Abstract
Introduction
There is evidence suggesting a detrimental effect of asymptomatic carotid artery stenosis on cognitive function even in the absence of ischemic cerebral lesions. Hypoperfusion has been suggested as pathophysiological mechanism causing cognitive impairment. We aimed to assess cognitive performance and cerebral perfusion changes in patients with carotid artery stenosis without ischemic lesions by arterial spin labeling (ASL) and contrast enhanced (CE) perfusion MRI before and after revascularization therapy.
Methods
17 asymptomatic patients with unilateral high-grade (≥70%) carotid artery stenosis without evidence of structural brain lesions underwent ASL and CE perfusion MRI and cognitive testing (MMSE, DemTect, Clock-Drawing Test, Trail-Making Test, Stroop Test) before and 6–8 weeks after revascularization therapy by endarterectomy or stenting. Multiparametric perfusion maps (ASL: cerebral blood flow (ASL-CBF), bolus arrival time (ASL-BAT); CE: cerebral blood flow (CE-CBF), mean transit time (CE-MTT), cerebral blood volume (CE-CBV)) were calculated and analyzed by vascular territory. Relative perfusion values were calculated.
Results
Multivariate analysis revealed a significant impact of revascularization therapy on all perfusion measures analyzed. At baseline post-hoc testing showed significant hypoperfusion in MCA borderzones as assessed by ASL-CBF, ASL-BAT, CE-MTT and CE-CBV. All perfusion alterations normalized after revascularization. We did not observe any significant correlation of cognitive test results with perfusion parameters. There was no significant change in cognitive performance after revascularization.
Conclusion
We found evidence of traceable perfusion alterations in patients with high grade carotid artery stenosis in the absence of structural brain lesions, which proved fully reversible after revascularization therapy. In this cohort of asymptomatic patients we did not observe an association of hypoperfusion with cognitive performance.
Keywords: Magnetic resonance imaging, Perfusion imaging, Carotid artery stenosis, Carotid artery stenting, Cognitive function
Highlights
-
•
In patients with high grade unilateral carotid artery stenosis there was a significant impact of revascularization therapy on brain perfusion
-
•
All perfusion alterations normalized after revascularization.
-
•
We did not observe any significant correlation of cognitive test results with perfusion parameters.
-
•
There was no significant change in cognitive performance after revascularization.
1. Introduction
Stenosis of the internal carotid artery (ICA) without incidence of ipsilateral stroke, TIA or retinal infarction is considered asymptomatic. However, there is evidence suggesting clinical relevance of asymptomatic carotid artery stenosis and even carotid atherosclerosis beyond the risk of stroke, by increasing the risk for cognitive decline and dementia. (Mathiesen et al., 2004; Johnston et al., 2004; Wendell et al., 2012; Lal et al., 2017) This does not only apply to dementia cases classified as vascular dementia but does also to Alzheimer's Disease, which has shown to have overlapping risk factors and pathophysiological findings with vascular dementia. (Arvanitakis et al., 2016; Heller and Hines, 2017).
Cerebral Hypoperfusion and silent brain infarction are hypothesized to be major pathophysiological mechanisms leading to cognitive decline associated with carotid artery stenosis. (Mathiesen et al., 2004; Johnston et al., 2004; Arvanitakis et al., 2016; De La Torre, 2004; Vermeer et al., 2003) Previous studies reported a significant association of carotid or intracranial artery disease with cognitive impairment after correction for the presence of silent infarction, possibly indicating an effect of chronic hypoperfusion alone. (Mathiesen et al., 2004; Arvanitakis et al., 2016; Scherr et al., 2012) Results, however, are contradictory. Data from patients without structural brain lesions is scarce. Two recent studies evaluating cognitive performance and cerebral perfusion using either CT- or MRI-perfusion measurements did not demonstrate a significant association of hypoperfusion and cognitive impairment in patients with asymptomatic carotid stenosis. (Chen et al., 2012; Wang et al., 2017a) Thus, it remains unclear whether hypoperfusion alone without evidence of infarction is sufficient to cause cognitive decline.
Two standard procedures are available for intervention in carotid stenosis: carotid artery stenting (CAS) and carotid endarterectomy (CEA). Several studies have assessed the effect of revascularization therapies on cognitive performance, again with contradictory results. For both procedures, a benefit on cognitive performance was demonstrated. (Chen et al., 2012; Kougias et al., 2015; De Rango et al., 2008; Wang et al., 2017b) However, a decline in cognitive performance after revascularization therapy was also reported. (Nanba et al., 2012; Chida et al., 2009).
To evaluate brain perfusion by MRI, invasive contrast enhanced (CE) and non-invasive arterial spin labeling (ASL) methods are available. ASL perfusion uses blood as an endogenous contrast agent and does not require gadolinium-based contrast agents. Thus, the “contrast medium” in ASL decays with the T1 of blood and ASL has been reported to overestimate cerebral hypoperfusion. (Nael et al., 2013) Nevertheless, using innovative approaches like measurements at multiple inflow times and background suppression, ASL has been validated against CE-perfusion MRI in steno-occlusive disease. (Martin et al., 2015)
We aimed to assess cognitive performance and its relationship with cerebral perfusion in patients without evidence of brain infarction at baseline and after revascularization by CEA or CAS. Furthermore, we compared the performance of CE and ASL perfusion MRI in characterizing brain perfusion alterations in high-grade asymptomatic carotid artery stenosis.
