Abstract
Radiation‐induced lung injury (RILI) is a common complication in radiotherapy of thoracic tumors and limits the therapeutic dose of radiation that can be given to effectively control tumors. RILI develops through a complex pathological process, resulting in induction and activation of various cytokines, infiltration by inflammatory cells, cytokine‐induced activation of fibroblasts, and subsequent tissue remodeling by activated fibroblasts, ultimately leading to impaired lung function and respiratory failure. Increasing evidence shows that mesenchymal stem cells (MSCs) may play a main role in modulating inflammation and immune responses, promoting survival and repair of damaged resident cells and enhancing regeneration of damaged tissue through soluble paracrine factors and therapeutic extracellular vesicles. Therefore, the use of the MSC‐derived secretome and exosomes holds promising potential for RILI therapy. Here, we review recent progress on the potential mechanisms of MSC therapy for RILI, with an emphasis on soluble paracrine factors of MSCs. Hypotheses on how MSC derived exosomes or MSC‐released exosomal miRNAs could attenuate RILI are also proposed. Problems and translational challenges of the therapies based on the MSC‐derived secretome and exosomes are further summarized and underline the need for caution on rapid clinical translation. stem cells translational medicine 2019;8:344–354
Keywords: Mesenchymal stem cells, Secretome, Exosome, Radiation pneumonitis, Lung fibrosis
Significance Statement.
Although it has been reported that soluble cytokines based on MSC therapy that could attenuate RILI, the mechanism of MSC‐based secretome therapy for RILI is still not fully understood. This review summarized the recent progress regarding the potential mechanisms of MSCs therapy for RILI, with an emphasis on MSC‐secreted cytokines and miRNAs as a safe and, effective cell‐free therapy, which may be helpful to accelerate the strategy from bench to bedside.
Introduction
Radiotherapy is an effective and important strategy for cancer treatment that may extend the survival time of patients by improving localized inhibition of tumor development 1. However, radiation‐induced lung injury (RILI) is a common adverse effect, with a lethality of up to 15%, and limits the therapeutic dose of radiation that can be administered to control tumors 2. RILI is a complex pathological process, resulting in an early radiation pneumonitis (RP) and late radiation‐induced lung fibrosis (RILF) 2. Symptomatic RP occurs in ∼5%–50%, ∼5%–10%, and ∼1%–5% of patients irradiated for cancers of the lung, mediastinal lymphatics, and breast, respectively 3, 4. Pneumonitis is characterized by shortness of breath, cough, and fever; however, patients with severe RP have almost 50% mortality 5. RLIF is a chronic, progressive, and fatal interstitial pulmonary disease with a poor prognosis, and poor response to available medical therapies 6. The rate of RLIF, which can continue to evolve around 1 year after radiotherapy, is reportedly up to 70%–80% in regions that use high‐dose radiotherapy 7. Therefore, RILI has become a focus of prevention and treatment in biomedical research.
Currently, RP can be treated with steroids but abrupt withdrawal may activate latent injury to the lung 8. Amifostine (WR‐2721) remains the only agent currently in clinical use as a radioprotector, which can scavenge free radicals, protect DNA, and accelerate repair 9. However, the radioprotective effects of chemical compounds, including amifostine, are short‐term, and associated with side effects such as nausea, vomiting, diarrhea, and hypotension 10, 11, thereby limiting their clinical use. The biological growth factors and cytokines such as IL‐7, IL‐11, granulocyte‐colony stimulating factor, macrophage‐colony stimulating factor, and keratinocyte growth factor have been used to alleviate radiation‐induced damage. However, success with these compounds has also been limited 11. Lycopene, as a naturally occurring dietary carotenoid, can protect against γ‐radiation induced DNA damage and antioxidant status in rats 12. However, there are still key considerations that need to be addressed in evaluating a potential antioxidant. Similarly, the signaling inhibitors, including TLR agonists CBLB502 and the STAT3 signaling inhibitor WP1066, can alleviate RP, but their toxicity and side effects still need to be considered before clinical application 9, 13. In addition, although lung transplantation is the most useful intervention for treating RILF, it is limited by the lack of available donated lungs and transplantation‐related complications 14, 15.
Therefore, a new and more effective therapeutic strategy based on the pathological mechanisms of RILI is urgently needed. Mesenchymal stem cells (MSCs), as a population of multipotent cells, can modulate the inflammation response, promote survival and repair of damaged resident cells, and enhance regeneration of damaged tissue 16, and thus show potential for clinical utility. Moreover, their advantages include convenient isolation and culture, low immunogenicity, regenerative and multiple differentiation abilities, and potent immunosuppressive effects 17, 18, 19, 20, 21, 22. These beneficial properties make MSC therapy a promising candidate for the treatment of RILI. Although preclinical studies showed therapeutic effects of MSCs for RILI therapy 23, many hurdles still exist for translating the therapeutic promise of MSCs in preclinical studies to the clinical setting 24, 25. To overcome those concerns of MSC‐based therapy, several studies showed that MSC‐derived conditioned medium (CM) recapitulated many of the therapeutic properties of the parent cells, suggesting that the development of cell‐free strategies based on using components of MSC‐derived CM, such as soluble factors, and extracellular vesicles, such as exosomes, merits further investigation 25, 26. Indeed, accumulating evidence shows that the therapeutic effects of MSCs are due to their capacity to secrete paracrine factors 26, 27, 28. In this review, we have summarized the potential mechanisms underlying MSC therapy for radiation‐induced pulmonary events in the lungs, with an emphasis on the importance of specific secreted soluble paracrine cytokines. Additionally, based on currently published data, we have predicted a potential therapeutic role for miRNAs shuttled by exosomes from MSCs. These data may help to support the therapeutic strategy of using the MSC‐derived secretome and exosomes to treat RILI and to accelerate this strategy from bench to bedside.
The Potential of MSC Therapy for RILI Based on the Secretion of Soluble Factors
Modulation of Expression Levels of Inflammatory and Fibrotic Cytokines
For decades, the biologic response to radiation has been reported to start with the generation of reactive oxygen species (ROS) 29. Similarly, RILI, as a radiation‐associated pulmonary complication, is different from other types of acute lung injury because it starts with radiation‐induced energy deposition and the generation of ROS, followed by a series of biologic responses, including a cascade of subsequent inflammatory events, angiogenesis, programmed cell death, autophagy, production of extracellular matrix, and crosstalk of activated signal transduction pathways, ultimately leading to lung fibrosis and respiratory failure 3, 30, 31. In this process, the release of various cytokines is considered to play a major role in the pathogenesis of RILI 30, 32, 33, including proinflammatory cytokines such as interleukin‐1α (IL‐1α), IL‐1β, IL‐6, and profibrogenic cytokines such as transforming growth factor β1 (TGF‐β1). Indeed, MSCs have been reported to inhibit ROS and reduce oxidative stress, playing an antifibrotic role and reducing proinflammatory responses by regulating the release of various cytokines in many experimental models 34, 35, 36, 37. Moreover, increasing evidence suggests that gene‐modified MSCs or MSC‐expressed cytokines play a key role in activating anti‐inflammatory and antifibrotic signaling or neutralizing proinflammatory and profibrotic cytokines via secreting paracrine cytokines, superoxide dismutases, or soluble inflammatory cytokine receptors. These activities may aid in repairing damaged lung tissue and in tissue regeneration following RILI.
Inhibition of Lung Myofibroblasts by MSC Secretion of Hepatocyte Growth Factor and Prostaglandin E2
Radiation can stimulate lung fibroblasts to secrete cytokines, undergo hyperplasia, and differentiate into myofibroblasts 38, 39, 40. Myofibroblasts can further promote the synthesis of additional collagens, leading to excessive deposition and abnormal remodeling of the extracellular matrix, which is a hallmark of RILI 39, 40, 41. Zhang et al. reported that human umbilical cord MSCs (hucMSCs) can attenuate RILI by inhibiting myofibroblastic differentiation of human lung fibroblasts 42. However, whether the inhibition involves the secretome of MSCs was unclear. Dong et al. further showed that human adipose tissue‐derived MSCs can downregulate levels of TNF‐α and TGF‐β1 by stimulating secretion of hepatocyte growth factor (HGF) and prostaglandin E2 (PGE2) 43.
