Abstract
Since their discovery in 2006, induced pluripotent stem cells (iPSCs) have opened up a world of possibilities for regenerative medicine and novel cell‐based therapeutics. Now, over a decade later, robust reprogramming and expansion and differentiation protocols have been developed, and iPSC‐derived cells have been used in a wide variety of small and large animal models to treat many different diseases. Furthermore, the first iPSC derivatives are on their way into clinical trials. In this line, (i) GMP‐compliant generation, cultivation, and differentiation, (ii) preclinical efficacy and safety, as well as (iii) ethical and regulatory compliance of stem cell research represent important aspects that need to be evaluated for proper clinical translation of iPSCs and their derivatives. In this review article, we provide an overview of the current advances and challenges of the clinical translation of iPSC‐derived blood cells and highlight the most pressing problems that have to be overcome in the next years. stem cells translational medicine 2019;8:332–339
Keywords: Induced pluripotent stem cells, Blood, Macrophages, Erythrocytes, Platelets, Upscaling, Clinical translation, GMP
Significance Statement.
Given the groundbreaking discovery of induced pluripotent stem cells (iPSCs), use of these cells has been envisioned for future cell‐based therapies. Nowadays, iPSCs have been differentiated toward a variety of different effector cells, and iPSC derivates have also reached clinical translation. Although clinical transfer of iPSC‐derived hematopoietic cells remains as of yet sparse, the study describes the recent advances in hematopoietic differentiation of iPSC and provides a current perspective on the GMP‐compliant generation of suitable hematopoietic cells.
Introduction
Given their potential to continuously regenerate and differentiate into all cell types of an organism, pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) or induced PSCs (iPSCs) represent a highly promising source for new cell‐based therapies. Following the pioneering studies by Takahashi and Yamanaka 1, murine and human iPSCs have been used to derive a multitude of therapeutically active cell types, thus laying the foundation for new treatment concepts in regenerative medicine (Fig. 1). After over a decade of research, the effectiveness and feasibility of iPSC‐derivatives have been proven in small and large animal models, building the foundation for clinical application 2. In this review article, we will give an overview of the ongoing efforts to translate iPSC‐derived blood cells toward clinical use, with the main focus on hematopoietic stem cells (HSCs) and macrophages, highlighting some of the problems that have to be overcome in the upcoming years.
Figure 1.
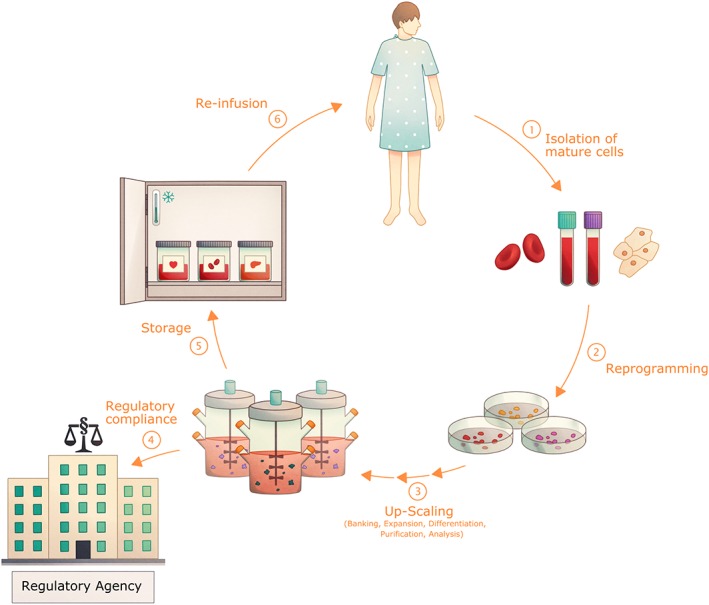
The clinical translation of induced pluripotent stem cell (iPSC)‐derived cells. iPSCs and their derived progeny hold great potential for their use in regenerative and personalized medicine. To achieve this aim, mature cells, for example, blood cells or fibroblasts, are collected from a patient (1) and reprogrammed into iPSCs using a GMP‐compliant protocol (2). After this, the cells have to go through several processes (3) including but not limited to: banking for future use and testing, expansion and differentiation in sufficient numbers via upscaling, for example, in bioreactors, purification, and functional analysis. Cells also have to go through tests by regulatory agencies regarding safety and compliance (4). Following certain cell release criteria, the cell products (5) can potentially be stored and infused back into the patient (6).
