Abstract
Background
Neutrophil elastase (NE) has been linked to lung neutrophil dysfunction in bronchiectasis and cystic fibrosis (CF), making NE inhibition a potential therapeutic target. NE inhibitor trials have given mixed result perhaps because not all patients have elevated airway NE activity.
Methods
We tested whether a single baseline sputum NE measurement or a combination of clinical parameters could enrich patient populations with elevated NE activity for “personalised medicine”. Intra- and interindividual variations of total and active NE levels in induced sputum from patients with CF or bronchiectasis were monitored over 14 days. Patients with established CF and bronchiectasis (n=5 per group) were recruited. NE was measured using three different methods: one total and two active NE assays. Subsequently, we analysed the association between clinical parameters and NE from a large bronchiectasis cohort study (n=381).
Results
All three assays showed a high degree of day-to-day variability (0–233% over 14 days). There were strong correlations found between all assays (p<0.0001). Despite high day-to-day variability, patients could be stratified into “high” or “low” groups based on moderate cut-off levels. In the bronchiectasis cohort study, factors most associated with high sputum NE levels were: Pseudomonas aeruginosa infection (β-estimate 11.5, 95% CI −6.0–29.0), sputum colour (β-estimate 10.4, 95% CI 4.3–16.6), Medical Research Council dyspnoea score (β-estimate 6.4, 95% CI 1.4–11.4) and exacerbation history (β-estimate 3.4, 95% CI 1.4–5.3). Collectively, P. aeruginosa infection, sputum colour and exacerbation frequency provided the greatest specificity for “high” NE (98.7%, 95% CI 7.0–99.6%).
Conclusion
These results show that patients with bronchiectasis and CF can be effectively divided into “high” or “low” groups, based on sputum NE assays or clinical inclusion criteria.
Short abstract
NE at baseline can enrich studies for “high” NE patients, while in bronchiectasis, inclusion of patients with Pseudomonas aeruginosa, purulent sputum and frequent exacerbations provides a high degree of certainty of including patients with high NE http://ow.ly/mPTS30nI8ec
Introduction
Bronchiectasis is a chronic disease characterised by cough, sputum production, neutrophilic airway inflammation and bacterial infection [1–3]. Bronchiectasis is a heterogeneous disease, being caused by a range of disorders including cystic fibrosis (CF) and presenting with a wide spectrum of clinical phenotypes [2, 4, 5].
Development of new treatments for bronchiectasis is essential in view of the high burden of the disease [6, 7]. Such development has been highly challenging and several randomised clinical trials in bronchiectasis have failed to reach their primary end-points [8–11]. The difficulty in achieving positive results in bronchiectasis trials is thought to be due to disease heterogeneity, arguing for a personalised or stratified medicine approach [3]. Neutrophil elastase (NE) has long been implicated in the pathophysiology of bronchiectasis [12–14]. NE inhibition is considered a promising therapeutic approach in bronchiectasis and CF [15].
In a study of 381 patients with bronchiectasis, NE activity was predictive of future risk of exacerbations and lung function decline over 4 years [16]. This study, however, identified that a third of the cohort (132 out of 381 subjects) did not have detectable sputum NE activity [16].
NE inhibition is likely to only be an effective therapy in individuals with active NE in the airways [17–19]. The high frequency of individuals without significantly elevated NE may help to explain the mixed results of prior studies of inhibitors [20, 21]. AZD9668 was tested in 38 patients with bronchiectasis. The primary outcome of reducing sputum neutrophils was not met, but there was a significant 100 mL increase in forced expiratory volume in 1 s (FEV1) and a trend to improvement in St George's Respiratory Questionnaire score [20]. A study of 94 patients with BAY85-8501 also showed no evidence of efficacy [21].
These studies did not enrich for patients with elevated sputum NE either using biomarkers or clinical characteristics. There are a number of examples in respiratory medicine where drugs that were thought to be ineffective were shown to be effective when targeted to the appropriate patient population using biomarkers or clinical parameters [22, 23].