Endogenous HGF/c‐Met signaling plays important roles in tissue repair 44, and the expression of HGF and c‐Met can be upregulated by exogenous stimuli such as MSCs and gene‐modified MSCs 45. MSC‐secreted HGF probably attenuates EMT in type II AECs by increasing intracellular levels of Smad7 upon binding to c‐Met and by upregulating the expression of matrix metalloproteinases‐1, ‐3, and ‐9 in injured sites in a PI3K/Akt/p70‐dependent manner, thereby promoting apoptosis of myofibroblasts 46, 47, 48. Wang et al. further showed that HGF‐modified MSC therapy can increase endogenous HGF/c‐Met expression in a mouse RILI model 49. S1P/S1PR1 may also participate in the HGF/c‐Met‐mediated process 49, 50, 51, suggesting that HGF‐modified MSCs may exert anti‐inflammatory and antifibrotic effects via paracrine secretion of HGF.
The cytokine PGE2 is secreted by MSCs to reprogram host macrophages to increase their anti‐inflammatory IL‐10 production 52. Moreover, PGE2 can inhibit TGF‐β1‐induced activation and fibroblast proliferation, thereby reducing the production of the fibrosis marker α‐SMA and collagens by elevating intracellular cAMP levels, and PGE2 also induces apoptosis in myofibroblasts by increasing the activity of the PTEN protein, which blocks the PI3K/Akt signaling pathway 53, 54, 55, 56, 57, 58, These findings suggest the potential application of MSCs in RILI therapy via activation of anti‐inflammatory pathways and inhibition of profibrotic signaling in a paracrine factor‐associated manner.
Protection of Injured Cells against ROS by MSC Secretion of Superoxide Dismutases
RILI starts with radiation‐induced energy deposition and generation of ROS 3, 30, 31. Inflammatory diseases are also accompanied by excessive production of ROS and depletion of endogenous antioxidants, but antioxidant enzymes such as superoxide dismutase 1 (SOD1, Cu/Zn SOD) are known to be very effective scavengers of ROS 59. It was reported that MSC‐secreted SOD1 protected lungs from radiation‐induced endothelial cell damage 60. Similarly, SOD3 or manganese superoxide dismutase‐modified MSCs also have more anti‐inflammatory and antifibrotic effects on RILI, compared with nongene‐modified MSCs 61, 62. This effect of superoxide dismutase is probably based on their ability to catalyze the dismutation of the superoxide radical into oxygen and hydrogen peroxide, thereby protecting injured cells against ROS generated during RILI 29, 35, 63, 64.
MSC Secretion of Soluble Factors that Inhibit Proinflammatory Signaling and Immune Cell Activation
Expression levels of the key proinflammatory cytokines IL‐1 and TNF‐α in the lung correlate with the development of pulmonary injury in rodents exposed to radiation 33, 65; however, MSC‐secreted interleukin 1 receptor antagonist (IL1RN) can function as a competitive inhibitor of IL‐1α and IL‐1β, and block the production and/or activity of TNF‐α and IL‐1α signaling in lung tissue 66, suggesting the possibility of using MSC‐secreted IL1RN as a paracrine mediator for treating RILI. Moreover, increased NF‐κB activity often triggers inflammation‐related pathologies including RILI 67, but Yagi et al. reported that human MSCs can neutralize TNF‐α by secreting significant quantities of soluble TNF receptor 1, which consequently blocks activation of NF‐κB by TNF‐α 68. Such neutralization of TNF‐α would contribute to the anti‐inflammatory effect of MSC therapy on TNF‐α/NF‐κB signaling in RILI via a paracrine manner. A similar effect was found in TGF‐β type II receptor‐modified MSCs and MSC‐conditioned medium 26, 69. Notably, Xue et al. reported that only 0.1% of lung cells are derived from transplanted MSCs, a level too low to support the observed protective effects. Other studies have also reported that MSCs can repair injured lung tissues without significant engraftment or differentiation in some situations 26, 70, suggesting that other factors, including paracrine cytokines (Table 1), may be mainly involved in regulating anti‐inflammatory responses and repair mechanisms.
Table 1.
Summary of soluble paracrine factors of MSC derived secretome for RILI therapy
| Soluble factors | Species | Role for RILI therapy | References |
|---|---|---|---|
| sTβR | Murine MSCs | Reduces TNF‐α, IFN‐γ, IL‐6, TGF‐β | 26 |
| HGF | Human MSCs | Reduces TNF‐α, IFN‐γ, IL‐6, TGF‐β, and inhibits myofibroblasts | 43, 49 |
| PGE2 | Murine MSCs | Increases their anti‐inflammatory IL‐10 production | 43, 52 |
| SOD1 | Murine MSCs | Scavenges ROS | 60 |
| SOD3 | Human MSC | Reduces collagen deposition, inflammatory cell infiltration, and oxidative stress | 61 |
| MnSOD | Human MSCs | Attenuates lung inflammation, ameliorates lung damage, and protects the lung cells from apoptosis | 62 |
| IL1RN | Murine MSCs | Inhibits IL‐1α and IL‐1β,and reduces TNF‐α | 65, 66 |
Like infectious, thermal, or physical damage, radiation‐induced damage of lung tissue can lead to the activation of the immune system 60. Infiltration of innate and adaptive immune cells is a common response of normal tissues to ionizing radiation in the lung 71. The pneumonitic phase is characterized by the recruitment of immune cells and a subsequent cascade of cytokines/chemokines that results in various degrees of lung inflammation after ionizing radiation 71. MSCs not only have a reparative and regenerative ability via the secretion of paracrine cytokines, including EGF, FGF, PDGF, TGF‐β, VEGF, HGF, Ang‐1, KGF, SDF‐1, IGF‐1, and others 72, but also have immunosuppressive properties through the secretion of IL‐10, TSG6, IL‐6, LIF, PGE2, HO‐1, and other cytokines 72. These immunosuppressive cytokines can minimize organ damage caused by inflammation and cells activated by the immune system via inhibiting activation and proliferation of immune cells, including T cells, B cells, NK cells dendritic cells, monocyte, macrophages, and neutrophils 71, 72, 73. Therefore, we hypothesize that MSCs have strong potential for treating RILI by secreting various paracrine cytokines that regulate inflammatory and fibrotic responses and immunomodulatory actions in injured lung tissue. Interestingly, Chen et al. reported that preactivation of MSCs with TNF‐α, IL‐1β, and nitric oxide can enhance paracrine effects on radiation‐induced injury by a heme oxygenase‐1 dependent mechanism 74. Similarly, Block et al. reported that MSCs can be activated by UV‐irradiated fibroblasts to secrete stanniocalcin‐1, a peptide hormone that modulates calcium metabolism 75. The antiapoptotic effect of stanniocalcin‐1 secretion by MSCs was also observed in a coculture system with injured lung cancer epithelial cells, suggesting that the inflammatory environment is probably a key factor in regulating the paracrine responses of MSCs 75. However, although utilizing the paracrine functions of MSCs to treat RILI shows considerable promise, further investigation of the paracrine‐associated mechanisms of MSCs in RILI therapy is still needed.
The Potential of MSC Therapy for RILI Based on the Release of Extracellular Vesicles
Advantages of Using MSC‐Derived Exosomes Compared with MSCs
In addition to secreting an array of soluble cytokines that could attenuate RILI (Fig. 1), MSCs also release large numbers of extracellular vesicles (EVs) that mediate tissue repair and anti‐inflammatory effects in lung pathogenesis 66. Indeed, exogenously administered MSCs may exert some of their complex paracrine anti‐inflammatory, antifibrogenic actions and proregenerative roles through released EVs 66. Therefore, there is growing interest in the possibility of using EVs derived from cultured MSCs as a safe and, effective cell‐free therapy 76, especially because of the potential carcinogenic effect of administering MSCs 77. EVs are typically categorized based on their biogenesis. The 3 main classes of EVs are exosomes, microvesicles and apoptotic bodies 66, all of which are enclosed by a lipid bilayer and which range from 30 to 2,000 nm in diameter depending on the biogenesis pathway 66. The term “exosome” refers to an endosome‐derived subclass of membrane microvesicles with a diameter of 50–100 nm, that are components of the secretome of multiple cell types, including MSCs 66, 78. Exosomes are important facilitators in cell‐to‐cell interactions by impacting multiple signaling pathways 66, 78, and their contents include proteins, miRNA and lipids 79. Compared with MSCs, MSC‐derived exosomes present exciting advantages 25. Firstly, exosomes are vesicles with a lipid bilayer membrane that protects a complex cargo of enzymes, cytokines and genetic material 80. They can transfer their cargo to target cells due to various proteins present on the vesicle surfaces that have binding affinity for cellular surfaces. Secondly, exosomes can travel freely through blood due to their small size and can easily fuse with cells due to their surface structures 80. In contrast, after systemic delivery, only a small number of MSCs arrive at the target site, and only a small percentage of those cells can integrate into the tissue and exert their functions for a short time. Thirdly, exosomes do not express MHC I or II antigens whereas MSCs can be induced to express higher levels of MHC II with inflammation 25. Fourthly, exosomes can be loaded with chemotherapeutics, specific proteins, metabolites, or RNAs including miRNAs and siRNAs 81.