Promises and Ethical Concerns of Human iPSC
To broaden the clinical use of iPSC‐derived cell types, current efforts focus on three cornerstones, which are dedicated to (i) GMP‐compliant generation, cultivation, and differentiation of human iPSCs, (ii) preclinical efficacy and safety, as well as (iii) ethical and regulatory compliance of stem cell research. Especially, the latter has recently been considered in more detail, as human iPSCs are derived from adult‐type somatic donor cells. Given the unique features of iPSCs for unlimited self‐renewal and indefinite differentiation toward various cell types, these cells can be used for research purposes or be translated into clinical practice many years after their generation. Furthermore, iPSCs contain the individual genetic fingerprint of the donor, which can be (mis)used for in depth research of the personal genetic code. Although human iPSCs can nowadays be generated within days or weeks from many different donors, the history of Henrietta Lacks should act as a memorial to follow certain national and international guidelines on stem cell research. The International Society of Stem Cell Research has also become aware of these prerequisites and has published a compendium of “Guidelines for Stem Cell Research and Clinical Translation” (see also 3). Besides recent landmark studies on the therapeutic success of iPSC‐derivatives, such guidelines are necessary to protect the rights of the cell donors (e.g., by written informed consent) and to fulfill (pre)clinical standards (e.g., by preclinical efficacy and safety studies) before an iPSC‐derived cell therapeutic reaches individual patients. Given the rapid medical progress in the field of stem cell research and regenerative medicine, national stem cell societies (e.g., the German Stem Cell Network) also provide knowledge on regulatory compliance, with the aim to use the iPSC technology for disease modeling, drug discovery, and also clinical translation.
Scalable Generation and Maintenance of iPSCs as a Prerequisite for the Clinical Translation
Since their discovery in 2006, the concept of “reprogramming” was quickly transferred from the murine to the human system 4 and then expanded toward different starting cell sources with various different reprogramming techniques 5, 6, 7, 8, 9, 10, 11 (for a more in depth overview, see 12). The original protocol is based on introducing the four transcription factors (TFs), OCT4, SOX2, KLF4, and c‐Myc, via viral vectors; although it remains the basis of iPSC generation 1, other factors and methods have also been explored. After their establishment, iPSCs were quickly compared to the “gold standard” ESCs and it was found that they are remarkably similar. However, subtle differences in transcriptomic and epigenetic profiling were discovered 13, 14. How critical these differences are, as well as the use of different starting materials, the donor age, and reprogramming method are still debated 13, 15, 16, 17 (for a more in depth overview of the influence of donor age and of genetic variability, see 18 and 19, respectively). Furthermore, the genetic integrity and stability of iPSCs are of concern as the reprogramming process and subsequent culturing are associated with DNA damages including double strand breaks and other genomic aberrations 20. As such aberrations are passed down to any nucleated differentiated progeny, they could present a major safety issue. Despite these concerns, iPSCs are pushed toward clinical application because of their great therapeutic potential. However, the main difficulties still persist in GMP‐compliant generation and maintenance of iPSCs, translation of cultivation and differentiation protocols toward industrial standards, and the definition of safety standards.
As the original reprogramming technique used viral vector integration, the risk of genotoxicity and nonintegrating viral and nonviral reprogramming methods have been successfully developed. For the viral reprogramming, adenovirus and sendai virus have been predominantly used 6, 21, whereas for the nonviral methods, mRNA, protein, plasmid, and transposon‐based methods have been successfully employed 12. Most of these methods, however, trade‐off higher security for lower efficiency in comparison to the traditionally used lenti‐ or gamma‐retroviral methods 22. In hand with the nonintegrating reprogramming, also GMP‐grade generation and maintenance protocols were developed 23. This includes chemically defined and xeno‐free media but also feeder‐free cultivation of iPSCs. Many systems have been successfully developed so far, Wiley et al., for example, used sendai virus‐based reprogramming and culturing on recombinant human laminin 24, whereas Baghbaderani et al. used plasmid‐based reprogramming and the L7 matrix from Lonza 23, 25. Both groups demonstrate fully GMP‐compliant protocols ready for clinical translation. As there is no standardized method up until this point, much work is still needed toward establishing robust and reliable protocols. The next step after the GMP‐compliant reprogramming and culturing is the translation toward industrial‐scale production. The aforementioned protocols work with traditional monolayer culture, but for a clinical application, up‐scaling to bioreactor technology is required. Various groups have shown that cultivation of iPSCs as pluripotent aggregates in bioreactors is possible 26, 27, although the bioreactors were still inoculated with cells initially derived from feeder‐based cultivation, and continuous passaging of the pluripotent aggregates remains problematic. The most important hurdle that remains is the connection of all the different parts to achieve a combined, all‐in‐one GMP‐compliant reprogramming and maintenance protocol that can be transferred to the bioreactor and ideally be linked to various differentiation protocols. One of the few cell types for which robust and combined protocols in a bioreactor system have already been established are cardiomyocytes 28, 29, but for the majority of other cell types including the blood cells robust protocols remain sparse. Furthermore, the produced cells need to be linked to clinical use: Even though protocols for cardiomyocytes are already well established, the application of these cells in the clinic is still in the very beginning 30. In summary, both the GMP‐compliant reprogramming as well as the maintenance of iPSCs have been established. However, main challenges remain in connecting the protocols, up‐scaling of the processes, and achieving standards and guidelines for all steps involved.
