Abstract
One of the fundamental limitations in assessing potential efficacy in Central Nervous System (CNS) transplantation of stem cells is the capacity for monitoring cell survival and migration noninvasively and longitudinally. Human glial‐restricted progenitor (hGRP) cells (Q‐Cells) have been investigated for their utility in providing neuroprotection following transplantation into models of amyotrophic lateral sclerosis (ALS) and have been granted a Food and Drug Administration (FDA) Investigational New Drug (IND) for intraspinal transplantation in ALS patients. Furthermore, clinical development of these cells for therapeutic use will rely on the ability to track the cells using noninvasive imaging methodologies as well as the verification that the transplanted GRPs have disease‐relevant activity. As a first step in development, we investigated the use of a perfluorocarbon (PFC) dual‐modal (19F magnetic resonance imaging [MRI] and fluorescence) tracer agent to label Q‐Cells in culture and following spinal cord transplantation. PFCs have a number of potential benefits that make them appealing for clinical use. They are quantitative, noninvasive, biologically inert, and highly specific. In this study, we developed optimized PFC labeling protocols for Q‐Cells and demonstrate that PFCs do not significantly alter the glial identity of Q‐Cells. We also show that PFCs do not interfere with the capacity for differentiation into astrocytes either in vitro or following transplantation into the ventral horn of the mouse spinal cord, and can be visualized in vivo by hot spot 19F MRI. These studies provide a foundation for further preclinical development of PFCs within the context of evaluating Q‐Cell transplantation in the brain and spinal cord of future ALS patients using 19F MRI. stem cells translational medicine 2019;8:355–365
Keywords: MRI, Cell tracking, Fluorine‐19, 19F, Perfluorocarbon, Stem cell, Transplantation
Significance Statement.
This study demonstrates the feasibility of incorporating perfluorocarbon (PFC) into human glial‐restricted progenitors for in vivo 19F magnetic resonance imaging (MRI). Central to the use of PFC for clinical trials of transplantation into patients with diseases of the brain and spinal cord, including ALS, is the demonstration that PFCs do not significantly alter the glial identity of the Q‐Cell product. Finally, PFCs did not interfere with the capacity for differentiation into astrocytes either in vitro or following transplantation into the ventral horn of the mouse spinal cord and can be visualized in the brain using 19F MRI following transplantation. These studies form the foundation for future 19F MRI imaging studies of Q‐Cells using large animal and, eventually, human clinical trials.
Introduction
There are several “stem cell” transplantation studies in amyotrophic lateral sclerosis (ALS) occurring worldwide, using a variety of different strategies for administration of transplanted cells (intraparenchymally 1, 2, intrathecally 3, 4, and intravenously 3). Thus far, only 1 clinical trial has provided an assessment of the anatomical distribution of these cells in vivo in ALS patients through the use of superparamagnetic iron oxide (SPIO) as an magnetic resonance imaging (MRI) contrast agent for labeled, transplanted cells 3. The development of a robust stem cell imaging biomarker that can serially assess stem cell survival, migration, and localization within gray or white matter, would be a significant advancement in this field. The survival of stem cells transplanted in mouse models of ALS can be serially followed using bioluminescent imaging 5, but this modality cannot be used in humans.
Fluorine‐19 cell labeling has emerged as a recent alternative method for MRI‐based cell tracking 6, 7, 8, 9. 19F has several attractive properties, among which is that it is 100% naturally abundant and there is negligible 19F background signal in tissues, thus allowing for “hot spot” MR imaging of transplanted cells 10. Imaging 19F‐labeled cells can be performed after 1H MRI within the same MRI session, and using 1H/19F MRI overlays, the anatomical localization of these cells can be accurately determined 11. PFCs are also chemically inert and stable, are not metabolized, and are excreted by normal clearance mechanisms 11. Previous work has demonstrated that 19F‐labeled neural stem cells (NSCs) could be readily identified in the mouse striatum using serial MR imaging over at least a 2‐week period post‐transplantation 9.
Now that 19F MRI cell tracking is entering the clinic 12, 13, 14, central to the further use of perfluorocarbon (PFC)‐labeled cells in human clinical trials is the demonstration that PFCs do not significantly alter the fundamental properties of the cells following labeling. In this study, we used several methods to optimize the most appropriate strategy for loading human glial‐restricted progenitors (hGRPs) with the commercial PFC probe CS‐1000 in order to maximize the 19F signal while minimizing cellular toxicity. We demonstrate only minor effects of 19F CS‐1000 on hGRP survival, proliferation, and capacity for differentiation. We also show that CS‐1000 labeled Q‐Cells can successfully integrate in the rodent spinal cord and retain CS‐1000 within their cytoplasm without leakage of the compound to adjacent host cells for at least 7 days after transplantation. We also confirmed that labeled cells can be readily visualized with 19F MRI following transplantation into mouse brain.
Materials and Methods
Derivation and Culture of hGRPs
Human GRPs (Q‐Cells) were derived using methods described previously 15, 16. Following derivation, Q‐Cells were cultured in vitro on polyornithine and laminin‐coated flasks according to the protocols provided by Q Therapeutics, Inc., and as described by Sandrock et al. 16. Q‐media is used for the maintenance of Q‐Cells and is composed of Dulbecco's modified Eagle medium/F12‐containing l‐glutamine supplemented with antibiotic/antimycotic (Corning Life Sciences, Acton, MA), N1 supplement (Sigma–Aldrich, St. Louis, MO), 0.01% human serum albumin (Baxter, Deerfield, IL), 20 ng/ml basic fibroblast growth factor (Peprotech, Rocky Hill, NJ), 10 ng/ml platelet‐derived growth factor‐AA (Peprotech) and 5 ng/ml NT‐3 (Peprotech). Q‐Cells were expanded and either prepared for experiments or frozen using serum free freezing medium (Biowhittaker Profreeze freezing medium [Lonza Walkersville, Inc., Walkersville, MD]) as described previously 16. Frozen cells were stored at −80°C and transferred to liquid nitrogen storage the following day.
Q‐Cells have been solely derived from deidentified tissue collected under protocols that have been Institutional Review Board (IRB)‐reviewed and deemed exempt as cadaveric tissue. Tissues were collected only after the donor had been counseled on the potential use of the donated tissue and subsequently executed an informed consent specific to that tissue donation and collection, in compliance with FDA regulations.