Figure 1.
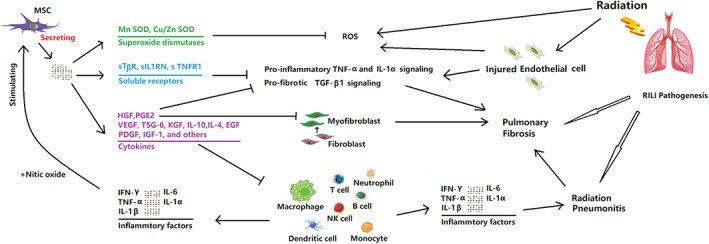
Mesenchymal stem cells (MSCs) regulate inflammatory signaling, fibrotic response and immune cells to attenuate radiation‐induced lung injury (RILI) via secreting an array of soluble factors. Radiation causes delayed damage to resident lung cells, leading primarily to the injury; however, MSCs can protect injured lung cells against ROS via secreting superoxide dismutases including SOD1 and SOD3. Radiation can also stimulate lung fibroblasts to differentiate into myofibroblasts. Myofibroblasts can further promote the synthesis of additional collagens, leading to excessive deposition and abnormal remodeling of the extracellular matrix, which is a hallmark of RILI. MSCs may inhibit lung myofibroblasts via secreting HGF and PGE2. Moreover, radiation can also activate proinflammatory signaling pathways and trigger the recruitment of various immune cells into the lung, such as monocytic cells, neutrophils, and lymphocytes. MSCs can inhibit proinflammatory signaling and immune cell activation via secreting soluble receptors and various cytokines including sTβR, sIL1RN, TNFR1, VEGF, KGF, EGF, IL‐10, TSG6, IL‐6, HGF, PGE2, and so forth.
Modulation of Expression Levels of RILI‐Related Inflammatory Cytokines by MSC‐Derived Extracellular Vesicles
RP differs from other pulmonary pneumonitis arising from other causes such as allergic pneumonitis, chemical pneumonitis, or pneumonia with viral, bacterial, fungal, or parasitic origins 9. Accumulating studies show that RP is a type of inflammatory reaction involving high expression levels of proinflammatory cytokines such as IL‐1α,IL‐1β, IL‐6, and others, which may play a main role in RILI progression and can be regulated by MSC application 23, 26, 33, 35, 49, 82. However, the immunomodulatory ability of MSCs cannot be sufficiently explained by the effects of only 1 secreted factor 83, 84, 85, and therefore it is likely that this ability results from the synergism of multiple factors. MSC‐exosome shuttling of multiple immunomodulatory proteins is an ideal pathway for this synergism 86, 87, 88, and consistent with this, MSC exosomes can attenuate levels of the proinflammatory cytokines IL‐1β, IL‐6, TNF‐α and induce high levels of the anti‐inflammatory IL‐10 in vitro 89. These released cytokines are also closely involved in RILI pathogenesis 33. Similar anti‐inflammatory effects were also reported in other studies of MSC‐exosome applications 90, 91, 92. Moreover, Wen et al. showed that both murine and human MSC‐derived extracellular vesicles can reverse radiation damage 93. Blazquez et al. further reported that exosomes derived from human adipose MSCs can inhibit the differentiation and activation of T cells and reduce IFN‐γ production by stimulated T cells in vitro 94. Based on these findings, we hypothesize that MSC exosomes could have therapeutic potential for RILI.
Modulation of Expression Levels of RILI‐Related Inflammatory Cytokines by MSC Release of Exosomal miRNAs
Changes in miRNAs after radiation have been reported in lung cancer patients undergoing radiotherapy in the clinic and animal studies 95, 96, suggesting that miRNAs may function during the pathologic process of RILI. Information approximately miRNA changes in the lung after radiation will facilitate a better understanding of the mechanism(s) of injury. Indeed, a growing number of studies show that using MSC exosome‐shuttled miRNAs can treat multiple inflammatory diseases by regulating levels of the proinflammatory cytokines IL‐1β, IL‐6, TNF‐α, and others 97. Li et al. further reported that MSC exosome‐shuttled miRNA‐181c can attenuate levels of IL‐1β and, TNF‐α and induce high levels of IL‐10 via targeting the TLR4/p65 signaling pathway 98, and therefore 1 possible strategy would be to use MSC‐released exosomal miRNAs to treat RILI. Indeed, increasing evidence has suggested that MSCs release exosomal miRNAs as vital extracellular communicators to mediate the regenerative and immunomodulatory effects that prevent inflammatory and fibrogenic activity in injured tissue, including: miR‐let‐7b targeting TLR4 99; miR‐21, miR‐23a, and miR‐145 targeting TGF‐β2 100; miR‐125b targeting Smad2 100; and miR‐let‐7c targeting TGF‐βR1 101. These targets of MSC‐released miRNAs are closely involved in production of various cytokines or in relevant inflammatory pathways in RILI, again strongly suggesting the potential of MSC‐released miRNAs for RILI therapy. Moreover, evidence indicates that highly abundant miRNAs shuttled by hucMSCs play a major role in preventing inflammatory and fibrogenic activity 100, 102 (Fig. 2), further supporting the potential of MSC‐released miRNAs for RILI therapy (Table 2).
Figure 2.
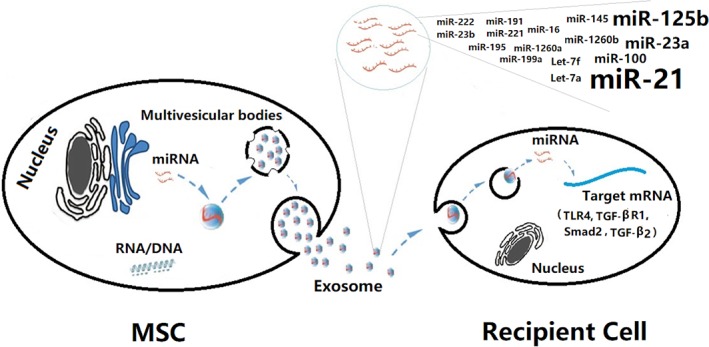
The anti‐inflammatory and antifibrogenic potential of mesenchymal stem cells (MSCs) released exosomal miRNAs. In the RILI microenvironment, MSCs actively release miRNAs by exosomal transportation, which are taken up by recipient cells including injured endothelial cells, immune cells, myofibroblast, fibroblast, and so forth. These activities can downregulate IL‐β, IL‐6, and TNF‐α by targeting the mRNA of proinflammatory and profibrogenic genes including TLR4, TGF‐β, Smad2, and so forth, and then attenuate radiation‐induced lung injury.
Table 2.
Anti‐inflammatory or antifibrotic effects of top 18 abundant miRNAs in umbilical cord MSCs‐derived exosomes
| MicroRNA | Targets | Effects on inflammation or fibrosis | References |
|---|---|---|---|
| miR‐21‐5p | PTEN, PDCD4 | Dampens NF‐κB/TNFα signaling, induces IL‐10 expression | 143 |
| miR‐125b‐5p | Smad2 | Inhibits TGF‐β2/SMAD2 pathway | 100 |
| miR‐23a‐3p | TGFβ2 | Inhibits TGF‐β2/SMAD2 pathway | 100 |
| miR‐100‐5p | mTOR | Modulates the expression of IL‐6 | 144, 145 |
| Let‐7f‐5p | IL‐6 | Targets IL6 to inhibit inflammation | 146 |
| Let‐7a‐5p | LIN28B, TGFBR1 | Targets Lin28B to regulate IL6 and NF‐κB pathway | 147, 148, 149 |
| miR‐145‐5p | Smad3 | Negatively regulates proinflammatory cytokine release from in COPD by targeting SMAD3 | 150 |
| miR‐1260b | Smad4 | Regulates TGF‐β pathway via targeting Smad4 | 151, 152 |
| miR‐1260a | COL1A1 | Targets the fibrosis marker COL1A1 | 153, 154 |
| miR‐199a‐3p | COX2 | Targets COX2 to block TNF‐α pathway | 149, 155 |
| miR‐16‐5p | Smad3 | Decreases IL‐1β, TNF‐α, and NF‐κB | 156, 157 |
| miR‐195‐5p | DLL1 | Inhibits notch‐induced IL‐22 secretion | 158, 159 |
| miR‐191‐5p | STX3 | Inhibits secretion of IL‐1α, IL‐1β, IL‐12b, and CCL4 via targeting STX3 | 160, 161 |
| miR‐221‐3p | SDF1 | Prevents IL‐1β‐induced ECM degradation | 162 |
| miR‐222‐3p | IRF‐2, ICAM‐1 | Inhibits inflammation via targeting IRF‐2, ICAM‐1 | 163, 164 |
| miR‐23b‐3p | PTEN | Inhibits PTEN to promote the phosphorylation of Akt which leads to a decrease in proinflammatory cytokine production | 165, 166 |
| miR‐3,120‐5p | Hsc70 | Inhibits HSC70‐triggered activation of TLR signaling and inflammatory cytokine production via target HSC 70 | 167, 168 |
| miR‐214‐3p | EZH1, EZH2 | Prevents fibrosis‐associated genes in myofibroblasts via targeting EZH1 and EZH2 | 169 |
Problems and Translational Challenges of the Therapies Based on MSC‐Derived Secretome and Exosomes
The evidence above indicates the therapeutic potential of the MSC paracrine secretome and exosomes for RILI; however, the problems and translational challenges of the MSC secretome and exosomes have to be addressed before the therapies can be routinely applied in RILI patients.