The Differentiation Potential of Human iPSCs into Hematopoietic Stem Cells
Considering their three‐lineage (mesoderm, ectoderm, and endoderm) differentiation potential, human iPSCs have been shown to give rise to almost all cell types of the human organism, highlighting their attractiveness for the field of regenerative medicine. This also holds true for the hematopoietic lineage, in which the HSC gives rise to all mature blood cells in a hierarchical process. Given the high therapeutic value of HSC transplantation in clinical practice, great efforts have been put into the generation of HSCs from PSC sources. And while substantial improvements have been made in the generation of a variety of mature blood cells, the in vitro generation of HSCs from PSCs is still fraud with problems. The main hallmarks such as engraftment in secondary recipients and multilineage reconstitution currently remain mostly unmet. Although many protocols are published, they vary greatly in both methods used and the outcome. So far, only genetic modification of the original iPSC starting material allowed the generation of iPSC‐derived functional HSCs, whereas iPSC‐derived HSC‐like cells from differentiation cultures employing “only” cytokine administration, small molecule‐guided activation of signaling pathways, or cocultivation with endothelial/stromal cells are missing critical functionality 31, 32. One promising approach to overcome this problem is the forced overexpression of defined TFs during the differentiation process. In the human system, Sandler et al. showed that the overexpression of FOSB, GFI1, RUNX1, and SPI1 in endothelial cells together with a coculture with E4EC vascular niche cells is able to produce multipotent progenitor cells that can reconstitute primary and secondary recipients 33. An alternative approach comes from the Daley lab, that used the inducible overexpression of the TFs ERG, HOXA9, RORA, SOX4, and MYB (EARSM) in CD34+ CD45+ myeloid precursors derived from human PSCs (hPSCs). Following this approach, they were able to generate engraftable multilineage progenitors with myeloid and erythroid differentiation potential 34. Of note, the additional knockdown of the epigenetic modifier and polycomb group protein EZH1 unlocked lymphoid potential in vitro 35. In addition, also the overexpression of MLL‐AF4 only has shown the generation of engraftable iPSC‐derived blood cells; however, transplanted cells showed a myeloid bias and leukemic transformation at later timepoints 36. Similarly, a screen of 26 TF candidates after hPSC differentiation in hemogenic endothelium discovered seven TFs (ERG, HOXA5, HOXA9, HOXA10, LCOR, and RUNX1) that were sufficient to generate hematopoietic stem and progenitor cells that engraft in primary and secondary recipients and generate myeloid, B and T cell lineages 37. Here, the overexpression of these TFs is combined with a preceding morphogen‐based differentiation protocol to first generate hemogenic endothelium, which is then converted into HSCs. With similar success Rafii and coworkers converted murine endothelial cells into HSCs via the transient overexpression of the TFs Fosb, Gfi1, Runx1, and Spi1 and coculture with an inductive vascular niche 38. Another approach is performed by Suzuki et al. 39 and Amabile 40, for example, who successfully generated HSCs via teratoma formation. However, this approach has clear limitations with respect to clinical translation. Even though great advances have been made, the clinical translation of in vitro generated transgene‐free HSCs remains out of reach for the moment. This might be explained by the complex hematopoietic embryonic development, which proceeds through two distinct stages: a primitive and a definitive hematopoietic program. Whereas these programs are spatially and temporarily separated in the developing embryo, they are simultaneously induced during iPSC differentiation (also reviewed in 41). Certainly, specific factors and signaling pathways are still missing to instruct the developing HSPCs to a definitive, long‐term engraftable HSC. Because of these problems, many researchers have turned their attention toward the generation of further differentiated cells instead. Here, our understanding of the ontogeny of these cells in vivo has been the crucial guiding plan toward their in vitro generation.
Generation of Therapeutically Active Macrophages from Human iPSC
Macrophages have become an increasingly interesting cell type for in vitro generation and clinical translation, as insights into their function and ontogeny have been unveiled. Several recent publications have shown that macrophages from different organs (Fig. 2), also called tissue resident macrophages (TRMs), are of embryonic origin and originate from progenitors, which seed the different tissues before birth. Furthermore, many TRM populations have been shown to self‐maintain independent of monocyte influx as, for example, the microglia in the brain, alveolar macrophages (AMs) in the lung, or the Kupffer cells in the liver (as also reviewed elsewhere 42). Given their remarkable self‐renewal and plasticity combined with their crucial role in a wide variety of diseases such as hereditary alveolar proteinosis 43 and mendelian susceptibility to mycobacterial disease 44, 45, the in vitro generation of macrophages can lead to new insights into their role in pathophysiology 46, 47, while creating possible clinical applications.
