Abstract
Objective
Small cell carcinoma of ovary, hypercalcemic type is a rare malignancy with a dismal prognosis. The diagnosis is often confused with many other tumors.
Case Report
We describe a rare case of ovarian small cell carcinoma of hypercalcemic type in an adolescent. She presented with abdominal pain, awareness of mass and vomiting. She underwent exploratory laparotomy and right ovarian excision. The detailed histopathological examination including immunohistochemistry was suggestive of ovarian small cell carcinoma of hypercalcemic type. She had progressive disease on chemotherapy and ultimately died within 2 years of diagnosis. Due to rarity of this neoplasm and its aggressive nature, the optimal treatment regimen has not been established.
Conclusion
We report this case because of its rare occurrence leading to clinical and diagnostic challenges and need to explore effective treatment options to improve survival in these patients.
Keywords: Adolescent, Chemotherapy, Hypercalcemia, Rhabdoid, Small cell carcinoma
Introduction
Ovarian small cell carcinoma of hypercalcemic type (SCCOHT) is an uncommon and highly aggressive malignancy. It is an undifferentiated neoplasm, and origin of tumor cells still remains an enigma. It typically affects adolescents and young women with age range of 5–71 years [1–3]. The youngest reported case was 14 months old [4]. Dickersin et al. in 1982 first described this entity [5]. Two-third of these tumors are associated with paraneoplastic hypercalcemia [5]. A wide range of differential diagnostic entities can be entertained while diagnosing this tumor. SMARCA4/BRG1 mutations are involved in the pathogenesis of these tumors [6]. Herein, we report a rare case of small cell carcinoma of the ovary in an adolescent girl.
Case Presentation
A 16-year-old female presented with complaints of pain abdomen and awareness of abdominal mass for one month and vomiting for 10 days. Physical examination revealed a large abdomino-pelvic mass reaching above umbilicus. Laboratory examination consisting of hematology, liver and renal function tests including serum calcium levels was within normal limits. Ultrasound of the abdomen was suggestive of a large complex solid cystic lesion measuring 15.5 × 11.3 cm extending from pelvis toward abdominal cavity with moderate ascites and right pleural effusion. The patient underwent exploratory laparotomy with right salpingo-oophorectomy and omentectomy. External iliac lymph nodes were also removed, and a peritoneal biopsy was taken. Since the tumor was highly vascular and was of large size, only debulking could be done. Grossly, the entire ovary was replaced by predominantly solid tumor with few cystic areas. Low-power microscopy revealed a densely cellular tumor arranged in solid sheets, nests, islands with large areas of multifocal necrosis. These tumor cells showed high nuclear–cytoplasmic ratio and hyperchromatic nuclei. Prominent peritheliomatous pattern was noted. Focally, clusters of larger tumor cells with moderate dense eosinophilic cytoplasm were noted, suggestive of rhabdoid differentiation (Fig. 1). On immunohistochemistry (IHC), CD99, epithelial membrane antigen (EMA), WT-1 and p53 positivity were noted. SMARCB1/INI1 expression was retained and BRG1 showed complete loss of expression. Neuron-specific enolase and CD56 showed focal strong positivity. Vimentin, smooth muscle actin (SMA) and weak desmin positivity were noted in the admixed clusters of larger rhabdoid tumor cells. Inhibin A, calretinin, chromogranin, CD45, S-100, cytokeratin, placental alkaline phosphatase (PLAP) and α-fetoprotein (AFP) were negative. Based on morphology and IHC panel, a diagnosis of ovarian small cell carcinoma of hypercalcemic type (ovarian rhabdoid tumor) was offered. External iliac lymph nodes (five out of five), omentum and peritoneal biopsy showed metastatic deposits. The patient was started on chemotherapy with BEP regimen in standard doses (bleomycin 30 IU on days 1, 8 and 15; etoposide 100 mg/m2 on days 1–5, cisplatin 20 mg/m2 on days 1–5, each given intravenously; every 21 days). The patient tolerated chemotherapy well. After 4 cycles, repeat CECT chest, abdomen and pelvis was done which revealed a large abdomino-pelvic mass measuring 8.4 × 9.5 cm. There was resolution in ascites and pleural fluid with presence of pleural, abdominal wall and left epiphrenic deposits. The patient was started on second-line chemotherapy with paclitaxel (175 mg/m2) and carboplatin (in a dose equivalent to an area under the curve [AUC] of 5) given intravenously every 21 days, but the disease progressed on chemotherapy and patient ultimately died of disease within 2 years of the diagnosis.