The Tumor‐Promoting Effect of MSC‐Derived Soluble Factors
Although results from clinical studies using MSCs for the treatment of various lung diseases show that MSC treatment in some patients is safe 103, the carcinogenic potential of MSCs has been the subject of strong controversy for some time 77, 104. Indeed, in addition to giving rise to cancer themselves, MSCs can also secrete a plethora of paracrine cytokines to promote tumor progression 104, 105. For example, cell proliferation is a key process of tumor growth in tumor progression, and MSCs can promote this process by releasing IL‐6 106, IL‐8 107, MCP‐1 108, CXCL16 109, and glutamine 110, by activating the JAK2‐STAT3 pathway 106 and AMPK/mTOR‐mediated NF‐κB signaling 107, and through a β1‐integrin‐dependent mechanism 111. Moreover, angiogenesis, as an essential component of tumor growth and survival, can also be promoted by key vasculogenic factors secreted by MSCs, including CXCL1 112, CXCL8 112, GDNF 113, VEGF 114, and TGF‐β 114. In addition, tumor cell invasion and migration are further malignant behaviors in tumor progression, and MSCs can promote these behaviors by producing and releasing IL‐6 115, IL‐8 115, CCL5 116, IL‐17B 117, soluble NRG1 118, β2‐microglobulin 119, FGF10 120, VEGFC 120, MMPs 120, and nitric oxide 121, by activation of NF‐κΒ, STAT3, PI3K/AKT signaling 117, 122, and in part through the Wnt pathway 120. In addition to promoting tumor progression, MSCs can also elicit drug resistance in tumors by secreting CXCL1 and by activation of the STAT3 pathway 123, 124, and further promote tumor stemness by producing Gremlin‐1, BMP2, BMP4, CXCL7, CXCL12, and others 114.
The Tumor‐Promoting Effect of MSC‐Derived Exosomes
Despite the fact that exosomes do not elicit acute immune rejection and lack the potential to directly form tumors, MSC‐derived exosomes are also capable of inducing physiological processes in tumor development, for example, proliferation, angiogenesis, metastasis, and drug resistance. For example, MSC‐derived exosomes can promote these processes in gastric cancer cells via activation of ERK, the PKB pathway 125, and Hedgehog signaling 126. Moreover, MSC‐derived exosomes can promote EMT effects via the FGF19‐FGFR4 axis in nasopharyngeal carcinoma 127 and by TGF‐β1 signaling in lung cancer 128. In addition, such exosomes can also deliver mir‐222/223 or mir‐9 to induce drug resistance in tumors 125. Similarly, Dong et al. showed that MSC‐derived EVs can promote lung cancer growth by transferring mir‐410, which is probably involved in PTEN downregulation 129. On the other hand, MSC‐derived exosomes are reported to exert proapoptotic functions in hepatoma 130, Kaposi's sarcoma 130 and ovarian tumors 130, suggesting a complex role for MSC‐derived exosomes in tumor progression and one that warrants further investigation.
Hurdles in Clinical Translation
Compared with the risk of tumor‐promoting effect of MSCs, the following major translational challenges should be considered seriously before clinical application of the MSC paracrine secretome and exosomes in the future.
First, according to the European and United States regulatory bodies, acquisition and processing of MSCs need to be in accordance with Good Manufacturing Practices (GMPs), which demand a high level of standardization, regarding the isolation of MSCs, the culture medium and serum for MSCs, and the use of closed‐system bioreactors. GMP standards also demand stringent and standardized quality control measures for MSC production with reference to microbiological safety and the absence of any transformation due to genetic instability 77. In addition, lack of a comparative characterization of murine and human MSCs may also limit the direct translation of preclinical animal model findings to clinical trials 24. The etiology and progression of human inflammatory diseases are multifactorial, and animal models of inflammatory diseases do not fully represent human inflammatory diseases 24. Translation of cellular or biological therapy from an animal model of inflammatory disease to human inflammatory disease remains a challenge. Additionally, MSCs derived from mice and humans are not identical in their capacity to suppress inflammation or in their mechanisms of action due to specific species differences 24. For these reasons, preclinical animal studies with murine MSCs cannot precisely predict the outcome of human MSC‐based clinical trials. Moreover, differences in MSC source, preparation, and handling methods may affect the quality and therapeutic efficacy of the cellular product and subsequently affect the clinical outcome 24. For example, cryopreserved MSCs exhibited attenuated biodistribution and immunosuppressive properties compared with actively growing MSCs in cultures in vitro. The effects of MSC transplantation may also be limited not only by the transplantation site of MSC injection but also by the number of transplanted MSCs 131. Indeed, it has been reported that <1% of MSCs survive for more than 1 week after systemic administration 132, 133. Thus, many issues should be considered when translating the therapeutic promise of MSCs in preclinical studies to the clinical setting. Another big concern regarding MSC therapy for RILI is perhaps the potential for fibrosis. Yan et al. reported that Flk‐1 + MSCs injected into the lung immediately after irradiation could differentiate into functional lung cells, but those injected at a later stage after irradiation may be involved in fibrosis development 134, possibly because the TGF‐β1 level is markedly increased in the middle and later stages after irradiation. The dramatic change in the microenvironment of the injured lung might also inhibit differentiation of transplanted MSCs into lung epithelial cells and induce them to differentiate into myofibrocytes, which then participate in lung fibrosis.
Second, to understand the mechanisms of how cytokines are expressed during RILI and how they modulate the therapeutic effects of stem cells are a significant challenge due to the myriad complex interactions of paracrine factors in the secretome of MSCs. In addition, despite the fact that the secretome of MSCs is normally present in conditioned medium, the components of conditioned medium do not only reflect the secretome because the medium also contains proteins that are released during cell death. Avoiding leakage of intracellular proteins from dead cells is thus a challenge in the careful optimization of the secretome from MSCs. Furthermore, the production and concentration of secreted molecules in quantities sufficient for clinical administration are also challenging. Other limitations of secretome therapy include tissue transport, pharmacokinetics, and protein stability.
Third, as with the use of MSCs, the defined and standardized methods used to isolate and identify exosomes will also be required, according to the International Society of Extracellular Vesicles 135. Thus, a universally accepted protocol for exosome isolation, large‐scale GMP production guidance, as well as validated methods for quantifying and evaluating the potency of exosomes are lacking. According to preclinical studies, the amount of MSC EVs required to produce the equivalent effect of MSCs in lung injury is generally 10 times higher 25. Therefore, if the average dose of MSCs is 10 × 106 cells per kilogram per body weight, the number of MSCs required to generate enough exosomes may be greater than 100 × 106 of MSC per kilogram, which may make the production costs prohibitive. In addition, expanding big batches of MSCs for exosome production will impact the costs of derivation, testing, and validation, because the biological properties of MSCs may become altered with repeated passages 25. Although a potential approach to significantly increasing extracellular vesicle production could be the use of bioreactor systems to culture MSCs, different bioreactor culture conditions may impact the content and therapeutic efficacy of EVs, including the build‐up of metabolic byproducts, pH balance, hydrodynamic shear stress, and oxygen supply 25. The very significant barriers above underline the need for caution on rapid clinical translation. Although clinical effects of using MSC exosomes have been explored in phase I and II trials in lung diseases 136, the latent side effects, based on the recipients’ long‐term follow‐up, was unclear. In addition, the uptake mechanisms for exosomal miRNAs and the mechanisms that regulate incorporation of a particular miRNA into exosomes are still unclear, so the best methods for confirming their safety and proving their efficacy in vitro and especially in vivo still need to be resolved. Finally, other issues related to clinical application of these therapies, including ethical, legal, technical and regulatory concerns, are also challenging.
