Abstract
Periodontitis is a widespread disease characterized by inflammation‐induced progressive damage to the tooth‐supporting structures until tooth loss occurs. The regeneration of lost/damaged support tissue in the periodontium, including the alveolar bone, periodontal ligament, and cementum, is an ambitious purpose of periodontal regenerative therapy and might effectively reduce periodontitis‐caused tooth loss. The use of stem cells for periodontal regeneration is a hot field in translational research and an emerging potential treatment for periodontitis. This concise review summarizes the regenerative approaches using either culture‐expanded or host‐mobilized stem cells that are currently being investigated in the laboratory and with preclinical models for periodontal tissue regeneration and highlights the most recent evidence supporting their translational potential toward a widespread use in the clinic for combating highly prevalent periodontal disease. We conclude that in addition to in vitro cell‐biomaterial design and transplantation, the engineering of biomaterial devices to encourage the innate regenerative capabilities of the periodontium warrants further investigation. In comparison to cell‐based therapies, the use of biomaterials is comparatively simple and sufficiently reliable to support high levels of endogenous tissue regeneration. Thus, endogenous regenerative technology is a more economical and effective as well as safer method for the treatment of clinical patients. stem cells translational medicine 2019;8:392–403
Keywords: Periodontal regeneration, Cell transplantation, Cell homing, Biomaterials, Tissue engineering, Endogenous regeneration
Significance Statement.
Stem cells play a crucial role in the regeneration of the tooth/supporting tissue that is lost or damaged because of the periodontal diseases. The review summarizes the translational potential of the developed regenerative approaches using either culture‐expanded or host‐mobilized stem cells for periodontal regeneration. To facilitate the translation of ex vivo manipulated stem cells into clinical use, concerted research endeavors should be placed on making cell therapy and tissue engineering more practical and economical as well as safer. To enable the use of endogenous cells for therapeutics, the current and future designs should focus on directing more cells to the site of injury and making the target site more suitable for cell differentiation and new tissue growth.
Introduction
Periodontitis, an oral disease with a high prevalence worldwide, affects the function of teeth and constitutes one of the main oral health burdens 1. An epidemiological survey has suggested that more than half of all adults are affected by periodontal disease to varying degrees 2, 3, 4, and a remarkable surge (25.4% increase) in the prevalence rates of periodontal disease was observed from 2005 to 2015 5, 6. Periodontitis can consistently disrupt tooth‐investing tissues and lead to tooth loss if left untreated 5, 7. Periodontitis is also closely associated with the occurrence and prognosis of various systemic diseases, including cardiovascular diseases, cancer, obesity, diabetes, and chronic nephritis 8, 9, 10, 11. Therefore, the exploration of effective and safe periodontal therapies that can be translated into the clinic is an urgent health need worldwide.
The ambitious purpose of periodontal therapy is to regenerate multiple periodontal tissues, including the alveolar bone, cementum, and periodontal ligament (PDL) in the damaged periodontium 12. Although nonsurgical periodontal therapies (e.g., scaling and root planning) can prevent disease progression by physically removing the pathogens and necrotic tissues, only a small amount of periodontal tissue can be regenerated at the treated sites 7. The application of technologies such as guided tissue regeneration (GTR) for periodontal surgery can erratically restore the alveolar bone and soft tissues, but the overall outcomes are not necessarily satisfactory and show a lack of clinical predictability 13. Although new biomaterials and growth factors have enriched the methods for managing periodontal defects, clinical trials have revealed that their efficacy is still controversial, and the structural and functional regeneration of lost periodontal structures remains challenging 12.
Stem cells can self‐renew and differentiate into multiple cell types and thus have tremendous therapeutic potential. The identification of stem cells from human PDL tissues, termed PDL stem cells (PDLSCs), in 2004, led to a new era of research on periodontal regeneration 14. Since then, other stem cells have been found to possess the ability to form multiple periodontal tissues under appropriate induction conditions 15. In addition to their regenerative potential, the ability of stem cells to undergo immunomodulation plays an equally important role in achieving a successful outcome (reviewed in 16). Today, the use of stem cells is considered as a mainstream strategy for periodontal treatment, particularly for complete regeneration of the periodontal complex, which implies not only the reconstruction of appropriate alveolar bone but also the induction of cementogenesis along the root surfaces with the oriented insertion of newly formed PDL tissue 13, 17, 18.
Based on therapeutics using ex vivo‐expanded stem cells, the regeneration of the periodontal complex has been demonstrated to be feasible in a variety of models tested (reviewed in 17, 18). However, in vitro cell culture places a heavy financial burden on patients and is associated with multiple other difficulties, including an insufficient stem cell source that is available for use, time‐consuming culture procedures, and safety issues 19, 20. To accelerate the clinical use of stem cell technology, the mobilization/homing of resident stem cells for regeneration based on endogenous healing mechanisms has become a new concept in regenerative medicine, which we herein definitively term endogenous regeneration medicine (ERM) 21, 22, 23, 24. ERM is particularly promising in periodontal research because of the high incidence rate of periodontitis, and mounting evidence indicates that endogenous stem cells can be directed to the periodontium to exert regenerative and immunomodulating functions; this strategy is similar to or more effective than the use of transplanted foreign stem cells (e.g., see 25, 26). In the future, ERM could offer a safer as well as more effective and economical method for periodontal regeneration than current cell‐based therapies. In this concise review, we summarize the current periodontal regenerative approaches based on either in vitro cell‐material design (cell delivery and transplantation) or in vivo cell‐material interactions (cell recruitment and homing; Fig. 1) and highlight the most recent evidence supporting their translational potential toward widespread use in the clinic for combating highly prevalent periodontal diseases.
Figure 1.
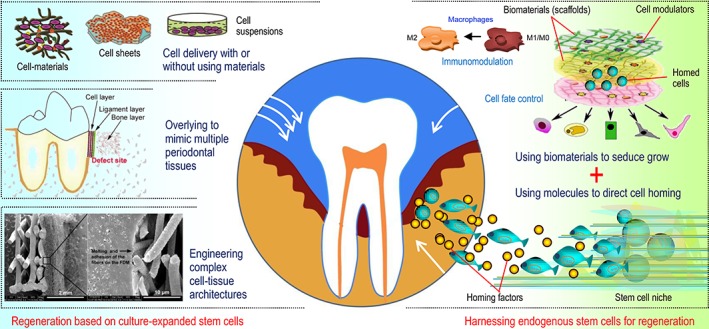
Periodontal regeneration can potentially be achieved via either in vitro designed cell‐material constructs for transplantation to the area of damage, where the transplants undergo remodeling and revascularization to integrate with the host tissue, or in vivo manipulation of the cell‐material interplay at the target site, where biomaterials and molecules coax the recruitment of endogenous stem cells to regrow new tissues.
Stem Cell Delivery Shows Promise for Periodontal Healing
Any cell type with an enormous proliferative capacity and a multipotent nature, particularly stem cells, can be used to replenish destroyed cells under certain conditions 27, 28. The discovery and therapeutic application of stem cells have offered a new concept for periodontal regeneration. The current stem cell‐based therapies in periodontics rely mainly on the delivery of culture‐expanded cells to the periodontal defect to enhance wound healing, and many elegant studies have documented positive outcomes using either intraoral or extraoral stem cells (reviewed in 29, 30).
