulnerability to stressors which results in multiple impairments across different systems (Figure 1). It results, at least in part, from the unbalance between biological and chronological age. Individuals who have a lower functional capacity and few physiological reserves are at higher risk for homoeostatic disruption in case of stress such as ACS. Frailty has multiple contributors including age-related loss of muscle mass, reduced nutritional intake, low physical activity, cardiovascular and non-cardiovascular disease. Frailty is also associated with alterations in several biomarkers as well as with increased levels of C-reactive protein and of cystatin C or with low vitamin D and albumin.[4],[5] Albumin is associated with the nutritional status and it was one of the first impaired biomarker in patients at risk of frailty.[5] Resistance and autonomy in walking, time to walk 5–10 meters and time spent in physical activity are markers of frailty, and these show an independent and strong association with the cumulative occurrence of death and hospitalizations. Thus, since frailty is a composite of multiple deficits, it is not surprising that its assessment requires a comprehensive evaluation. This comprehensive evaluation varies according to clinical setting and aims. Generally, a complete frailty assessment includes: (1) questions about daily activities; (2) questionnaire about physical autonomy and/or cognitive and emotional status; (3) laboratory parameters; and (4) objective measurements of physical performance. Cardiologists, in their daily practice, tend to identify frailty clinically from the “end of the bed”, using subjective approaches such as the “eye ball test”. However, this approach is unreliable and leads to several biases. Frailty requires standardized methods providing quantitative estimates and reliable information. To achieve the result, several tools and scales have been developed. The large majority has been validated in other clinical conditions rather than ACS. Nevertheless, some tests and scales can be translated to the ACS setting (Table 1). Below, we reported a brief description of the main scales available to assess frailty in older ACS patients. Some of them can be calculated with data obtained by the chart review or by interview and their application is feasible at the hospital admission (Table 1). On the contrary, other tests and scales, requiring objectivation of the physical performance (i.e., walking, grip strength, chair rise, etc.), can be performed only after mobilization and these are ideal for the assessment before the discharge (Table 1).
Table 1. Main frailty tools used in ACS setting and the prognostic value of frailty assessed by these tests.
| Brief description | Pros | Cons | Prognostic implication in ACS | |
| Scales based on interview, without objective assessment of physical performance | ||||
| FRAIL scale | Five items about fatigue, resistance, ability to walk for one block, concomitant illnesses and loss of weight. Frailty is defined by the presence of 3 or more criteria. | Multidomain evaluation Inclusion of comorbidities |
Time required. Few evidences in ACS. Lacking inclusion of relevant laboratory data. |
Predictor of 6-monthall-cause death.[6] |
| Frailty Index | Evaluation based on 32 different variables about symptoms, signs, disabilities, diseases, and laboratory measurements obtaining final point with a threshold of 0.25 defining frailty. | Multidomain evaluation | Time required. | Association with long-term mortality and myocardial Infarction.[7] |
| CFS | Nine frailty levels ranging from 1 (very fit) to nine (terminally ill); the degree of frailty can be assessed by simple questions, according to the description encoded for every level. | Ease Convenience Speed practical |
Biased by subjective considerations. Lacking multidomain evaluation. Lacking inclusion of relevant clinical and laboratory data. |
Association with increased risk of in-hospital and 1-month death and increased length of stay.[1],[8]–[11] |
| Scales with objective assessment of physical performance | ||||
| Fried criteria | Fvie criteria: unintentional weight loss > 4.5 kg in the past year, exhaustion, physical activity, walk time and grip strength (frailty is defined by the presence of three or more criteria). | Strong evidence of frailty identification Multidomain evaluation |
Need of training with a geriatrician Time required. Lacking inclusion of relevant clinical and laboratory data. |
Strong predictor of mortality and myocardial infarction.[12]–[14] |