For the in vivo 19F MRI studies, we used SV40 immortalized Q‐Cells which were prepared as described previously 17. This allowed us to generate larger numbers of cells required for these studies.
Optimization of Labeling Q‐Cells with PFC
A dual‐modal (i.e., MRI and fluorescence) tracer agent was used to label cells (CS‐1000 DM Green or CS‐1000 DM Red, Celsense, Inc., Pittsburgh, PA) in culture. Cells were first labeled using the specified manufacturer's instructions and as described previously 18. Cells were labeled with CS‐1000 DM Green and CS‐1000 DM Red along with 1% bovine serum albumin (BSA).
Immunocytochemistry
Q‐Cells were passaged onto chamber slides and labeled with CS‐1000 DM Green at 37°C. Following incubation, subsets of cells were immediately fixed and another subset of cells were incubated for 96 hours (and then fixed) with normal growth media supplemented with 10% fetal bovine serum (FBS) to induce in vitro differentiation. After fixation, the cells were stained for various markers including, but not limited to, glial progenitor markers, neural stem cell markers, neuronal markers, and astroglial markers. A detailed list of antibodies used is given in Supporting Information Table S1. One coverslip was used per condition. Five fields of view at ×20 were randomly selected, and the number of positive cells was counted for each well. Four wells for each condition were analyzed and the statistical analysis was performed using a one way analysis of variance (Prism Software) and a significance level of p < .05.
Resazurin Assay for Assessment of Cell Survival
A resazurin assay was used in order to determine cell proliferation and cell survival in control groups of Q‐Cells as well as Q‐Cells that were labeled with varying concentrations of fluorescently labeled CS‐1000. The experimental conditions were as follows: Q‐media control (culture media and growth factors only), 1% BSA control (culture media, growth factors, and 1% BSA), 1 mg/ml CS‐1000 DM Green (culture media, growth factors, 1% BSA, 1 mg/ml CS‐1000 DM Green), and 5 mg/ml CS‐1000 DM Green (culture media, growth factors, 1% BSA, 5 mg/ml CS‐1000 DM Green). Following a 24‐hour incubation period, media was removed from all wells and fresh Q‐media with growth factors was added along with the resazurin solvent (10%; Sigma–Aldrich). After an incubation period of approximately 3.5 hours, the supernatant was collected and transferred to a 96‐well assay plate. The fluorescence was measured at 590 nm using a FLUOstar OPTIMA fluorospectrometer 19.
Flow Cytometry
Flow cytometry experimental conditions were as follows: Q‐media control (Q‐media and growth factors), 1% BSA control (received culture media, growth factors, and 1% BSA), 1 mg/ml CS‐1000 DM Green (Q‐media, growth factors, 1% BSA, and 1 mg/ml CS‐1000 DM Green), and 5 mg/ml CS‐1000 DM Green (Q‐media, growth factors, 1% BSA, 5 mg/ml CS‐1000 DM Green). Following incubation, cells were washed twice with phosphate‐buffered saline (PBS), lifted from culture flasks using TrypLE and DNase and then centrifuged for 7 minutes at 300g. The pellets were then resuspended in 1% BSA in PBS.
hGRP Transplantation
Spinal cord transplantation: Q‐Cells were incubated for 1 week with 1 mg/ml CS‐1000 DM Green. At the time of transplantation, cells were suspended in basal medium at a concentration of 7.5 × 104 cells per microliter. Mice harboring an ALS transgene (B6SJL‐Tg[SOD1* G93A]1Gur/J: Stock #002726) were immunosuppressed by intraperitoneal administration of FK‐506/Rapamycin (1 mg/kg per each; LC Laboratories, Woburn, MA) daily beginning 5 days before grafting and continuously until sacrifice. Immunosuppressed animals received labeled cell transplants at 50–60 days of age. Each mouse received 2 grafts (bilaterally at C5) of 1.5 × 105 cells per site in 2 μl basal medium into the ventral horn. Cells were delivered using a 10 μl Hamilton Gastight syringe with an attached 30‐gauge 45° beveled needle (Hamilton, Reno, NV). The injection pipette was secured to a manual micromanipulator (World Precision Instruments, Sarasota, FL) attached to an 80° tilting base 20. The tip was lowered to a depth of 0.75 mm below the surface of the cord and was held in place for 2 minutes before and after cell injection. Cells were delivered under the control of a microsyringe pump controller (World Precision Instruments) at a rate of 1 μl/minute 21. Animals were placed on a heating pad for recovery until awake from the anesthetic and then transferred to their home cages.
Brain transplantation: SV40 immortalized Q‐Cells (SV40 Q‐Cells) were incubated for 4 days with 10 mg/ml CS‐1000 DM Green. At the time of transplantation, cells were suspended at 1.5 × 105 cells per microliter PBS. Two male, 7‐week‐old immunodeficient Rag2(−/−) mice were anesthetized using 1.5% isoflurane gas. Each mouse received a bilateral graft in the striatum containing 1.5 × 106 cells per site in 10 μl PBS. CS‐1000 labeled SV40 Q‐Cells were injected in the right and unlabeled SV40 Q‐Cells in the left striatum. Cells were injected using a 10 μl Hamilton gastight syringe with an attached 30‐gauge 45° beveled needle (Hamilton). The injection pipette was secured to a manual micromanipulator (World Precision Instruments). The tip was lowered into the striatum and was held in place for 5 minutes before and after cell injection. The stereotaxic coordinates used were AP = +0.6 mm, ML = ±1.7 mm, and DV = −2.6 mm relative to bregma. Cells were delivered using a microsyringe pump controller (World Precision Instruments) at a rate of 0.2 μl/minute.
Post‐Mortem Tissue Analysis
Animals were sacrificed 1 week following transplantation in the spinal cord. Mice were anesthetized using an anesthetic cocktail (acepromazine maleate [0.7 mg/kg], ketamine [95 mg/kg], and xylazine [5 mg/kg]) and then transcardially perfused with ice‐cold 0.9% saline, followed by ice‐cold 4% paraformaldehyde (Fisher Scientific, Pittsburgh, PA). Spinal cords were removed and cryoprotected in a 30% sucrose/0.1 M phosphate buffer at 4°C for 3 days. Tissue was embedded in tissue freezing media (Triangle Biomedical Sciences, Durham, NC), snap frozen on dry ice, and stored at −80°C until processed. Spinal cord tissue blocks were cut in transverse planes at 20 μm thicknesses. Sections were collected on glass slides and stored at −20°C until analyzed. Subsets of spinal cord slices were collected in Tris‐buffered saline (TBS) for free‐floating immunohistochemistry 21. Animals with transplanted cells in the striatum were perfused immediately after MRI which was conducted 24 hours after transplantation. Brains were removed and cryoprotected as described above and cut in coronal sections at 30 μm thickness.