Figure 2.
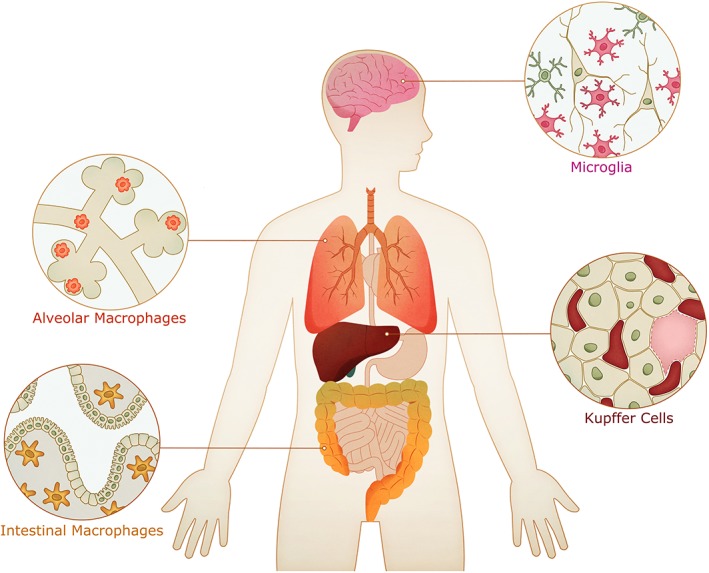
Localization of different macrophage subsets in different organs. Tissue macrophages play an important role in tissue homeostasis and can act as regulators in the innate immunity. Prominent examples for macrophages in different tissues are microglia in the brain, Kupffer cells in the liver, alveolar macrophages in the lung, and the intestinal macrophages. Considering the individual turnover and the ontogeny of the different macrophage subsets, generation and transplantation of induced pluripotent stem cell‐derived macrophages might be a future therapeutic approach for different diseases in which tissue macrophages are impaired.
The generation of human macrophages from PSCs started out with a variety of complex differentiation methods regularly including coculturing with other cells such as mouse bone marrow cells, complex cytokine cocktails, and lengthy purification steps. In 2008, however, the group of William James published a more streamlined and efficient protocol to derive macrophages from ESCs. In short, embryoid bodies (EBs) are formed and subjected to directed differentiation via M‐CSF/IL‐3, and the subsequently produced cells can be harvested from the supernatent 48. The resulting cells are of high purity, transcriptionally closely related to their in vivo counterparts, and seem to be fully functional. Because of its simplicity and efficiency, this protocol has since become a standard for differentiating macrophages and meanwhile been further advanced to generate macrophages and granulocytes from human iPSCs 49, 50. In addition, similar techniques have been developed to also generate microglia‐like cells from human iPSCs, further demonstrating the potential to produce various macrophage subsets 51, 52. With a well‐established protocol in hand, current efforts are mainly focused on defined, GMP‐compliant, and large‐scale production of these cells, to push their use toward the clinical application. Here, additional steps have often been introduced to the original protocol, mainly a mesoderm priming step after EB formation, which often includes the addition of Stem Cell Factor (SCF), Vascular Endothelial Growth Factor (VEGF), Bone morphogenetic protein 4 (BMP‐4) 53. This step seems to be made necessary when using a feeder‐free cultivation of the iPSCs as either a monolayer or as aggregates. Further modifications often include single EB formation with a defined cell numbers to make the process more reproducible and a switch to media that are suitable for clinical translation such as X‐Vivo, E8/E6 or mTeSR medium. With large parts of the GMP compliant and defined production of these cells established, further efforts are now concentrated on the large‐scale production of these cells and establishing a link to the GMP‐compliant cultivation and maintenance of iPSCs to have an all‐in‐one large‐scale production protocol. Regarding the function of the iPSC‐derived macrophages, the question remains whether they rather resemble an adult‐type monocyte/macrophage or a primitive embryonic‐type macrophage phenotype. Some evidence seems to be pointing toward the latter 52, 53, but how important this is for future clinical application of the cells still has to be shown. As of yet, the connection of the ontogeny and function of different macrophage subsets is poorly understood, and the question persists whether adult and embryonic macrophages have the same functionality. Furthermore, macrophages are one of the few known cell types that exist both from embryo and adult origin in the adult organism. Given the tendency of most PSC‐derived cells to be more of primitive (embryonic/fetal) than definitive origin, macrophages are an interesting cell type to study the functional differences between these cells. In fact, recent studies suggest a more primitive origin of murine and human iPSC‐derived macrophages 53, 54, 55. Given the MYB‐independent origin of murine TRMs 56, a MYB‐independent and RUNX1‐dependent development has been shown for human iPSC‐derived macrophages 53.