| SHAREFI | Six items: exhaustion, appetite, physical activity, ambulation, resistance and measurement of grip strength. | Multidomain evaluation Speed practical |
Self-reported. Lacking inclusion of relevant clinical and laboratory data. |
Association with early complications and survival.[15] |
| EFS | Questions and tasks about nutrition, symptoms, mood and physical performance and it ranges from 0 (not frail) to 17 (very frail). | Multidomain evaluation | Time required. Lacking inclusion of relevant laboratory data. |
Association with length of stay, 1-year mortality and undertreatment.[16],[17] |
| Green score | Scale of four items (physical activity, serum albumin, gait speed, grip strength) ranging from 0 to 12. | Multidomain evaluation including laboratory data | Lacking inclusion of clinical data such ad comorbidities. | Predictive value for all-cause death and death/re-infarction.[14] |
| Measurements of physical performance | ||||
| Grip strength | Assessment of the force of the flexor muscles of the fingers, wrist and forearm by a dynamometer. | Ease Convenience Speed practical |
Lacking multidomain evaluation. Lacking inclusion of relevant clinical and laboratory data. |
Predictive value for cardiac death, all-cause death and hospital admission for heart failure.[18],[19] |
| Gait Speed | Evaluation of walking speed with a usual pace for some meters, the length of five to ten meters is the most used. Slow gait speed is defined as a value ≤ 0.8 m/s. | Ease Convenience Speed practical |
Lacking multidomain evaluation. Lacking inclusion of relevant clinical and laboratory data. |
Predictive value for 1-year mortality and hospital Readmission.[20] |
| SPPB | Assessment of lower limb function according to three tests: standing balance, usual walking speed and standing chair. The score ranges from 0 (worst performance) to 12 (best performance). Physical performance is considered reduced with a SPPB score ≤ 9. | Multidomain evaluation of physical performance | Need of training with geriatrician. Lacking inclusion of relevant clinical and laboratory data. |
Ongoing study about its usefulness in ACSpatients.[25] |
ACS: acute coronary syndrome; CFS: clinical frailty scale; EFS: Edmonton frail scale; SHARE-FI: Survey of Health, Ageing and Retirement in Europe Frailty Instrument; SPPB: short physical performance battery.
2.1. Scales based on interview and chart review
The FRAIL scale, the Frailty Index and the Clinical Frailty Scale (CFS) are based on simple questions of standardized questionnaire (Table 1). They assess frailty quantifying the accumulation of deficits. The FRAIL scale is based on five questions about fatigue, resistance, ability to walk for one block, concomitant illnesses and loss of weight. Alegre, et al.[6] used the FRAIL scale to assess frailty in a population of older adults admitted for non-ST segment elevation myocardial infarction (NSTEMI), and the study demonstrated that frailty evaluated by this scale predicted the 6-month all-cause mortality. The Frailty Index score calculates deficits based on symptoms, signs, disabilities, diseases, and laboratory measurements. It has been developed including 32 different variables. Myers, et al.[7] demonstrated the association between Frailty Index, hospitalization and long-term mortality after myocardial infarction (MI). The computation of these scales requires around 15–20 minutes and these have been tested mainly in patients with a diagnosis of NSTEMI. As compared to the above-mentioned tools, the CFS is easier and quicker (Table 1). It is based on few and fast questions and it has been created by Rockwood, et al.[1] The CFS classifies the patient in nine potential categories: very fit, well, managing well, vulnerable, mildly frail, moderately frail, severely frail, very severely frail, terminally ill. The identification of the category is based on the judgment of the physician in agreement with the ability to work, to walk, to complain symptoms and to need help for outside activities, keeping house and personal care. Different studies showed its prognostic value in some cardiovascular conditions such as coronary artery disease and aortic stenosis undergoing interventional procedures.[8]–[11] It is better for conditions where time is lacking (i.e., ST-segment elevation MI) and where non-geriatric personnel is present. However, CFS should not be considered a multidimensional evaluation, because it is biased by subjective considerations and it lacks of the evaluation of relevant clinical and laboratory data.