The aims of our study were: 1) to determine if sputum NE activity was stable over time to establish if it could be used for population enrichment in clinical trials of NE inhibition, 2) to compare different assays and sample types to identify the optimal method for enriching a clinical trial population, and 3) to study the association of clinical parameters and baseline NE to identify the optimal inclusion criteria for a future trial.
Methods
In the first part of the study we aimed to determine the optimal patient population for a future stratified clinical trial, defined as patients with consistently elevated sputum NE levels.
This study enrolled 10 bronchiectasis and CF patients (n=5 per group) at Ninewells Hospital (Dundee, UK). All patients were adults aged ≥18 years and were clinically stable at the time of recruitment, defined by the absence of acute antibiotic treatment for at least 4 weeks and the absence of exacerbation symptoms at the screening visit. Both groups were required to have daily sputum production and be able to produce sputum at screening. Exclusion criteria for both groups were: inability to give informed consent, immunodeficiency, active mycobacterial infection, current smoker/ex-smoker of <1 year, active allergic bronchopulmonary aspergillosis (ABPA), pregnancy/breastfeeding, long-term oxygen therapy and FEV1 <25% predicted. Specific inclusion criteria for CF subjects were: a diagnosis of CF with two disease-causing CF transmembrane conductance regulator mutations. Specific inclusion criteria for bronchiectasis subjects were: idiopathic or post-infective bronchiectasis confirmed on computed tomography (CT). Exclusion criteria were: active sarcoidosis, α1-antitrypsin deficiency, poorly controlled asthma and active malignancy. The study was approved by the local research ethics committee (16/ES/0047) and all patients provided written informed consent.
Sputum was induced using 3% hypertonic saline for up to 20 min. Samples of sputum were obtained on days 1 (baseline), 3, 5, 7 and 14 after enrolment. 24 h sputum collection was conducted on days 2–3, 4–5, 6–7 and 13–14 for measurement of 24 h sputum NE.
Availability of data and materials
Access to data held by the European Bronchiectasis Registry can be accessed through: www.bronchiectasis.eu/dataaccess.
NE measurement
Sputum samples were processed through dilution with 8 times volume PBS followed by centrifugation at 3000×g for 15 min. The repeatability of three different NE assays was tested: 1) an activity-based fluorescence resonance energy transfer (FRET) assay according to the protocol of Korkmaz et al. [24], 2) an activity-based immunoassay (ProteaseTag ELISA; ProAxsis, Belfast, UK) [25] and 3) a total NE ELISA (AssayMax; Assaypro, St Charles, MO, USA), which measures total protein rather than activity. For the FRET assay, the intra-assay coefficient of variation was 5.6% and the interassay coefficient of variation was 8.8%. For the total NE assay, the intra-assay coefficient of variation was 14.5% and interassay coefficient of variation was 10.6%. For the activity-based immunoassay, the intra-assay coefficient of variation was 7.1% and interassay coefficient of variation was 7%.
Bronchiectasis cohort study
The objective of this analysis was to determine in a large patient cohort whether combinations of clinical parameters (inclusion criteria for a hypothetical trial) could be used to identify patients with elevated sputum NE using the ProteaseTag immunoassay.
Patients were recruited to a longitudinal observational study (the TAYBRIDGE registry) at Ninewells Hospital [26]. The study was approved by the local research ethics committee (12/ES/0059) and all patients gave written informed consent. Inclusion criteria were: age ≥18 years, high-resolution CT-confirmed bronchiectasis and clinical symptoms consistent with bronchiectasis. Exclusion criteria were: inability to give informed consent, active mycobacterial infection, active ABPA, malignancy, CF or pulmonary fibrosis with traction bronchiectasis.
Baseline data were used to evaluate the association of sputum NE concentration and clinical parameters. Severity of disease was evaluated with the Bronchiectasis Severity Index (BSI) [27]. Chronic bacterial infection was defined as the growth of pathogens from sputum on at least two occasions 3 months apart during the previous 12 months [28]. Exacerbation frequency was defined according to British Thoracic Society criteria [29, 30].