2. Methods
2.1. Patients
We prospectively recruited patients with high-grade carotid artery stenosis without evidence of brain lesions at the University Medical Center Hamburg-Eppendorf using the following inclusion criteria: 1. Age over 50 years; 2. Unilateral internal carotid artery stenosis ≥70% according to NASCET criteria (Moneta et al., 1993; Staikov et al., 2002) planned to undergo either carotid artery stenting or carotid endarterectomy; 3. No visible structural brain lesions on cranial CT or MRI imaging other than minimal leukoaraiosis deemed unspecific by a board certified radiologist; 4. No history of stroke, dementia or depression; 5. No significant neurological symptoms, no disability – patients had to be able to carry out usual activities in their daily life without support; 6. Written informed consent.
Exclusion criteria were as follows: 1. Any contraindications to MRI scan (e.g. metal implants or claustrophobia); 2. Severe systemic or neuropsychiatric disease; 3. History of cognitive impairment; 4. Contraindications against Gadolinium application (e.g., previous adverse events or renal failure).
Each case was discussed in an interdisciplinary board meeting involving vascular surgeons, neurologists and neuroradiologists to determine the optimal treatment modality (CAS or CEA). (Rimmele et al., 2017) CEA and CAS were performed according to institutional standards. After intervention, patients were transferred to an intensive care unit or intermediate care unit for at least 24 h. Postinterventional blood pressure management maintained a systolic pressure between 120 and 160 mmHg. As a standard, all patients received aspirin, and patients who underwent CAS additionally received clopidogrel for 12 weeks. Clinical and demographical data were collected from medical records.
The local ethics committee (Ethikkommission der Ärztekammer Hamburg) approved the study protocol in accordance with and based on German law and ICH-GCP.
2.2. Cognitive assessment
All patients underwent cognitive assessment within 10 days before and 6 to 10 weeks after revascularization procedures. Neuropsychological assessment was based on the CERAD (Consortium to Establish a Registry for Alzheimer's Disease) test battery. We used the Mini Mental State Examination (MMSE) (Folstein et al., 1975) and DemTect (Kalbe et al., 2004) to evaluate global cognition. MMST has a maximum of 30 points, values of 24 or below indicate cognitive impairment. DemTect is a screening test for mild cognitive impairment including a word list task with immediate and delayed recall, a number transcoding task, a word fluency task and digit span reverse task. A maximum of 18 points can be achieved in DemTect, values of 13 an below indicate mild cognitive impairment, values of 8 or below indicate dementia. In addition, we administered the trail-making test (Bowie and Harvey, 2006) and Stroop test to assess information processing speed and cognitive flexibility. Normative scores based on the performance on a healthy population can be calculated for Trail making and stroop tests. (Bowie and Harvey, 2006; Oswald and Fleischmann, 1997) For the present analysis we used the time values patients required to complete the trail making and stroop tests before and after revascularization only and did not perform comparison with data from healthy populations.
2.3. Magnetic resonance imaging
Patients received structural and perfusion MRI within 10 days before and 6 to 8 weeks after revascularization procedures. If possible, an additional MRI was performed within 3 days after the procedure to detect possible short term hyperperfusion. All MRI scans were acquired on a 3T Siemens Scanner (Skyra, Siemens, Erlangen, Germany).
The MRI protocol included T1 MPRage (flip angle = 9°, TR = 2500 ms, TE = 2.12 ms, slice thickness = 0.9 mm, inversion time 1100 ms, matrix = 232 × 288, FOV = 193 × 293 mm), Time of Flight angiography of extracranial carotid arteries to determine placement of ASL labeling planes, T2 FLAIR (flip angle = 150°, TR = 9000 ms, TE = 90 ms, slice thickness = 5 mm, inversion time = 2500 ms, matrix = 320 × 270, FOV = 194 × 230 mm), CE-perfusion weighted imaging (PWI) (flip angle = 90°, TR = 1920 ms, TE = 30 ms, slice thickness = 4 mm, matrix = 128 × 128, FOV = 240 × 240 mm), during dynamic acquisition, a single dose of 0.1 mmol/kg of gadolinium contrast agent (DOTAREM) was injected using an automatic injection pump and ASL-PWI. For the ASL acquisition a 3D-GRASE readout technique was employed (Günther et al., 2005) for image readout with a matrix size of 64x48x20 for a FOV of 256mmx192mmx80mm resulting in an isotropic nominal resolution of 4 mm. Echo train was split up into 2 segments inplane yielding an EPI factor 25 (has to be odd) and a turbo factor 20. Multi-PLD pseudo-continuous ASL was used with 10 different PLDs ranging evenly from 300 ms to 3000 ms with 300 ms increment. pCASL bolus duration was 1500 ms.
2.4. Image processing
ASL cerebral blood flow (CBF) and Bolus arrival time (BAT) maps were generated using the BASIL toolbox from the Functional MRI of the Brain Software Library (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl) applying both spatial smoothing and fitting of the macrovascular component, CE-PWI maps of CBF, cerebral blood volume (CBV) and mean transit time (MTT) were generated using an in-house developed software tool for automated perfusion analysis (ANTONIA).(Forkert et al., 2014) Voxels for detection of the arterial input function were selected manually contralaterally to the carotid artery stenosis in distal ICA or proximal M1 segments.
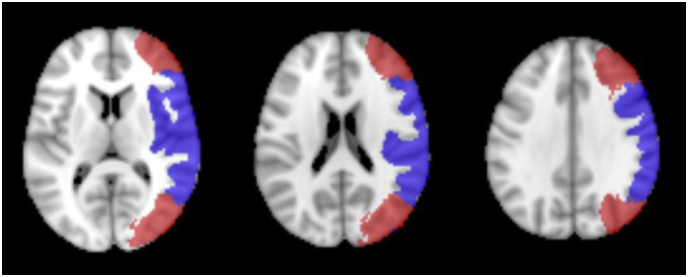
For quantitative analysis cortical masks of arterial flow territories in the anterior circulation, (i.e. branches of carotid arteries) including middle (MCA) and anterior (ACA) cerebral arteries were created in Montreal Neurological Institute (MNI) space based on an atlas published by Tatu et al. (Tatu et al., 1998) Additionally, based on these masks an “MCA border zone” mask including the marginal areas of MCA-PCA and MCA-ACA territories, and an “MCA core” mask, covering the core of the MCA territory not included in the border zone mask, were created. See Fig. 1 for MCA territory maps.