Tissue sections were blocked for 1 hour in 10% goat serum with 0.3% Triton‐X, incubated overnight with primary antibody at 4°C in 2% goat serum with 0.3% Triton‐X, washed in 1× TBS and then incubated with secondary antibody (Alexa Fluor, Life Technologies at 1:500 or Jackson Immunoresearch at 1:200) for 2 hours at room temperature followed by further washing in 1× TBS. Tissue sections were mounted with Prolong Gold with DAPI (Life Technologies). Primary antibodies used were antihuman nuclear antigen (HuNA; Millipore, #AB5541, 1:100) and antiglial fibrillary acidic protein (GFAP, Clontech, 1:400). Images were acquired on a Zeiss fluorescence microscope using a Hamamatsu C11440 Orca Flash 4.0 LT digital CMOS camera.
19F MRI
In vivo MRI of mice injected with CS‐1000 DM Green labeled SV40 Q‐Cells was performed 24 hours after cell transplantation using a 11.7 T Bruker horizontal bore magnet. Mice were anesthetized with ketamine/xylazine (100/10 mg/kg) to circumvent 19F background signal arising from isoflurane gas anesthesia. The body temperature was maintained at 37°C, and animals were monitored using a respiratory sensory pad (SA Instruments, Stony Brook, NY). Imaging was performed using a transceiver 20 mm surface coil tunable to both 1H and 19F frequencies. 1H images were acquired by using a FLASH sequence with the following parameters: repetition time (TR) = 100 milliseconds; echo time (TE) = 1.45 milliseconds; number of averages (NA) = 16; slice thickness = 1 mm; matrix = 196 × 196; and field of view [FOV]) = 2.2 cm × 2.2 cm. 19F images were also acquired using a FLASH sequence with the following parameters: TR = 100 milliseconds; TE = 0.83 milliseconds; NA = 1800; slice thickness = 5 mm; matrix = 32 × 32; and FOV = 2.2 cm × 2.2 cm.
Results
Efficiency of CS‐1000 DM Green Labeling
In order to determine the efficiency of CS‐1000 DM Green labeling in Q‐Cells, we incubated cells with 1 mg/ml CS‐1000 DM Green over a 1‐week period. Figure 1 demonstrates that 100% of Q‐Cells are labeled with CS‐1000 DM Green (Fig. 1A,C). No change in morphology was noted when comparing labeled cells with Q‐Cells incubated with 1% BSA alone. Following a 96‐hour incubation with 10% FBS to induce astrocyte differentiation, high power confocal microscopic examination revealed perinuclear localization of CS‐1000 DM Green in a Q‐Cell derived astrocyte as evaluated by GFAP immunostaining (Fig. 1D‐H).
Figure 1.
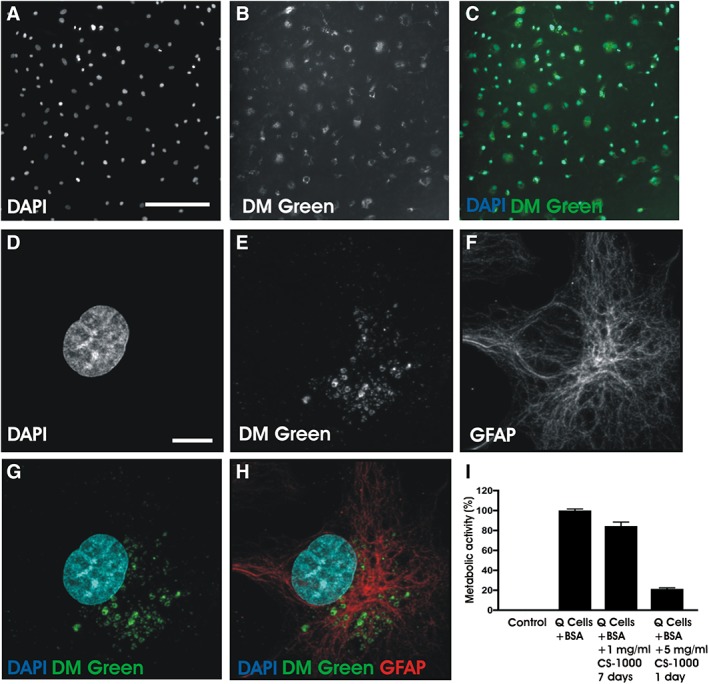
Optimizing CS‐1000 DM green labeling of Q‐Cells. (A–C): Fluorescence microscopy demonstrates that 100% of Q‐Cells exhibit CS‐1000 DM green labeling without changes in cell morphology. Scale bar = 200 μm. (D–H): Immunohistochemistry shows perinuclear localization of internalized CS‐1000 DM green in a differentiated Q‐Cell expressing glial fibrillary acidic protein. Scale bar = 10 μm. (I): Survival of Q‐Cell survival as determined by a resazurin reduction assay following incubation with 2 concentrations of CS‐1000 DM green of varying duration.
Cell Survival of CS‐1000 DM Green‐Labeled Cells
We examined the most appropriate concentrations of fluorescent PFC that would maximize survival of Q‐Cells. In order to aid in the endocytosis of CS‐1000 DM Green, we added 1% BSA to the incubation medium. The incubation of CS‐1000 DM Green at a concentration of 1 mg/ml for 1 week resulted in nearly 100% viability of Q‐Cells at this time when compared with unlabeled Q‐Cells. Higher concentrations of fluorinated CS‐1000 DM Green (5 mg/ml) for even shorter time periods of 1 day resulted in a greater than 60% reduction in survival as determined by the resazurin reduction assay (Fig. 1I).
Flow Cytometry
We observed similar fluorescence intensities for the labeling paradigm of 1 mg/ml CS‐1000 DM Green for 7 days and the 5 mg/ml CS‐1000 DM Green incubated for 1 day (Fig. 2A). Longitudinal analysis during day 2, 4, and 6 days of incubation with CS‐1000 DM Green demonstrated that the label is gradually and homogeneously internalized over time (Fig. 2B).
Figure 2.