Statistical analysis
Repeatability statistics used were the coefficient of variation and the within-subject standard deviation. Mean differences between groups were compared with the t-test. Correlations utilised linear regression. For predictive tests we calculated sensitivity, specificity and positive/negative predictive values. To identify clinical parameters associated with sputum NE we used a multiple linear regression with sputum NE as a linear dependent variable. We used 20 µg·mL−1 as a pre-defined cut-off of “high” NE based on our prior work [11]. A sensitivity analysis was performed looking at predictive statistics for cut-offs defined by the lower and upper 95% confidence intervals of the biomarker distribution. For analysis of clinical characteristics associated with NE, a multivariable logistic regression with a binary dependent variable of NE >20 µg·mL−1 was used in this analysis, with an exploratory analysis using a higher cut-off of 50 µg·mL−1. p<0.05 was considered statistically significant.
Results
Repeatability of sputum NE as a biomarker (CF and bronchiectasis)
The characteristics of the five patients with bronchiectasis and five patients with CF are shown in supplementary tables S1 and S2. Patients with bronchiectasis were predominantly elderly, three were treated with chronic macrolide therapy and four were never-smokers. FEV1 ranged from 45% to 110% predicted. Using the BSI, two patients were classified as severe, two were moderate and one was mild.
The adult CF patients had an age range of 21–38 years and had 0–6 exacerbations per year. The majority were receiving prophylactic oral antibiotic therapy.
Repeatability of sputum NE quantification
To investigate whether a single “point-of-care” or laboratory measurement of sputum NE could be used for stratification in a trial we examined whether a single measure in induced or 24 h sputum could identify patients with consistently high or low levels of sputum NE.
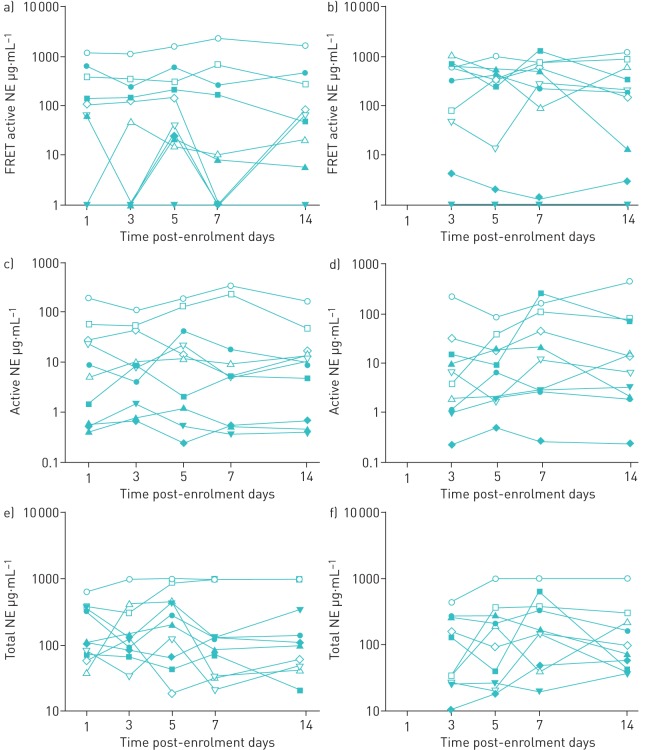
The repeatability data are shown in figure 1 for each assay. All of the assays showed a high degree of day-to-day variability. No patients had exacerbations or changes in clinical status during the study to explain these changes. We observed no benefit of 24 h sputum collections, in terms of reducing variability, compared with a single-point induced sputum.
FIGURE 1.
Day-to-day variability in the measurement of sputum neutrophil elastase (NE) measured using three different assays at the same time-points: a, b) fluorescence resonance energy transfer (FRET) active NE, c, d) active NE (ProteaseTag) and e, f) total NE. Open symbols: individual cystic fibrosis subjects (n=5); solid symbols: individual bronchiectasis subjects (n=5). a, c, e) Induced sputum samples taken at days 1, 3, 5, 7 and 14 post-enrolment. b, d, f) 24 h sputum collections commencing on days 2, 4, 6 and 13 and completed on days 3, 5, 7 and 14.