First in vivo data of how these cells could be used in the clinical application came, for example, from macrophage transplantation in the context of hereditary pulmonary alveolar proteinosis (herPAP) 57, 58. Because of the absence of AMs and therefore an empty niche, it has been shown that pulmonary macrophage transplantation of human iPSC‐derived macrophages can also lead to stable long‐term engraftment, adaption toward an AM phenotype, and thereby improvement of disease parameters in a humanized mouse model of herPAP 54. Although very much similar, the concept of HSC‐derived macrophage transplantation is now on its way toward clinical translation and might also be a useful application in other macrophage‐related diseases and even infectious diseases.
Clinical Translation of Human iPSC and Future Directions
As introduced before, human iPSCs have been differentiated into a multitude of different blood cells; however, the clinical translation of iPSC‐derived cell products is still impeded. One major hurdle is our insufficient knowledge about the in vivo functionality of iPSC‐derived blood cells as well as the lack of scalable differentiation protocols allowing to generate therapeutically relevant quantities of effector cells. Another potential concern relates to the contamination of the cell product with residual undifferentiated iPSCs, which can form unwanted teratomas. To circumvent this problem, improved differentiation protocols in combination with rigorous purification strategies are currently underway. As an alternative, the addition of safety switches, which are based on suicide genes, for example, the herpes simplex virus thymidine kinase or the inducible Caspase 9 system, may be used to efficiently deplete tumor (including teratoma)‐forming cells 59, 60. Application of such a system has recently shown to eradication approximately 95% of iPSCs, making this system an attractive safety‐backup option 59. Although current preclinical lab work relates to GMP‐compliant generation, maintenance, and differentiation as well as safety concerns, one major question remaining is which iPSC line(s) should be used to generate the therapeutic cell product. First studies promised iPSC technology to provide autologous cell products and tissues for patient‐specific cell‐based therapies; however, this scenario is extremely labor and cost intensive, suggesting the clinical use of allogenic iPSCs. To provide suitable target cells, either genetically modified “universal” iPSCs, which, for example, expresses artificial HLA molecules 61, or the use of human iPSCs, which can be obtained from iPSC‐banks, is interesting approache. Indeed, S. Yamanaka has started recently to establish a GMP‐grade iPSC‐bank in Japan (CiRA's iPSC bank for regenerative medicine), preferentially using material from HLA‐homozygous donors. This approach offers the advantage that 5 to 10 allogenic donor lines would be sufficient to match 30%–50% of the Japanese population 62, 63. Using HLA‐homozygous donors greatly reduces the numbers of iPSC lines needed to cover a given population, but potential donors would have to be identified performing large screenings or using established data from HSC donors/cord blood banks. While this scenario may be applicable for Japan, more diverse populations (e.g., Brazil, United States, or India) are expected to require more iPSC lines to match the majority of their population 64, 65. As an alternative approach, a single iPSC line, previously genetically manipulated to escape immune rejection would allow for a universal iPSC‐based cell therapy approach, which can in principle be applied for all patients and thus drastically reduce the costs for cell therapy. Going this line, recent studies could already demonstrate feasibility by disrupting the beta‐2 microglobulin gene‐locus and introducing a single‐chain HLA‐E molecule into PSCs. Derivatives from these iPSCs were able to escape T cell‐mediated rejection and were resistant to NK‐cell lysis, highlighting their potential as allogenic alternatives 61.
Although no clinical trials employing iPSC‐derived hematopoietic cells have been conducted so far, two other iPSC‐derived products have been used to treat the first patients. The worldwide first clinical trial using an autologous iPSC‐derived cell product has been launched in 2014 in Japan. Here, the team of Masayo Takahashi from RIKEN and Kobe City Medical Center initiated a clinical trial to treat neovascular age‐related macular degeneration by transplanting iPSC‐derived retinal pigment epithelial (RPE) sheets (UMIN‐CTR #, UMIN000011929). To achieve this aim, the team generated a GMP‐grade autologous iPSC line from patient's fibroblasts using nonintegrating episomal vectors. After vigorous safety testing of the iPSCs and the iPSC‐derived cell product, the first patient has been treated in September 2014, with an intact RPE cell sheet 1 year post transplantation 2. However, given new regulations in Japan's regenerative medicine law 66, the team of Takahashi has changed the strategy, aiming to use allogeneic donor cells instead 67, 68. Unfortunately, one adverse event was reported for one of the patients earlier this year, which, however, was most likely related to the surgical procedure and not the iPSC product itself 69. Besides this pioneering trial, only one more interventional trial using allogeneic iPSC‐derived mesenchymal stem cells (MSCs) is currently being conducted by the Australian Company Cynata Therapeutics. In this trial, the investigator aims to use mesenchymoangioblast‐derived MSCs for the treatment of steroid‐resistant acute graft‐versus‐host disease (Clinical Trials.gov: NCT02923375). Currently, all eight patients treated so far have demonstrated at least a partial response, while no treatment‐related serious adverse events or safety concerns have been observed 70. The outcomes of these two trials will certainly be helpful for further clinical translation of iPSC‐based therapies.
