Abstract
Dithranol is one of the important topical agents for the treatment of psoriasis, a chronic inflammatory skin disease with aberrant differentiation of keratinocytes. However, its application is troublesome and inconvenient because of its associated side effects, including staining, burning sensation, irritation, and necrotizing effect on the diseased cells as well as on the normal cells. The purpose of the current investigation was to explore the potential of poly(amido) amine (PAMAM) dendrimers in the topical delivery of dithranol through a novel microsponge based gel. Generation-4 (G4) dendrimers were incorporated into the microsponge based gel formulation by quasi-emulsion solvent diffusion method with varying concentration of polymers, and evaluated for the morphology of the formulation, encapsulation efficiency and skin irritation potential. Percentage yield of the formulation was found to be 66.28%, whereas encapsulation efficiency was ranged between 71.33% to 49.21%, and an average particle size was ranged between 28 ± 1.12 μm to 130 ± 1.01 μm. Surface morphology of developed microsponge was confirmed by scanning electron microscopy, revealed micro-porous nature. The optimized microsponge formulation was found to be stable and recorded non-irritant during cutaneous application of the experimental animals. Further, the pharmacokinetic outcomes of study were showed prolong penetration of the drug through the skin, equivalent to the marketed formulation of dithranol. Therefore, it could be conferred that the microsponge formulation of the PAMAM entrapped dithranol can produce prolonged efficacy without producing toxicities to the skin, and thus can effectively be projected in the treatment of diseases like psoriasis.
Keywords: Pharmaceutical science, Materials science
1. Introduction
Psoriasis, a chronic and even multisystem inflammatory disorder of skin or joints, is generally characterized by hyper-proliferation and aberrant differentiation of keratinocytes [1, 2]. Psoriasis is the most common autoimmune disorder in the USA and affects equally to men and women, however the severity was found more in men than women [3, 4, 5]. Prevalence of psoriasis is captured as a global concern by the World Health Organisation, and it has been estimated that more than 125 million patients are suffering with psoriasis worldwide [6]. Occurrence of psoriasis is increasing gradually, from 0·12% at age 1 year to 1·2% at age 18 years [5]. Recently, several guidelines has been established in the management of psoriasis, such as International European guidelines [7], North American guidelines [8], German S3 guidelines [9], where it has been designed to treat this disease with topical therapies first, to avoid the systemic toxicities of the drugs. Among the topical agents available for the treatment of this disease, dithranol (1,8-dihydroxy-9-anthrone) is a potential agent, where application of the agent is made on well-defined plaques of the diseased skin. Effectivity of dithranol in psoriasis is reflected by inhibition of granulocyte function, keratinocyte hyper-proliferation, as well as by immunosuppression [10]. It has also been reported that reduction of mitotic activity of the epidermal hyperplasia resulted in normalization of epidermal cell proliferation and keratinization [11]. The drug also inhibits DNA synthesis to exert its anti-proliferative action, simultaneously, anti-inflammatory role of the drug leads towards healthy skin [11, 12]. Advancement of research with dithranol reported that the effective role of the drug is due to accumulating the drug on mitochondrial membrane to interfere oxidative phosphorylation to restrict energy transfer in epidermal cells and thereby slows down excessive cell division in psoriatic condition [13]. Application of this agent relieves the patients for prolonged period with a low rate of recurrence [4]. However, degradative products of dithranol in presence of oxygen, light, basic pH, and heavy metals, generates free radicals to cause harm to the biological tissues and irritate deeply [14]. It has also been suggested that the generated free radicals by the drug further exerts its positive action through inhibiting psoriasis. Not only this, the generated superoxide anions can induce adaptive mechanism for the increased tolerance of the drug [10]. Alternatively the oxidised products of dithranol by the free radicals, i.e., danthron and dithranol dimer, are dark brown in colour, which produce staining on the patients skin and clothes without producing any efficacy on psoriatic skin [15].
Therefore, researchers are continuously trying to deliver this potential therapy precisely on the skin avoiding the reported toxicities of the drug. Nanotechnological application in medicine have perceived a great progression in this field since past few decades, which has targeted to overcome several limitations in conventional therapy by providing superior effectiveness and desired safety properties to attract patients acceptability [16, 17, 18]. Irregular and erratic pharmacokinetic parameters of most of the conventional therapy has effectively been overcome through adaptation of novel nanocarrier platform [19, 20]. Dendrimers, one of the leading nanocarrier, are widely explored, highly branched, multifunctional, nano-sized, monodisperse polymer with special physicochemical properties and exterior functional surface groups [21, 22]. Whereas, microsponges is an unique delivery technology for the controlled release of topical agents, which shows chemical affinity towards oil glands of the skin, thus resides on the skin for longer period [23]. Encapsulation of drug-entrapped nanocarrier in the microsponge could effectively deliver the drug slowly for prolonged period without allowing the drug component to degrade.