Single‐cell suspensions prepared in vitro or cells suspended in medium can be directly injected into the site of injury easily, and this method is simple and minimally invasive 31. Bone marrow‐derived mesenchymal stem cells (BMMSCs) locally injected into mouse periodontal defects exert anti‐inflammatory and immunomodulatory effects at the target site and contribute to the regeneration of new tissue 32. In addition, an early‐phase study also showed that in the clinic, the transplantation of expanded autologous fibroblasts might be efficacious for curing papillary insufficiency following a papilla priming procedure 33. However, there are drawbacks associated with the injection of cell suspensions, such as an insufficient cell supply following an injection, poor engraftment, spread of injected cells to surrounding healthy tissue, and loss of cell fate control (reviewed in 31).
The therapeutic outcomes of delivered cells at the transplanted sites could potentially be improved with the aid of cell sheet/pellet technology, a scaffold‐free approach for cell transplantation (reviewed in 20, 34). In this context, confluent cells that are cultured/expanded are harvested as intact cell sheets, and monolayer or stacked cell sheets are notably easier to deliver and transplant than fluid cells and result in minimal cell loss/damage (reviewed in 35). Because stem cells are delivered together with their extracellular matrix (ECM) produced during ex vivo culture, cells with cell‐cell and cell‐matrix contacts are expected to remain engrafted for a long time after their introduction into the body without changes in their viability and function, which would lead to enhanced tissue regeneration compared with that observed with the injection of cells alone. In fact, the transplantation of cell sheets instead of fluid cells has revealed improvements in animal periodontitis with bone defects (e.g., see 36, 37).
In many cases, stem cell sheets are transplanted into the damaged periodontium with bone substitutes, such as hydroxyapatite/tricalcium phosphate (TCP; e.g., see 38, 39), bovine bone (e.g., see 40), synthetic hydrogels (e.g., see 41), and their composites (e.g., see 42), to increase the stability of the cells within the defect (reviewed in 35).
Irrespective of the animal model used and cell/defect type, substantial evidence from preclinical animal studies indicates that the local delivery of foreign cellular materials can benefit the outcome of periodontal disease management (reviewed in 43). Although human case studies (e.g., see 44) and randomized clinical trials (e.g., see 40) have demonstrated the feasibility and safety of stem cells, more well‐designed human studies should be performed to examine the beneficial effects of stem cells on periodontal regeneration. In addition, future translational research is still needed to identify cell populations with highly regenerative potential and to optimize the delivery methods. In addition, the therapeutic procedure should be sufficiently practical for use in a clinical setting.
Periodontal Tissue Engineering Via in vitro Cell‐Material Design
Given that the periodontium represents a unique complex architecture surrounding and supporting the tooth, the complete regeneration of hybrid periodontal tissues in humans remains limited and unpredictable, at least in current clinical practice, which shows that this achievement is a hard‐fought goal even with the aid of stem cell transplantation. To achieve complex periodontal regeneration, several advanced engineering approaches based on in vitro cell‐material design have paved the way for preclinical animal studies and offer state‐of‐the‐art therapeutic avenues for functional periodontal regeneration (reviewed in 18). Without a doubt, the combination of stem cells with well‐organized biomaterials that present and release robust signaling molecules might support a higher level of tissue regrowth than the use of cells alone.
Inspiration From the Bone‐PDL‐Cementum Apparatus
Insight into the physiogenesis and anatomical structure of the periodontium provides a solid basis for understanding the process of tissue destruction as well as for advancing the design of more effective cell‐based therapeutic strategies 45. The periodontium, which supports and anchors the teeth in their alveolar sockets, has a well‐arranged structure and can be divided into four main vital components, that is, gingiva, root cementum, PDL, and alveolar bone 45, 46 (Fig. 2). As the main part of this apparatus, the periodontal complex (i.e., bone‐PDL‐cementum) is hierarchically organized into the final three components affiliated with periodontal tissues 18 and characterized by periodontal fibers that are inserted into the root cementum and adjoining alveolar bone. Because of the interfacial interconnection of this multitissue complex, the periodontium can support and stabilize the tooth by distributing and absorbing forces as well as serve as a barrier to defend against dissimilar invading pathogens 45, 47, 48, 49. Consequently, the periodontal complex is considered the cornerstone of the periodontal structure and plays pivotal roles in restoring and maintaining the function of periodontal tissues. When the integrity of the periodontal complex is compromised by periodontitis, complete regeneration of the complex is very difficult to achieve through the local administration of stem cells alone because of the multiple tissue types and complex structures involved 50, 51. Taking inspiration from the anatomical structure of the periodontium, researchers hypothesize that the regeneration of the periodontal complex can benefit from specific cell‐material designs and have used the concept of tissue engineering to recreate the native bone‐PDL‐cementum apparatus.
Figure 2.
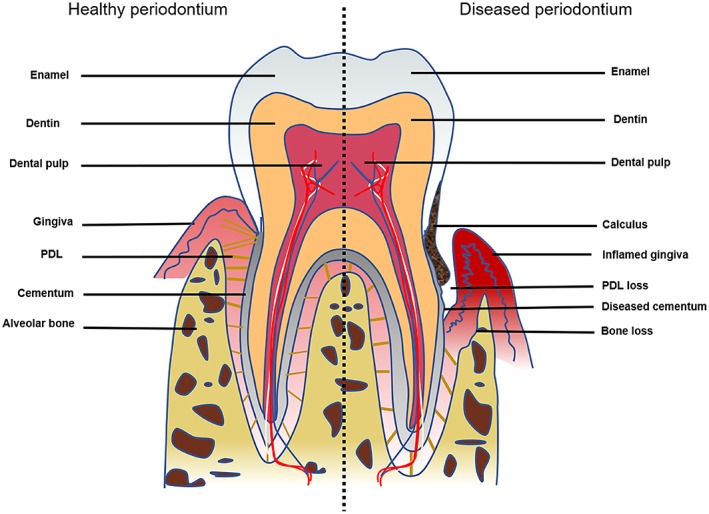
Schematic representation of the periodontium containing the intact periodontal complex (i.e., bone‐PDL‐cementum apparatus). As a result of disease (e.g., periodontitis), damage to the periodontium leads to the loss of multiple periodontal tissues surrounding and supporting the tooth. Abbreviation: PDL, periodontal ligament.
Overlaying to Mimic Multiple Periodontal Tissues
Given that the veritable regeneration of periodontal tissue involves reestablishment of the bone‐PDL‐cementum apparatus, the simple combination of in vitro‐cultured stem cells and biomaterials cannot realistically be used to achieve this goal 13, 52. However, it is possible to regain the periodontal hybrid tissues by layering materials and cells to mimic the different tissue layers involved in the periodontium 18. The vertical stacking of three‐layered PDLSC sheets, woven poly(glycolic acid), and porous β‐TCP in an orderly manner based on this concept and their placement into the three‐wall periodontal defects of a canine resulted in newly formed bone and cementum interspersed with the aligned collagen fibers (Fig. 3A) 53. Similarly, cell sheets comprising PDLSCs and/or jaw BMMSCs have been multilayered to regenerate a complex periodontium‐like architecture 54. Recently, a “sandwich” tissue engineering complex was constructed by adding a layer of mineralized membrane on each side of the collagen membrane. After seeding with gingival fibroblasts, this complex was implanted into periodontal defect areas in dogs, and simultaneous neogenesis of ligamentous and osseous structures was achieved (Fig. 3B) 55. Although the development of bilayered cell constructs serve as a promising strategy to simultaneously regenerate multiple periodontal tissues, the orchestrated use of multiple regenerated tissues to reconstruct the periodontal complex with micron‐scaled tissue compartmentalization as well as osseous and ligamentous interfacial structures with systematic tooth‐supporting functions remains a major clinical challenge.