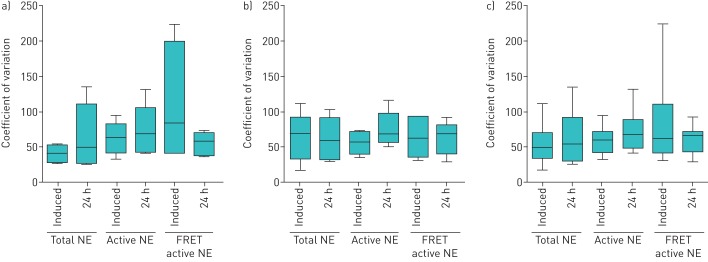
Figure 2 shows the median coefficient of variation per subject. NE values from visit to visit varied by 0% to 233% within individuals. 0% refers to one individual with undetectable NE activity using the FRET active NE assay at each time-point. This individual was excluded in calculating the variability of the assay. There was no clear difference in repeatability between bronchiectasis and CF.
FIGURE 2.
Coefficient of variation within subjects for each neutrophil elastase (NE) assay: total NE, active NE (ProteaseTag) and fluorescence resonance energy transfer (FRET) active NE. a) Bronchiectasis, b) cystic fibrosis and c) both populations combined. Plots show median with interquartile range (boxes) and range (whiskers). One subject with undetectable FRET NE activity (and therefore 0% variation) at all time-points was not included in calculating the coefficient of variation.
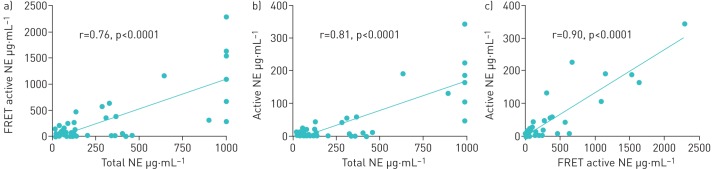
Despite the high variability observed there was a distinction between “high” and “low” patients as defined by sputum NE. A previous publication using the ProteaseTag immunoassay defined “high” as >20 µg·mL−1 as this cut-off was associated with clinical outcomes [11]. Based on the strong correlation between the three assays (figure 3) we determined that 176 µg·mL−1 using the total NE ELISA and 138 µg·mL−1 using the FRET NE assay correspond to the 20 µg·mL−1 cut-off for which clinical data were available.
FIGURE 3.
Intercorrelation between different neutrophil elastase (NE) assays in subjects with bronchiectasis and cystic fibrosis. FRET: fluorescence resonance energy transfer. a) FRET active NE versus total NE, b) active NE (ProteaseTag) versus total NE and c) active NE (ProteaseTag) versus FRET active NE.
All subjects with NE levels <20 µg·mL−1 continued to have levels <20 µg·mL−1 at follow-up. This was used to generate predictive statistics as shown in table 1. In addition, predictive statistics are shown to identify patients above the lower and upper 95% confidence intervals for each assay. Across the whole population the mean levels were: total NE 271.3 µg·mL−1 (95% CI 180–362.6 µg·mL−1), FRET active NE 267 µg·mL−1 (95% CI 130.9–404.4 µg·mL−1) and active NE 36.9 µg·mL−1 (95% CI 16.9–56.9 µg·mL−1).
TABLE 1.