Fig. 1.
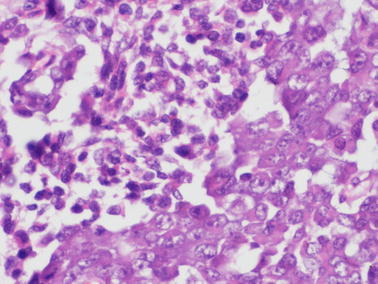
A tumor showing small round cells with a focus showing larger tumor cells with moderate dense eosinophilic cytoplasm suggestive of rhabdoid differentiation (H&E x40X)
Discussion
Small cell carcinoma of ovary, hypercalcemic type (SCCOHT) is an uncommon aggressive neoplasm with unknown histogenesis and wide differential diagnosis. The presentation is unilateral in majority of the cases [1–5]. Hypercalcemia is present at the time of diagnosis in approximately 60% of those with SCCOHT [1]. Histopathological examination of SCCOHT exhibits diffuse cellular sheets of small round cells with scanty cytoplasm, hyperchromatic nuclei with irregular coarse chromatin with small but readily visible nucleoli. Follicle-like structures containing eosinophilic fluid are observed in 80% of cases. Approximately 50% of the cases exhibit large cell variant with moderate-to-abundant eosinophilic cytoplasm [1, 2, 8]. Immunohistochemically, tumor cells are positive for EMA, cytokeratin, WT1, calretinin, CD10 and p53. Desmin, S100 and inhibin are negative. Negative staining with CD99, desmin, NB84, alpha-inhibin and TTF1 may help in differentiating from intra-abdominal desmoplastic small round cell tumor, primitive neuroectodermal tumor, neuroblastoma, rhabdomyosarcoma, sex cord–stromal tumor and metastatic pulmonary small cell carcinoma [7]. SMARCB1/INI 1 expression was retained in tumor nuclei in the present case. Loss of SMARCA4 (BRG1) protein expression has been reported to be highly sensitive and specific for SCCOHT, and loss of SMARCB1/INI 1 expression has been seen in a minority of the cases [7, 8].
Due to rarity and rapid fatal course of disease, treatment strategies have not been established and there is paucity of the literature on the effective treatment regimens. However, most patients have undergone aggressive surgical resection followed by multi-agent chemotherapy, but the results are unsatisfactory. The benefit and efficacy of chemotherapy is difficult to explicate as large variety of chemotherapy regimens have been used with limited success. In majority of the studies, platinum-based combination chemotherapy has been used. Various chemotherapy regimens used in various studies are: cisplatin/carboplatin and etoposide (EP); bleomycin, etoposide and cisplatin (BEP); vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin and etoposide (VPCBAE); carboplatin and paclitaxel; cyclophosphamide, cisplatin, doxorubicin and etoposide (CPAE); cisplatin, adriamycin and cyclophosphamide (CAP); irinotecan and cisplatin; cisplatin, cyclophosphamide and etoposide [1–4, 9]. The long-term responses and overall survival in ovarian small cell carcinomas have been observed with semi-intensive VPCBAE regimen [9]. Dose intensive chemotherapy with stem cell transplantation has also been investigated in a prospective trial in 27 patients of ovarian small cell carcinomas by Pautier et al. [10]. Our patient received BEP followed by paclitaxel and carboplatin, but she ultimately died due to progressive disease. The role of radiotherapy is still not established. Few of the studies have shown improved survival with addition of radiotherapy [1–3, 5]. Radiotherapy was not considered in our case due to the presence of ascites and disseminated disease.
Conclusion
Ovarian small cell carcinoma is a rare neoplasm with poor prognosis. Diagnosis is based on histopathological examination and immunohistochemistry. The present case depicts the characteristics of aggressive ovarian small cell carcinomas with detailed histopathological and immunohistochemical features. Due to rarity and aggressive disease course, optimal treatment of ovarian small cell carcinomas is not established. Aggressive multimodal treatment comprising of surgery, chemotherapy and radiotherapy should be considered in order to improve survival and outcome.