Figure 3.
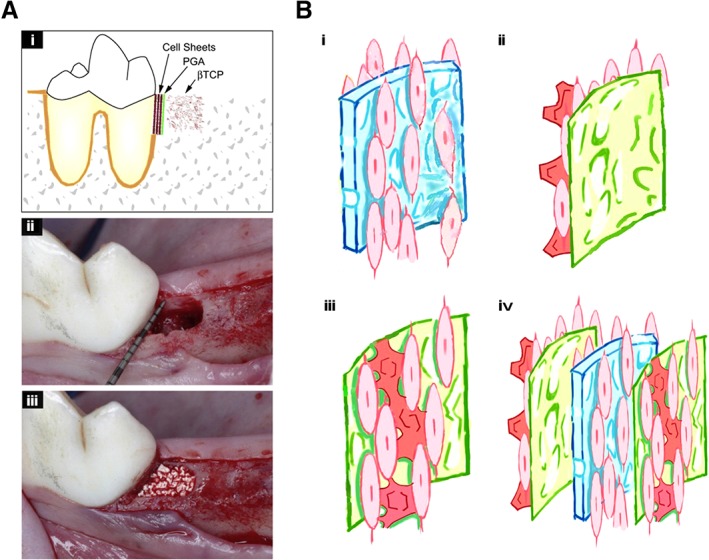
Overlaying cell sheets and biomaterials to mimic multiple periodontal tissues. (A): Three‐layered cell sheets together with woven PGA and porous β‐TCP were used to repair three‐wall infrabony defects in dogs 53 (please refer to the original source for more information). (B): Schematic representation of a generated sandwich complex including (i) an engineered membrane (Bio‐Gide collagen membrane seeded with cells on both sides and cultured without the addition of mineralization‐induction medium) and (ii) two mineralized membranes (a cellular small intestinal submucosa in which cells are seeded on one side and cultured in mineralization‐induction medium for 8 days); (iii and iv) a sandwich structure was obtained by placing a cell‐seeded periodontal membrane between the two cell‐seeded/mineralized membranes 54 (please refer to the original source for more details). Abbreviations: PGA, polyglycolic acid; β‐TCP, β‐tricalcium phosphate.
Engineering Approaches to Reconstruct Periodontal Complex Interfaces and Architectures
Recent advances in biomaterials technology have enabled the engineering of periodontal scaffolds with triphasic tissue interfaces and structures (e.g., see 56). In particular, computer‐aided design and three‐dimensional (3D) printing now allow the generation of compartmentalized hybrid scaffolds with biomimetic interfaces in 3D space (e.g., see 57). These scaffolds with spatial constructs can specifically regulate cell behaviors and influence the orientations of multiple tissues and thus provide an essential foundation for the formation of oriented ligamentous tissues and their successful incorporation into the new bone and cementum (reviewed in 17). In this context, biomimetic hybrid scaffolds containing bone‐specific and PDL‐specific polymer compartments are used to engineer human tooth‐ligament interfaces 56, and the engineering of bone‐ligament complexes has successfully been achieved with fiber‐guiding scaffolds (Fig. 4A) 56. Biphasic scaffolds that mimic the bone compartment by using a fused deposition modeling scaffold and mimic the PDL compartment by using an electrospun membrane have been reported, and the combination of these scaffolds and multiple PDLSC sheets allows the in vivo regeneration of complex periodontal structures (Fig. 4B) 58. Furthermore, the coating of the bone compartment with a calcium phosphate layer increased the osteoconductivity of seeded osteoblasts 59.
Figure 4.
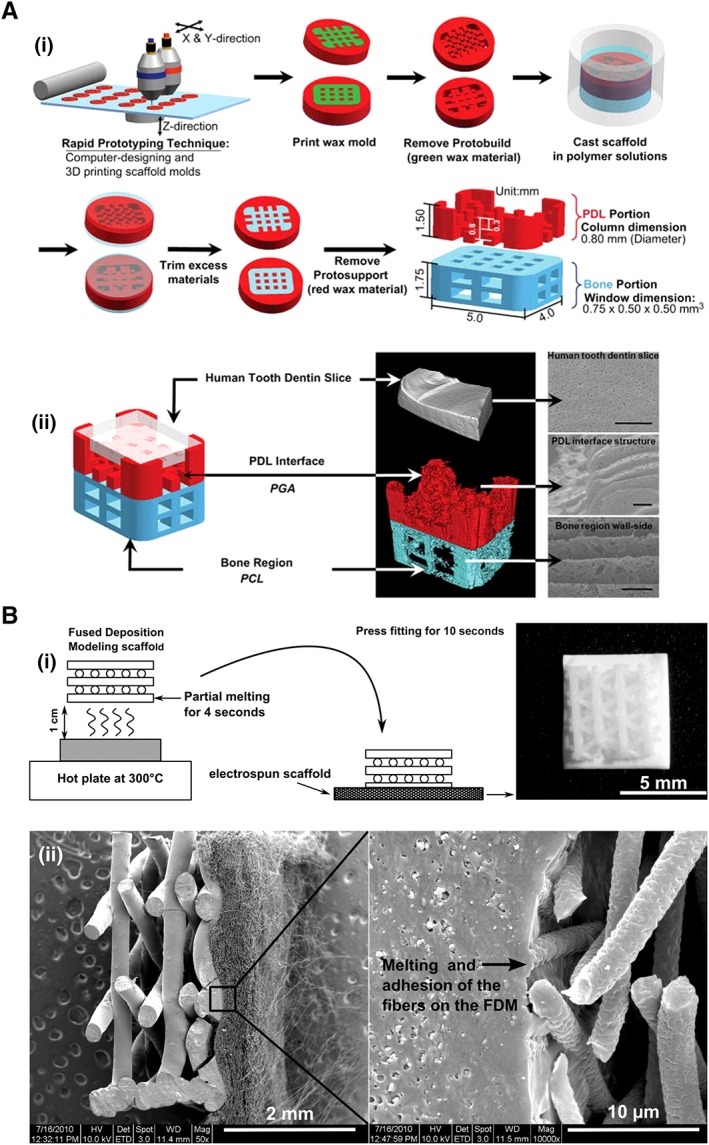
Engineering of biomimetic materials and architectures to reconstruct the periodontal complex. (A): Graphical illustration of the fabrication of a biphasic scaffold mimicking the tooth‐ligament‐bone complex using multiscale computational design and polymeric architecture manufacturing 56 (please refer to the original source for fabrication details). (B): Schematic representation of the fabrication (i) of a biphasic scaffold (ii) composed of the bone compartment (left side, a FDM scaffold) and periodontal compartment (right side, a membrane with electrospun fibers); this scaffold can be applied for the simultaneous regeneration of the bone‐ligament complex when combined with stem cell sheets 58 (please refer to the original source for more information). Abbreviations: 3D, three‐dimensional; FDM, fused deposition modeling; PCL, polycaprolactone; PGA, polyglycolic acid; PDL, periodontal ligament.