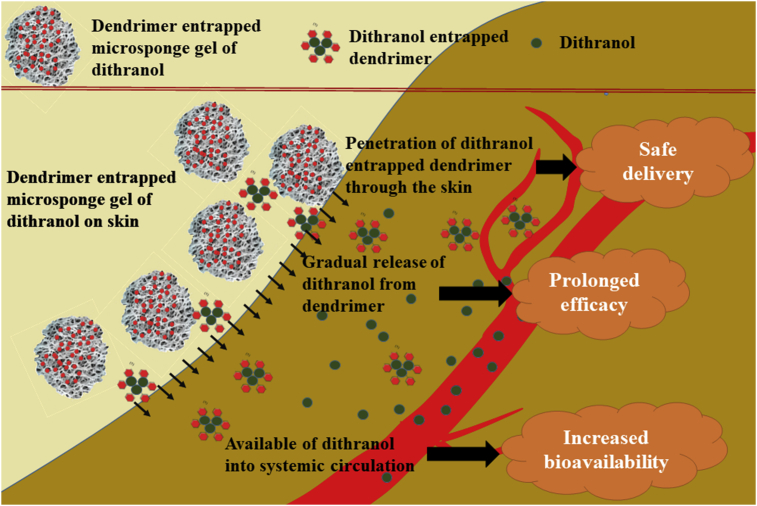
Taking into consideration the inherent advantages of drug delivery, to avoid formation of oxidative inert products of dithranol, to improve solubility of the drug and permeability through skin, the present study envisaged formulation development and evaluation of microsponge based gel of dendrimer-entrapped dithranol for topical delivery to bring new generation topical application. The schematic representation of the objective of our present work has been represented in Fig. 1.
Fig. 1.
Schematic representation of improved penetration of dendrimer entrapped microsponge gel of dithranol through skin.
2. Materials and methods
2.1. Materials
Dithranol was received as gift sample from Lifecare Innovation Pvt. Ltd, Lucknow. PAMAM G-4 dendrimer with surface amino groups (G4-NH2) was received from Nano Synthons, whereas sodium acetate, high performance liquid chromatography (HPLC) water, and potassium di-hydrogen phosphate were obtained from Merck Limited, India. Acetonitrile, and HPLC grade methanol were obtained from Qualigens Fine Chemicals Pvt. Ltd., India, and ethyl cellulose, polyvinyl alcohol (PVA), dichloromethane and Carbopol934 were obtained from S. D. Fine Chem. Ltd., India. All the chemicals used in the experiments were of analytical grade.
2.2. Methods
2.2.1. Preparation of microsponges of dithranol
The microsponge was formulated by adopting quasi-emulsion solvent diffusion method, where the method consisted of two phases, the inner phase and outer phase [23]. Initially, the inner phase was developed by dissolving drug (dithranol), G4-NH2 PAMAM dendrimer, and ethyl cellulose in dichloromethane. Whereas, the outer phase was prepared by dissolving sodium meta-bisulphate in distilled water (100 mL), and 5 mg of PVA was added to the aqueous solution. Finally, the inner phase was added to the outer phase with vigorous agitation at room temperature. Stirring leads to the formulation of discrete emulsion globules called quasi-emulsion globules. The stirring was continued up to 6 h until the insoluble rigid microsponges was formed. Then the solution was filtered to separate the microsponges. The microsonges were then dried in an air-heated oven at 40 °C. Details of composition and coding were provided in Table 1.
Table 1.
Composition of different microsponge formulations in various batches.
| Ingredient | Formulation code |
||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| Dithranol (%) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| PAMAM dendrimer (%) | 0.05 | 0.05 | 0.05 | 0.05 | - |
| Ethyl cellulose (mg) | 50 | 100 | 150 | 200 | 250 |
| Polyvinyl alcohol (mg) | 5 | 5 | 5 | 5 | 5 |
| Dichloromethane (ml) | 10 | 10 | 10 | 10 | 10 |
| Sodium metabisulphate (mg) | 1 | 1 | 1 | 1 | 1 |
| Distilled water (ml) | 100 | 100 | 100 | 100 | 100 |
| Drug: polymer | 1:1 | 1:2 | 1:3 | 1:4 | 1:4 |
2.2.2. Evaluation of microsponges
2.2.2.1. Percentage yield
The percentage yields of all formulated batches were evaluated by measuring the weight of final product following drying with respect to the initial combined weight of the components used for developing the solid dispersions [23].
2.2.2.2. Encapsulation efficiency (EE)
To evaluate the EE, specific measured quantity of microsponge (10 mg) was dissolved in dichloromethane (10 mL). To obtain the drug in solution, it was then filtered using Whatman filter paper (No 44). Then, 1 mL of filtrate was diluted to 10 mL with solvent to measure the absorbance at 257 nm. The drug content was determined from the standard curve [24].
2.2.2.3. Evaluation of dendrimer-drug interaction
2.2.2.3.1. Evaluation of dendrimer-drug interaction by UV spectroscopy
UV spectra of drug, dendrimer and the developed formulation were recorded by using UV spectrophotometer (Systronics) using the λmax in the range of 180–400nm.
2.2.2.3.2. Evaluation of dendrimer-drug interaction by FTIR spectroscopy
FTIR spectra of drug, dendrimer and final formulations were checked using KBr disc method.
2.2.2.4. Determination of particle size and zeta potential
Particle size and zeta potential of the optimized batch was determined using Malvern particle size analyzer, where the specified samples were placed in a clear disposable zeta cells. The results were recorded at 25 °C temperature. Before changing the sample during each experiment, cuvettes were washed with methanol and rinsed using the sample to be measured [25].
2.2.2.5. Morphology and surface topography
Scanning electron microscopy (SEM) was used to determine the morphology and surface topography of formulated microsponges. Before examining the samples under LEO 430 SEM analyser, all the samples were coated with gold–palladium alloy under vacuum.