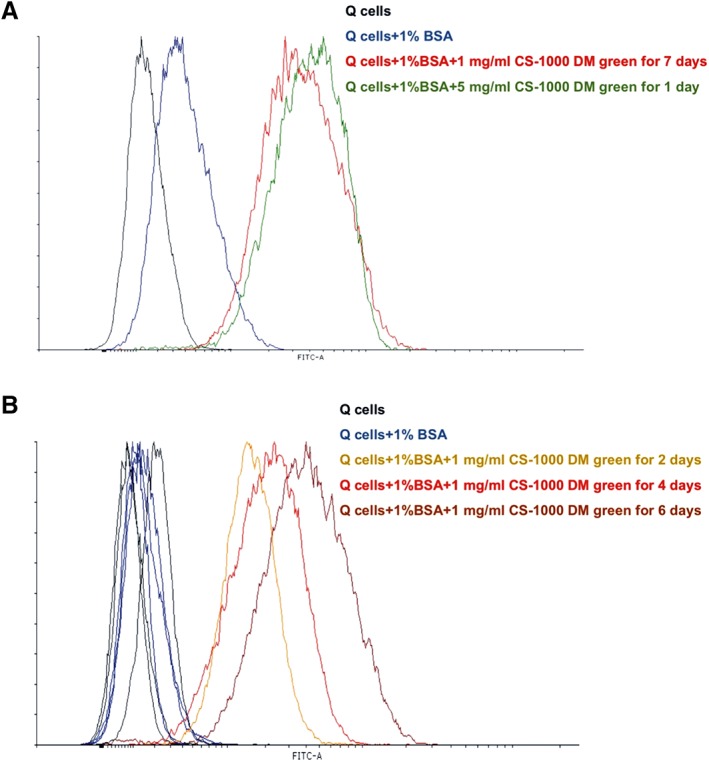
Flow cytometry of CS‐1000 DM green‐labeled cells. (A): Adding 1% bovine serum albumin (BSA) produces a minimal increase in autofluorescence (blue) compared with medium alone (black). Incubation of Q‐Cells with 1 mg/ml CS‐1000 DM green for 7 days (red) and 5 mg/ml CS‐1000 DM green (green) for 1 day demonstrate similar fluorescence labeling intensities. (B): Fluorescence intensity increases homogeneously over time. Adding 1% BSA again produces a minimal increase in autofluorescence (blue) compared with incubation in medium without BSA (black). Incubation of Q‐Cells with 1 mg/ml CS‐1000 DM green over time shows increasing fluorescence intensity over time (2 days [orange], 4 days [red] and 6 days [dark red]).
Analysis of hGRP Phenotype Following CS‐1000 DM Green Labeling
Important to the application of PFC‐labeled cells in clinical use is an analysis of the effects of PFC on markers of cell identity both before and after differentiation. Q‐Cells are defined by the expression of the glial progenitor cell‐surface marker A2B5 16. Analysis of undifferentiated Q‐Cells maintained in Q‐media showed that 68.6% ± 1.25% of cells expressed A2B5. Incubation of cells with 1% BSA did lower the percentage of A2B5 expressing cells. Q‐Cells incubated for 1 week with 1 mg/ml of CS‐1000 DM Green resulted in a similar percentage of cells showing A2B5 immunoreactivity which was not significantly different from either Q‐Cells alone, or Q‐Cells incubated with 1% BSA for 1 week (p > .05; Fig. 3A). We also used the expression of nestin as a marker for neural stem cell identity. Nestin immunostaining was noted in 68.2% ± 1.05% Q‐Cells, 69.1% ± 6.0% of those incubated with 1% BSA, and a modest reduction in nestin immunostaining to 51.7% ± 1.6% in Q‐Cells incubated with 1% BSA + 1 mg/ml of CS‐1000 DM Green (p < .05; Fig. 3B).
Figure 3.
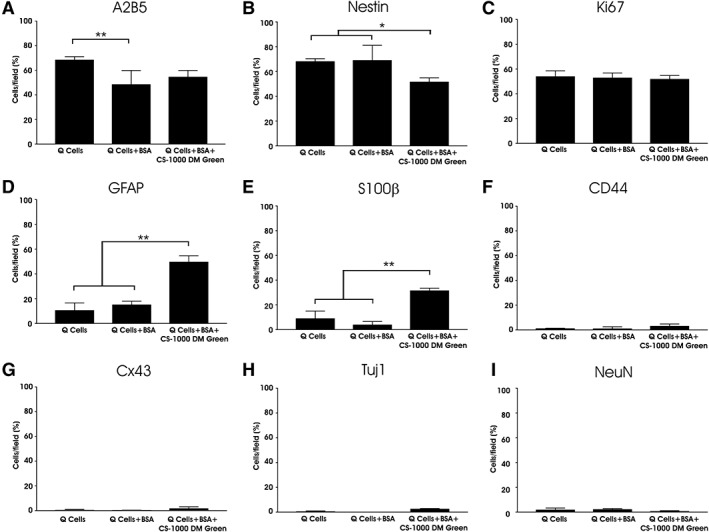
Expression of glial markers by CS‐1000 DM green labeled Q‐Cells. The majority of Q‐Cells express markers of multipotency including the glial‐restricted progenitor marker A2B5 (A) and nestin (B). Cell division is not affected by CS‐1000 DM green labeling as seen with Ki67 staining (C). Incubation of Q‐Cells with CS‐1000 DM green results in an increase in GFAP (D) and S100β expression (E). Immunostaining for the astrocyte progenitor marker CD44 is low among all groups (F) as is the astrocyte gap junction protein Cx43 (G). Neuronal markers Tuj1 (H) and NeuN (I) were expressed only rarely among the 3 labeling conditions (*, p < .05; **, p < .01).