Using 3D printing and directional freeze‐casting techniques, a fiber‐guiding hybrid scaffold has been designed to spatiotemporally control the morphogenesis, integration, and functionalization of various tissues 60. Animal experiments have shown that this customized fiber‐guiding scaffold can accurately adapt to defect sites and thus successfully guide cell/tissue directionality during regeneration and facilitate the formation of a more stable ligament‐ligand complex with a rapidly maturing matrix 61. More recently, a similar “3D‐patterned, periodontal mimic multiphasic architecture” approach was reported, taking his technology one step closer to clinical translation 62. The aforementioned fiber‐guiding scaffold has been approved by the U.S. Food and Drug Administration for clinical use 60. Collectively, 3D‐patterned multiphasic complexes have enabled the reconstruction of periodontal complex architectures for periodontal tissue engineering strategies, but when translated to clinical use, the complexity of tissue‐engineered constructs must be kept to a minimum to ensure cost‐effectiveness and ease of production.
Crucial Barriers to Progress
In vitro stem cell‐material designs can mimic the anatomy of the periodontium, and different biomaterials, such as the bone graft materials and cell sheets, particularly barrier membranes, could be applied for this strategy. The goal of periodontal tissue engineering is to reinstate the normal function of the diseased periodontium to support the teeth, whereas the triad would require stem cells, biomaterials, and infection control. The available data demonstrate the capacity of stem cells to regenerate lost periodontal tissues in animal models (Table 1); however, the efficacy of cell‐based interventions in humans remains to be confirmed 43. Although experimental data have allowed the initiation of clinical trials in periodontal cell therapy, proper consideration of the cell source, material type, and regulatory concerns is crucial to facilitate clinical translation 63. For periodontal tissue engineering, regeneration in response to overengineered constructs should be investigated in larger animals, including rodents, because the anatomy of the dentoalveolar architecture in larger animals more closely resembles that of humans 64. In addition, the experimentally generated defects need to accurately mimic the pathophysiology of periodontitis in humans. In many cases, the periodontal defects generated in the currently used animal studies do not sufficiently reflect the inflammatory status of human periodontitis.
Table 1.
Selected examples based on culture‐expanded cells and/or biomaterial design toward in vivo periodontal regeneration
| Concept | Cells | Materials | Animal models | Cell‐material construct and transplantation | Outcomes | References |
|---|---|---|---|---|---|---|
| Cell transplantation without exogenous biomaterials | Allogeneic BMMSCs | No materials | Experimental periodontitis in rats | Injection of cell solution without additional materials | Cell injection significantly enhanced periodontal tissue regeneration. | 32 |
| Allogeneic DPSCs | No materials | Experimental periodontitis in miniature pigs | Transplantation of cell sheets without additional materials | Cell sheet treatment led to more bone regeneration compared with that obtained with the injection of cells alone | 36 | |
| Overlaying to mimic multiple periodontal tissues | Autologous PDLSCs | HA/TCP | Experimental periodontitis in miniature pigs | Transplantation of a combination of cells and materials | Materials containing PDLSCs significantly improved periodontal tissue regeneration, as determined through clinical and radiological evaluations | 37 |
| Autologous GMSCs | HA‐sECM | Experimental periodontal defects in miniature pigs | Transplantation of a combination of GMSCs and IL‐1ra‐releasing HA‐sECM | GMSCs in conjunction with HA‐sECM (either with or without IL‐1ra) showed significant potential for periodontal regeneration. | 41 | |
| Autologous PDL‐derived cells | Woven PGA (cell sheet support) and β‐TCP (defect fill) | Three‐wall periodontal defects in canines | Multilayered cell sheets supported with woven PGA were applied to the dental root surfaces, and the remaining defect area was filled with porous β‐TCP | The transplantation of three‐layered cell sheets resulted in the regeneration of both new bone and cementum connecting with well‐oriented collagen fibers | 53 | |
| Allogeneic PDLSCs and jaw BMMSCs | TDM/CBB | Immunodeficient mice | Ectopic transplantation of TDM/CBB material‐coated composite cell sheets | The combination of PDLSC and JBMMSC sheets facilitated the regeneration of complex periodontium‐like structures. | 54 | |
| Autologous gingival fibroblasts | Bio‐Gide collagen membrane and SIS segments | Experimental periodontal defects in beagles | Transplantation of a sandwich tissue‐engineered complex (Fig. 2B) | Periodontal defects were completely repaired by the sandwich tissue‐engineered complex 10 days after the operation | 55 | |
| Engineering to reconstruct periodontal complex interfaces and architectures | Heterogenic gingival fibroblasts | Composite hybrid PCL/PGA scaffolds (Fig. 3A) | Immunodeficient mice | Ectopic transplantation of biomimetic hybrid scaffolds (with or without ad‐BMP7‐modified cells) | In vivo regeneration of tooth dentin‐ligament‐bone complexes | 56 |
| Heterogenic DPSCs | Multiphase scaffolds (PCL/HA) | Immunodeficient mice | Ectopic transplantation of DPSC‐seeded multiphase region‐specific microscaffold with spatiotemporal delivery of BMP2, CTGF, and amelogenin | In vivo regeneration of multiphase periodontal tissues | 57 | |
| Heterogenic osteoblasts and PDLSCs | A biphasic scaffold composed of an FDM scaffold (PCL/β‐TCP) and an electrospun membrane (PCL) (Fig. 3B) | Immunodeficient rats | Ectopic transplantation of a biphasic scaffold composed of a bone compartment (seed with osteoblasts) and a periodontal compartment (combined by PDLSC sheets) | In vivo simultaneous regeneration of the alveolar bone/PDL complex | 58 | |
| Heterogenic osteoblasts and PDL cells | Second‐generation biphasic scaffold incorporating an osteoconductive bone compartment through a CaP coating and replacing the periodontal compartment with a thin melt electrospun scaffold with larger pores | Immunodeficient rats | Ectopic transplantation of a modified biphasic scaffold (optimized from Fig. 3B) with osteoblasts and PDL cell sheets | In vivo regeneration of the complex hierarchical periodontal structure | 59 |
Abbreviations: β‐TCP, β‐tricalcium phosphate; ABSCs, alveolar bone stem/progenitor cells; ad‐BMP7, adenovirus‐encoding murine bone morphogenetic protein‐7; BMMSCs, bone marrow mesenchymal stem cells; CaP, calcium phosphate; CBB, ceramic bovine bone; CEMP‐1, cementum protein 1; CTGF, connective tissue growth factor; DPSCs, dental pulp stem cells; FDM, fused deposition modeling; GMSCs, gingival mesenchymal stem cells; HA, hydroxyapatite; JBMMSC, jaw bone marrow mesenchymal stem cell; PCL, polycaprolactone; PDL, periodontal ligament; PDLSCs, periodontal ligament stem cells; PGA, polyglycolic acid; sECM, synthetic extracellular matrix; SIS, small intestinal submucosa; TCP, tricalcium phosphate; TDM, treated dentin matrix.