2.2.3. Development of microsponge based gel
Gel formulation of the developed microsponge was prepared using Carbopol 934. Measured amount of Carbopol 934 was uniformly dispersed in aqueous media using propeller and allowed to hydrate overnight. Later, the microsponge formulation of dithranol was incorporated to the above media with constant stirring. This final mixture was neutralized slowly via addition of triethanolamine with constant stirring until formation of gel [23]. Detailed composition and coding of the formulations were depicted in Table 2.
Table 2.
Composition of different microsponge gel formulation.
| Components | Formulation code |
||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |
| Dithranol microsponges (mg) | 10 | 10 | 10 | 10 | 10 |
| Carbopol 934 (mg/g) | 30 | 60 | 90 | 120 | 150 |
| Triethanolamine (ml) | 1 | 1 | 1 | 1 | 1 |
| NaOH (2% w/v) to adjust pH 6.8 | q.s. | q.s. | q.s. | q.s. | q.s. |
| Distilled water (ml) | 10 | 10 | 10 | 10 | 10 |
2.2.4. Characterization of microsponge gel formulation
2.2.4.1. Measurement of pH
The pH of gel formulation was determined by using digital pH meter. Five grams of developed gel was dissolved in 45 mL of distilled water at 27 °C and stored for 2 h for measurement of pH of solution [23].
2.2.4.2. Spreadability studies
Expression of spreadability is determined by specific time required by the slides to slip off from the placed gel under a specific weight. Here, the spreadability was determined by wooden block and glass slide apparatus. It consists of two slides, the upper movable slide and lower non-movable slide. About 20 gm weight of gel was placed on the pan and time was noted for upper slide to separate completely from the fixed lower slide. Spreadability was then calculated by using the formula:
Where, S = Spreadability; W= Weight (in g) tide to upper slide; L = Length (in cm) of glass slide; T = Time (in s) taken to separate the slide completely from each other.
2.2.4.3. Drug content
Weighed quantity of microsponge gel was dissolved in 100 ml phosphate buffer solution (pH 7.4) and was shaken for 2 h on mechanical stirrer in order to promote solubility of the incorporated drug. This drug solution was then filtered using millipore filter paper (0.45 μm) and measured spectrophotometrically following suitable dilution at 257 nm against phosphate buffer (pH 7.4) as a blank.
2.2.4.4. Determination of viscosity
Viscosity of developed microsponge gel was determined by using Brookfield viscometer at room temperature using Spindle no 7 at 50 rpm. The dial scale reading multiplied by specific factor reflected the viscosity of the sample in centipoises.
2.2.5. In vitro drug release
A weighed quantity of formulation (6 mg/mL) was used in this release assessment. The sample was placed in a dialysis bag and suspended in a beaker of phosphate buffer saline at 37 °C under gentle magnetic stirring (100 rpm). At pre-determined timings, a specific volume of the sample was drawn with a replacement of equal volume of fresh medium [26]. The amount of dithranol released was estimated spectrophotometrically. The cumulative release percentage (CR%) of drug at each time point was determined using the following equation:
Where, Mi and Mt are the initial amount of drug encapsulated in the formulation and the amount of drug released at the time t, respectively.
2.2.6. Stability studies
All the selected formulations were subjected to stability testing as per ICH conditions at 40 ± 2 °C and 75 ± 5% relative humidity. All selected formulations were analysed at an interval of 30, 60 and 90 days to determine changes in appearance, pH and drug content [27].
2.2.7. Acute dermal toxicity studies
The dermal toxicity study of the developed microsponge gel was performed according to the Organization for Economic Corporation and Development guidelines [28]. Healthy young adult Wister rats of either sex were used in this experiment, where the animals were acclimatized under standard laboratory conditions, temperature at 25 ± 2 °C and 55 ± 5% relative humidity to the laboratory conditions for 7 days prior to the initiation of any test. The animal experiments were approved by the animal ethical committee and we followed the associated guidelines throughout the study. Animals were divided into 3 groups with 3 animals in each (n = 3). Fur of the experimental animals were carefully removed from the dorsal section of body surface a 24 h prior to the test with the help of thioglycolate containing hair removing cream, as thioglycolate salts are considered to be the safer depilatory agent without affecting skin characteristics [29]. Treatment design of different animal groups are mentioned in Table 3.
Table 3.
Treatment strategies of different groups of animals.
| Group | Design | Treatment |
|---|---|---|
| First | Control | Gel base, without drug |
| Second | Formulation | Formulated microsponge gel |
| Third | Standard | Marketed formulation, Derobin® |
All the animals were observed for a period of 14 days for any changes in fur, eyes, sleep pattern, central nervous system activity, behavior pattern, toxic reactions and time of death occurring during the dermal toxicity study.