The absence of tumor formation, secondary to rapid proliferation and cell division, within the CNS is important in establishing the safety of such cells with reference to their translational capacity for ALS treatment following transplantation. Incubation of Q‐Cells with 1% BSA + 1 mg/ml of CS‐1000 DM Green for 1 week resulted in positive Ki67 immunostaining of 51.9% ± 1.43% for labeled cells compared with 54.1% ± 2.23% for unlabeled cells and 53.1% ± 1.87% for cells labeled with 1% BSA only, with no statistical difference among the 3 groups. These data suggest that there is a substantial population of cells, consistent with their progenitor identity, that continue to divide but that the presence of CS‐1000 DM Green does not increase this phenomenon (Fig. 3C). GFAP has been identified in vitro as a property acquired by astrocytes during maturation but is also present in immature cells to some degree 22. Prior to differentiation, 10.7% ± 2.93% Q‐Cells stained positively for GFAP similar to those incubated with 1% BSA (15.2% ± 1.41%). Incubation with 1 mg/ml of CS‐1000 DM Green increased the number of GFAP+ cells to 49.8% ± 2.43% (p < .01; Fig. 3D), possibly suggesting an initiation of glial maturation following exposure to CS‐1000 DM Green. Similarly, less than 10% of Q‐Cells without exposure to CS‐1000 DM were positive for S100β, whereas 31.6% ± 0.9% of Q‐Cells labeled with CS‐1000 DM Green expressed S100β (p < .01; Fig. 3E). There was little expression of the astrocyte gap junction protein Cx43 or the astrocyte progenitor marker CD44 for any of the 3 conditions (Fig. 3F,G). As expected, few undifferentiated Q‐Cells expressed the neuronal proteins Tuj1 or NeuN (Fig. 3H,I). Supporting Information Table S2 lists the percentage of cells that express these neural markers.
CS‐1000 DM Green Labeling Does Not Affect Differentiation of hGRP into Glial Cell Subtypes
Q‐Cells were differentiated into astrocytes by adding 10% FBS to the medium. Following a 4‐day incubation of Q‐Cells with 10% FBS for all 3 groups, we noted a significant reduction in the glial progenitor marker A2B5 across all 3 groups which is consistent with a transition to a more mature glial identity (Fig. 4A). Interestingly, nestin immunoreactivity remained elevated across the 3 groups (Fig. 4B). As expected following differentiation, the number of Ki67 positive cells was reduced in the presence of 10% FBS but was unaffected by the incubation of 1 mg/ml of CS‐1000 DM Green when compared with differentiated, but unlabeled, Q‐Cells (Fig. 4C). Most notable was the increase in GFAP immunoreactivity observed for Q‐Cells (88.4% ± 2.9%) that is comparable to those Q‐Cells incubated with 1 mg/ml of CS‐1000 DM Green (80.0% ± 1.41%; Fig. 4D). These data suggest that CS‐1000 DM Green does not influence the potential of labeled Q‐Cells to fully differentiate into mature astrocytes. Similar changes were seen with S100β with all 3 conditions yielding similar percentages of cells expressing this glial marker (Fig. 4E). Consistent with a transition toward an astrocyte lineage, we also appreciated a significant increase in CD44 as an indicator of astrocyte progenitor identity (Fig. 4F). The expression of the astrocyte gap junction protein Cx43 was also increased across all 3 groups following differentiation (Fig. 4G). The addition of 10% FBS, either alone or in combination with 1 mg/ml of CS‐1000 DM Green, did not induce neuronal differentiation, but seemed to provoke a small decrease in neuronal markers expression levels, which were already very low (<3% of total population; Fig. 4H,I). Supporting Information Table S2 lists the percentage of cells that express these neural markers for given conditions both before and after differentiation of Q‐Cells into an astrocyte lineage. A representative example of the immunocytochemical staining for these astrocytic markers following differentiation of Q‐Cells labeled with CS‐1000 DM Green into appropriate subtypes showing the perinuclear localization of CS‐1000 DM Green can be seen in Figure 5 and Supporting Information Video S1.
Figure 4.
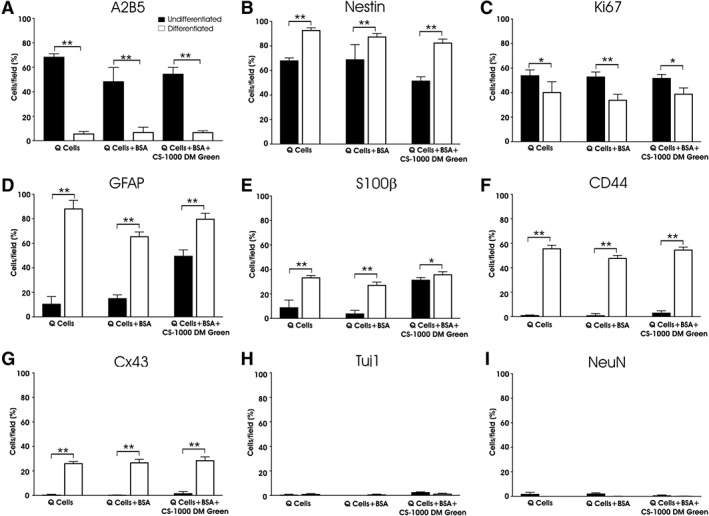
Q‐Cells differentiate into appropriate glial subtypes following induction with fetal bovine serum (FBS) and are unaffected by CS‐1000 DM green labeling. A2B5 immunostaining is reduced following induction of astrocyte differentiation among all groups (A) with the majority of cells still retaining nestin expression as a marker of progenitor cell identity (B). Cellular proliferation (Ki67 immunostaining) is reduced following FBS incubation (C). Increased expression of the astrocyte proteins glial fibrillary acidic protein (D) and S100β (E) are particularly noticeable along with an increase in the astrocyte progenitor marker CD44 (F) and the astrocyte gap junction protein Cx43 (G). Rare neuronal phenotype expression (Tuj1 [H] and NeuN [I]) are observed after differentiation (*, p < .05; **, p < .01).
Figure 5.