Conclusion
Given the pioneering work of the aforementioned clinical trials and long‐standing expertise of transfusion medicine in cell‐based therapies, iPSC‐derived hematopoietic cells represent a promising cell type for the initiation of further iPSC‐based clinical trials. Especially, the recent advances in GMP‐compliant generation and differentiation of human iPSCs in combination with upscaling approaches of hematopoietic differentiation put iPSC‐derived blood cells on the right path toward possible clinical translation. While various preclinical studies could already demonstrate the efficacy and safety of various iPSC‐derived blood cells, more work is still needed to generate transgene‐free HSCs for clinical application. Here, maybe the transplantation of macrophages into different tissues could be the forefront of clinical translation (Fig. 1). As mentioned earlier, dysfunction of macrophages has been associated with a variety of diseases entities, and for some, macrophage transplantation has been proven feasible and long lasting. Also highlighting recent efforts in the generation of other mature and immature cells, it will be a fascinating time to see when and how iPSCs will be translated into clinical application and furthermore which hematopoietic cell types will be applied first.
Author Contributions
K.H. and M.A.: manuscript writing, final approval of manuscript; N.L.: conception/design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
We thank Juliette Nowak for critical review of the manuscript. The authors were supported by grants from the Deutsche Forschungsgemeinschaft: Cluster of Excellence REBIRTH (Exc 62/3) and (LA3680/2‐1). N.L. is further supported by the Else Kröner‐Fresenius‐Stiftung (EKFS) (2015_92) and the Federal Ministry of Education and Research (01EK1602A). M.A. is supported by the EKFS (2016_A146), whereas K.H. is supported by a scholarship from the Hannover Biomedical Research School, REBIRTH PhD Program “Regenerative Sciences.”
References
- 1. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2. Mandai M, Watanabe A, Kurimoto Y et al. Autologous induced stem‐cell–derived retinal cells for macular degeneration. N Engl J Med 2017;376:1038–1046. 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 3. Daley GQ, Hyun I, Apperley JF et al. Setting global standards for stem cell research and clinical translation: The 2016 ISSCR guidelines. Stem Cell Rep 2016;6:787–797. 10.1016/j.stemcr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu J, Vodyanik MA, Smuga‐Otto K et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5. Shi Y, Desponts C, Do JT et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small‐molecule compounds. Cell Stem Cell 2008;3:568–574. 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6. Ban H, Nishishita N, Fusaki N et al. Efficient generation of transgene‐free human induced pluripotent stem cells (iPSCs) by temperature‐sensitive Sendai virus vectors. Proc Natl Acad Sci USA 2011;108:14234–14239. 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kogut I, McCarthy SM, Pavlova M et al. High‐efficiency RNA‐based reprogramming of human primary fibroblasts. Nat Commun 2018;9:745 10.1038/s41467-018-03190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grabundzija I, Wang J, Sebe A et al. Sleeping Beauty transposon‐based system for cellular reprogramming and targeted gene insertion in induced pluripotent stem cells. Nucleic Acids Res 2013;41:1829–1847. 10.1093/nar/gks1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim D, Kim C‐H, Moon J‐I et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteinsa. Cell Stem Cell 2009;4:472–476. 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stadtfeld M, Nagaya M, Utikal J et al. Induced pluripotent stem cells generated without viral integration. Science 2008;322:945–949. 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia F, Wilson KD, Sun N et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods 2010;7:197–199. 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. González F, Boué S, Belmonte JCI. Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat Rev Genet 2011;12:231–242. 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 13. Kim K, Zhao R, Doi A et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol 2013;29:1117–1119. 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parrotta E, De Angelis MT, Scalise S et al. Two sides of the same coin? Unraveling subtle differences between human embryonic and induced pluripotent stem cells by Raman spectroscopy. Stem Cell Res Ther 2017;8:271 10.1186/s13287-017-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim K, Doi A, Wen B et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010;467:285–290. 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okano H, Nakamura M, Yoshida K et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res 2013;112:523–533. 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 17. Miura K, Okada Y, Aoi T et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 2009;27:743–745. 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 18. Strässler ET, Aalto‐Setälä K, Kiamehr M et al. Age is relative‐impact of donor age on induced pluripotent stem cell‐derived cell functionality. Front Cardiovasc Med 2018;5:4 10.3389/fcvm.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang G, Zhang Y. Genetic and epigenetic variations in iPSCs: Potential causes and implications for application. Cell Stem Cell 2013;13:149–159. 