2.2. Scales mixing interview and measurements of physical performance
The below-mentioned scales are characterized by the presence of questions investigating daily activities and attitudes and at least one item related to objective assessment of physical function (Table 1). The first and most used scale in this category is represented by the Simplified Fried criteria for frailty. It includes five main criteria: unintentional weight loss > 4.5 kg in the past year, exhaustion, physical activity, walk time and grip strength.[12] The score ranges from 0 to 5. A subject is defined frail in presence of three or more criteria. Previous studies showed that frailty assessed by the Fried score was associated to long-term mortality and re-infarction in ACS patients.[13],[14] The Survey of Health, Ageing and Retirement in Europe Frailty Instrument (SHARE-FI) is a mix of questions about exhaustion, appetite, physical activity, ambulation, resistance and measurement of grip strength (two times for both hands). Alonso Salinas GL and colleagues found that SHARE-FI was an independent predictor of both major bleeding and a combination of death, MI and stroke, and they found that the rate of mortality was impressively higher in frail subjects as identified by SHARE-FI (8.5% vs. 0.8%, P = 0.004).[15] The Edmonton Frail Scale (EFS) is an easy and fast scale including questions and tasks about nutrition, symptoms, mood and physical performance. Blanco, et al.[16] demonstrated that EFS had a strong and independent prognostic value for all-cause mortality in ACS patients. Graham, et al.[17] demonstrated that EFS was associated with increased length of hospitalization and 1-year mortality in a cohort of older ACS patients. The Green score considers serum albumin level, gait speed, physical activity assessed by the evaluation of activities of daily living and grip strength: it demonstrated a strong predictive value in terms of all-cause death and re-infarction adding important information to GRACE score.[14] Other scales combining questions and assessment of physical performance are available (i.e., Columbia, Bern, etc.). However, these scales did not substantially different from the previous ones described and these have been less investigated in ACS patients.
2.3. Measurements of physical performance
The overlap between physical performance and frailty is important. Thus, it is not surprising that measurements of physical performance alone can be used as surrogates of more complex scales. The most validated and simple measurements are the grip strength, the gait speed and the Short Physical Performance Battery (SPPB). Grip strength assesses the force of the flexor muscles of the fingers, wrist and forearm by a dynamometer. Previous studies demonstrated that this index correlates with the nutritional status of the subject, with the capacity of functional recovery post-surgery and it is an index of physical performance.[18] A recent meta-analysis showed that in patients with cardiac disorders, grip strength predicted cardiac death, all-cause death and hospital admission for heart failure.[19] Similar considerations can be done for the gait speed. It is a single parameter, but it is strongly related to poor physical performance, frailty and outcomes.[20] A slowed down gait speed may reflect decreased organ system functions due to frailty and comorbidities. A slow gait speed has an important prognostic impact in older adults with MI in terms of 1-year mortality and hospital readmission.[20] The SPPB is a more complete evaluation of the limb function. It includes three tests: standing balance, usual walking speed and standing chair.[21],[22] The test showed a strong and independent ability to predict mortality, morbidity and hospitalization in different clinical settings, including patients with cardiovascular disease.[23]–[25]
3. When to assess frailty
The prevalence of frailty varies significantly, depending by the definition, timing of assessment and type of the population. Frailty status ranges from 4% to 59% in community-dwelling populations, and it has a higher prevalence in nursing homes.[26] Obviously, in this specific context, the timing of frailty assessment does not matter, and it does not influence findings. On the contrary, the timing of the assessment becomes crucial in patients with cardiac disease. The correct timing is strictly related to the purpose of the assessment. One example of ideal scenario is represented by older adults with symptomatic aortic stenosis undergoing transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement (SAVR). There is an increasing recognition that some patients simply fail to derive a benefit in terms of functional capacity, morbidity, or mortality after TAVI or SAVR. With ongoing scrutiny of the economic implications, accurately identifying the subgroup of patients in whom intervention is likely to be futile remains a priority. In these subjects, frailty assessment could optimize the selection of patients requiring intervention or could sway potential SAVR candidates towards TAVI. Both interventions are “elective” procedures. The treatment's decision is taken in multidisciplinary meetings. Frailty assessment can be performed before the intervention. In addition, recent data by Afilalo J and colleagues standardized the frailty assessment. The Authors compared several tools in a large cohort of older adults undergoing TAVI or SAVR (n = 1020), they identifying the Essential Frailty Toolset (EFT) as the best scales for risk stratification.[27] The EFT is a brief 4-item scale encompassing lower-extremity weakness (time to perform 5 chair rises), cognitive impairment (as defined as a score < 24 points on the Mini-Mental State Examination), anemia (< 13 g/dL in male and < 12 g/dL in females), and hypoalbuminemia (< 3.5 g/dL). The EFT was the strongest predictor of 1-year death (adjusted OR = 3.72, 95% CI: 2.54–5.45) and of 1-year worsening disability (adjusted OR = 2.13, 95% CI: 1.57–2.87).[27] All these considerations are not transferable to older ACS patients. Major obstacles to a recommendation for screening include determining what should be done if it is detected, ensuring that no harm results from labeling a patient as frail. The pivot of current ACS management is the invasive management. Thus, we may speculate about two possible timing for frailty assessment: the first one before invasive treatment and the second one between coronary artery angiography and discharge. The implications are important. A frailty assessment before invasive management is mainly directed to support or not the choice of the invasive strategy. On the contrary, a frailty assessment after angiography (coronary revascularization) can improve the risk stratification, and it also can drive a tailored approach for older patients. In the following chapter, the above-mentioned implications are discussed.