Acknowledgement
We acknowledge Dr. Kirti Gupta, PGIMER, Chandigarh for helping in work-up of this case and Dr. Simranjeet Singh for his involvement in the treatment of this case.
Dr Divya Khosla
is working as Assistant Professor in the Department of Radiotherapy and Oncology at Government Medical College and Hospital, Sector 32, Chandigarh, from November 2012 till date. She did MD (Radiation Oncology) from Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, in the year 2009 and completed senior residency from the same institute in July 2012. She received ‘Young Radiation Oncologist Fellowship Award in Advanced Radiation Techniques’ and worked in UPMC Pittsburgh, USA, for 1 month in October 2012. She received travel grant for ‘The European Society For Medical Oncology (ESMO) Preceptor-ship on GI Neuroendocrine tumors Meeting’ at Singapore in November 2012. She has a total of 48 publications in international and national journals, and she has contributed two chapters in books.
Author’s contributions
DK, NG and AB analyzed and interpreted the patient data, especially clinical and histopathological data. AD did the primary surgery and helped in preparing the manuscript. AKP, KD and DK helped in the management of the patient and final approval of the contents of the manuscript. NG and AK did all the literature search, immunohistochemistry and primary preparation of the manuscript. All authors read and approved the final manuscript.
References
- 1.Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol. 1994;18:1102–1116. doi: 10.1097/00000478-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Harrison ML, Hoskins P, du Bois A, Quinn M, Rustin GJ, Ledermann JA, et al. Small cell of the ovary, hypercalcemic type–analysis of combined experience and recommendation for management. A GCIG study. Gynecol Oncol. 2006;100:233–238. doi: 10.1016/j.ygyno.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Callegaro-Filho D, Gershenson DM, Nick AM, Munsell MF, Ramirez PT, Eifel PJ, et al. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): a review of 47 cases. Gynecol Oncol. 2016;140:53–57. doi: 10.1016/j.ygyno.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florell SR, Bruggers CS, Matlak M, Young RH, Lowichik A. Ovarian small cell carcinoma of the hypercalcemic type in a 14 month old: the youngest reported case. Med Pediatr Oncol. 1999;32:304–307. doi: 10.1002/(SICI)1096-911X(199904)32:4<304::AID-MPO13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Dickersin GR, Kline IW, Scully RE. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer. 1982;49:188–197. doi: 10.1002/1097-0142(19820101)49:1<188::AID-CNCR2820490137>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Agaimy A, Thiel F, Hartmann A, Fukunaga M. SMARCA4-deficient undifferentiated carcinoma of the ovary (small cell carcinoma, hypercalcemic type): clinicopathological and immunohistochemical study of 3 cases. Ann Diagn Pathol. 2015;19:283–287. doi: 10.1016/j.anndiagpath.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Senekjian EK, Weiser PA, Talerman A, Herbst AL. Vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide in the treatment of small cell carcinoma of the ovary. Cancer. 1989;64:1183–1187. doi: 10.1002/1097-0142(19890915)64:6<1183::AID-CNCR2820640603>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Clarke BA, Witkowski L, Ton Nu TN, Shaw PA, Gilks CB, Huntsman D, et al. Loss of SMARCA4 (BRG1) protein expression as determined by immunohistochemistry in small-cell carcinoma of the ovary, hypercalcaemic type distinguishes these tumors from their mimics. Histopathology. 2016;69:727–38. doi: 10.1111/his.12988. [DOI] [PubMed] [Google Scholar]
- 9.Wallbillich JJ, Nick AM, Ramirez PT, Watkins JL, Euscher ED, Schmeler KM. Vinblastine, cisplatin, cyclophosphamide, bleomycin, doxorubicin, and etoposide (VPCBAE) in the management of three patients with small-cell carcinoma of the ovary. Gynecol Oncol Case Rep. 2012;2:58–60. doi: 10.1016/j.gynor.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pautier P, Ribrag V, Duvillard P, Rey A, Elghissassi I, Sillet-Bach I, et al. Results of a prospective dose-intensive regimen in 27 patients with small cell carcinoma of the ovary of the hypercalcemic type. Ann Oncol. 2007;18:1985–1989. doi: 10.1093/annonc/mdm376. [DOI] [PubMed] [Google Scholar]