Summary and Future Perspectives
RILI is a major limiting factor in the application of thoracic radiation and a major obstacle to the use of advanced dose escalation modalities and ablative hypofractionation radiotherapy regimens. Therefore, developing alternative strategies to protect the lungs from RILI is essential. However, there are still existing drawbacks in the application of various therapeutic drugs for RILI therapy. MSCs, as an immunomodulatory and regenerative tool, exhibit considerable promise for targeting RILI pathogenesis by secreting paracrine cytokines, as demonstrated in a growing number of studies. However, tumor‐promoting features of the MSC paracrine secretome may limit the translational application of MSCs for clinical RILI patients. Indeed, it has been reported that MSCs may possess a distinct tropism to tumors after systemic administration 137, 138. And some studies have demonstrated that MSCs that are cultured for a long‐term may exhibit some neoplastic transformation 139, 140. The progression of existing tumors may be problematic due to direct effect on the tumor or surrounding stroma as a result of reducing inflammation and promoting tumor evasion from the immune system. Thus, the limitations of tumorigenic potential of MSCs in clinical trials should be also considered. Furthermore, studies should focus on more clearly identifying factors responsible for the therapeutic effects of MSCs. These efforts will help to develop more effective protein‐based conditioning approaches. Modification of the secretion profile to augment the therapeutic effects of the secretome may be achieved via physical, physiological, and pharmaceutical preconditioning of stem cells including hypoxia induction, treatment with disease‐specific drugs, small molecules, specific growth factors/cytokines, and cellular reprogramming/genetic manipulation strategies.
Compared with MSCs, although MSC‐derived exosomes exhibit many advantages for treating lung diseases 25, the promoting and proapoptotic role of MSC‐derived exosomes on tumor progression raises controversy with reference to their suitability for clinical translation. Fortunately, it was recently reported that miRNAs are transported by exosomes in lung disease 136, suggesting that, instead of native exosomes, exosomes modified by the expression of different miRNAs will be a promising alternative for RILI therapy. In particular, taking advantage of miRNAs that can inhibit both cancer and inflammation will be a better alternative for RILI therapy based on MSC‐derived exosome cargo.
In addition, MSC‐derived exosomes contain not only miRNA but also lipids 79 and long noncoding RNAs, which regulate multiple signaling pathways related to inflammation 141. Moreover, immune cells can interact with various classes of lipids to regulate the plasticity of macrophages and T lymphocytes 142. Whether MSC‐derived exosomes can attenuate RILI via shuttled long noncoding RNAs, lipids, or both is unknown. Thus, the progress made so far on the potential of MSCs in RILI therapy suggests that the MSC‐derived secretome and exosomes function in RILI through a variety of mechanisms but that further research is needed to understand their potential.
Author Contributions
S.X.: manuscript preparation, financial support; C.L.: literature search; S.X. and H.L.J.: conception and design, manuscript revision, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Contributor Information
Siguang Xu, Email: xusiguang@qq.com.
Hong‐Long Ji, Email: james.ji@uthct.edu.
References
- 1. Jeremic B, Fidarova E, Sharma V et al. The International Atomic Energy Agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol 2015;116:21–26. [DOI] [PubMed] [Google Scholar]
- 2. Graves PR, Siddiqui F, Anscher MS et al. Radiation pulmonary toxicity: From mechanisms to management. Semin Radiat Oncol 2010;20:201–207. [DOI] [PubMed] [Google Scholar]
- 3. Marks LB, Yu X, Vujaskovic Z et al. Radiation‐induced lung injury. Semin Radiat Oncol 2003;13:333–345. [DOI] [PubMed] [Google Scholar]
- 4. Marks LB, Bentzen SM, Deasy JO et al. Radiation dose‐volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta V. Radiation pneumonitis and pulmonary fibrosis in non‐small‐cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5–24. [DOI] [PubMed] [Google Scholar]
- 6. Epperly MW, Guo H, Gretton JE et al. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:213–224. [DOI] [PubMed] [Google Scholar]
- 7. Takeda A, Kunieda E, Takeda T et al. Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumor recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2008;70:1057–1065. [DOI] [PubMed] [Google Scholar]
- 8. Castellino RA, Glatstein E, Turbow MM et al. Latent radiation injury of lungs or heart activated by steroid withdrawal. Ann Intern Med 1974;80:593–599. [DOI] [PubMed] [Google Scholar]
- 9. Rajan Radha R, Chandrasekharan G. Pulmonary injury associated with radiation therapy—Assessment, complications and therapeutic targets. Biomed Pharmacother 2017;89:1092–1104. [DOI] [PubMed] [Google Scholar]
- 10. Devine A, Marignol L. Potential of amifostine for chemoradiotherapy and radiotherapy‐associated toxicity reduction in advanced NSCLC: A meta‐analysis. Anticancer Res 2016;36:5–12. [PubMed] [Google Scholar]
- 11. Arora R, Gupta D, Chawla R et al. Radioprotection by plant products: Present status and future prospects. Phytother Res 2005;19:1–22. [DOI] [PubMed] [Google Scholar]
- 12. Srinivasan M, Sudheer AR, Pillai KR et al. Lycopene as a natural protector against gamma‐radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim Biophys Acta 2007;1770:659–665. [DOI] [PubMed] [Google Scholar]
- 13. Liu Z, Lei X, Li X et al. Toll‐like receptors and radiation protection. Eur Rev Med Pharmacol Sci 2018;22:31–39. [DOI] [PubMed] [Google Scholar]
- 14. Ghafoori P, Marks LB, Vujaskovic Z et al. Radiation‐induced lung injury assessment, management, and prevention. Oncology 2008;22:37–47. [PubMed] [Google Scholar]
- 15. Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med 2011;208:1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horie S, Laffey JG. Recent insights: Mesenchymal stromal/stem cell therapy for acute respiratory distress syndrome. Anesthesiology 2016;5:238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penfornis P, Pochampally R. Isolation and expansion of mesenchymal stem cells/multipotential stromal cells from human bone marrow. Methods Mol Biol 2011;698:11–21. [DOI] [PubMed] [Google Scholar]
- 18. Rasini V, Dominici M, Kluba T et al. Mesenchymal stromal/stem cells markers in the human bone marrow. Cytotherapy 2013;15:292–306. [DOI] [PubMed] [Google Scholar]
- 19. Lindner U, Kramer J, Rohwedel J et al. Mesenchymal stem or stromal cells: Toward a better understanding of their biology? Transfus Med Hemother 2010;37:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobs SA, Roobrouck VD, Verfaillie CM et al. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 2013;91:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golemovic M, Skific M, Cepulic BG. Mesenchymal stem cells: Immunomodulatory properties and clinical application. Lijec Vjesn 2012;134:42–49. [PubMed] [Google Scholar]
- 22. Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res Ther 2016;7:82–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang X, Jiang X, Qu C et al. Intravenous delivery of adipose‐derived mesenchymal stromal cells attenuates acute radiation‐induced lung injury in rats. Cytotherapy 2015;17:560–570. [DOI] [PubMed] [Google Scholar]
- 24. Chinnadurai R, Ng S, Velu V et al. Challenges in animal modelling of mesenchymal stromal cell therapy for inflammatory bowel disease. World J Gastroenterol 2015;21:4779–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monsel A, Zhu YG, Gudapati V et al. Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther 2016;16:859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue J, Li X, Lu Y et al. Gene‐modified mesenchymal stem cells protect against radiation‐induced lung injury. Mol Ther 2013;21:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Agostino B, Sullo N, Siniscalco D et al. Mesenchymal stem cell therapy for the treatment of chronic obstructive pulmonary disease. Expert Opin Biol Ther 2010;10:681–687. [DOI] [PubMed] [Google Scholar]
- 28. Weiss DJ, Kolls JK, Ortiz LA et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riley PA. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 1994;65:27–33. [DOI] [PubMed] [Google Scholar]
- 30. Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation‐induced lung fibrosis. Curr Drug Targets 2013;14:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation‐induced late normal tissue injury: Therapeutic implications. Curr Med Chem 2009;16:130–143. [DOI] [PubMed] [Google Scholar]
- 32. Johnston CJ, Williams JP, Okunieff P et al. Radiation‐induced pulmonary fibrosis: Examination of chemokine and chemokine receptor families. Radiat Res 2002;157:256–265. [DOI] [PubMed] [Google Scholar]