Table 2. Prevalence of frailty and adjusted risk of mortality for older patients following ACS and PCI.
| Study | Frailty definition | Frailty prevalence | Follow up | Adjusted risk of all-cause mortality |
| Alegre, et al.[6] | FRAIL scale | 27.3% | 6 months | HR = 3.82 (95% CI: 1.80–8.11) |
| Ekerstad, et al.[8] | CFS | 48.5% | 30 days | OR = 2.17 (95% CI: 1.28–3.67) |
| Ekerstad, et al.[9] | CFS | 48.5% | 1 yr | HR = 4.3 (95% CI: 2.4–7.8) |
| Kang, et al.[10] | CFS | 43.2% | 4 months | HR = 5.39 (95% CI: 1.48–19.69) |
| Myers, et al.[11] | Frailty index | 5.1% | 13 yrs | HR = 2.02 (95% CI: 1.46–2.79) |
| Sanchis, et al.[14] | Green score | 47.0% | 25 months | HR = 1.25 (95% CI: 1.15–1.36) |
| Salinas, et al.[15] | SHARE-FI | 37.9% | 30 days | Not applicable |
| Blanco, et al.[16] | EFS | 20.8% | 1 yr | HR = 4.03 (95% CI: 2.02–8.04) |
| Graham, et al.[17] | EFS | 30.0% | 1 yr | HR = 3.49 (95% CI: 1.08–7.61) |
| Matsuzawa, et al.[20] | Gait speed | 33% | 5.5 yrs | HR = 0.71 (95% CI: 0.62–0.82) for increase of0.1 m/ second in gait speed |
For all outcomes, the comparator is older people defined as fit. ACS: acute coronary syndrome; CI: confidence interval; CFS: clinical frailty scale; EFS: Edmonton frail scale; HR: hazard ratio; PCI: percutaneous coronary intervention; SHARE-FI: Survey of Health, Ageing and Retirement in Europe Frailty Instrument.
4. Why to assess frailty
As reported in Table 3, randomized clinical trials about strategies or treatments and frailty status in older ACS adults are missing. Below, we speculate about potential applications of frailty assessment.
Table 3. Proposals for daily clinical implications of frailty assessment in older ACS patients.