Harnessing Endogenous Stem Cells Via in vivo Cell‐Material Interaction
Based on the body's self‐regeneration potential, a new avenue for enhancing periodontal regeneration is to use endogenous stem cells. This concept makes periodontal regenerative therapy safer, simpler and easier to accept 21, reduces the high cost of cell culture, storage, and transplantation, and provides a bright future for the clinical application of new regenerative approaches. However, under most circumstances, the regeneration ability of diseased tissue is quite weak, and the lost/damaged periodontium might not be able to repair itself 65. The overscale defect and impaired regenerative capacity caused by aging and other systemic diseases also add to the local inflammatory microenvironment. Harnessing endogenous stem cells to defect sites via in vivo cell‐material interactions is the first goal of ERM. Based on this concept, multiple scaffolds combined with growth factors, antibodies, and drugs have been designed and applied to enhance cell homing and tissue regeneration 24, 66.
Self‐Healing Potential of Periodontal Tissues
The periodontium possesses a staggering self‐healing potential with the help of proper treatment and guidance. For example, at the early stage of periodontitis, removing the dental plaque can cure the infected/damaged periodontal tissues 8. Various degrees of periodontal repair/regeneration have been observed after GTR treatment, but the outcome is unpredictable 13. Thus, endogenous regeneration of the periodontium is feasible under certain circumstances. The factors affecting periodontium regeneration are quite complex, and the correct type and a sufficient number of cells, a beneficial microenvironment with biological signals and suitable scaffolding matrices, are all critical for regeneration. Therefore, the current strategies for enhancing self‐healing focus on directing resident cells for target trafficking and on coaxing these cells to grow new tissues 24.
Directing Endogenous Stem Cells to the Periodontium
The directing of endogenous cells to an injury site in response to biological signals (including but not limited to chemokines, growth factors, cytokines, and cell‐adhesive molecules) is a process known as directed stem cell homing 67. Clearly, the recruitment of sufficient cell populations to a diseased site is the first step to achieve successful regeneration. Many chemoattractants and growth factors, such as stromal cell‐derived factor‐1α (SDF‐1α) and stem cell factor, can be used to attract stem cells and recruit the patient's own stem cells for self‐repair (reviewed in 22). However, directly injected factors have a very short half‐life and high expenditure, and using carriers or scaffolds for growth factor delivery is a practical strategy 68. In vitro results have shown that SDF‐1α‐loaded gelatin sponges could release their cargo for up to 35 days and enhance bone/PDL regeneration 69. Because the therapeutic activity of SDF‐1α is negatively affected by dipeptidyl peptidase‐IV (DPP‐IV), the DPP‐IV inhibitor parathyroid hormone (PTH) has been used for SDF‐1α protection, and PTH/SDF‐1α cotherapy has been found to increase the migration of reparative cells to rat periodontal defects 70. In addition to the growth factor‐loaded construct, platelet‐rich plasma (PRP) and platelet‐rich fibrin are warehouses of various native growth factors that can also be used for facilitated stem cell homing and periodontal regeneration 71. The combination of platelet‐rich preparation and native matrix has been used for tooth root regeneration, and the results showed that the cementum‐PDL complex can be regenerated on the construct placed in a tooth socket by homing stem cells 72. Moreover, decellularized ECM, which retains the structural components of native tissue and contains an abundant variety of signals and growth factors, can also direct endogenous cell homing and manipulate stem cell differentiation (reviewed in 40, 73). Compared with artificial materials, native matrices induce less inflammatory responses and make therapies safer and more effective (reviewed by 73, 74). In fact, many biomaterials or their modified forms have already paved the way for clinical periodontal regeneration. The creation of more specific designs or the incorporation of selected homing factors would largely enhance their capacity to recruit host cells and thus further induce endogenous tissue regeneration.
Inducing Growth of the Periodontium
After the stem cells reach their target site, the promotion of their differentiation into the periodontium through growth factor delivery and biomaterial design is another basic step required for periodontal regeneration (reviewed in 24). In a local microenvironment targeted for regeneration, macrophages (Mϕs) are one of the predominant immunological regulators 75. Mϕs have two different phenotypes, M1 and M2: during the endogenous regenerative process, M1 plays an elimination role in the initial inflammatory stage, whereas M2 plays an inflammation regulatory role in the later stage. Hence, immunomodulatory cytokines, such as interleukin‐4 (IL‐4), have been used to promote M1‐to‐M2 transformation 76. For example, the systemic injection of IL‐4 has been found to regulate the polarization of Mφs during the orthodontic process and hence attenuate tooth root resorption 77. To achieve a better effect, a specifically devised carrier has been used to stabilize this cytokine and protect it from denaturation and degradation, leading to a prolonged modulation of macrophage polarization. This strategy has resulted in an enhanced repair of the rat mandibular periodontal fenestration defect 78. In fact, the polymorphism of immune‐regulating cytokines (IL‐4 and IL‐13) has been demonstrated to be associated with the susceptibility of chronic periodontitis 79, 80.
As mentioned above, biomaterials mimicking the properties of the host tissue to avoid immune detection could benefit periodontal regeneration. For example, ECM‐mimicking devices, such as an electrospun nanofibrous scaffold 81 or a nanocomposite hydrogel 82, could benefit bone regeneration. In prospect, these ECM‐mimicking biomaterials not only provide a scaffold for cell homing but also create a friendly immune environment for correct periodontal regeneration.
The presentation and release of signaling molecules (such as growth factors) are also important for facilitating stem cell homing, proliferation, and differentiation. In this regard, growth/differentiation factor‐5 (e.g., see 83), fibroblast growth factor‐2 (FGF‐2; e.g., see 84), platelet‐derived growth factor‐BB (e.g., see 55), and many other factors have already been used alone or in combination with various biomaterials for local cell manipulation and periodontal regeneration. Throughout the native regenerative response, different growth factors play multiple roles in specific release sequences and dosages; thus, the sequential release of multiple growth factors to mimic the normal biology of periodontal regeneration can lead to an enhanced outcome (e.g., see 85, 86). The use of growth factors to modulate the local regenerative microenvironment and enhance the endogenous healing process of the periodontium has been reviewed elsewhere 22, 24; please refer to those articles for more information.
Along with the required growth factors, the implantation of tissue‐/structure‐mimicking biomaterials would also benefit the recreation of a suitable local matrix microenvironment. An interesting example that supports the proof‐of‐concept that anatomically shaped that tooth scaffolds can be used for periodontal regeneration by cell homing 25 is the trilayered scaffold reported by Sowmya et al.87 for concurrent regeneration of the three types of periodontal tissues; each layer was specifically designed to contain chitin/poly(lactic‐co‐glycolic acid) (chitin‐PLGA) and/or nanobioactive glass ceramic (nBGC) components. A layer composed of chitin‐PLGA/nBGC loaded with recombinant human cementum protein‐1 was applied to generate cementum, and similar components combined with PRP were included to regenerate the bone. For PDL regeneration, a chitin‐PLGA hydrogel was loaded with recombinant human FGF‐2. The assessment of this scaffold using a rabbit periodontal defect model showed that the cell‐free construct induced complete regeneration of the hybrid tissues in the periodontium (Fig. 5) 87. Based on available data, chemoattractive constructs have been proven effective in the treatment of periodontal defects, and further research should consider the translation of these proof‐of‐concept studies to clinical use.
Figure 5.