Predictive statistics for the ability of a single baseline induced sputum measurement of neutrophil elastase (NE) (activity or total protein as indicated) to predict a NE measurement above the predefined threshold at follow-up or at any stage during the study
| Outcome and test | Sensitivity % | Specificity % | PPV % | NPV % |
| “High” NE at day 14 | ||||
| FRET active NE | 100 | 100 | 100 | 100 |
| Active NE (ProteaseTag) | 100 | 75 | 50 | 100 |
| Total NE ELISA | 100 | 85.7 | 75 | 100 |
| “High” NE at any time-point | ||||
| FRET active NE | 66.7 | 100 | 100 | 66.7 |
| Active NE (ProteaseTag) | 80 | 100 | 100 | 83.3 |
| Total NE ELISA | 66.7 | 100 | 100 | 66.7 |
| Above lower 95% CI at day 14 | ||||
| FRET active NE | 100 | 85.7 | 75 | 100 |
| Active NE (ProteaseTag) | 100 | 75 | 50 | 100 |
| Total NE ELISA | 100 | 85.7 | 75 | 100 |
| Above lower 95% CI at any time-point | ||||
| FRET active NE | 80 | 100 | 100 | 83.3 |
| Active NE (ProteaseTag) | 80 | 100 | 100 | 83.3 |
| Total NE ELISA | 60 | 80 | 75 | 66.7 |
| Above upper 95% CI at day 14 | ||||
| FRET active NE | 100 | 100 | 100 | 100 |
| Active NE (ProteaseTag) | 100 | 88.9 | 50 | 100 |
| Total NE ELISA | 100 | 100 | 100 | 100 |
| Above upper 95% CI at any time-point | ||||
| FRET active NE | 66.7 | 100 | 100 | 87.5 |
| Active NE (ProteaseTag) | 100 | 100 | 100 | 100 |
| Total NE ELISA | 100 | 87.5 | 66.7 | 100 |
PPV: positive predictive value; NPV: negative predictive value; FRET: fluorescence resonance energy transfer.
These data suggest that NE measurement can effectively exclude patients likely to rarely have elevated NE, which may be useful for stratification in clinical trials.
Clinical parameters to select patients for a randomised controlled trial of NE inhibition
This analysis included 381 patients with bronchiectasis. Median (interquartile range) age was 67 (58–74) years and FEV1 was 71.4% (49.4–90.9%) predicted. The median exacerbation frequency was 1 per year. 60 patients (15.7%) were chronically infected with P. aeruginosa.
Based on the prior results, two candidate cut-offs were identified for elevated sputum NE for inclusion in a stratified trial: high (>20 µg·mL−1) and very high (>50 µg·mL−1). Logistic regression analysis of predictors of these NE levels is shown in table 2.
TABLE 2.
Multivariable analysis of determinants of neutrophil elastase (NE) activity in bronchiectasis
| Linear regression analysis | Logistic regression analysis to predict sputum NE >20 µg·mL−1 | Logistic regression analysis to predict sputum NE >50 µg·mL−1 | ||||
| β-estimate (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Pseudomonas aeruginosa infection | 11.5 (−6.0–29.0) | 0.2 | 2.17 (0.88–5.33) | 0.09 | 0.98 (0.28–3.38) | 0.9 |
| Sputum colour (4-point scale) | 10.4 (4.3–16.6) | 0.001 | 2.13 (1.50–3.03) | <0.0001 | 2.80 (1.70–4.62) | <0.0001 |
| MRC dyspnoea score | 6.4 (1.4–11.4) | 0.01 | 1.57 (1.18–2.09) | 0.002 | 2.43 (1.56–3.78) | <0.0001 |
| Exacerbation history (per 1 event) | 3.4 (1.4–5.3) | 0.001 | 1.07 (0.96–1.19) | 0.2 | 1.16 (1.02–1.32) | 0.03 |
| Radiological extent (per additional lobe) | 1.5 (0.1–2.9) | 0.03 | 1.12 (1.05–1.21) | 0.002 | 1.09 (0.98–1.20) | 0.1 |
| Male | 1.1 (−8.5–10.6) | 0.8 | 0.82 (0.40–1.45) | 0.5 | 0.61 (0.27–1.40) | 0.2 |
| Infection with other organisms | 0.4 (−10.6–11.4) | 0.9 | 1.23 (0.61–2.46) | 0.6 | 0.69 (0.22–2.16) | 0.5 |
| Age (per year) | 0.1 (−0.3–0.5) | 0.7 | 1.01 (0.99–1.04) | 0.4 | 1.01 (0.98–1.05) | 0.5 |
| FEV1 % pred | −0.1 (−0.3–0.1) | 0.3 | 0.98 (0.97–1.00) | 0.05 | 0.99 (0.98–1.01) | 0.5 |
MRC: Medical Research Council; FEV1: forced expiratory volume in 1 s.