2.2.8. Pharmacokinetic studies
To evaluate the pharmacokinetic profile of the gel formulation, the animals were divided into three groups with 6 animals each. The animals were fasted overnight with free access to water before study. Gel equivalent to 0.5 mg/kg of microsponge gel was applied topically in the test animals, whereas animals in the standard group received marketed formulation, Derobin® at a dose of 1.15 mg/kg. Predetermined blood samples (0.5mL) were collected by puncture of retro-orbital plexus of the experimental animals at time intervals of 0.0, 3.0, 6.0, 9.0, 12.0, 15.0, 18.0, 21.0 and 24.0 h post application of gel and collected in 1.5 mL polypropylene tube containing potassium EDTA. Plasma samples were prepared by centrifuging sample blood at 8000 rpm for 5 min. Supernatant was collected and stored at –20 °C till HPLC analysis [30, 31]. The analysis of the samples was carried out using chromatographic system from Shimadzu Corporation (Japan). Analysis of dithranol was performed at room temperature on a C18 column (4.6 mm × 250 mm ID, 5 μm particle size) under isocratic condition using the mobile phase consisted of 0.1% formic acid and methanol (30:70, v/v). For isocratic elution, the flow rate of the mobile phase was kept at 0.8 mL/min with themax at 257nm. Cmax and Tmax were obtained from the plasma concentration time profiles. The area under plasma concentration time curve (AUC0–24h) was calculated using trapezoidal rule.
3. Result and discussion
3.1. Development and evaluation of microsponge
Dithranol is highly susceptible agent for oxidation, as well as poorly permeable through the horny layer of the skin. Keeping in view the advantages of dendritic drug delivery system, to control oxidation of the drug and to increase permeability, the drug was encapsulated in PAMAM G4 dendrimer in this current experiment.
Further, microsponges were developed by quasi-emulsion solvent diffusion method using the different concentration of incorporated polymer. EE of all the batches was found to be in the range between 49.21% to 71.33%, where the percentage yield was about 56.96–72.41. Data presented in Table 4 reflected that the results of EE largely dependent on drug and polymer ratio, where, it is inversely proportional to each other. Thus, increase in drug to polymer ratio in the preparative mixture had been resulted in decreased EE. Additionally, percentage yield was found to be directly proportional to the drug, polymer ratio. It has been suggested that the increase production yield at a higher drug: polymer ratio reduces diffusion of dichloromethane from the concentrated solutions to the aqueous media, which ultimately helps in increased production yield via providing additional time to form the droplets [27]. Data on encapsulation efficiency, and percentage yield was in agreement with previous reports by other researchers [23, 27].
Table 4.
Representation of evaluation parameters of different microsponge formulations (n = 3).
| Formulation code | Drug: Polymer | Encapsulation Efficiency | Percentage Yield | Mean particle size diameter (μm) ± SD |
|---|---|---|---|---|
| F1 | 1:1 | 71.33 ± 5.451 | 56.96 ± 3.236 | 130 ± 1.01 |
| F2 | 1:2 | 69.81 ± 6.372 | 61.11 ± 4.352 | 115 ± 1.00 |
| F3 | 1:3 | 62.32 ± 5.553 | 66.28 ± 3.894 | 100 ± 1.01 |
| F4 | 1:4 | 59.76 ± 7.731 | 69.03 ± 6.386 | 50 ± 1.06 |
| F5 | 1:5 | 49.21 ± 4.949 | 72.41 ± 5.225 | 28 ± 1.12 |
Compatibility study between drug and dendrimer was confirmed by the UV spectrophotometry analysis and FTIR analysis. The outcome of the UV spectrometry experiment represents behavior of the drug-dendrimer complex similar to the pure drug. FTIR spectroscopic analysis results did not provide any additional peak or any disappearance of existing peaks, revealing no interaction between the drug and dendrimer.
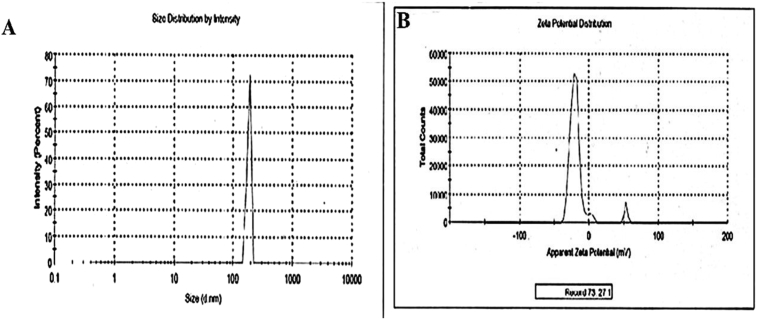
Furthermore, the particle sizes of the microsponges were about 28 ± 1.12 μm to 130 ± 1.01 μm. Data presented in Table 4 portrayed that the particle sizes, similar to EE of the product, depended on drug: polymer ratio, where it is inversely proportional to each other. This could be explained that at higher drug: polymer ratio, the available polymer was more and thus increase the polymer wall thickness, leading to increased sizes of the microsomes. Conversely, zeta potential of optimized formulations was shown potential stability of the formulated microsponge gel dispersion. Result demonstrated that zeta potential of the formulation tends to be more negative and thus repels to each other, to establish more stability. As per literature, the general separating line between an unstable and a stable suspension is generally taken as +30 or -30mV with particles having surface charge outside of these limits are considered stable [32].
Microsponge composition of F3 was found to have optimized results than other compositions, where the particle size, percent yield and EE of F3 batch were 100 ± 1.01 μm, 66.28%, and 62.32%, respectively. Therefore, among the five developed formulation batches, F3 was selected to evaluate further. Particle size distribution and surface charge of the F3 microsponge formulation were represented in Fig. 2.
Fig. 2.
A) Particle size distribution of optimized microsponge; B) Zeta potential of optimized micosponge.