| Why | When | How | Data |
| To guide invasive strategy | At hospital admission | Scales based on interview or chart review (i.e., FRAIL scale, CFS, EFS, Frailty Index, SHARE-FI) | No RCT. Invasive strategy is related to better outcomes in older ACS patients, but in frail subjects there could be a lack of benefit. |
| To improve risk stratification | At hospital admission At hospital discharge |
Scales based on interview or chart review (i.e., FRAIL scale) Scales including physical performance assessment ( i.e., SPPB, Green score) |
No RCT. FRAIL scale and Green score showed to have important predictive value, outperforming GRACE score alone. |
| To guide complete revascularization | Before hospital discharge | Scales based on interview or chart review ( i.e., CFS, EFS, Frailty Index, FRAIL scale). Scales including physical performance assessment ( i.e., Fried score, SHARE-FI, gait speed, SPPB). |
Ongoing RCT. Age and multivessel disease emerged as independent predictor of ischemic events; ischemic events could be related to the fact that only few patients with a multivessel disease receive a complete revascularization. |
| To guide DAPT lenght | At hospital discharge | Scales based on interview or chart review. Scales including physical performance assessment (i.e., gait speed, SPPB). |
No RCT. Age is related to high bleeding risk but also to high ischemic risk; no data about frailty implications. |
| To improve physical performance | At hospital discharge | Scales including physical performance assessment ( i.e., gait speed, handgrip strength, SPPB). | Ongoing RCT. Low physical performance is related to poor prognosis in older patients with cardiovascular diseases. |
| To improve nutritional status | At hospital discharge | Scales based on interview or chart review ( i.e., FRAIL scale, EFS). Scales including physical performance and nutritional status assessment (Fried score, SPPB, gait speed, handgrip strength, SHARE-FI, Green score). |
No RCT. Vitamin supplementation could prevent sarcopenia and improve older adults' prognosis. |
CFS: clinical frailty score; DAPT: dual antiplatelet therapy; EFS: Edmonton frailty scale; GRACE: Global risk of adverse cardiac events; RCT: randomized clinical trials; SHARE-FI: Survey of Health, Ageing and Retirement in Europe Frailty Instrument; SPPB: short physical performance battery.
4.1. To guide the invasive strategy
First minutes after admission to hospital are crucial in the decision-making process about invasive strategy in patients presenting ST-segment elevation ACS (STE-ACS). The assessment of frailty is not feasible in patients with STE-ACS undergoing primary PCI. The benefits of percutaneous reperfusion are largely demonstrated, also in older adults.[28] Frailty identification may not alter the effectiveness associated with primary PCI in terms of hard endpoints, symptom control, independence, and quality of life. The TRatamiento del Infarto Agudo de miocardio eN Ancianos (TRIANA) study pooled its data with those from two previous trials.[29] The analysis confirmed the presence of a benefit coming from primary PCI in patients aged 70 years old and over.[29] Observational registries showed that revascularization in older STEMI patients reduces 30-day, 1-year and 5-year mortality.[17],[20],[30] Different considerations can be argued for patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS). Although the temporal window is limited, we can suppose a frailty assessment with scales based on interview and chart review. The purpose of the frailty assessment should be the identification of patients with a burden of comorbidities and illness overcoming the benefit of invasive management. The multicenter randomized After Eighty Study demonstrated a reduction of the composite primary endpoint including death, stroke, MI, need for urgent revascularization 1.5 years after the index event in patients randomized to invasive strategy.[30] More recently, the MOSCA trial included 106 patients with NSTE-ACS aged ≥ 70 years with a high degree of comorbidity. Patients were randomized to an invasive or conservative strategy. Although the invasive strategy tended to improve three-month outcomes in terms of mortality and of morbidity or ischaemic events (reinfarction or post-discharge revascularization), this benefit declined during the follow-up. After 2.5 years, no significant differences were observed.[31] The Impacto de la fragiLidad y Otros síNdromes GEriátricos en el manejo y pronóstico Vital del ancianO con Síndrome Coronario Agudo sin elevación de segmento ST (LONGEVO-SCA) registry included unselected NSTE-ACS patients aged ≥ 80 years. The authors found that an invasive strategy was independently associated with better outcomes in very elderly patients with NSTE-ACS. Nevertheless, the finding was strongly related to the frailty status, with a lack of benefit in patients with established frailty (as defined by the FRAIL scale).[6] Thus, the hypothesis that in older patients with a high degree of frailty, the benefit of an invasive strategy might be weakened by the weight of comorbidities is still unsolved. Larger studies and randomized trials of frail patients with NSTE-ACS are mandatory to clarify this issue.