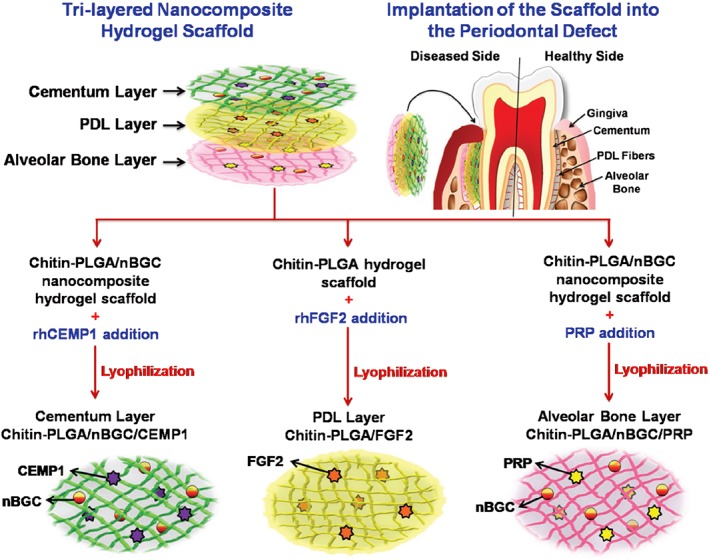
Schematic representation of the formation of a trilayered nanocomposite hydrogel scaffold (each layer incorporates different growth factors or preparation‐containing growth factors) for the simultaneous regeneration of multiple periodontal tissues 87 (please refer to the original source for more information). Abbreviations: CEMP1, cementum protein‐1; FGF‐2, fibroblast growth factor‐2; PDL, periodontal ligament; PLGA, poly(lactic‐co‐glycolic acid); PRP, platelet‐rich plasma; nBGC, nanobioactive glass ceramic; rhCEMP1, recombinant human cementum protein‐1; rhFGF, recombinant human fibroblast growth factor.
Collectively, the data show that periodontal regeneration via in vivo cell‐material interactions motivates the self‐healing potential of the host. The biomaterials themselves and the incorporated signaling molecules that are used to recreate a proper in vivo milieu are all key to initiating the process of self‐regeneration (reviewed in 24, 66). The cascade of the mobilization of stem cells from their niches, directed cell homing, and the propagation and differentiation of the recruited cells once they reach the site of injury can potentially result in the functional regeneration of multiple periodontal tissues and their unique architectures (Fig. 6; e.g., see 78, 88). Substantial work using developmental biology insights is still needed to direct the design of next‐generation biomaterials that actively work together with nature's own regeneration and healing mechanisms based on biological disciplines.
Figure 6.
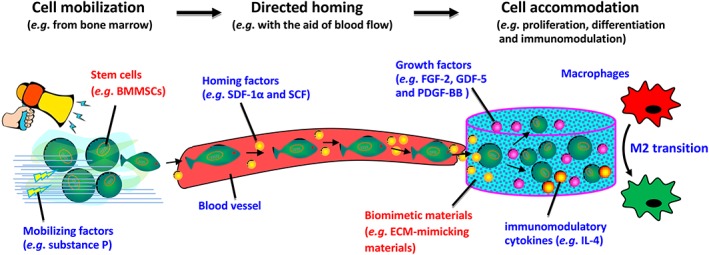
Schematic representation of the mobilization of stem cells from their niche (e.g., bone marrow) using cell mobilizing factors such as substance P, directed cell movement with the aid of blood flow, and cell homing factors such as SDF‐1α and SCF and the regulation of stem cell fate (e.g., cell proliferation and differentiation) once they reach the site of injury, normally via the design of materials such as the generation of ECM‐mimicking biomaterials and the presentation of a wide variety of growth factors, such as FGF‐2, GDF‐5, and PDGF‐BB and immunomodulatory cytokines, such as IL‐4. Abbreviations: BMMSCs, mesenchymal stem cells derived from bone marrow; ECM, extracellular matrix; FGF‐2, fibroblast growth factor‐2; GDF‐5, growth/differentiation factor‐5; IL‐4, interleukin‐4; PDGF‐BB, platelet‐derived growth factor‐BB; SCF, stem cell factor; SDF‐1α, stromal‐derived factor‐1α.
Conclusion
Based on the principles of tissue engineering, the use of cells, biomaterials, and growth factors for periodontal regeneration has been proposed as a new concept for the treatment of periodontal disease. Although bioengineered cellular materials represent the current state‐of‐the‐art treatment and have resulted in curative successes in preclinical scenarios, the effective regeneration of human periodontal tissue lost/damaged because of periodontitis needs continuous improvements. Endeavors aiming to bridge the gap between the promising results obtained with animal models and the reality of clinical treatments indicate that clinical success can be facilitated by avoiding the use of external stem cells. Therefore, studies have established endogenous regenerative techniques involving material design and/or cytokine presentation that permit the patient's own cells to be harnessed for therapeutics 89. An evolving body of work in this topic corroborates that the recreation of a suitable microenvironment at the injured site can instruct the homing of resident stem cells and can induce the periodontium itself to regenerate. In the future, better control of the local microenvironment for stem cell homing and accommodation will most likely render high levels of periodontal tissue regeneration a clinical reality, even for mature periodontal teeth.
Periodontal tissue regeneration is a complex healing cascade that arises from a coordinated interplay among stem cells, biomaterials, and the host immune system 90. In this concise review, we illustrate the possible approaches based on the therapeutic potential of stem cells that might enhance periodontal wound healing and regeneration and highlight the advantages and disadvantages of culture‐expanded and host‐mobilized cell populations in clinical translation. However, stem cell therapies and tissue engineering in the arena of periodontics remain in their infancy, irrespective of the cell source or route from which the cells are obtained. Although a number of clinical observations have delivered promising outcomes, there is still no perfect cell regenerative paradigm ready for translation to the clinic. The translation of a laboratory‐based periodontal regenerative approach to the clinic requires a thorough understanding of the mechanism underlying the cell‐based regeneration progress, a targeted cell‐material design for a given task, and a potential dichotomy between the need for a biomimetic design and cost‐effective production, because sophisticated constructs are normally required for producing complex tissue.
Author Contributions
X.‐Y.X., X.L., and F.‐M.C.: conception/design, collection and/or assembly of data, structure and figure design, data analysis and interpretation, manuscript writing and revision, final approval of manuscript; J.W., X.‐T.H., and H.‐H.S.: data collection, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
F.‐M.C. is supported by the National Key Research and Development Program of China (2018YFC1105204), the National Natural Science Foundation of China (81530050), the Shaanxi Key Scientific and Technological Innovation Team (2017KCT‐32), and the Changjiang Scholars Program of the Ministry of Education of the People's Republic of China (2016). We apologize for not citing many relevant contributions because of the space limitations.
Contributor Information
Hai‐Hua Sun, Email: sunhaihua1972225@163.com.
Fa‐Ming Chen, Email: cfmsunhh@fmmu.edu.cn.