10.1016/j.stem.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turinetto V, Orlando L, Giachino C. Induced pluripotent stem cells: Advances in the quest for genetic stability during reprogramming process. Int J Mol Sci 2017;18 (pii): E1952. 10.3390/ijms18091952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fink KD, Crane AT, Lévêque X et al. Intrastriatal transplantation of adenovirus‐generated induced pluripotent stem cells for treating neuropathological and functional deficits in a rodent model of Huntington's disease. Stem Cells Translational Medicine 2014;3:620–631. 10.5966/sctm.2013-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature 2012;481:295–305. 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baghbaderani BA, Tian X, Neo BH et al. cGMP‐manufactured human induced pluripotent stem cells are available for pre‐clinical and clinical applications. Stem Cell Rep 2015;5:647–659. 10.1016/J.STEMCR.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiley LA, Burnight ER, DeLuca AP et al. cGMP production of patient‐specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep 2016;6:30742 10.1038/srep30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shafa M, Yang F, Fellner T et al. Human‐induced pluripotent stem cells manufactured using a current good manufacturing practice‐compliant process differentiate into clinically relevant cells from three germ layers. Front Med 2018;5:69 10.3389/fmed.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zweigerdt R, Olmer R, Singh H et al. Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc 2011;6:689–700. 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]
- 27. Shafa M, Sjonnesen K, Yamashita A et al. Expansion and long‐term maintenance of induced pluripotent stem cells in stirred suspension bioreactors. J Tissue Eng Regen Med 2012;6:462–472. 10.1002/term.450. [DOI] [PubMed] [Google Scholar]
- 28. Kempf H, Andree B, Zweigerdt R. Large‐scale production of human pluripotent stem cell derived cardiomyocytes. Adv Drug Deliv Rev 2016;96:18–30. 10.1016/J.ADDR.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 29. Fluri DA, Tonge PD, Song H et al. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods 2012;9:509–516. 10.1038/nMeth.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wehman B, Siddiqui OT, Mishra R et al. Stem cell therapy for CHD: Towards translation. Cardiol Young 2015;25:58–66. 10.1017/S1047951115000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy M, Awong G, Sturgeon CM et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep 2012;2:1722–1735. 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 32. Choi KD, Vodyanik MA, Togarrati PP et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep 2012;2:553–567. 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandler VM, Lis R, Liu Y et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature 2014;511:312–318. 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Doulatov S, Vo LT, Chou SS et al. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage‐restricted precursors. Cell Stem Cell 2013;13:459–470. 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vo LT, Kinney MA, Liu X et al. Regulation of embryonic haematopoietic multipotency by EZH1. Nature 2018;553:506–510. 10.1038/nature25435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan Y‐T, Ye L, Xie F et al. Respecifying human iPSC‐derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci USA 2018;115:2180–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sugimura R, Jha DK, Han A et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2017;545:432–438. 10.1038/nature22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lis R, Karrasch CC, Poulos MG et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature 2017;545:439–445. 10.1038/nature22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki N, Yamazaki S, Yamaguchi T et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther 2013;21:1424–1431. 10.1038/mt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amabile G. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121:1–3. doi:1 10.1182/blood-2012-06-434407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ackermann M, Liebhaber S, Klusmann J‐H et al. Lost in translation: Pluripotent stem cell‐derived hematopoiesis. EMBO Mol Med 2015;7:1388–1402. 10.15252/emmm.201505301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoeffel G, Ginhoux F. Fetal monocytes and the origins of tissue‐resident macrophages. Cell Immunol January 2018;330:5–15. 10.1016/j.cellimm.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 43. Suzuki T, Sakagami T, Young LR et al. Hereditary pulmonary alveolar proteinosis: Pathogenesis, presentation, diagnosis, and therapy. Am J Respir Crit Care Med 2010;182:1292–1304. 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McClean CM, Tobin DM. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog Dis 2016;74:ftw068 10.1093/femspd/ftw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hetzel M, Mucci A, Blank P et al. Hematopoietic stem cell gene therapy for IFNγR1 deficiency protects mice from mycobacterial infections. Blood 2018;131:533–545. 10.1182/blood-2017-10-812859. [DOI] [PubMed] [Google Scholar]