Fig. 1.
Multi-Slice image of MCA core (blue) and borderzone (red) masks used for perfusion analysis in MNI-2 mm space. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Territory masks were registered to individual space and the respective cortical perfusion values were recorded using tools from the Functional MRI of the Brain Software Library (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl). As previously suggested we calculated relative perfusion values for each arterial territory and perfusion measure (relative perfusion = perfusion ipsilateral/perfusion contralateral) facilitate longitudinal comparisons. (Martin et al., 2015)
2.5. Statistical analysis
All statistical analysis was performed using SPSS 21. Correlations were calculated using Spearman's ranked correlation coefficient. To account for repeated measures in comparisons between different time-points we used linear mixed models to analyze effects on relative perfusion values, as fixed effects time point (baseline and 6–8 weeks after revascularization, for subgroup analysis only additionally 2–3 days after revascularization), preexisting hypertension, vascular territories, grade of stenosis and method of revascularization were tested, individual intercept was included as random effect. First-order autoregressive (AR1) was chosen as covariance structure. We applied paired t-Test as post-hoc test to compare relative perfusion values between time points where appropriate.
A one sample t-Test was used to evaluate mean relative perfusion deviations at baseline and follow-up. Bonferroni correction was used to adjust for multiple testing, P values ≤.05 were considered significant.
3. Results
Overall, 30 patients were enrolled. Of these, 7 patients did not complete the imaging protocol or were lost to follow-up (5 due to withdrawal of consent, 2 due to periprocedural complications [pneumonia, myocardial infarction] requiring prolonged hospitalization). Due to insufficient image quality for analysis, 3 patients had to be excluded at baseline and another 3 on follow-up. Thus, 17 patients with complete follow-up were finally included in the analysis. Of these, 12 patients received an additional MRI within 3 days after the procedure.
Demographical data, medical history and cognitive test scores of 17 patients at baseline included in the analysis are displayed in Table 1.
Table 2.
Perfusion parameters in MCA core and borderzone territories at baseline in 17 patients included in the final analysis.
| ASL Perfusion Imaging |
||||||
|---|---|---|---|---|---|---|
| Parameter | CBF | BAT | ||||
| Territory | MCA core |
MCA borderzone |
MCA core |
MCA borderzone |
||
| Mean SD |
0.972 ± 0.107 |
0.905 ± 0.118 |
1.051 ± 0.049 |
1.053 ± 0.038 |
||
| p-value | p = .301 | P = .004 | P = .001 | p < .001 | ||
|
CE Perfusion Imaging |
||||||
|---|---|---|---|---|---|---|
| Parameter | CBF | MTT | CBV | |||
| Territory | MCA core |
MCA borderzone |
MCA core |
MCA borderzone |
MCA core |
MCA borderzone |
| Mean SD |
1.004 ± 0.044 |
1.017 ± 0.039 |
1.098 ± 0.123 |
1.077 ± 0.076 |
1.11 ± 0.149 |
1.089 ± 0.11 |
| p-value | P = .694 | P = .081 | P = .006 | P = .001 | P = .008 | P = .004 |
Table 1.
Demographical data, medical history at baseline and cognitive test results at baseline and at follow-up after 6–8 weeks of 17 patients included in the final analysis. There were no significant changes in cognitive test results after revascularization.
| Age | 66.9 years (±10.4; 50–87) | |
|---|---|---|
| Sex | 5 women, 12 men | |
| Hypertension | 16 (80%) | |
| Diabetes | 5 (25%) | |
| Hypercholesterolemia | 9 (45%) | |
| Active smoking | 8 (40%) | |
| Education | 15.1 years (±3.7; 12–24) | |
| At baseline | After 6–8 weeks | |
| MMSE | 28 (25–30) | 28 (25–30) |
| DemTect | 17 (8–18) | 16 (12–18) |
| Trail-Making-Test part A | 41.6 ± 11.8 s | 37.3 ± 8.8 s |
| Trail-Making-Test part B | 112.2 ± 55.1 s | 90.4 ± 52.4 s |
| Stroop-Test | 50.3 ± 17.7 s | 47.9 ± 14.7 s |
Mean or median values are given where appropriate, number in parenthesis are standard deviation, minimum-maximum range or percentages. MMST: Mini Mental State Examination.
There were no significant differences in age, sex, side of stenosis, CEA or CAS treatment, duration of education and prevalence of hypertension, diabetes, hyperlipoproteinemia or smoking between patients analyzed and excluded.