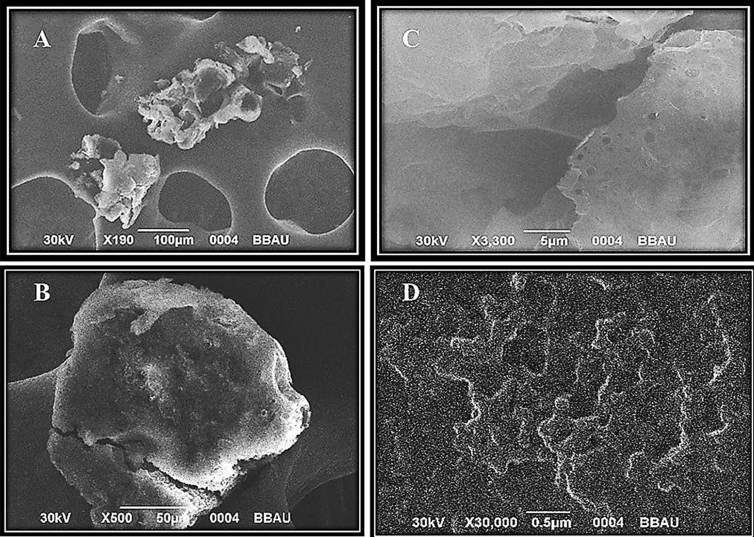
3.2. Morphology and surface topography of microsponges
Optimized F3 microsponge sample was then subjected to SEM analysis to assess the morphology and surface topography. The captured images of SEM analysis were represented in Fig. 3 (A to D). From the SEM photomicrographs, it could be observed that the formed microsponges were uniform and spherical in shape with highly porous nature, where no drug crystals on the surface could be observed. Moreover, it can be observed that the distinctive structures were comprised of spherical cavity encircling a rigid shell of the polymer. Therefore, it could be inferred that the shape of the microsponge are spherical as a single entity, which is porous in nature.
Fig. 3.
SEM images of F3 microsponge formulation coded in different resolution under (a) X 190, (b) X 500, (c) X 3,300, (D) X 30,000.
3.3. Evaluation of microsponge based gel
The pH of all the developed gel formulations, corresponding to increased concentration of Carbopol with F3 microsponge formulation, were in the range of 5.2–6.8 indicating suitability for skin application (Table 5). Spreadability of formulated gels were also measured within the range of 4.7–5.7 g cm/sec. The value of spreadibility indicates that the gel could be easily spreadable by small amount of shear. Drug content of all the batches were in the 52.39–71.32%.
Table 5.
Representation of various evaluation parameters for the different microsponge gel formulations (n = 3).
| Formulation code | pH | Spreadability (g.cm/sec) | Homogenicity | Viscosity (cps) | Drug Content (%) |
|---|---|---|---|---|---|
| GL-1 | 6.5 | 5.4 ± 1.145 | Good | 2874 ± 374 | 71.32 ± 2.463 |
| GL-2 | 6.8 | 5.5 ± 1.212 | Good | 2051 ± 287 | 63.26 ± 3.216 |
| GL-3 | 6.5 | 5.7 ± 0.985 | Very good | 2463 ± 256 | 65.91 ± 3.146 |
| GL-4 | 7.0 | 5.1 ± 1.347 | Good | 2641 ± 562 | 68.17 ± 5.463 |
| GL-5 | 5.2 | 4.7 ± 1.159 | Poor | 1946 ± 348 | 52.39 ± 4.572 |
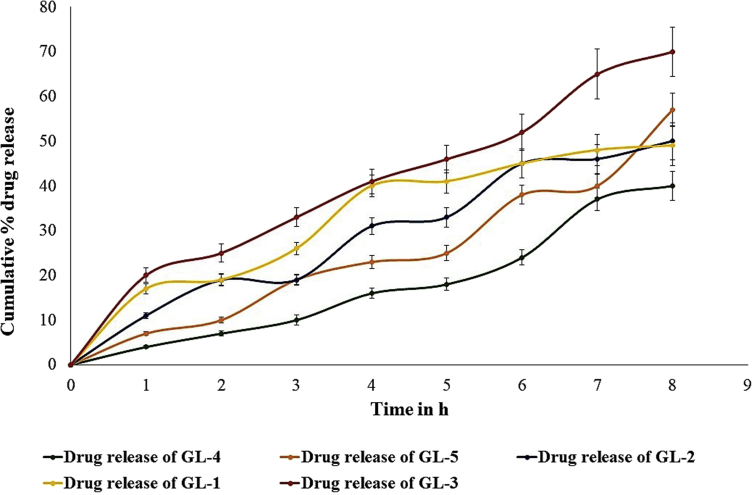
3.4. In vitro drug release of different formulation
The release pattern of drug from the microsponge gel was found to have a correlation to the percentage of incorporated Carbopol in it. Increase in Carbopol content resulted in decreased drug release from the gel formulation. Thickening of the gel matrix offers extended diffusion path for the entrapped drug, and ultimately led to decreased drug release. However, cumulative drug release of all the batches were found to be within the range of 40.33%–70.02% at the end of 8 h. Graphical representation of the comparative release of drug from the formulated microsponge batches (GL-1 to GL-5) had been depicted in Fig. 4.
Fig. 4.
In vitro comparative drug release of profile of formulated microsponge gels.
3.5. Stability studies
The developed microsponge gel formulation found to be stable upon storage of 12 weeks. No significant changes observed in their physical appearance, pH, rheological properties, drug content and drug release profiles after the incubation at specified condition for 12 weeks (data not shown).