- 33. Xia C, Chang P, Zhang Y et al. Therapeutic effects of bone marrow‐derived mesenchymal stem cells on radiation‐induced lung injury. Oncol Rep 2016;35:731–738. [DOI] [PubMed] [Google Scholar]
- 34. Maria AT, Toupet K, Bony C et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl‐induced systemic sclerosis. Arthritis Rheumatol 2016;68:1013–1025. [DOI] [PubMed] [Google Scholar]
- 35. Oh JY, Ko JH, Lee HJ et al. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells 2014;32:1553–1563. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Li D, Liu X et al. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS‐induced acute lung injury in rats. J Inflamm 2012;9:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klein D, Schmetter A, Imsak R et al. Therapy with multipotent mesenchymal stromal cells protects lungs from radiation‐induced injury and reduces the risk of lung metastasis. Antioxid Redox Signal 2016;24:53–69. [DOI] [PubMed] [Google Scholar]
- 38. Martin M, Lefaix JL, Pinton P et al. Temporal modulation of TGF‐beta1 and beta‐actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res 1993;134:63–70. [PubMed] [Google Scholar]
- 39. Martin M, Lefaix J, Delanian S. TGF‐beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000;47:277–290. [DOI] [PubMed] [Google Scholar]
- 40. Rodemann HP, Bamberg M. Cellular basis of radiation‐induced fibrosis. Radiother Oncol 1995;35:83–90. [DOI] [PubMed] [Google Scholar]
- 41. Hu Y, Peng J, Feng D et al. Role of extracellular signal‐regulated kinase, p38 kinase, and activator protein‐1 in transforming growth factor‐beta1‐induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung 2006;184:33–42. [DOI] [PubMed] [Google Scholar]
- 42. Zhang C, Zhu Y, Zhang Y et al. Therapeutic potential of umbilical cord mesenchymal stem cells for inhibiting myofibroblastic differentiation of irradiated human lung fibroblasts. Tohoku J Exp Med 2015;236:209–217. [DOI] [PubMed] [Google Scholar]
- 43. Dong LH, Jiang YY, Liu YJ et al. The anti‐fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci Rep 2015;5:8713–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu J, Pan G, Liang T et al. HGF/c‐Met signaling mediated mesenchymal stem cell‐induced liver recovery in intestinal ischemia reperfusion model. Int J Med Sci 2014;11:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kearns‐Jonker M, Dai W, Gunthart M et al. Genetically engineered mesenchymal stem cells influence gene expression in donor cardiomyocytes and the recipient heart. J Stem Cell Res Ther 2012;1:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shukla MN, Rose JL, Ray R et al. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol 2009;40:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mizuno S, Matsumoto K, Li MY et al. HGF reduces advancing lung fibrosis in mice: A potential role for MMP‐dependent myofibroblast apoptosis. FASEB J 2005;19:580–582. [DOI] [PubMed] [Google Scholar]
- 48. Singh S, Saraiva L, Elkington PT et al. Regulation of matrix metalloproteinase‐1, −3, and −9 in Mycobacterium tuberculosis‐dependent respiratory networks by the rapamycin‐sensitive PI3K/p70(S6K) cascade. FASEB J 2014;28:85–93. [DOI] [PubMed] [Google Scholar]
- 49. Wang H, Yang YF, Zhao L et al. Hepatocyte growth factor gene‐modified mesenchymal stem cells reduce radiation‐induced lung injury. Hum Gene Ther 2013;24:343–353. [DOI] [PubMed] [Google Scholar]
- 50. Matloubian M, Lo CG, Cinamon G et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355–360. [DOI] [PubMed] [Google Scholar]
- 51. Sanna MG, Wang SK, Gonzalez‐Cabrera PJ et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2006;2:434–441. [DOI] [PubMed] [Google Scholar]
- 52. Nemeth K, Leelahavanichkul A, Yuen PS et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med 2009;15:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okunishi K, Sisson TH, Huang SK et al. Plasmin overcomes resistance to prostaglandin E2 in fibrotic lung fibroblasts by reorganizing protein kinase A signaling. J Biol Chem 2011;286:32231–32243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moore BB, Peters‐Golden M, Christensen PJ et al. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP‐1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol 2003;284:342–349. [DOI] [PubMed] [Google Scholar]
- 55. Walker NM, Badri LN, Wadhwa A et al. Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med 2012;185:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomas PE, Peters‐Golden M, White ES et al. PGE(2) inhibition of TGF‐beta1‐induced myofibroblast differentiation is Smad‐independent but involves cell shape and adhesion‐dependent signaling. Am J Physiol Lung Cell Mol Physiol 2007;293:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White ES, Atrasz RG, Dickie EG et al. Prostaglandin E(2) inhibits fibroblast migration by E‐prostanoid 2 receptor‐mediated increase in PTEN activity. Am J Respir Cell Mol Biol 2005;32:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bauman KA, Wettlaufer SH, Okunishi K et al. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 2010;120:1950–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kost OA, Beznos OV, Davydova NG et al. Superoxide dismutase 1 nanozyme for treatment of eye inflammation. Oxid Med Cell Longev 2015;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Klein D, Steens J, Wiesemann A et al. Mesenchymal stem cell therapy protects lungs from radiation‐induced endothelial cell loss by restoring superoxide dismutase 1 expression. Antioxid Redox Signal 2017;26:563–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wei L, Zhang J, Yang ZL et al. Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis. Cytotherapy 2017;19:586–602. [DOI] [PubMed] [Google Scholar]
- 62. Chen HX, Xiang H, Xu WH et al. Manganese superoxide dismutase gene‐modified mesenchymal stem cells attenuate acute radiation‐induced lung injury. Hum Gene Ther 2017;28:523–532. [DOI] [PubMed] [Google Scholar]
- 63. Halliwell B. Free radicals and antioxidants: Updating a personal view. Nutr Rev 2012;70:257–265. [DOI] [PubMed] [Google Scholar]
- 64. Kim J, Kil IS, Seok YM et al. Orchiectomy attenuates post‐ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J Biol Chem 2006;281:20349–20356. [DOI] [PubMed] [Google Scholar]
- 65. Johnston CJ, Piedboeuf B, Rubin P et al. Early and persistent alterations in the expression of interleukin‐1 alpha, interleukin‐1 beta and tumor necrosis factor alpha mRNA levels in fibrosis‐resistant and sensitive mice after thoracic irradiation. Radiat Res 1996;145:762–767. [PubMed] [Google Scholar]
- 66. Ortiz LA, Dutreil M, Fattman C et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haase MG, Klawitter A, Geyer P et al. Sustained elevation of NF‐kappaB DNA binding activity in radiation‐induced lung damage in rats. Int J Radiat Biol 2003;79:863–877. [DOI] [PubMed] [Google Scholar]
- 68. Yagi H, Soto‐Gutierrez A, Navarro‐Alvarez N et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 2010;18:1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ikushima H, Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat Rev Cancer 2010;10:415–424. [DOI] [PubMed] [Google Scholar]
- 70. Fujita Y, Kosaka N, Araya J et al. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol Med 2015;21:533–542. [DOI] [PubMed] [Google Scholar]
- 71. Wirsdorfer F, Jendrossek V. The role of lymphocytes in radiotherapy‐induced adverse late effects in the lung. Front Immunol 2016;7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ma S, Xie N, Li W et al. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res 2016;9:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen H, Min XH, Wang QY et al. Pre‐activation of mesenchymal stem cells with TNF‐alpha, IL‐1beta and nitric oxide enhances its paracrine effects on radiation‐induced intestinal injury. Sci Rep 2015;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75. Block GJ, Ohkouchi S, Fung F et al. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin‐1. Stem Cells 2009;27:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rani S, Ryan AE, Griffin MD et al. Mesenchymal stem cell‐derived extracellular vesicles: Toward cell‐free therapeutic applications. Mol Ther 2015;23:812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nicolay NH, Lopez Perez R, Debus J et al. Mesenchymal stem cells—A new hope for radiotherapy‐induced tissue damage? Cancer Lett 2015;366:133–140. [DOI] [PubMed] [Google Scholar]
- 78. Lee C, Mitsialis SA, Aslam M et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia‐induced pulmonary hypertension. Circulation 2012;126:2601–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mobius MA, Thebaud B. Stem cells and their mediators—Next generation therapy for bronchopulmonary dysplasia. Front Med 2015;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rosca AM, Rayia DM, Tutuianu R. Emerging role of stem cells‐derived exosomes as valuable tools for cardiovascular therapy. Curr Stem Cell Res Ther 2017;12:134–138. [DOI] [PubMed] [Google Scholar]