4.2. To improve risk stratification
A pooled meta-analysis of 22 randomized trials showed that older patients have a worse long-term outcome after PCI and that traditional cardiac risk factors play a less important role compared to the young patients.[32],[33] This finding indirectly supports the need of a different risk stratification in older ACS adults. The Global Risk of Cardiac Events (GRACE) is the most validated risk score and international guidelines strongly support its use in daily practice.[34] Age is one of the most important item of the GRACE risk score. Nevertheless, the discrepancy between chronological and biological age has been well-established and the GRACE risk score cannot capture it. Then, it is not surprising that some studies showed that the assessment of frailty and/or physical performance may improve risk stratification in terms of short-term and long-term prognosis. Alegre, et al.[6] demonstrated that in the LONGEVO-SCA population, the predictive model including age, a score of comorbidity (Charlson Comorbidity Index), GRACE score and FRAIL scale significantly outperformed the ability of GRACE score for predicting 6-month death (AUC = 0.75, 95% CI: 0.68–0.82, P = 0.003). Sanchis, et al.[14] underlined the important additive information of Green score to GRACE score, in terms of all-cause death with a median follow-up time is 25 (31–72) months, with a shift in AUC from 0.6 to 0.64. Investigators from the ICON1 study recently demonstrated that Fried score associated with some clinical variables (hypertension, Killip class, age, ability of dressing self, extracoronary vascular disease) better predicts 1-year mortality when compared to the GRACE score alone.[35]
4.3. To guide complete revascularization
In a large real-life registry cohort of more than 50.000 ACS elderly patients (≥ 65 years) from the Medicare database, multivessel disease was present in more than half of the cases.[36] Older ACS patients were largely undertreated, since 80% of the study population received only culprit lesion treatment. Similar findings were confirmed by the 2-year analysis of the Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug Coated Stent versus the Gazelle Bare Metal Stent in Patients With High Risk of Bleeding (LEADERS FREE) trial.[37] Age and multivessel disease emerged as independent predictors of ischemic events (HR = 1.56, 95% CI: 1.23–1.97 and HR = 1.66, 95% CI: 1.27–2.18, respectively). The authors noted that ischemic events could be related to the fact that 62% of patients had multivessel disease, while only 22% of patients received multivessel revascularization. However, if older adults and/or frail ACS patients may benefit from a complete revascularization is unknown. Data from younger study population supports this hypothesis, but further investigations are mandatory. The Functional Versus Culprit-only Revascularization in Elderly Patients With Myocardial Infarction and Multivessel Disease (FIRE, NCT03772743) trial will enrol older adults aged 75 years and over admitted to hospital for MI. The trial is designed to show the superiority of a functional-guided complete revascularization over the culprit only treatment. Frailty and physical performance will be systematically evaluated in all patients to investigate potential benefit across subgroups of the study population.
4.4. To optimize strategies of secondary prevention
Dual antiplatelet therapy (DAPT) should be maintained for at least 12 months after ACS, except for subjects at higher bleeding risk (6 months). In accordance with available tool for bleeding risk stratification (i.e., PRECISE-DAPT score), older adults are always considered at high bleeding risk.[38] However, no prospective studies confirmed a benefit from shortened DAPT regimen and frailty was missing as covariate in models of risk stratification. Finally, although poor physical performance is more common in older ACS patients, many studies investigating the effectiveness of cardiac rehabilitation after ACS excluded older subjects for concomitant comorbidities and/or poor compliance.[39] Nevertheless, older ACS patients are those who could mostly benefit from physical activity interventions and/or nutritional support. This issue has been investigated by the Physical Activity Intervention for Elderly Patients with Reduced Physical Performance after Acute Coronary Syndrome.[40] The study included ACS patients aged ≥ 70 years with reduced physical performance at hospital discharge and 1-month later. The subjects were randomized to health education program vs. a physical activity intervention mixing few (n = 4) early supervised sessions and home-based exercises. The aim was to assess safety and effectiveness of the physical intervention in terms of physical performance, daily activities, anxiety/depression and quality of life, and the analyses are still ongoing. Similarly, considering the relevant role of malnutrition, vitamin D, albumin and hemoglobin values in the frailty status and prognosis, future researches investigating the effects of nutritional supplements should be highly desirable. Indeed, literature data demonstrated that vitamin supplementation could prevent the occurrence of sarcopenia in older patients[41], but as far as we know, there are no studies about these supplementation specifically targeting older ACS patients.