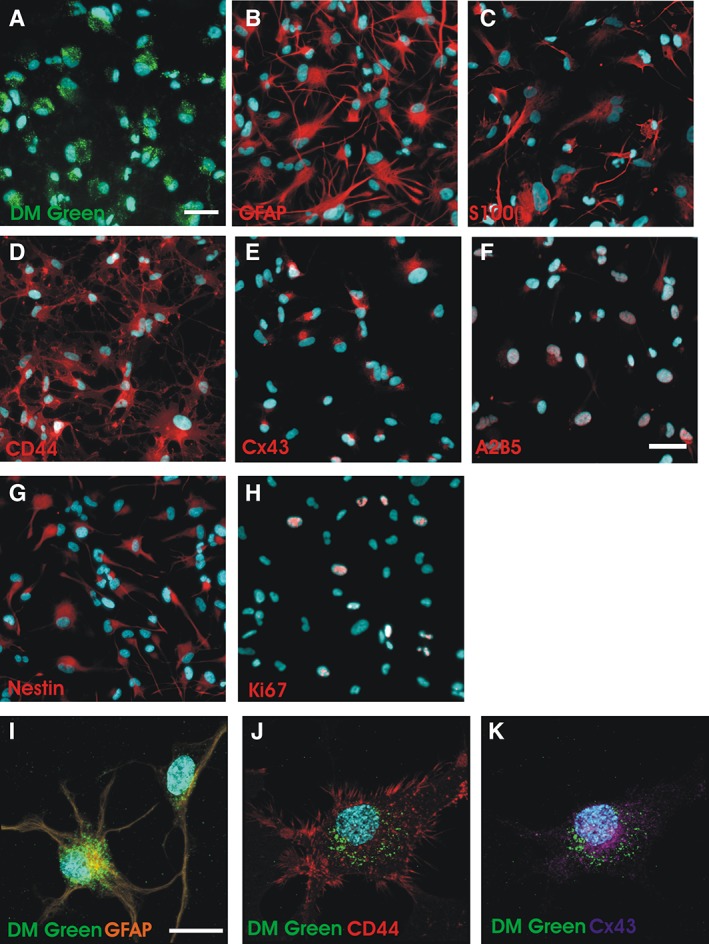
Immunohistochemical analysis of Q‐Cells after labeling with CS‐1000 DM green (A) Q‐Cells were incubated with 1 mg/ml CS‐1000 DM green for 1 week followed by incubation with 10% fetal bovine serum (FBS) for 96 hours (scale bar = 50 μm). (B–E): Q‐Cells differentiate into astrocytes and express appropriate astrocytic markers. (F, G): A2B5 and nestin immunostaining is still present after 4 days of FBS exposure suggesting a transition from progenitor to more mature astrocyte identity. Scale bar = 50 μm. (H): Few Ki67+ phenotypes are observed in differentiated Q‐Cells. (I–K): Confocal microscopy shows perinuclear DM green localization in differentiating astrocytes. Scale bar = 25 μm.
Analysis of CS‐1000 DM Green‐Labeled Cells After Transplantation into the Spinal Cord
In order to investigate whether the CS‐1000 DM Green label would persist inside Q‐Cells following transplantation into the ventral horn of the cervical spinal cord, we incubated undifferentiated Q‐Cells with CS‐1000 DM Green at 1 mg/ml for 1 week. One week after transplantation, we observed CS‐1000 DM Green‐labeled Q‐Cells primarily near the site of transplantation which colocalized with the expression of human cytoplasmic marker (Fig. 6). We observed that the vast majority of CS‐1000 DM Green could be identified in a perinuclear distribution in transplanted Q‐Cells 1 week following transplantation into the spinal cord (Supporting Information Video S2). We did not observe any host cells labeled with CS‐1000 DM Green through possible transfer from dying Q‐Cells.
Figure 6.
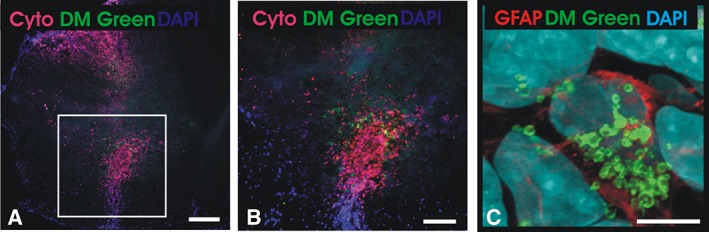
Spinal cord transplantation of CS‐1000 DM green‐labeled Q‐Cells. (A): Antihuman cytoplasmic antibody (Cyto) staining shows localization of CS‐1000 DM green‐labeled Q‐Cells within the ventral horn of the cervical spinal cord (square). Scale bar = 200 μm. (B): Higher magnification shows colocalization of DM green with human transplanted cells. Scale bar = 100 μm. (C): Antihuman specific GFAP immunostaining shows perinuclear localization of DM green in a transplanted Q‐Cell without transfer of label to host cells. Scale bar = 20 μm.
In Vivo 19F MRI
In order to examine whether Q‐Cells labeled with CS‐1000 DM Green would allow for an in vivo signal that can be visualized by MRI we first performed sensitivity measurements for 19F detection using labeled SV40 Q‐Cell phantoms composed of 2% agarose +0.1% sodium azide (data not shown). We used SV40 Q‐Cells to generate the larger numbers of transplanted cells required for imaging studies. We also found that they tolerated higher labeling concentrations of CS‐1000 DM Green compared with Q‐Cells. We determined the required concentration for labeling cells with CS‐1000 DM Green to be up to 10 mg/ml and the number of cells required to be 1 million to be detectable with 19F MRI using a clinically applicable scan duration time (NA). Since the size of the mouse spinal cord (unlike in humans) would not allow for injecting a sufficient amount cells to obtain an adequate signal, we chose the brain striatum as engraftment site for in vivo 19F MRI. Having optimized the imaging parameters, CS‐1000 DM Green labeled SV40 Q‐Cells were transplanted into the right striatum and unlabeled immortalized SV40 Q‐Cells into the left striatum as control. Twenty‐four hours after transplantation, the mice were imaged, with a resulting strong 19F signal from labeled SV40 Q‐Cells but no signal from unlabeled SV40 Q‐Cells (Fig. 7). The 19F MRI results were confirmed with histology, with the presence or absence of green fluorescence from the DM Green dye matching the 19F MRI results.
Figure 7.
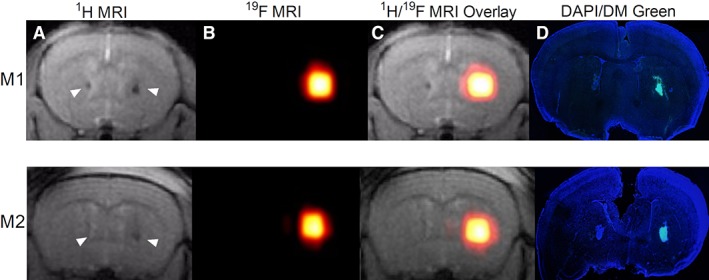
In vivo 19F MRI of immortalized Q‐Cells transplanted in the brain striatum. Representative examples are shown for 2 different animals (M1 and M2). (A): T2‐weighted coronal 1H MRI showing the 2 sites of transplantation (arrows) with CS‐1000 DM green labeled SV40 Q‐Cells on the right and unlabeled SV40 Q‐Cells on the left. (B): 19F MRI showing that signal is observed only for labeled cells 24 hours following transplantation. (C): 1H/19F MRI overlays. (D): Histological validation of MRI results. Green fluorescence is observed from labeled cells only, colocalizing with the 19F signal seen on MRI.