- 81. Melzer C, von der Ohe J, Hass R. Concise review: Crosstalk of mesenchymal stroma/stem‐like cells with cancer cells provides therapeutic potential. Stem Cells 2018;36:951–968. [DOI] [PubMed] [Google Scholar]
- 82. Chen Y, Williams J, Ding I et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol 2002;12:26–33. [DOI] [PubMed] [Google Scholar]
- 83. Kang HS, Habib M, Chan J et al. A paradoxical role for IFN‐gamma in the immune properties of mesenchymal stem cells during viral challenge. Exp Hematol 2005;33:796–803. [DOI] [PubMed] [Google Scholar]
- 84. Liu H, Lu K, MacAry PA et al. Soluble molecules are key in maintaining the immunomodulatory activity of murine mesenchymal stromal cells. J Cell Sci 2012;125:200–208. [DOI] [PubMed] [Google Scholar]
- 85. Forest VF, Tirouvanziam AM, Perigaud C et al. Cell distribution after intracoronary bone marrow stem cell delivery in damaged and undamaged myocardium: Implications for clinical trials. Stem Cell Res Ther 2010;1:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lai RC, Tan SS, Teh BJ et al. Proteolytic potential of the MSC exosome proteome: Implications for an exosome‐mediated delivery of therapeutic proteasome. Int J Proteomics 2012;2012:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- 88. Zhang B, Yin Y, Lai RC et al. Immunotherapeutic potential of extracellular vesicles. Front Immunol 2014;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang B, Yin Y, Lai RC et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 2014;23:1233–1244. [DOI] [PubMed] [Google Scholar]
- 90. Nong K, Wang W, Niu X et al. Hepatoprotective effect of exosomes from human‐induced pluripotent stem cell‐derived mesenchymal stromal cells against hepatic ischemia‐reperfusion injury in rats. Cytotherapy 2016;18:1548–1559. [DOI] [PubMed] [Google Scholar]
- 91. Tofino‐Vian M, Guillen MI, Perez Del Caz MD et al. Microvesicles from human adipose tissue‐derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem 2018;47:11–25. [DOI] [PubMed] [Google Scholar]
- 92. Chen W, Huang Y, Han J et al. Immunomodulatory effects of mesenchymal stromal cells‐derived exosome. Immunol Res 2016;64:831–840. [DOI] [PubMed] [Google Scholar]
- 93. Wen S, Dooner M, Cheng Y et al. Mesenchymal stromal cell‐derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016;30:2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Blazquez R, Sanchez‐Margallo FM, de la Rosa O et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol 2014;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tomasik B, Chalubinska‐Fendler J, Chowdhury D et al. Potential of serum microRNAs as biomarkers of radiation injury and tools for individualization of radiotherapy. Transl Res 2018;201:71–83. [DOI] [PubMed] [Google Scholar]
- 96. Shin S, Cha HJ, Lee EM et al. Alteration of miRNA profiles by ionizing radiation in A549 human non‐small cell lung cancer cells. Int J Oncol 2009;35:81–86. [PubMed] [Google Scholar]
- 97. Ti D, Hao H, Fu X et al. Mesenchymal stem cells‐derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci 2016;59:1305–1312. [DOI] [PubMed] [Google Scholar]
- 98. Li X, Liu L, Yang J et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates miR‐181c attenuating burn‐induced excessive inflammation. EBioMedicine 2016;8:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ti D, Hao H, Tong C et al. LPS‐preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome‐shuttled let‐7b. J Transl Med 2015;13:308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fang S, Xu C, Zhang Y et al. Umbilical cord‐derived mesenchymal stem cell‐derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor‐beta/SMAD2 pathway during wound healing. Stem Cells Translational Medicine 2016;5:1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang B, Yao K, Huuskes BM et al. Mesenchymal stem cells deliver exogenous microRNA‐let7c via exosomes to attenuate renal fibrosis. Mol Ther 2016;24:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Qian X, Xu C, Fang S et al. Exosomal microRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis C virus infection. Stem Cells Translational Medicine 2016;5:1190–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Geiger S, Hirsch D, Hermann FG. Cell therapy for lung disease. Eur Respir Rev 2017;26:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rhee KJ, Lee JI, Eom YW. Mesenchymal stem cell‐mediated effects of tumor support or suppression. Int J Mol Sci 2015;16:30015–30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Haarer J, Johnson CL, Soeder Y et al. Caveats of mesenchymal stem cell therapy in solid organ transplantation. Transpl Int 2015;28:1–9. [DOI] [PubMed] [Google Scholar]
- 106. Wei HJ, Zeng R, Lu JH et al. Adipose‐derived stem cells promote tumor initiation and accelerate tumor growth by interleukin‐6 production. Oncotarget 2015;6:7713–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu XB, Liu Y, Wang GH et al. Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR‐mediated NF‐kappaB activation. Sci Rep 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jia XH, Du Y, Mao D et al. Zoledronic acid prevents the tumor‐promoting effects of mesenchymal stem cells via MCP‐1 dependent recruitment of macrophages. Oncotarget 2015;6:26018–26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Takiguchi G, Nishita M, Kurita K et al. Wnt5a‐Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16‐CXCR6 axis. Cancer Sci 2016;107:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tang K, Hu L, Ma J et al. Brief report: Human mesenchymal stem‐like cells facilitate floating tumorigenic cell growth via glutamine‐ammonium cycle. Stem Cells 2015;33:2877–2884. [DOI] [PubMed] [Google Scholar]
- 111. Widder M, Lutzkendorf J, Caysa H et al. Multipotent mesenchymal stromal cells promote tumor growth in distinct colorectal cancer cells by a beta1‐integrin‐dependent mechanism. Int J Cancer 2016;138:964–975. [DOI] [PubMed] [Google Scholar]
- 112. Wang Y, Liu J, Jiang Q et al. Human adipose‐derived mesenchymal stem cell‐secreted CXCL1 and CXCL8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells 2017;35:2060–2070. [DOI] [PubMed] [Google Scholar]
- 113. Zhong Z, Gu H, Peng J et al. GDNF secreted from adipose‐derived stem cells stimulates VEGF‐independent angiogenesis. Oncotarget 2016;7:36829–36841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ghaderi A, Abtahi S. Mesenchymal stem cells: Miraculous healers or dormant killers? Stem Cell Rev 2018;14:722–733. [DOI] [PubMed] [Google Scholar]
- 115. Ma F, Chen D, Chen F et al. Human umbilical cord mesenchymal stem cells promote breast cancer metastasis by interleukin‐8‐ and interleukin‐6‐dependent induction of CD44(+)/CD24(−) cells. Cell Transplant 2015;24:2585–2599. [DOI] [PubMed] [Google Scholar]
- 116. Zhong W, Tong Y, Li Y et al. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF‐kappaB signaling by paracrine CCL5. Oncotarget 2017;8:73693–73704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bie Q, Zhang B, Sun C et al. IL‐17B activated mesenchymal stem cells enhance proliferation and migration of gastric cancer cells. Oncotarget 2017;8:18914–18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. De Boeck A, Pauwels P, Hensen K et al. Bone marrow‐derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 2013;62:550–560. [DOI] [PubMed] [Google Scholar]
- 119. Wang J, Yang W, Wang T et al. Mesenchymal stromal cells‐derived beta2‐microglobulin promotes epithelial‐mesenchymal transition of esophageal squamous cell carcinoma cells. Sci Rep 2018;8:5422–5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen D, Liu S, Ma H et al. Paracrine factors from adipose‐mesenchymal stem cells enhance metastatic capacity through Wnt signaling pathway in a colon cancer cell co‐culture model. Cancer Cell Int 2015;15:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang L, Sun J, Liu Z et al. Mesenchymal stem cells regulate cytoskeletal dynamics and promote cancer cell invasion through low dose nitric oxide. Curr Mol Med 2014;14:749–761. [DOI] [PubMed] [Google Scholar]
- 122. Zhang X, Hu F, Li G et al. Human colorectal cancer‐derived mesenchymal stem cells promote colorectal cancer progression through IL‐6/JAK2/STAT3 signaling. Cell Death Dis 2018;9:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yeh WL, Tsai CF, Chen DR. Peri‐foci adipose‐derived stem cells promote chemoresistance in breast cancer. Stem Cell Res Ther 2017;8:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang W, Zhong W, Yuan J et al. Involvement of Wnt/beta‐catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 2015;6:42276–42289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sharma A. Role of stem cell derived exosomes in tumor biology. Int J Cancer 2018;142:1086–1092. [DOI] [PubMed] [Google Scholar]
- 126. Qi J, Zhou Y, Jiao Z et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem 2017;42:2242–2254. [DOI] [PubMed] [Google Scholar]