Finally, to select the optimum formulation for the next experiments, we had compared the obtained parameters of the five gels. Among these, GL-3 has shown better cumulative release of drug (70.02 ± 5.505%), and also shown superior spreadability as compared to other batches. Considering all evaluating parameters, batch GL-3 was selected as the final batch for animal studies.
3.6. Acute dermal toxicity study
The present work conducted to investigate the dermal toxicity of the developed GL-3 microsponge gel. The rats were treated with an equivalent dose of dithranol (0.5%) in formulated microsponge gel and in marketed formulation. The formulations were applied on shaved area of 1 cm2 at the dorsal surface. During the period of 14 days observation, the animals in the test treatment group did not show any pronounced toxic symptoms. Thus, there was no symptoms of inflammation, irritation redness of the skin, edema, and rashes on the test treatment groups were observed, and did not show the any type of the cracks in skin.
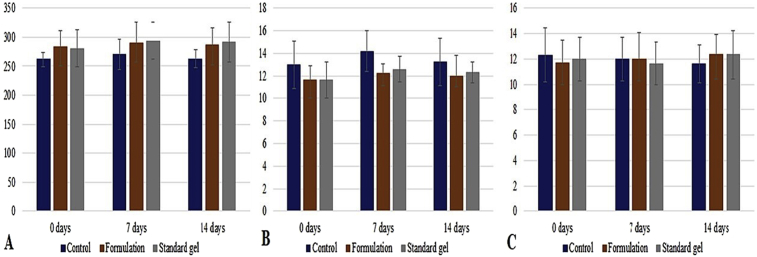
Simultaneously, no increased body temperature of any groups of animals was noticed during the observatory period. Further, the hairs of the experimental animals began growing following 7 days of experiment. However, grown hairs were again removed to observe the cutaneous results properly to report the experimental outcomes for the entire 14 days observatory period. During the experimental process, we recorded the change in body weight, consumption of food and water (Fig. 5). Food consumption in all the groups of animals were normal and no significant differences observed as compared to the animals in control group, however water consumption in animals of formulation treated animals was increased as compared to the control group and standard treated animal group. Additionally, in dermal toxicity study, the body weight of each animal was increased in each group as compared to control group. Overall, there were no significant changes observed for any changes among the various groups.
Fig. 5.
Representation of changes in (a) body weight (in gm), (b) consumption of food (in gm), and (c) consumption of water (in mL) in all groups of animals.
3.7. Pharmacokinetic studies
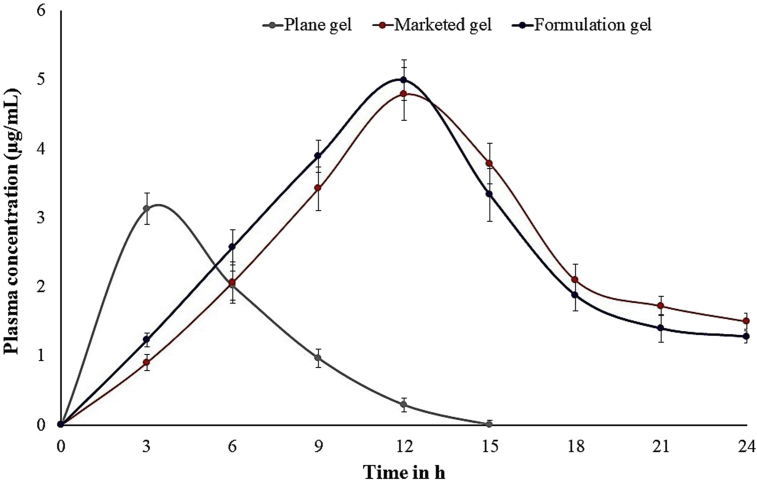
The plasma drug concentration profiles of pure drug and the complex of dendrimeric dithranol microsponge gel formulation were presented in Fig. 6. Calculated Cmax for the drug in the experimental animal groups of developed formulation, standard product and plane gel were found to be 4.1 μg/mL, 4.3 μg/mL, and 3.2 μg/mL, respectively at the respective Tmax at 3 h, 12 h, and 12 h, respectively. Thus, it is well understood that the entrapped drug in the dendritic structure within the microsponge gel formulation showed a controlled release of dithranol. Furthermore, for the developed dendrimer containing microsponge formulations, it was observed that a steady state flux in 3 h and the effective concentration maintained until 18 h. The area under the plasma concentration curve of the drug for 24 h duration (AUC0–24h) was found to be 32 μg h/mL, 34 μg h/mL, 19 μg h/mL, for developed formulation, standard product and plane gel, respectively.
Fig. 6.
Plasma concentration of different gel and marketed gel.
4. Conclusions
Finally, it can be concluded that this polymeric microsponge gel formulations of dithranol was successfully developed by the quasi-emulsion solvent diffusion method adopted. Such developed formulation is found to prevent auto-oxidation of the antipsoriatic drug to avoid the major limitations of the conventional treatment. Further, solubility enhancement of the hydrophobic drug could avoid the major hindrance in achieving satisfactory bioavailability. Microsponge gel of dithranol could provide advantages of reduced side effects, increased elegance, enhanced formulation flexibility, and modify drug release. From the current study, it can be established that the incorporated dendrimer in the formulation aided in increasing permeability of the drug across the skin. The study also concludes that the developed formulation showed its greater physicochemical properties like spreadability, viscosity, and homogeneity. A controlled release profile of the drug from the formulation onto the skin over a prolonged period of time was also found to be safe and useful by the experimental animals. Thus, projecting towards its application, it will enable to treat external psoriatic diseased skin, by topical delivery of microsponges based gel containing dithranol-dendrimer complex to increase the solubility, control oxidation and permeability dithranol through skin with extended release, reduced irritation and improved patient compliance, improved thermal, physical, and chemical stability.