Discussion
In this study, we examined several key parameters for successful labeling of hGRPs with CS‐1000 DM Green in an effort to define the concentration and duration of incubation with the label that would produce maximum survival of cells and to examine how labeling of GRPs would influence their glial identity both before and after differentiation into astrocytes.
GRPs can be identified and isolated in embryonic rodents at E11.5–E.13.5 using the cell surface antigen A2B5 23. A human homolog of these cells has also been identified using this same surface antigen in human fetal brain and spinal cord tissue 16. These cells can also be sorted with antibodies to the cell surface marker A2B5. GRPs are multipotent cells which can either differentiate into astrocytes or oligodendrocytes, but not neurons, under appropriate conditions 23.
We have previously demonstrated that rodent GRPs can be transplanted into the ventral horn of the cervical spinal cord of SOD1G93A rats, a preclinical model of ALS. These cells survive for long periods of time and differentiate primarily into astrocytes. We have also demonstrated that these cells have a number of astrocyte properties and can express glutamate transporters. The transplantation of these cells into the SOD1G93A rat model of ALS resulted in improved survival, a delay in disease progression, and a reduction in the rate of limb weakness. Mechanistically, this effect appeared to be related to the capacity of these cells to reduce glutamate excitotoxicity through glutamate transport 24.
Using hGRPs or Q‐Cells, we have also established that these cells can be transplanted into the SOD1G93A mouse model of ALS and can also survive for the duration of disease. Like their rodent homologues, these cells also differentiate into astrocytes although less efficiently and over a longer time course than occurs with rodent GRPs 25. hGRPs have also been used in transplantation studies of spinal cord injury 26, 27, 28 and demyelinating lesions of the spinal cord 29. Given these findings, efforts have been made to translate these findings in rodent models of neurological disease to patients. However, one of the challenges with stem cell transplantation studies into the CNS is that the capability for evaluating the status of cells and their migration in the CNS is limited. Therefore, developing imaging strategies to noninvasively identify transplanted cells would offer significant clinical advantages as it would allow us to verify that the cells have been targeted to the appropriate anatomical region. Furthermore, cell survival and migration could also be assessed.
In the past, GRPs have been labeled with SPIOs 30, but it is sometimes difficult to discriminate between labeled cells and other sources of contrast arising from magnetic susceptibility effects associated with traumatic injury or hemorrhage 14. There is also concern that SPIOs may persist following cell death and be taken up by host macrophages—leading to false‐positive imaging results approximately the persistence of transplanted cells 30. Although this potential pitfall also holds for PFCs, we were unable to identify host cells containing CS‐1000 DM Green. PFCs have a number of potential benefits that make them appealing for clinical use. They enable cell quantitation, are safe (biologically inert), and since they are tracers rather than contrast agents they cannot be confused with other sources of contrast 9, 10.
Important to the translational potential of using PFCs in cell transplantation therapeutics, we have demonstrated that Q‐Cells are readily able to take up CS‐1000 DM Green with an efficiency of nearly 100% of all cells being labeled. We did note that Q‐Cells were somewhat more sensitive to higher doses of CS‐1000 DM Green than have been used previously in neural stem cells where doses of up to 5 mg/ml for 24 hours were well tolerated 18. However, we found that high concentrations over short times were toxic. We were able to maximize concentration and cell survival by using lower concentrations of CS‐1000 DM Green over longer timeframes. We also attempted to load Q‐Cells with CS‐1000 DM Green while in suspension but this resulted in poor cell survival (data not shown).
Similar to our previous data in the study of undifferentiated Q‐Cells, we found that CS‐1000 DM Green did not affect the capacity of Q‐Cells to differentiate into astrocytes and express markers associated with a continuum of glial maturation in vitro. Our previous studies examined Q‐Cell transplantation in SOD1G93A mouse models over longer timeframes of weeks to months. This paradigm was used to establish the potential for therapeutic efficacy. However, our current in vivo analysis was not intended as a therapeutic study but rather a short‐term analysis of cell survival and to establish whether labeled Q‐Cells would retain CS‐1000 DM Green labeling after transplantation. Confocal microscopy demonstrated that CS‐1000 DM Green has a perinuclear localization without the presence of CS‐1000 DM Green outside transplanted cells. Cell retainment of CS‐1000 DM Green at least at this early in vivo time point suggests that the use of CS‐1000 would allow for general localization and assessment of Q‐Cell survival following transplantation into the host spinal cord. Finally, we showed that hGRPs can be reliably imaged using 19F MRI, allowing for specific noninvasive localization of these cells following transplantation into the brain.
Conclusion
Taken together, this study demonstrates the feasibility of incorporating PFC into human GRPs for in vitro as well as in vivo labeling. Central to the use of PFC for clinical trials of transplantation into patients with diseases of the brain and spinal cord, including ALS, is the demonstration that PFCs do not significantly alter the glial identity of the Q‐Cell product. Finally, PFCs did not interfere with the capacity for differentiation into astrocytes either in vitro or following transplantation into the ventral horn of the mouse spinal cord and can be visualized in the brain using 19F MRI following transplantation. These studies form the foundation for future 19F MRI imaging studies of Q‐Cells using large animal and, eventually, human clinical trials.
Author Contributions
J.‐P.R.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; U.H., S.G., A.A., A.T.: collection and assembly of data, data analysis and interpretation; M.K.: conception and design, collection and assembly of data, data analysis and interpretation; J.T.C.: conception and design, provision of study materials, data analysis and interpretation, manuscript writing; J.W.M.B., N.J.M.: conception and design, financial support/collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.C. is Vice President of Research & Development and holds ownership interests in Q Therapeutics, Inc. Q Therapeutics provided the Q‐Cells used in this study. J.B. declared grant funding from Philips Health care, and a grant form Weinberg Physics, LLC. N.M. declared research funding from Q Therapeutics. The other authors indicated no potential conflicts of interest.
Supporting information
Supplemental Video 1 Q‐Cells incubated with 1 mg/ml CS‐1000 DM Green for 7 days followed by 10% FBS for 4 days results in astrocytic differentiation as demonstrated by GFAP immunostaining accompanied by CS‐1000 DM green labeling in a perinuclear distribution.