- 46. Neehus A‐L, Lam J, Haake K et al. Impaired IFNγ‐signaling and mycobacterial clearance in IFNγR1‐deficient human iPSC‐derived macrophages. Stem Cell Rep 2017;10:7–16. 10.1016/j.stemcr.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lachmann N, Happle C, Ackermann M et al. Gene correction of human induced pluripotent stem cells repairs the cellular phenotype in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2014;189:167–182. 10.1164/rccm.201306-1012OC. [DOI] [PubMed] [Google Scholar]
- 48. Karlsson KR, Cowley S, Martinez FO et al. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture‐free differentiation in M‐CSF and IL‐3. Exp Hematol 2008;36:1167–1175. 10.1016/j.exphem.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lachmann N, Ackermann M, Frenzel E et al. Large‐scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep 2015;4:282–296. 10.1016/j.stemcr.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ackermann M, Kuhn A, Kunkiel J et al. Ex vivo generation of genetically modified macrophages from human induced pluripotent stem cells. Transfus Med Hemother 2017;44:135–142. 10.1159/000477129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haenseler W, Sansom SN, Buchrieser J et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal‐co‐culture‐specific expression profile and inflammatory response. Stem Cell Rep 2017;8:1727–1742. 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takata K, Kozaki T, Lee CZW et al. Induced‐pluripotent‐stem‐cell‐derived primitive macrophages provide a platform for modeling tissue‐resident macrophage differentiation and function. Cell Immun 2017;47:183–198.e6 [DOI] [PubMed] [Google Scholar]
- 53. Buchrieser J, James W, Moore MD et al. Human induced pluripotent stem cell‐derived macrophages share ontogeny with MYB‐independent tissue‐resident macrophages. Stem Cell Rep 2017;8:1388–1402. 10.1016/j.stemcr.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Happle C, Lachmann N, Ackermann M et al. Pulmonary transplantation of human induced pluripotent stem cell‐derived macrophages ameliorates pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2018;198:350–360. 10.1164/rccm.201708-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Litvack ML, Wigle TJ, Lee J et al. Alveolar‐like stem cell‐derived Myb(−) macrophages promote recovery and survival in airway disease. Am J Respir Crit Care Med 2016;193:1219–1229. 10.1164/rccm.201509-1838OC. [DOI] [PubMed] [Google Scholar]
- 56. Schulz C, Perdiguero EG, Chorro L et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012;336:86–90. 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 57. Suzuki T, Arumugam P, Sakagami T et al. Pulmonary macrophage transplantation therapy. Nature 2014;514:450–454. 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Happle C, Lachmann N, Kuljec J et al. Pulmonary transplantation of macrophage progenitors as effective and long‐lasting therapy for hereditary pulmonary alveolar proteinosis. Sci Transl Med 2014;6:250ra113–250ra113. 10.1126/scitranslmed.3009750. [DOI] [PubMed] [Google Scholar]
- 59. Itakura G, Kawabata S, Ando M et al. Fail‐safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Rep 2017;8:673–684. 10.1016/j.stemcr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ou W, Li P, Reiser J. Targeting of herpes simplex virus 1 thymidine kinase gene sequences into the OCT4 locus of human induced pluripotent stem cells. Lako M, ed. PLoS One. 2013;8:e81131. doi: 10.1371/journal.pone.0081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gornalusse GG, Hirata RK, Funk SE et al. HLA‐E‐expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol 2017;35:765–772. 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cyranoski D. Japanese man is first to receive “reprogrammed” stem cells from another person. Nature 2017. 10.1038/nature.2017.21730. Accessed June 13, 2018. [DOI] [Google Scholar]
- 63. Center for iPS Cell Research and Application, Kyoto University . iPS Cell Stock For Regenerative Medicine. Available at https://www.cira.kyoto‐u.ac.jp/e/research/stock.html. Accessed June 13, 2018.
- 64. Solomon S, Pitossi F, Rao MS. Banking on iPSC—Is it doable and is it worthwhile. Stem Cell Rev 2015;11:1–10. 10.1007/s12015-014-9574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andrews PW, Cavagnaro J, Deans R, et al. Harmonizing standards for producing clinical‐grade therapies from pluripotent stem cells. Nat Biotechnol 2014. 328. 2014;32:724–726. [DOI] [PubMed] [Google Scholar]
- 66. Konomi K, Tobita M, Kimura K et al. New Japanese initiatives on stem cell therapies. Cell Stem Cell 2015;16:350–352. 10.1016/j.stem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 67. RIKEN, Center for Developmental Biology . Launch of New Clinical Research Using Allogeneic iPSCs. Available at http://www.cdb.riken.jp/en/news/2017/topics/0217_10174.html. Accessed June 18, 2018.
- 68. Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol 2015;339:890–891. [DOI] [PubMed] [Google Scholar]
- 69. The Japan Times . First Serious Adverse Reaction to iPS‐Derived Retinal Cell Transplant Reported. Available at https://www.japantimes.co.jp/news/2018/01/17/national/science‐health/first‐serious‐reaction‐ips‐derived‐retinal‐cell‐transplant‐reported‐kobe/#.Wye6aGdciuS. Accessed June 18, 2018.
- 70. Excellent Cohort: A Data From Final Primary Evaluation Period in Cynata GvHD Phase 1 Clinical Trial. Available at https://globenewswire.com/news‐release/2018/02/27/1395934/0/en/Excellent‐Cohort‐A‐Data‐From‐Final‐Primary‐Evaluation‐Period‐in‐Cynata‐GvHD‐Phase‐1‐Clinical‐Trial.html. Accessed June 13, 2018.