3.1. Perfusion analysis
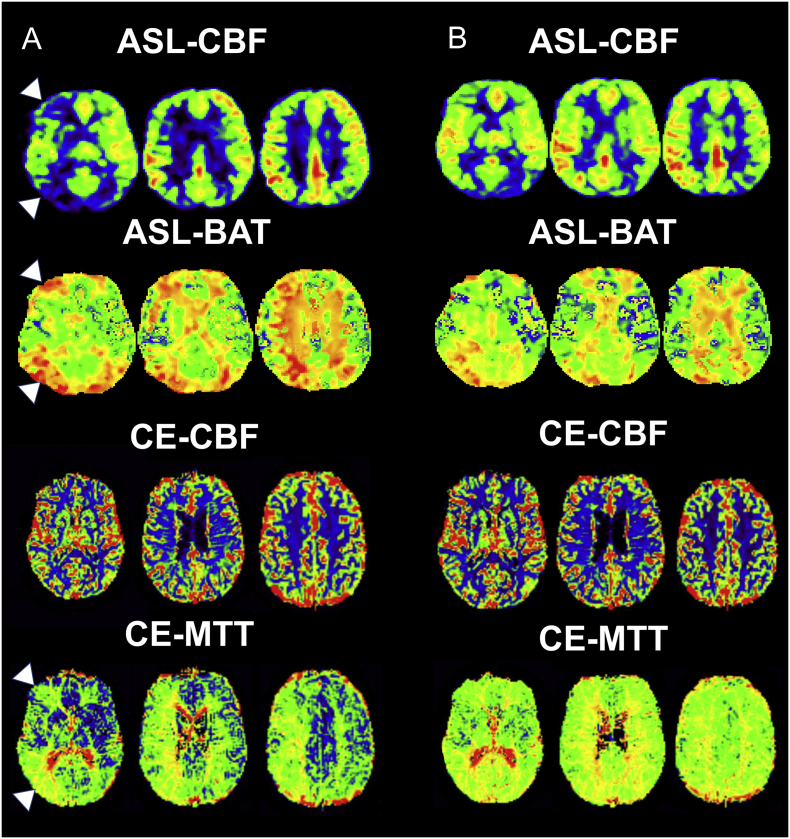
Data of 17 patients was available for follow-up analysis after revascularization (5 women, mean age 66.9 years ±10,4). Of these, 12 patients underwent TEA and 5 patients CAS. See Fig. 2 for an example of of ASL and CE perfusion imaging at baseline and after revascularization. There were no significant differences in age, sex, side of stenosis, years of education and prevalence of hypertension, diabetes, hyperlipoproteinemia or smoking between patients undergoing TEA or CAS.
Fig. 2.
Multi-slice ASL and CE Perfusion maps of a patient with right-sided ICA stenosis before (A) and 6 weeks after (B) endarterectomy. Before revascularization there is a notable increase of ASL-CBF and decrease of ASL-BAT and CE-MTT in the right MCA borderzones (indicated by white arrows), as opposed to ASL-CBF there is no visible change on CE-CBF maps. After TEA hypoperfusion is markedly reduced.
Multivariate mixed model analysis showed a significant effect of time point (i.e. baseline and after revascularization) on all perfusion measures (ASL: relative cerebral blood flow (rCBF), relative bolus arrival time (rBAT); CE: rCBF, relative mean transit time (rMTT), relative cerebral blood volume (rCBV)). There was also a significant effect of vascular territory on ASL-rBAT and ASL-rCBF, for CE-rMTT there was a trend towards significance (p = 0,082). There were no significant effects for grade of stenosis according to NASCET classification, hypertension or method of revascularization (i.e. TEA or CAS). Fig. 2 displays ASL and CE perfusion maps of a patient with right sided ICA stenosis before and after endarterectomy.
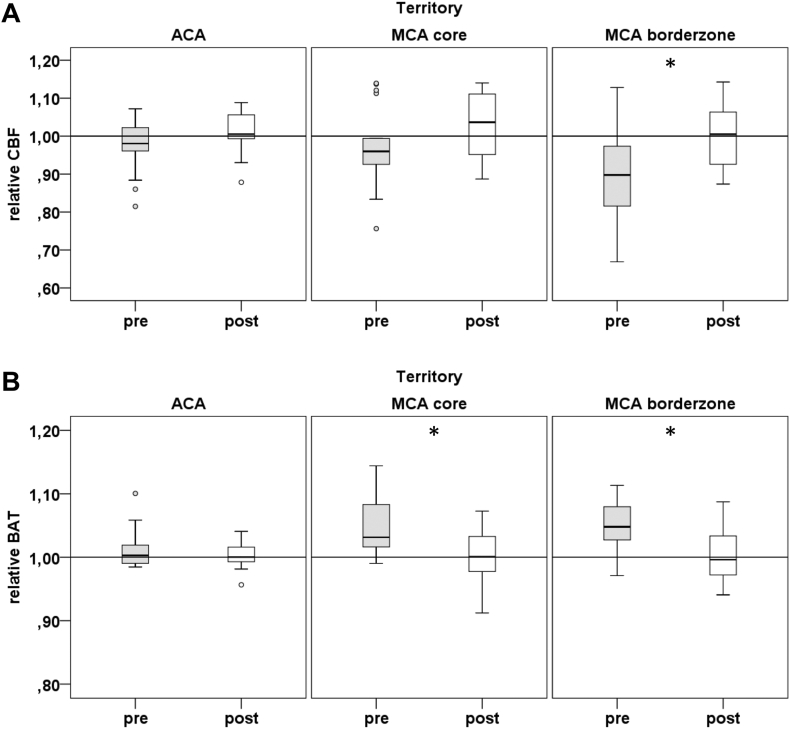
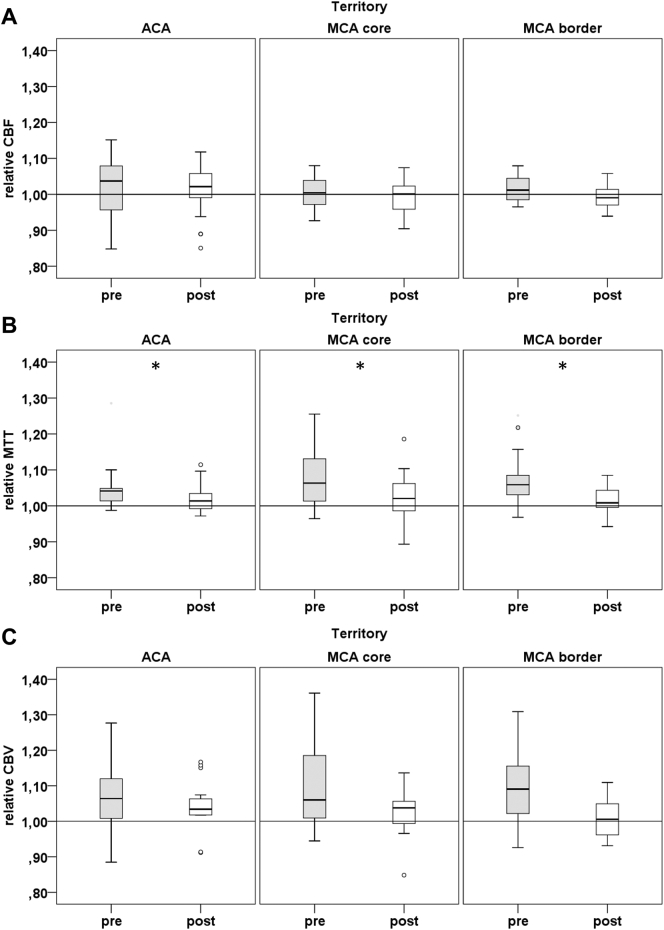
Post-Hoc testing using paired t-tests showed a significant increase of mean ASL rCBF from baseline to follow-up in MCA border territory (0.9 vs 1.00; P = .022). Mean ASL-rBAT significantly decreased after revascularization in MCA core (1.05 vs 1.00; p = .001) and MCA border (1.05 vs 1.01; p = .0004) territories. There was a significant decrease of mean CE rMTT from baseline to follow-up in ACA (1.05 vs 1.02; p = .042), MCA core (1.1 vs 1.02; p = .04) and MCA border (1.08 vs 1.02; P = .014) territories. Please refer to Fig. 3, Fig. 4 for details on perfusion changes.
Fig. 3.
Relative ASL-perfusion measures at baseline and after revascularization in ACA, MCA core and border territories. A: ASL relative cerebral blood flow B: ASL Bolus Arrival Time. pre indicates baseline, post 6–8 weeks post intervention. * indicates territories with significant changes of perfusion after revascularization according to post-hoc paired T-Test.
Fig. 4.
Relative CE-perfusion measures at baseline and after revascularization in MCA core (4) and border (5) territories. (A) CE relative cerebral blood flow (CBF) (B) CE mean transit time (MTT) (C) CE cerebral blood volume (CBV). pre indicates baseline, post 6–8 weeks post intervention * indicates territories with significant changes of perfusion after revascularization according to post-hoc paired T-Test.