Supplemental Video 2 Q‐Cells (immunostained with anti‐human cytoplasmic antibody) incubated with 1 mg/ml CS‐1000 DM Green for 7 days and transplanted into mouse spinal cord shows integration in the ventral horn amongst host cell nuclei of the cervical spinal cord. Perinuclear DM Green is retained within Q‐Cells without evidence of transfer of DM Green into neighboring host cells.
Supplemental Table 1 Specifications of primary antibodies used in this study
Supplemental Table 2: Percentage immunopositive cells/field.
Acknowledgments
We are grateful to Dr. Chengyan Chu for assistance with brain transplantation studies, Irina Shats for post‐mortem evaluation, and Dr. Michael T. McMahon for his advice on the in vivo 19F MRI studies. This study was supported by the National Institutes of Health (R01 EB023647, R56 NS098520), the Maryland Stem Cell Research Fund (MSCRFII‐1706, MSCRFD‐3899), and the ALS Association (ALSA 16‐IIP‐252).
References
- 1. Tadesse T, Gearing M, Senitzer D et al. Analysis of graft survival in a trial of stem cell transplant in ALS. Ann Clin Trans Neurol 2014;1:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldman EL, Boulis NM, Hur J et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: Phase 1 trial outcomes. Ann Neurol 2014;75:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karussis D, Karageorgiou C, Vaknin‐Dembinsky A et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010;67:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrou P, Gothelf Y, Argov Z et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurol 2016;73:337–344. [DOI] [PubMed] [Google Scholar]
- 5. Srivastava AK, Gross SK, Almad AA et al. Serial in vivo imaging of transplanted allogeneic neural stem cell survival in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 2017;289:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noth U, Morrissey SP, Deichmann R et al. Perfluoro‐15‐crown‐5‐ether labelled macrophages in adoptive transfer experimental allergic encephalomyelitis. Artif Cells Blood Substit Immobil Biotechnol 1997;25:243–254. [DOI] [PubMed] [Google Scholar]
- 7. Ahrens ET, Flores R, Xu H et al. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 2005;23:983–987. [DOI] [PubMed] [Google Scholar]
- 8. Srinivas M, Morel PA, Ernst LA et al. Fluorine‐19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med 2007;58:725–734. [DOI] [PubMed] [Google Scholar]
- 9. Ruiz‐Cabello J, Walczak P, Kedziorek DA et al. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med 2008;60:1506–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bulte JW. Hot spot MRI emerges from the background. Nat Biotechnol 2005;23:945–946. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz‐Cabello J, Barnett BP, Bottomley PA et al. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 2011;24:114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahrens ET, Helfer BM, O'Hanlon CF et al. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine‐19 MRI. Magn Reson Med 2014;72:1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rose LC, Kadayakkara DK, Wang G et al. Fluorine‐19 labeling of stromal vascular fraction cells for clinical imaging applications. Stem Cells Translational Medicine 2015;4:1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulte JWM, Daldrup‐Link HE. Clinical tracking of cell transfer and cell transplantation: Trials and tribulations. Radiology 2018;289:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campanelli JT, Sandrock RW, Wheatley W et al. Expression profiling of human glial precursors. BMC Dev Biol 2008;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandrock RW, Wheatley W, Levinthal C et al. Isolation, characterization and preclinical development of human glial‐restricted progenitor cells for treatment of neurological disorders. Regen Med 2010;5:381–394. [DOI] [PubMed] [Google Scholar]
- 17. Janowski M, Jablonska A, Kozlowska H et al. Neonatal desensitization does not universally prevent xenograft rejection. Nat Methods 2012;9:856–858. author reply 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bible E, Dell'Acqua F, Solanky B et al. Non‐invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke‐damaged rat brain by (19)F‐ and diffusion‐MRI. Biomaterials 2012;33:2858–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed SA, Gogal RM Jr, Walsh JE. A new rapid and simple non‐radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J Immunol Methods 1994;170:211–224. [DOI] [PubMed] [Google Scholar]
- 20. Lepore AC, Rauck B, Dejea C et al. Focal transplantation‐based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 2008;11:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haidet‐Phillips AM, Doreswamy A, Gross SK et al. Human glial progenitor engraftment and gene expression is independent of the ALS environment. Exp Neurol 2015;264:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haidet‐Phillips AM, Roybon L, Gross SK et al. Gene profiling of human induced pluripotent stem cell‐derived astrocyte progenitors following spinal cord engraftment. Stem Cells Translational Medicine 2014;3:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao MS, Mayer‐Proschel M. Glial‐restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol 1997;188:48–63. [DOI] [PubMed] [Google Scholar]
- 24. Lepore AC, Rauck B, Dejea C et al. Focal transplantation‐based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 2008;11:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lepore AC, O'Donnell J, Kim AS et al. Human glial‐restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One 2011;6:e25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma 2013;30:1035–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin Y, Neuhuber B, Singh A et al. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma 2011;28:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexanian AR, Svendsen CN, Crowe MJ et al. Transplantation of human glial‐restricted neural precursors into injured spinal cord promotes functional and sensory recovery without causing allodynia. Cytotherapy 2011;13:61–68. [DOI] [PubMed] [Google Scholar]
- 29. Walczak P, All AH, Rumpal N et al. Human glial‐restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia 2011;59:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lepore AC, Walczak P, Rao MS et al. MR imaging of lineage‐restricted neural precursors following transplantation into the adult spinal cord. Exp Neurol 2006;201:49–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1 Q‐Cells incubated with 1 mg/ml CS‐1000 DM Green for 7 days followed by 10% FBS for 4 days results in astrocytic differentiation as demonstrated by GFAP immunostaining accompanied by CS‐1000 DM green labeling in a perinuclear distribution.
Supplemental Video 2 Q‐Cells (immunostained with anti‐human cytoplasmic antibody) incubated with 1 mg/ml CS‐1000 DM Green for 7 days and transplanted into mouse spinal cord shows integration in the ventral horn amongst host cell nuclei of the cervical spinal cord. Perinuclear DM Green is retained within Q‐Cells without evidence of transfer of DM Green into neighboring host cells.
Supplemental Table 1 Specifications of primary antibodies used in this study
Supplemental Table 2: Percentage immunopositive cells/field.