References
- 1. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809–1820. [DOI] [PubMed] [Google Scholar]
- 2. Carasol M, Llodra JC, Fernández‐Meseguer A et al. Periodontal conditions among employed adults in Spain. J Clin Periodontol 2016;43:548–556. [DOI] [PubMed] [Google Scholar]
- 3. Eke PI, Dye BA, Wei L et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol 2015;86:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dye BA. Global periodontal disease epidemiology. Periodontology 2000 2012;58:10–25. [DOI] [PubMed] [Google Scholar]
- 5. Darveau RP. Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010;8:481–490. [DOI] [PubMed] [Google Scholar]
- 6. GBD 2015 Disease and Injury Incidence and Prevalence Collaboratorsan . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernández‐Monjaraz B, Santiago‐Osorio E, Monroy‐García A et al. Mesenchymal stem cells of dental origin for inducing tissue regeneration in periodontitis: A mini‐review. Int J Mol Sci 2018;19:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 9. Beukers NGFM, van der Heijden GJMG, van Wijk AJ et al. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in The Netherlands. J Epidemiol Community Health 2017;71:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han M. Oral health status and behavior among cancer survivors in Korea using nationwide survey. Int J Environ Res Public Health 2018;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heikkilä P, But A, Sorsa T et al. Periodontitis and cancer mortality: Register‐based cohort study of 68,273 adults in 10‐year follow‐up. Int J Cancer 2018;142:2244–2253. [DOI] [PubMed] [Google Scholar]
- 12. Bartold PM, Gronthos S, Ivanovski S et al. Tissue engineered periodontal products. J Periodontal Res 2016;51:1–15. [DOI] [PubMed] [Google Scholar]
- 13. Chen FM, Jin Y. Periodontal tissue engineering and regeneration: Current approaches and expanding opportunities. Tissue Eng Part B Rev 2010;16:219–255. [DOI] [PubMed] [Google Scholar]
- 14. Seo BM, Miura M, Gronthos S et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364:149–155. [DOI] [PubMed] [Google Scholar]
- 15. Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. Those from other sources: Their biology and role in regenerative medicine. J Dent Res 2009;88:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Racz GZ, Kadar K, Foldes A et al. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol 2014;65:327–339. [PubMed] [Google Scholar]
- 17. Ivanovski S, Vaquette C, Gronthos S et al. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res 2014;93:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park CH, Kim KH, Lee YM et al. Advanced engineering strategies for periodontal complex regeneration. Materials 2016;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishijima M, Hirota M, Park W et al. Osteogenic cell sheets reinforced with photofunctionalized micro‐thin titanium. J Biomater Appl 2015;29:1372–1384. [DOI] [PubMed] [Google Scholar]
- 20. Owaki T, Shimizu T, Yamato M et al. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol J 2014;9:904–914. [DOI] [PubMed] [Google Scholar]
- 21. Yin Y, Li X, He XT et al. Leveraging stem cell homing for therapeutic regeneration. J Dent Res 2017;96:601–609. [DOI] [PubMed] [Google Scholar]
- 22. Li X, He XT, Yin Y et al. Administration of signalling molecules dictates stem cell homing for in situ regeneration. J Cell Mol Med 2017;21:3162–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian BM, He XT, Xu XY et al. Advanced biotechnologies toward engineering a cell home for stem cell accommodation. Adv Mater Technol 2017;2:1700022. [Google Scholar]
- 24. Wu R‐X, Xu X‐Y, Wang J et al. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl Mater Today 2018;11:144–165. [Google Scholar]
- 25. Kim K, Lee CH, Kim BK et al. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res 2010;89:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu H, Li M, Du L et al. Local administration of stromal cell‐derived factor‐1 promotes stem cell recruitment and bone regeneration in a rat periodontal bone defect model. Mater Sci Eng C 2015;53:83–94. [DOI] [PubMed] [Google Scholar]
- 27. Bassir SH, Wisitrasameewong W, Raanan J et al. Potential for stem cell‐based periodontal therapy. J Cell Physiol 2016;231:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu B, Liu Y, Li D et al. Somatic stem cell biology and periodontal regeneration. Int J Oral Maxillofac Implants 2013;28:e494–e502. [DOI] [PubMed] [Google Scholar]
- 29. Chen F‐M, Sun H‐H, Lu H et al. Stem cell‐delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012;33:6320–6344. [DOI] [PubMed] [Google Scholar]
- 30. Han J, Menicanin D, Gronthos S et al. Stem cells, tissue engineering and periodontal regeneration. Aust Dent J 2014;59:117–130. [DOI] [PubMed] [Google Scholar]
- 31. Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008;2:205–213. [DOI] [PubMed] [Google Scholar]
- 32. Du J, Shan Z, Ma P et al. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J Dent Res 2014;93:183–188. [DOI] [PubMed] [Google Scholar]
- 33. McGuire MK, Scheyer ET. A randomized, double‐blind, placebo‐controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J Periodontol 2007;78:4–17. [DOI] [PubMed] [Google Scholar]
- 34. Matsuura K, Utoh R, Nagase K et al. Cell sheet approach for tissue engineering and regenerative medicine. J Control Release 2014;190:228–239. [DOI] [PubMed] [Google Scholar]
- 35. Iwata T, Washio K, Yoshida T et al. Cell sheet engineering and its application for periodontal regeneration. J Tissue Eng Regen Med 2015;9:343–356. [DOI] [PubMed] [Google Scholar]
- 36. Hu J, Cao Y, Xie Y et al. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther 2016;7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu Y, Zheng Y, Ding G et al. Periodontal ligament stem cell‐mediated treatment for periodontitis in miniature swine. Stem Cells 2008;26:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu X, Ge S, Chen S et al. Human gingiva‐derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogs. Cells Tissues Organs 2013;198:428–437. [DOI] [PubMed] [Google Scholar]
- 39. Fu X, Jin L, Ma P et al. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodontol 2014;85:845–851. [DOI] [PubMed] [Google Scholar]
- 40. Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 2016;53:86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fawzy ESKM, Mekhemar MK, Beck‐Broichsitter BE et al. Periodontal regeneration employing gingival margin‐derived stem/progenitor cells in conjunction with IL‐1ra‐hydrogel synthetic extracellular matrix. J Clin Periodontol 2015;42:448–457. [DOI] [PubMed] [Google Scholar]
- 42. Liu Z, Yin X, Ye Q et al. Periodontal regeneration with stem cells‐seeded collagen‐hydroxyapatite scaffold. J Biomater Appl 2016;31:121–131. [DOI] [PubMed] [Google Scholar]
- 43. Tassi SA, Sergio NZ, Misawa MYO et al. Efficacy of stem cells on periodontal regeneration: Systematic review of pre‐clinical studies. J Periodontal Res 2017;52:793–812. [DOI] [PubMed] [Google Scholar]
- 44. Feng F, Akiyama K, Liu Y et al. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis 2010;16:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Jong T, Bakker AD, Everts V et al. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J Periodontal Res 2017;52:965–974. [DOI] [PubMed] [Google Scholar]
- 46. Bartold PM, McCulloch CA. Information generation and processing systems that regulate periodontal structure and function. Periodontology 2000 2013;63:7–13. [DOI] [PubMed] [Google Scholar]