Declarations
Author contribution statement
Pushpendra Kumar Tripathi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Bapi Gorain, Prashant Kesharwani: Analyzed and interpreted the data; Wrote the paper.
Hira Choudhury: Analyzed and interpreted the data.
Ayushi Srivastava: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Author (P.K. Triphati) acknowledge Baba Saheb Bheem Rao Ambedakar University, CIMAP Lucknow for extending their facility for successful completion of this project. Also, author (P. Kesharwani) acknowledge Jamia Hamdard and SERB, New Delhi for financial assistance.
References
- 1.Takeshita J., Gelfand J.M., Li P., Pinto L., Yu X., Rao P., Viswanathan H.N., Doshi J.A. Psoriasis in the US medicare population: prevalence, treatment, and factors associated with biologic use. J. Investig. Dermatol. 2015;135:2955–2963. doi: 10.1038/jid.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofny H. Morsy, Hasaballa A., Twisy H. Skin level of microRNA-369-3P in patients with psoriasis and its correlation with disease severity. J. Curr. Med. Res. Pract. 2017;2:32. [Google Scholar]
- 3.Lise M.L.Z., Baptista T.S.A., Petersen L.E., Bauer M.E., Ungaretti C.A.L., Torres E., Harter K., Staub H.L., Lise M.L.Z., Baptista T.S.A., Petersen L.E., Bauer M.E., Ungaretti C.A.L., Torres E., Harter K., Staub H.L. Subclinical atherogenesis in patients with mild psoriasis: a role for IL-6? Rev. Assoc. Med. Bras. 2017;63:747–752. doi: 10.1590/1806-9282.63.09.747. [DOI] [PubMed] [Google Scholar]
- 4.Savian A.L., Rodrigues D., Weber J., Ribeiro R.F., Motta M.H., Schaffazick S.R., Adams A.I.H., de Andrade D.F., Beck R.C.R., da Silva C.B. Dithranol-loaded lipid-core nanocapsules improve the photostability and reduce the in vitro irritation potential of this drug. Mater. Sci. Eng. C. 2015;46:69–76. doi: 10.1016/j.msec.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Boehncke W.-H., Schön M.P. Psoriasis. Lancet. 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 6.Psoriasis Around the World: the WHO Report, National Psoriasis Foundation, (n.d.).
- 7.Nast A., Spuls P.I., van der Kraaij G., Gisondi P., Paul C., Ormerod A.D., Saiag P., Smith C.H., Dauden E., de Jong E.M., Feist E., Jobling R., Maccarone M., Mrowietz U., Papp K.A., Reich K., Rosumeck S., Talme T., Thio H.B., van de Kerkhof P., Werner R.N., Dressler C. European S3-Guideline on the systemic treatment of psoriasis vulgaris - update Apremilast and Secukinumab - EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2017;31:1951–1963. doi: 10.1111/jdv.14454. [DOI] [PubMed] [Google Scholar]
- 8.Hsu S., Papp K.A., Lebwohl M.G., Bagel J., Blauvelt A., Duffin K.C., Crowley J., Eichenfield L.F., Feldman S.R., Fiorentino D.F., Gelfand J.M., Gottlieb A.B., Jacobsen C., Kalb R.E., Kavanaugh A., Korman N.J., Krueger G.G., Michelon M.A., Morison W., Ritchlin C.T., Stein Gold L., Stone S.P., Strober B.E., Van Voorhees A.S., Weiss S.C., Wanat K., Bebo B.F. National psoriasis foundation medical board, consensus guidelines for the management of plaque psoriasis. Arch. Dermatol. 2012;148:95. doi: 10.1001/archdermatol.2011.1410. [DOI] [PubMed] [Google Scholar]
- 9.Nast A., Boehncke W.-H., Mrowietz U., Ockenfels H.-M., Philipp S., Reich K., Rosenbach T., Sammain A., Schlaeger M., Sebastian M., Sterry W., Streit V., Augustin M., Erdmann R., Klaus J., Koza J., Muller S., Orzechowski H.-D., Rosumeck S., Schmid-Ott G., Weberschock T., Rzany B. Deutsche Dermatologische Gesellschaft (DDG), Berufsverband Deutscher Dermatologen (BVDD), S3 - guidelines on the treatment of psoriasis vulgaris (English version). Update. JDDG J. Der Dtsch. Dermatologischen Gesellschaft. 2012;10:S1–s95. doi: 10.1111/j.1610-0387.2012.07919.x. [DOI] [PubMed] [Google Scholar]
- 10.Kemény L., Ruzicka T., Braun-Falco O. Dithranol: a review of the mechanism of action in the treatment of psoriasis vulgaris. Skin Pharmacol. 1990;3:1–20. doi: 10.1159/000210836. http://www.ncbi.nlm.nih.gov/pubmed/2202336 [DOI] [PubMed] [Google Scholar]
- 11.Torsekar R., Gautam M.M. Topical therapies in psoriasis. Indian Dermatol. Online J. 2017;8:235–245. doi: 10.4103/2229-5178.209622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zithranol-RR. (Anthralin) dose, indications, adverse effects, interactions... from PDR.net, (n.d.). https://www.pdr.net/drug-summary/Zithranol-RR-anthralin-2822 (accessed December 19, 2018).