5. Conclusions
In summary, physicians and cardiologists should begin to familiarize with the role of frailty in older adults admitted to hospital for ACS. Its assessment should be considered in a complete framework of this population. Scales and timing of assessment can be tailored according to the clinical condition and presentation, the expertise of the center and the final aim (i.e., decision for invasive strategy, risk stratification, tailored treatment, specific programs of physical activity or nutrition supplemental). At the same time, current evidences are limited. No randomized clinical trials are available to guide the application of the frailty assessment in the daily clinical practice (Table 3). Studies on older adults, with strategies and treatments tailored on frailty status are an unmet clinical need, and these are clearly needed.
Footnotes
This article is part of “Prognostic and management of ACS in the elderly” Special Issue.
Guest Editors: Prof. Albert Ariza Solé
References
- 1.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Díez-Villanueva P, Arizá-Solé A, Vidán MT, et al. Recommendations of the Geriatric Cardiology Section of the Spanish Society ofCardiology for the assessment of frailty in elderly patients with heart disease. Rev Esp Cardiol (Engl Ed) 2019;72:63–71. doi: 10.1016/j.rec.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Bebb O, Smith FG, Clegg A, et al. Frailty and acute coronary syndrome: a structured literature review. Eur Heart J Acute Cardiovasc Care. 2018;7:166–175. doi: 10.1177/2048872617700873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchis J, Núñez E, Ruiz V, et al. Usefulness of clinical data and biomarkers for the identification of frailty after acute coronary syndromes. Can J Cardiol. 2015;31:1462–1468. doi: 10.1016/j.cjca.2015.07.737. [DOI] [PubMed] [Google Scholar]
- 5.Sujino Y, Tanno J, Nakano S, et al. Impact of hypoalbuminemia, frailty, and body mass index on early prognosis in older patients (≥ 85 years) with ST-elevation myocardial infarction. J Cardiol. 2015;66:263–268. doi: 10.1016/j.jjcc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Alegre O, Formiga F, López-Palop R, et al. An easy assessment of frailty at baseline independently predicts prognosis in very elderly patients with acute coronary syndrome. J Am Med Dir Assoc. 2018;19:296–303. doi: 10.1016/j.jamda.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Myers V, Drory Y, Gerber Y. Clinical relevance of frailty trajectory post myocardial infarction. Eur J Prev Cardiol. 2014;21:758–766. doi: 10.1177/2047487312462828. [DOI] [PubMed] [Google Scholar]
- 8.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur J Prev Cardiol. 2014;21:1216–1224. doi: 10.1177/2047487313490257. [DOI] [PubMed] [Google Scholar]
- 9.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 10.Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662–667. doi: 10.11909/j.issn.1671-5411.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimura T, Yamamoto M, Kano S, et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135:2013–2024. doi: 10.1161/CIRCULATIONAHA.116.025630. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Singh M, Rihal CS, Lennon RJ, et al. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–791. doi: 10.1016/j.ahj.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Alonso Salinas GL, Sanmartín Fernández M, Pascual Izco M, et al. Frailty predicts major bleeding within 30 days in elderly patients with acute coronary syndrome. Int J Cardiol. 2016;222:590–593. doi: 10.1016/j.ijcard.2016.07.268. [DOI] [PubMed] [Google Scholar]
- 16.Blanco S, Ferrières J, Bongard V, et al. Prognosis impact of frailty assessed by the Edmonton Frail Scale in the setting of acute coronary syndrome in the elderly. Can J Cardiol. 2017;33:933–939. doi: 10.1016/j.cjca.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Graham MM, Galbraith PD, O'Neill D, et al. Frailty and outcome in elderly patients with acute coronary syndrome. Can J Cardiol. 2013;29:1610–1615. doi: 10.1016/j.cjca.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardized approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 19.Pavasini R, Serenelli M, Celis-Morales CA, et al. Grip strength predicts cardiac adverse events in patients with cardiac disorders: an individual patient pooled meta-analysis. Heart. 