- 47. Lin JD, Jang AT, Kurylo MP et al. Periodontal ligament entheses and their adaptive role in the context of dentoalveolar joint function. Dent Mater 2017;33:650–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee JH, Pryce BA, Schweitzer R et al. Differentiating zones at periodontal ligament‐bone and periodontal ligament‐cementum entheses. J Periodontal Res 2015;50:870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park CH, Rios HF, Jin Q et al. Tissue engineering bone‐ligament complexes using fiber‐guiding scaffolds. Biomaterials 2012;33:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee J‐H, Lin JD, Fong JI et al. The adaptive nature of the bone‐periodontal ligament‐cementum complex in a ligature‐induced periodontitis rat model. Biomed Res Int 2013;2013:876316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silva N, Abusleme L, Bravo D et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci 2015;23:329–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iwata T, Yamato M, Ishikawa I et al. Tissue engineering in periodontal tissue. Anat Rec 2014;297:16–25. [DOI] [PubMed] [Google Scholar]
- 53. Iwata T, Yamato M, Tsuchioka H et al. Periodontal regeneration with multi‐layered periodontal ligament‐derived cell sheets in a canine model. Biomaterials 2009;30:2716–2723. [DOI] [PubMed] [Google Scholar]
- 54. Zhang H, Liu S, Zhu B et al. Composite cell sheet for periodontal regeneration: Crosstalk between different types of MSCs in cell sheet facilitates complex periodontal‐like tissue regeneration. Stem Cell Res Ther 2016;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu M, Wang J, Zhang Y et al. Mineralization induction of gingival fibroblasts and construction of a sandwich tissue‐engineered complex for repairing periodontal defects. Med Sci Monit 2018;24:1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park CH, Rios HF, Jin Q et al. Biomimetic hybrid scaffolds for engineering human tooth‐ligament interfaces. Biomaterials 2010;31:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee CH, Hajibandeh J, Suzuki T et al. Three‐dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A 2014;20:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vaquette C, Fan W, Xiao Y et al. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012;33:5560–5573. [DOI] [PubMed] [Google Scholar]
- 59. Costa PF, Vaquette C, Zhang Q et al. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol 2014;41:283–294. [DOI] [PubMed] [Google Scholar]
- 60. Park CH, Kim KH, Rios HF et al. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J Dent Res 2014;93:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park CH, Rios HF, Taut AD et al. Image‐based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng Part C Methods 2014;20:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rasperini G, Pilipchuk SP, Flanagan CL et al. 3D‐printed bioresorbable scaffold for periodontal repair. J Dent Res 2015;94:153S–157S. [DOI] [PubMed] [Google Scholar]
- 63. Lu H, Xie C, Zhao Y‐M et al. Translational research and therapeutic applications of stem cell transplantation in periodontal regenerative medicine. Cell Transplant 2013;22:205–229. [DOI] [PubMed] [Google Scholar]
- 64. Kantarci A, Hasturk H, van Dyke TE. Animal models for periodontal regeneration and peri‐implant responses. Periodontology 2000 2015;68:66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Polimeni G, Xiropaidis AV, Wikesjö UME. Biology and principles of periodontal wound healing/regeneration. Periodontology 2000 2006;41:30–47. [DOI] [PubMed] [Google Scholar]
- 66. Wu RX, Yin Y, He XT et al. Engineering a cell home for stem cell homing and accommodation. Adv Biosyst 2017;1:1700004. [DOI] [PubMed] [Google Scholar]
- 67. Pacelli S, Basu S, Whitlow J et al. Strategies to develop endogenous stem cell‐recruiting bioactive materials for tissue repair and regeneration. Adv Drug Deliv Rev 2017;120:50–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee K, Silva EA, Mooney DJ. Growth factor delivery‐based tissue engineering: General approaches and a review of recent developments. J R Soc Interface 2011;8:153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cai X, Yang F, Walboomers XF et al. Periodontal regeneration via chemoattractive constructs. J Clin Periodontol 2018;45:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang F, Du L, Ge S. PTH/SDF‐1α cotherapy induces CD90+CD34− stromal cells migration and promotes tissue regeneration in a rat periodontal defect model. Sci Rep 2016;6:30403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miron RJ, Zhang Y. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater 2018;75:35–51. [DOI] [PubMed] [Google Scholar]
- 72. Ji B, Sheng L, Chen G et al. The combination use of platelet‐rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng Part A 2015;21:26–34. [DOI] [PubMed] [Google Scholar]
- 73. Agmon G, Christman KL. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr Opin Solid State Mater Sci 2016;20:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Parmaksiz M, Dogan A, Odabas S et al. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed Mater 2016;11:022003. [DOI] [PubMed] [Google Scholar]
- 75. Yu Y, Wu RX, Yin Y et al. Directing immunomodulation using biomaterials for endogenous regeneration. J Mater Chem B 2016;4:569–584. [DOI] [PubMed] [Google Scholar]
- 76. Spiller KL, Nassiri S, Witherel CE et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1‐to‐M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015;37:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. He D, Kou X, Luo Q et al. Enhanced M1/M2 macrophage ratio promotes orthodontic root resorption. J Dent Res 2015;94:129–139. [DOI] [PubMed] [Google Scholar]
- 78. Hu Z, Ma C, Rong X et al. Immunomodulatory ECM‐like microspheres for accelerated bone regeneration in diabetes mellitus. ACS Appl Mater Interfaces 2018;10:2377–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen D, Zhang TL, Wang X. Association between polymorphisms in interleukins 4 and 13 genes and chronic periodontitis in a Han Chinese population. Biomed Res Int 2016;2016:8389020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang W, Xu P, Chen Z et al. IL‐13‐1112 polymorphism and periodontitis susceptibility: A meta‐analysis. BMC Oral Health 2018;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bottino MC, Thomas V, Janowski GM. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater 2011;7:216–224. [DOI] [PubMed] [Google Scholar]
- 82. Paul A, Manoharan V, Krafft D et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J Mater Chem B 2016;4:3544–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee J, Wikesjö UME. Growth/differentiation factor‐5: Pre‐clinical and clinical evaluations of periodontal regeneration and alveolar augmentation ‐ review. J Clin Periodontol 2014;41:797–805. [DOI] [PubMed] [Google Scholar]
- 84. Ogawa K, Miyaji H, Kato A et al. Periodontal tissue engineering by nano beta‐tricalcium phosphate scaffold and fibroblast growth factor‐2 in one‐wall infrabony defects of dogs. J Periodontal Res 2016;51:758–767. [DOI] [PubMed] [Google Scholar]
- 85. Chen FM, Chen R, Wang XJ et al. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials 2009;30:5215–5224. [DOI] [PubMed] [Google Scholar]
- 86. Duruel T, Çakmak AS, Akman A et al. Sequential IGF‐1 and BMP‐6 releasing chitosan/alginate/PLGA hybrid scaffolds for periodontal regeneration. Int J Biol Macromol 2017;104:232–241. [DOI] [PubMed] [Google Scholar]
- 87. Sowmya S, Mony U, Jayachandran P et al. Tri‐layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv Healthc Mater 2017;6:1601251. [DOI] [PubMed] [Google Scholar]
- 88. He XT, Li X, Xia Y et al. Building capacity for macrophage modulation and stem cell recruitment in high‐stiffness hydrogels for complex periodontal regeneration: Experimental studies in vitro and in rats. Acta Biomater 2018. in press. [DOI] [PubMed] [Google Scholar]
- 89. He XT, Wang J, Li X et al. The critical role of cell homing in cytotherapeutics and regenerative medicine. Adv Therap 2018;1:1800098 10.1002/adtp.201800098. [DOI] [Google Scholar]
- 90. Zhou LN, Bi CS, Gao LN et al. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis 2019;25:265–273. [DOI] [PubMed] [Google Scholar]