Additionally, baseline perfusion values were assessed by 1-sample t-test against 1 (indicating equal perfusion in both hemispheres). ASL-rBAT on the stenosis side was significantly increased in MCA core and MCA border territories, and ASL-rCBF was significantly reduced in the MCA border territory (see Table 2). CE-rMTT and CE-rCBV were significantly increased ipsilaterally to ICA stenosis. There were no significant changes in CE-rCBF at baseline.
Results of one sample t-test of relative perfusion values at baseline against 1 for ASL and CE perfusion measures, values are given for MCA core and border territories separately. SD: standard deviation, CBF: cerebral blood flow, AT: bolus arrival time, MTT: mean transit time, CBV: cerebral blood volume.
At follow-up, none of the relative perfusion measures differed significantly from 1 in MCA core and border territories, indicating the absence of a persisting significant difference in perfusion parameters between stenosis hemisphere and the non-stenosis hemisphere after revascularization.
3.2. Results of cognitive testing
After correction for multiple testing there were no significant correlations between perfusion measures and cognitive test results at baseline. This did not change when all 20 patients with complete data at baseline were analyzed. None of the cognitive test results changed significantly after revascularization. There was a tendency towards improvement in TMT-A results (p = .1, mean of paired differences = 4.28353, standard deviation of paired differences = 10.11178, effect size 0.42). Again, there were no significant correlations between perfusion measures and cognitive test results at follow-up.
3.3. Short term perfusion changes after revascularization
A subgroup of 12 patients underwent a short-term follow-up imaging. In 1 case image quality of ASL imaging was insufficient for evaluation.
In the remaining 11 patients, mixed model analysis using parameters described above revealed a significant effect of time point (i.e. baseline, 3 days after revascularization, 6–8 weeks after revascularization) on ASL-rBAT, CE-rMTT and CE-rCBV, there was a trend towards significance for ASR-rCBF.
Accordingly, post-hoc testing showed significant changes as compared to baseline after 2–3 days post intervention for ASL-rBAT in MCA core (1.04 ± 0.05 vs 0.99 ± 0.04; p = .025) and MCA border (1.05 ± 0.04 vs 1.01 ± 0.04; p = .005) territories and for CE-rMTT in MCA core (1.07 ± 0.08 vs 1.00 ± 0.04; p = .043) and MCA border (1.06 ± 0.07 vs 1.00 ± 0.03; p = .021) territories. There were no significant changes for CE-rCBV, even though there was a trend towards significance in MCA core (1.07 ± 0.12 vs 0.99 ± 0.05; p = .051) and MCA border (1.06 ± 0.1 vs 1.0 ± 0.05; p = .075) territories. We could not detect significant changes in this subgroup for ASL-rCBF. ACA territories did not show any significant short term changes in any perfusion measure. There were no significant changes of relative perfusion from short term (up to 3 days) to long term follow-up (6–10 weeks).
4. Discussion
We studied regional brain perfusion and cognitive performance in patients with carotid artery stenosis without ischemic lesions before and after revascularization. As a main result, we found characteristic alterations of brain perfusion in the territory of the carotid artery at baseline which were normalized after revascularization by TEA or CAS.