2018;0:1–8. doi: 10.1136/heartjnl-2018-313816. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa Y, Konishi M, Akiyama E, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik J, Simonsick E, Ferrucci L, et al. A SPPB assessing lower extremity function: association with self reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci. 1994;42:85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14:215–215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campo G, Pavasini R, Maietti E, et al. The frailty in elderly patients receiving cardiac interventional procedures (FRASER) program: rational and design of a multicenter prospective study. Aging Clin Exp Res. 2017;29:895–903. doi: 10.1007/s40520-016-0662-y. [DOI] [PubMed] [Google Scholar]
- 26.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 27.Afilalo J, Lauck S, Kim DH, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 28.de Boer MJ, Ottervanger JP, van ’t Hof AW, et al. Reperfusion therapy in elderly patients with acute myocardial infarction: a randomized comparison of primary angioplasty and thrombolytic therapy. J Am Coll Cardiol. 2002;39:1723–1728. doi: 10.1016/s0735-1097(02)01878-8. [DOI] [PubMed] [Google Scholar]
- 29.Bueno H, Betriu A, Heras M, et al. Primary angioplasty vs fibrinolysis in very old patients with acute myocardial infarction: TRIANA (Tratamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. 2011;32:51–60. doi: 10.1093/eurheartj/ehq375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tegn N, Adbelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomized controlled trial. Lancet. 2016;387:1057–1065. doi: 10.1016/S0140-6736(15)01166-6. [DOI] [PubMed] [Google Scholar]
- 31.Sanchis J, Ariza-Solé A, Abu-Assi E, et al. Invasive versus conservative strategy in frail patients with NSTEMI: the MOSCA-FRAIL clinical trial study design. Rev Esp Cardiol (Engl Ed) 2019;72:154–159. doi: 10.1016/j.rec.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Antonsen L, Jensen LO, Terkelsen CJ, et al. Outcomes after primary percutaneous coronary intervention in octogenarians and nonagenarians with ST-segment elevation myocardial infarction: from the Western Denmark heart registry. Catheter Cardiovasc Interv. 2013;81:912–919. doi: 10.1002/ccd.24591. [DOI] [PubMed] [Google Scholar]
- 33.Kvakkestad KM, Abdelnoor M, Claussen PA, et al. Long-term survival in octogenarians and older patients with ST-elevation myocardial infarction in the era of primary angioplasty: a prospective cohort study. Eur Heart J Acute Cardiovasc Care. 2016;5:243–252. doi: 10.1177/2048872615574706. [DOI] [PubMed] [Google Scholar]
- 34.Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091–1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batty J, Qiu W, Gu S, et al. One-year clinical outcomes in older patients with non-ST elevation acute coronary syndrome undergoing coronary angiography: an analysis of the ICON1 study. Int J Cardiol. 2018;274:45–51. doi: 10.1016/j.ijcard.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 36.Wang TY, McCoy LA, Bhatt DL, et al. Multivessel vs culprit-only percutaneous coronary intervention among patients 65 years or older with acute myocardial infarction. Am Heart J. 2016;172:9–18. doi: 10.1016/j.ahj.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Garot P, Morice MC, Tresukosol D, et al. 2-Year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol. 2017;69:162–171. doi: 10.1016/j.jacc.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 39.Makris GC, Lattimer CR, Lavida A, et al. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;44:569–575. doi: 10.1016/j.ejvs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Tonet E, Maietti E, Chiaranda G, et al. Physical activity intervention for elderly patients with reduced physical performance after acute coronary syndrome (HULK study): rationale and design of a randomized clinical trial. BMC Cardiovasc Disord. 2018;18:98–98. doi: 10.1186/s12872-018-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artaza-Artabe I, Sáez-López P, Sánchez-Hernández N, et al. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas. 2016;93:89–99. doi: 10.1016/j.maturitas.2016.04.009. [DOI] [PubMed] [Google Scholar]