- 13.McGill A., Frank A., Emmett N., Turnbull D.M., Birch-Machin M.A., Reynolds N.J. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005;19:1012–1014. doi: 10.1096/fj.04-2664fje. [DOI] [PubMed] [Google Scholar]
- 14.Mahrle G. Dithranol. Clin. Dermatol. 1997;15:723–737. doi: 10.1016/s0738-081x(97)00019-9. [DOI] [PubMed] [Google Scholar]
- 15.Kemény L., Ruzicka T., Braun-Falco O. Dithranol: a review of the mechanism of action in the treatment of psoriasis vulgaris. Skin Pharmacol. Physiol. 1990;3:1–20. doi: 10.1159/000210836. [DOI] [PubMed] [Google Scholar]
- 16.Kesharwani P., Gorain B., Low S.Y., Tan S.A., Ling E.C.S., Lim Y.K., Chin C.M., Lee P.Y., Lee C.M., Ooi C.H., Choudhury H., Pandey M. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018;136:52–77. doi: 10.1016/j.diabres.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Gorain B., Choudhury H., Pandey M., Mohd Amin M.C.I., Singh B. Dendrimers as effective carriers for the treatment of brain tumor, nanotechnology-based target. Drug Deliv. Syst. Brain Tumors. 2018:267–305. [Google Scholar]
- 18.Gorain B., Choudhury H., Pandey M., Kesharwani P. Paclitaxel loaded vitamin E-TPGS nanoparticles for cancer therapy. Mater. Sci. Eng. C. 2018;91:868–880. doi: 10.1016/j.msec.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury H., Gorain B., Chatterjee B., Mandal U.K., Sengupta P., Tekade R.K. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharmaceut. Des. 2017;23:2504–2531. doi: 10.2174/1381612822666161201143600. [DOI] [PubMed] [Google Scholar]
- 20.Choudhury H., Gorain B., Pandey M., Chatterjee L.A., Sengupta P., Das A., Molugulu N., Kesharwani P. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 2017;106:1736–1751. doi: 10.1016/j.xphs.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 21.V Mishra N.K.J., Kesharwani P. Publ. M/s Stud. Press LLC; USA: 2014. Functionalized Polymeric Nanoparticles for Delivery of Bioactives, Nanobiomedicine; pp. 91–123. [Google Scholar]
- 22.Gorain B., Tekade M., Kesharwani P., Iyer A.K., Kalia K., Tekade R.K. The use of nanoscaffolds and dendrimers in tissue engineering. Drug Discov. Today. 2017;22:652–664. doi: 10.1016/j.drudis.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Osmani R.A.M., Aloorkar N.H., Ingale D.J., Kulkarni P.K., Hani U., Bhosale R.R., Jayachandra Dev D. Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm. J. 2015;23:562–572. doi: 10.1016/j.jsps.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayur K., Ramesh K., Nitin J., Prashant P., Rajendra G., Jeevan N. Ethyl cellulose based microsponge delivery system for anti-fungal vaginal gels of tioconazole. J. Drug Deliv. Ther. 2013;3:14–20. [Google Scholar]
- 25.Gorain B., Choudhury H., Kundu A., Sarkar L., Karmakar S., Jaisankar P., Pal T.K.T.K. Nanoemulsion strategy for olmesartan medoxomil improves oral absorption and extended antihypertensive activity in hypertensive rats. Colloids Surfaces B Biointerfaces. 2014;115:286–294. doi: 10.1016/j.colsurfb.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury H., Gorain B., Karmakar S., Biswas E., Dey G., Barik R., Mandal M., Pal T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014;460:131–143. doi: 10.1016/j.ijpharm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 27.Moin A., Deb T.K., Osmani R.A.M., Bhosale R.R., Hani U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J. Basic Clin. Pharm. 2016;7:39–48. doi: 10.4103/0976-0105.177705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draft Updated Test Guideline 402 on Acute Dermal Toxicity. OECD GUIDELINE FOR TESTING OF CHEMICALS; 2015. [Google Scholar]
- 29.McIntyre F., McCloy R. Shaving patients before operation: a dangerous myth? Ann. R. Coll. Surg. Engl. 1994;76:3–4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2502186/pdf/annrcse01587-0009.pdf [PMC free article] [PubMed] [Google Scholar]
- 30.Gorain B., Choudhury H., Nandi U., Das A., Dan S., Pal T.K. Development and validation of an HPLC method for simultaneous detection and quantification of paracetamol and etodolac in human plasma and its application to a pharmacokinetic study. J. AOAC Int. 2013 doi: 10.1002/dta.419. [DOI] [PubMed] [Google Scholar]
- 31.Choudhury H., Gorain B., Das A., Ghosh B., Pal T. Development and validation of a sensitive HPLC-MS/MS-ESI method for determination of febuxostat: application to pharmacokinetic study. Curr. Anal. Chem. 2014;10:528–536. [Google Scholar]
- 32.Larsson M., Hill A., Duffy J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012;20:209–214. [Google Scholar]