At baseline, time-based perfusion measures CE-MTT and ASL-BAT were significantly increased in MCA border and core territory ipsilateral to ICA stenosis, indicating a prolonged passage of blood through and a delayed arrival at the affected brain region. Cerebral blood flow measured by ASL was significantly decreased in MCA borderzone only, i.e., in the watershed location (Cheng et al., 2010) known to be especially susceptible to hypoperfusion from upstream stenosis. CE-CBV as a marker of vascular dilatation was significantly increased in MCA border and core territories before revascularization.
These findings are largely in line with earlier results. Several previous studies using ASL perfusion imaging reported decreased CBF in anterior circulation territories after high-grade carotid artery stenosis. (Wang et al., 2017b; Marshall et al., 2017; Bokkers et al., 2008) Time-based perfusion measures like bolus arrival time from ASL may even be more sensitive to moderate hemodynamic effects. In a previous study no changes in CBF but alterations in timing parameters only were reported in 20 patients with high grade ICA stenosis. (Chen et al., 2017) Our findings point in the same direction with more prominent changes in the time-based measure bolus arrival time (ASL-BAT) as compared to CBF.
Using dynamic contrast-enhanced perfusion imaging, we observed increased CBV, while CBF did not show significant changes. An increase of CBV indicates vasodilatation to optimize tissue oxygenation in the presence of hypoperfusion. In a previous study, increased CE-MTT and CE-CBV were reported in patients with high grade carotid artery stenosis or occlusion. (Lythgoe et al., 2000)
The discrepancy of findings of reduced CBF measured by ASL while CE-MRI did not show reduced CBF in carotid artery stenosis may result from methodological differences between ASL and DSC-perfusion MRI. ASL has been described to overestimate CBF hypoperfusion, especially in circumstances of delayed inflow times. (Nael et al., n.d.) Furthermore, underestimation of CBF by ASL has been reported, when CE time to peak was increased in patients with steno-occlusive disease. (Martin et al., 2015) ASL uses labeled blood as an endogenous tracer, which decays with the T1 of blood. Thus, in a setting of vastly prolonged inflow times the ASL signal can be disproportionally reduced due to tracer decay. This effect could explain the discrepancy in CBF between ASL and CE perfusion measurements in our study. Furthermore, vasodilatation aims to stabilize CBF in a setting of reduced inflow. Vasodilatation leads to an increase in CBV. A sufficient vasodilatory response could up to a certain degree stabilize CBF values possibly explaining our unremarkable findings in CE-CBF. (Lythgoe et al., 2000).
Both ASL and CE perfusion measures offer useful information. To quantify perfusion in carotid artery stenosis time based measures (such as MTT or BAT) appear to be more sensitive than CBF. Both CE and ASL perfusion have distinct advantages depending on the setting and information required, but none seems to be clearly superior.
As second main finding, all significant perfusion deviations observed at baseline were normalized after revascularization by TEA or CAS. This observation is in line with pathophysiological assumptions and the physiological target of these interventions. Multivariate analysis revealed a significant impact of revascularization therapy on perfusion for all perfusion measures assessed regardless of vascular territories.
A beneficial effect of revascularization therapies has been described for both ASL (Ances et al., 2004; Yun et al., 2013) and CE (Wang et al., n.d.; Soinne et al., 2003) perfusion measurement. Changes of perfusion measures were more pronounced in MCA borderzone territory compared to the central MCA territory, which is in line with the known dynamics of cerebral perfusion, with the borderzone areas being furthest from arterial supply and thus most vulnerable to reductions in perfusion pressure. Supporting this hypothesis are findings that MCA borderzone infarction is associated with severe ICA stenosis or occlusion. (Torvik, 1984; Bogousslavsky and Regli, 1986; Mounier-Vehier et al., 1994) Additionally, earlier perfusion studies found similar evidence of delayed BAT or pronounced alterations in MCA borderzone areas. (Bokkers et al., 2008; Soinne et al., 2003)
In a subgroup of patients we were able to perform MRI 2–3 days after revascularization. Perfusion MRI obtained at this early time-point showed an immediate effect of recanalization on cerebral perfusion without relevant further changes within the next weeks. Thus, we assume, that the effect of revascularization on brain perfusion is instantaneous. A previous study described an increase in CBF within 4 days after CAS in 2013, but further follow-up imaging was not performed. In our sample we did not find evidence of further perfusion changes occurring in the later course beyond 3 days after revascularization.
Cerebral hyperperfusion after carotid revascularization has been described on postoperative CBF imaging due to failure of vascular autoregulation in long term adapted tissue after reperfusion. This has been hypothesized to cause impairment of cognitive function and there is evidence of postoperative white matter damage due to hyperperfusion. (Nanba et al., 2012) We did not detect any short term hyperperfusion after revascularization in this cohort. This might be due to small sample size. We included only patients without evidence of structural brain lesion possibly indicating a relatively unimpaired vascular autoregulation and thus low risk of postoperative hyperperfusion.
The patients in our sample did not show relevant abnormalities in cognitive tests at the group level. One patient suffered from mild cognitive impairment according to MMSE and DemTect scores. However, there were no significant association of perfusion measures and cognitive test results and no significant changes of cognitive test scores after revascularization. There was tendency towards improved cognitive processing speed as assessed by the trail making test part A. Based on these results a sample size of 46 patients would be required to achieve 80% power and a level of significance of 5% to prove an effect of revascularization. Further analysis in a larger cohort of patients with more sensitive and elaborate neuropsychological evaluation may thus be validated.