- 127. Shi S, Zhang Q, Xia Y et al. Mesenchymal stem cell‐derived exosomes facilitate nasopharyngeal carcinoma progression. Am J Cancer Res 2016;6:459–472. [PMC free article] [PubMed] [Google Scholar]
- 128. Zhao X, Wu X, Qian M et al. Knockdown of TGF‐beta1 expression in human umbilical cord mesenchymal stem cells reverts their exosome‐mediated EMT promoting effect on lung cancer cells. Cancer Lett 2018;428:34–44. [DOI] [PubMed] [Google Scholar]
- 129. Dong L, Pu Y, Zhang L et al. Human umbilical cord mesenchymal stem cell‐derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR‐410. Cell Death Dis 2018;9:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bruno S, Collino F, Deregibus MC et al. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 2013;22:758–771. [DOI] [PubMed] [Google Scholar]
- 131. Majka M, Sulkowski M, Badyra B et al. Concise review: Mesenchymal stem cells in cardiovascular regeneration: Emerging research directions and clinical applications. Stem Cells Translational Medicine 2017;6:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Eggenhofer E, Benseler V, Kroemer A et al. Mesenchymal stem cells are short‐lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 2012;3:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Song YS, Lee HJ, Doo SH et al. Mesenchymal stem cells overexpressing hepatocyte growth factor (HGF) inhibit collagen deposit and improve bladder function in rat model of bladder outlet obstruction. Cell Transplant 2012;21:1641–1650. [DOI] [PubMed] [Google Scholar]
- 134. Yan X, Liu Y, Han Q et al. Injured microenvironment directly guides the differentiation of engrafted Flk‐1(+) mesenchymal stem cell in lung. Exp Hematol 2007;35:1466–1475. [DOI] [PubMed] [Google Scholar]
- 135. Witwer KW, Buzas EI, Bemis LT et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2:134–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen J, Hu C, Pan P. Extracellular vesicle microRNA transfer in lung diseases. Front Physiol 2017;8:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kidd S, Spaeth E, Dembinski JL et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009;27:2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang H, Cao F, De A et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor‐bearing mice by bioluminescence imaging. Stem Cells 2009;27:1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rosland GV, Svendsen A, Torsvik A et al. Long‐term cultures of bone marrow‐derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 2009;69:5331–5339. [DOI] [PubMed] [Google Scholar]
- 140. Rubio D, Garcia‐Castro J, Martin MC et al. Spontaneous human adult stem cell transformation. Cancer Res 2005;65:3035–3039. [DOI] [PubMed] [Google Scholar]
- 141. Gao F, Yu L, Zhang D et al. Long noncoding RNAs and their regulatory network: Potential therapeutic targets for adult moyamoya disease. World Neurosurg 2016;93:111–119. [DOI] [PubMed] [Google Scholar]
- 142. Hubler MJ, Kennedy AJ. Role of lipids in the metabolism and activation of immune cells. J Nutr Biochem 2016;34:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Das A, Ganesh K, Khanna S et al. Engulfment of apoptotic cells by macrophages: A role of microRNA‐21 in the resolution of wound inflammation. J Immunol 2014;192:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Aungier SR, Ohmori H, Clinton M et al. MicroRNA‐100‐5p indirectly modulates the expression of Il6, Ptgs1/2 and Tlr4 mRNA in the mouse follicular dendritic cell‐like cell line, FL‐Y. Immunology 2015;144:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ye X, Luo H, Chen Y et al. MicroRNAs 99b‐5p/100‐5p regulated by endoplasmic reticulum stress are involved in abeta‐induced pathologies. Front Aging Neurosci 2015;7:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gong Z, Zhao S, Zhang J et al. Initial research on the relationship between let‐7 family members in the serum and massive cerebral infarction. J Neurol Sci 2016;361:150–157. [DOI] [PubMed] [Google Scholar]
- 147. Lee SI, Jeon MH, Kim JS et al. The gga‐let‐7 family post‐transcriptionally regulates TGFBR1 and LIN28B during the differentiation process in early chick development. Mol Reprod Dev 2015;82:967–975. [DOI] [PubMed] [Google Scholar]
- 148. Graf R, Munschauer M, Mastrobuoni G et al. Identification of LIN28B‐bound mRNAs reveals features of target recognition and regulation. RNA Biol 2013;10:1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Beachy SH, Onozawa M, Chung YJ et al. Enforced expression of Lin28b leads to impaired T‐cell development, release of inflammatory cytokines, and peripheral T‐cell lymphoma. Blood 2012;120:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. O'Leary L, Sevinc K, Papazoglou IM et al. Airway smooth muscle inflammation is regulated by microRNA‐145 in COPD. FEBS Lett 2016;590:1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hirata H, Hinoda Y, Shahryari V et al. Genistein downregulates onco‐miR‐1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br J Cancer 2014;110:1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yang G, Yang X. Smad4‐mediated TGF‐beta signaling in tumorigenesis. Int J Biol Sci 2010;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Li BB, Li DL, Chen C et al. Potentials of the elevated circulating miR‐185 level as a biomarker for early diagnosis of HBV‐related liver fibrosis. Sci Rep 2016;6:34157–34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cheuk BL, Cheng SW. Identification and characterization of microRNAs in vascular smooth muscle cells from patients with abdominal aortic aneurysms. J Vasc Surg 2014;59:202–209. [DOI] [PubMed] [Google Scholar]
- 155. Williams KC, Renthal NE, Gerard RD et al. The microRNA (miR)‐199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol 2012;26:1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ye EA, Liu L, Jiang Y et al. MiR‐15a/16 reduces retinal leukostasis through decreased pro‐inflammatory signaling. J Neuroinflammation 2016;13:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Li L, Jia J, Liu X et al. MicroRNA‐16‐5p controls development of osteoarthritis by targeting SMAD3 in chondrocytes. Curr Pharm Des 2015;21:5160–5167. [DOI] [PubMed] [Google Scholar]
- 158. Xu F, Zhu Y, He Q et al. Identification of microRNA‐regulated pathways using an integration of microRNA‐mRNA microarray and bioinformatics analysis in CD34+ cells of myelodysplastic syndromes. Sci Rep 2016;6:32232–32242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wei X, Wang JP, Hao CQ et al. Notch signaling contributes to liver inflammation by regulation of interleukin‐22‐producing cells in hepatitis B virus infection. Front Cell Infect Microbiol 2016;6:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mendes‐Silva AP, Pereira KS, Tolentino‐Araujo GT et al. Shared biologic pathways between alzheimer disease and major depression: A systematic review of microRNA expression studies. Am J Geriatr Psychiatry 2016;24:903–912. [DOI] [PubMed] [Google Scholar]
- 161. Naegelen I, Plancon S, Nicot N et al. An essential role of syntaxin 3 protein for granule exocytosis and secretion of IL‐1alpha, IL‐1beta, IL‐12b, and CCL4 from differentiated HL‐60 cells. J Leukoc Biol 2015;97:557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zheng X, Zhao FC, Pang Y et al. Downregulation of miR‐221‐3p contributes to IL‐1beta‐induced cartilage degradation by directly targeting the SDF1/CXCR4 signaling pathway. J Mol Med 2017;95:615–627. [DOI] [PubMed] [Google Scholar]
- 163. Duan M, Yao H, Hu G et al. HIV Tat induces expression of ICAM‐1 in HUVECs: Implications for miR‐221/−222 in HIV‐associated cardiomyopathy. PLoS One 2013;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Corsten M, Heggermont W, Papageorgiou AP et al. The microRNA‐221/−222 cluster balances the antiviral and inflammatory response in viral myocarditis. Eur Heart J 2015;36:2909–2919. [DOI] [PubMed] [Google Scholar]
- 165. Zaman MS, Thamminana S, Shahryari V et al. Inhibition of PTEN gene expression by oncogenic miR‐23b‐3p in renal cancer. PLoS One 2012;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kalantari P, Harandi OF , Agarwal S et al. miR‐718 represses proinflammatory cytokine production through targeting phosphatase and tensin homolog (PTEN). J Biol Chem 2017;292:5634–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Scott H, Howarth J, Lee YB et al. MiR‐3120 is a mirror microRNA that targets heat shock cognate protein 70 and auxilin messenger RNAs and regulates clathrin vesicle uncoating. J Biol Chem 2012;287:14726–14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Imamura Y, Wang PL. Salivary histatin 3 inhibits heat shock cognate protein 70‐mediated inflammatory cytokine production through toll‐like receptors in human gingival fibroblasts. J Inflamm 2014;11:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhu WS, Tang CM, Xiao Z et al. Targeting EZH1 and EZH2 contributes to the suppression of fibrosis‐associated genes by miR‐214‐3p in cardiac myofibroblasts. Oncotarget 2016;7:78331–78342. [DOI] [PMC free article] [PubMed] [Google Scholar]