As discussed earlier the relevance of hypoperfusion in the absence of structural brain lesions for cognitive performance is unclear. (Mathiesen et al., 2004; Arvanitakis et al., 2016; Scherr et al., 2012; Chen et al., 2012; Wang et al., 2017a) Our results could not prove a detrimental effect on cognitive performance by reduced perfusion alone in patients without evidence of structural cerebral lesions. This might be due to the small sample size in this study. We could prove a significant effect of ICA stenosis on cerebral perfusion, but we did not find any evidence of association with poor cognitive test results.
Our results add to the conflicting evidence on the effect of carotid revascularization on cognitive performance. The absence of relevant cognitive abnormalities in our cohort might explain the lacking effect of revascularization. Furthermore, our findings of increased CBV and unimpaired CBF on CE MRI without evidence of structural cerebral lesions indicate an autoregulatory response to hypoperfusion. These findings could imply a relevant compensation for detrimental effects of carotid artery stenosis and may thus explain the relative resilience to cognitive decline in our cohort.
It has been suggested that there might be a relevant benefit of intervention for carotid stenosis in those patients with clinically significant cognitive disorders. (Heller and Hines, 2017) Possibly hypoperfusion leads to deterioration in already impaired brain regions but can be compensated for in otherwise healthy tissue. Further studies in larger, more heterogenous collectives are needed to identify subgroups benefiting from intervention.
There are several limitations to our study. First, due to the elaborate test and imaging protocols only a relatively small number of patients could be included, leading to the risk of relevant selection bias. Follow-up testing and imaging was performed 6–8 weeks after the procedure, which might be too early to detect beneficial long term effects of restored perfusion. Another issue could be a lack of sensitivity in the cognitive tests applied to record minimal changes. This could be improved by use of more sensitive measures of executive and memory functions in a larger cohort of patients.
To summarize, in this cohort of patients with high grade carotid stenosis with no history cognitive impairment or structural brain lesions, we observed traceable alterations of brain perfusion in the hemisphere of the stenosis. These changes were more pronounced in the MCA borderzone areas. All alterations normalized after revascularization. These findings are in line with previous reports. Thus, we were able to underpin the pathophysiological processes of perfusion alteration in asymptomatic stenosis and of improvement by revascularization in this subgroup of patients without evidence of structural brain lesions. However, we did not observe any significant effect on cognition, neither of perfusion alterations, nor of revascularization, which may be due to small sample size and selection bias or improved resilience in our comparatively healthy cohort.
5. Author contribution statement
Julian Schröder, Marlene Heinze, Matthias Günther, Bastian Cheng, Tanja Schröder, Felix Fischer, Simon S. Kessner, Tim Magnus, Jens Fiehler, Axel Larena-Avellaneda, Christian Gerloff, Götz Thomalla made a substantial contribution to the concept and design, acquisition of data or analysis and interpretation of data, drafted the article or revised it critically for important intellectual content and approved the final version to be published.
Declarations of interest
This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Sonderforschungsbereich (SFB) 936, Project C2 (Götz Thomalla)
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Ances B.M., McGarvey M.L., Abrahams J.M. Continuous arterial spin labeled perfusion magnetic resonance imaging in patients before and after carotid endarterectomy. J. Neuroimaging. 2004;14:133–138. [PubMed] [Google Scholar]
- Arvanitakis Z., Capuano A.W., Leurgans S.E. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15:934–943. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogousslavsky J., Regli F. Unilateral watershed cerebral infarcts. Neurology. 1986;36:373–377. doi: 10.1212/wnl.36.3.373. [DOI] [PubMed] [Google Scholar]
- Bokkers R.P.H., Van Laar P.J., Van De Ven K.C.C. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. Am. J. Neuroradiol. 2008;29:1698–1703. doi: 10.3174/ajnr.A1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie C.R., Harvey P.D. Administration and interpretation of the trail making test. Nat. Protoc. 2006;1:2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Chen Y.-H., Lin M.-S., Lee J.-K. Carotid stenting improves cognitive function in asymptomatic cerebral ischemia. Int. J. Cardiol. 2012;157:104–107. doi: 10.1016/j.ijcard.2011.10.086. [DOI] [PubMed] [Google Scholar]
- Chen Y.F., Tang S.C., Wu W.C. Alterations of cerebral perfusion in asymptomatic internal carotid artery steno-occlusive disease. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-02094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Golsari A., Fiehler J. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. J. Cereb. Blood Flow Metab. 2010;31:36–40. doi: 10.1038/jcbfm.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida K., Ogasawara K., Suga Y. Postoperative cortical neural loss associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy 123I-iomazenil SPECT study. Stroke. 2009;40:448–453. doi: 10.1161/STROKEAHA.108.515775. [DOI] [PubMed] [Google Scholar]
- De La Torre J.C. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- De Rango P., Caso V., Leys D. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. 2008;39:3116–3127. doi: 10.1161/STROKEAHA.108.518357. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forkert N.D., Cheng B., Kemmling A. ANTONIA perfusion and stroke: a software tool for the multi-purpose analysis of MR perfusion-weighted datasets and quantitative ischemic stroke assessment. Methods Inf. Med. 2014;53:469–481. doi: 10.3414/ME14-01-0007. [DOI] [PubMed] [Google Scholar]
- Günther M., Oshio K., Feinberg D.A. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn. Reson. Med. 2005;54:491–498. doi: 10.1002/mrm.20580. [DOI] [PubMed] [Google Scholar]
- Heller S., Hines G. Carotid stenosis and impaired cognition: the effect of intervention. Cardiol. Rev. 2017;25:211–214. doi: 10.1097/CRD.0000000000000139. [DOI] [PubMed] [Google Scholar]
- Johnston S.C., O'Meara E.S., Manolio T.A. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann. Intern. Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- Kalbe E., Kessler J., Calabrese P. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int. J. Geriatr. Psychiatry. 2004;19:136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- Kougias P., Collins R., Pastorek N. Comparison of domain-specific cognitive function after carotid endarterectomy and stenting. J. Vasc. Surg. 2015;62:355–362. doi: 10.1016/j.jvs.2015.02.057. [DOI] [PubMed] [Google Scholar]
- Lal B.K., Dux M.C., Sikdar S. Asymptomatic carotid stenosis is associated with cognitive impairment. J. Vasc. Surg. 2017;66:1083–1092. doi: 10.1016/j.jvs.2017.04.038. [DOI] [PubMed] [Google Scholar]
- Lythgoe D.J., Østergaard L., Williams S.C. Quantitative perfusion imaging in carotid artery stenosis using dynamic susceptibility contrast-enhanced magnetic resonance imaging. Magn. Reson. Imaging. 2000;18:1–11. doi: 10.1016/s0730-725x(99)00112-5. [DOI] [PubMed] [Google Scholar]
- Marshall R.S., Asllani I., Pavol M.A. Altered cerebral hemodynamics and cortical thinning in asymptomatic carotid artery stenosis. PloS One. 2017;1:1–14. doi: 10.1371/journal.pone.0189727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.Z., Madai V.I., Von Samson-Himmelstjerna F.C. 3D GRASE pulsed arterial spin labeling at multiple inflow times in patients with long arterial transit times: comparison with dynamic susceptibility-weighted contrast-enhanced MRI at 3 Tesla. J. Cereb. Blood Flow Metab. 2015;35:392–401. doi: 10.1038/jcbfm.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen E.B., Waterloo K., Joakimsen O. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromso study. Neurology. 2004;62:695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- Moneta G.L., Edwards J.M., Chitwood R.W. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. J. Vasc. Surg. 1993;17(152–7) doi: 10.1067/mva.1993.42888. (discussion 157–9) [DOI] [PubMed] [Google Scholar]
- Mounier-Vehier F., Leys D., Godefroy O. Borderzone infarct subtypes: preliminary study of the presumed mechanism. Eur. Neurol. 1994;34:11–15. doi: 10.1159/000117001. [DOI] [PubMed] [Google Scholar]
- Nael K., Meshksar A., Liebeskind D.S. Periprocedural arterial spin labeling and dynamic susceptibility contrast perfusion in detection of cerebral blood flow in patients with acute ischemic syndrome. Stroke. 2013 doi: 10.1161/STROKEAHA.112.672956. Epub ahead of print 6 February 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba T., Ogasawara K., Nishimoto H. Postoperative cerebral white matter damage associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: a diffusion tensor magnetic resonance imaging study. Cerebrovasc. Dis. 2012;34:358–367. doi: 10.1159/000343505. [DOI] [PubMed] [Google Scholar]
- Oswald W.D., Fleischmann U.M. Hogrefe; Göttingen: 1997. Das Nürnberger-Alters-Inventar. [Google Scholar]
- Rimmele D.L., Larena-Avellaneda A., Alegiani A.C. Real-world experience of treatment decision-making in carotid stenosis in a neurovascular board. Neurology. 2017;89:399–407. doi: 10.1212/WNL.0000000000004151. [DOI] [PubMed] [Google Scholar]
- Scherr M., Trinka E., Mc Coy M. Cerebral hypoperfusion during carotid artery stenosis can lead to cognitive deficits that may be independent of white matter lesion load. Curr. Neurovasc. Res. 2012;9:193–199. doi: 10.2174/156720212801619009. [DOI] [PubMed] [Google Scholar]
- Soinne L., Helenius J., Tatlisumak T. Cerebral hemodynamics in asymptomatic and symptomatic patients with high-grade carotid stenosis undergoing carotid endarterectomy. Stroke. 2003;34:1655–1661. doi: 10.1161/01.STR.0000075605.36068.D9. [DOI] [PubMed] [Google Scholar]
- Staikov I.N., Nedeltchev K., Arnold M. Duplex sonographic criteria for measuring carotid stenoses. J. Clin. Ultrasound. 2002;30:275–281. doi: 10.1002/jcu.10078. [DOI] [PubMed] [Google Scholar]
- Tatu L., Moulin T., Bogousslavsky J. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- Torvik A. The pathogenesis of watershed infarcts in the brain. Stroke. 1984;15:221–223. doi: 10.1161/01.str.15.2.221. [DOI] [PubMed] [Google Scholar]
- Vermeer S.E., Prins N.D., den Heijer T. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Wang T., Xiao F., Wu G. Impairments in brain perfusion, metabolites, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients: an integrated MRI study. Neural Plast. 2017 doi: 10.1155/2017/8738714. Epub ahead of print 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Sun D., Liu Y. The impact of carotid artery stenting on cerebral perfusion, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients. Front. Neurol. 2017;8:1–7. doi: 10.3389/fneur.2017.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhou M, Zhou Y, et al. Effects of carotid endarterectomy on cerebral reperfusion and cognitive function in patients with high grade carotid stenosis: a perfusion weighted magnetic resonance imaging study. Eur. J. Vasc. Endovasc. Surg. Epub ahead of print 2015. DOI: 10.1016/j.ejvs.2015.03.032. [DOI] [PubMed]
- Wendell C.R., Waldstein S.R., Ferrucci L. Carotid atherosclerosis and prospective risk of dementia. Stroke. 2012;43:3319–3324. doi: 10.1161/STROKEAHA.112.672527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun T.J., Sohn C.-H., Han M.H. Effect of carotid artery stenting on cerebral blood flow: evaluation of hemodynamic changes using arterial spin labeling. Neuroradiology. 2013;55:271–281. doi: 10.1007/s00234-012-1104-y. [DOI] [PubMed] [Google Scholar]