Across all of these analyses, there was a consistent trend that purulent sputum colour, elevated Medical Research Council (MRC) dyspnoea score, more extensive radiological bronchiectasis and exacerbation frequency were associated with elevated NE activity, independent of other variables. P. aeruginosa infection also appeared to be strongly associated with elevated NE, but was nonsignificant in most models.
Based on the aforementioned analysis we generated a set of potential inclusion criteria approaches for clinical trials, and evaluated their sensitivity and specificity. As a guide to interpretation of the data, a high specificity indicates a low likelihood of including patients without NE >20 µg·mL−1 in a trial, which is desirable. A low sensitivity, however, indicates that a large number of individuals who could potentially benefit from therapy (because of elevated NE >20 µg·mL−1) would be excluded using these inclusion criteria. Our results suggest that stratifying on sputum colour provided the greatest degree of sensitivity, as only 6.7% of subjects had NE >20 µg·mL−1 with a sputum sample graded as mucoid, while P. aeruginosa infection provided high specificity as a single criterion. The results for various combinations of criteria are shown in table 3.
TABLE 3.
Sensitivity/specificity of different combinations of inclusion criteria for a hypothetical trial of neutrophil elastase (NE) inhibition aiming to enrol patients with >20 µg·mL−1 sputum NE activity at baseline
|
Sensitivity (95% CI) % |
Specificity (95% CI) % |
PPV (95% CI) % |
NPV (95% CI) % |
|
| Sputum colour grade ≥2 | 93.3 (86.6–97.3) | 46.5 (41.4–51.6) | 32.2 (29.9–34.6) | 96.2 (92.5–98.1) |
| Sputum colour grade 3/4 only | 58.7 (48.6–68.2) | 79.1 (73.8–83.7) | 51.3 (33.3–58.2) | 83.6 (80.1–86.6) |
| P. aeruginosa patients only | 37.5 (28.2–47.5) | 92.4 (88.6–95.3) | 65.0 (53.5–75.0) | 79.8 (77.2–82.1) |
| P. aeruginosa+grade 3/4 sputum and a history of ≥2 exacerbations per year | 24.0 (16.2–33.4) | 98.7 (97.0–99.6) | 83.3 (66.2–92.7) | 82.6 (81.0–84.1) |
| Sputum colour grade ≥2 and ≥2 exacerbations per year | 65.4 (55.4–74.5) | 76.1 (71.5–80.3) | 42.8 (37.3–48.4) | 89.0 (86.0–91.3) |
| Sputum colour grade ≥2, MRC dyspnoea score ≥3, ≥3 lobes involved on CT and ≥2 exacerbations per year | 37.5 (28.2–47.5) | 91.6 (88.4–94.2) | 54.9 (44.6–64.8) | 84.3 (82.2–86.2) |
| ≥3 exacerbations per year, ≥2 lobes involved on CT and infection with any organism (including P. aeruginosa) | 16.4 (9.8–24.9) | 91.3 (88.1–94.0) | 34.0 (23.0–47.0) | 80.0 (78.5–81.4) |
PPV: positive predictive value; NPV: negative predictive value; P. aeruginosa: Pseudomonas aeruginosa; MRC: Medical Research Council; CT: computed tomography.
Discussion
This aim of this study was to support the future development of NE inhibitors for the treatment of patients with bronchiectasis and CF by providing data to identify the patient population most likely to benefit from treatment. This concept of precision medicine is increasingly advocated across respiratory medicine, and has led to significant progress in areas such as cancer and severe asthma [31–34]. Bronchiectasis is a heterogeneous disease with multiple phenotypes/endotypes [2, 3, 35]. NE is thought to play a key pathophysiological role, but is not consistently elevated in all patients [16]. Inclusion of a relatively unselected group of patients with bronchiectasis would therefore not be expected to result in positive results, assuming that NE inhibition is not beneficial in patients without NE activity in sputum. We think this is a reasonable assumption, and is supported by unsuccessful clinical trials of NE inhibitors in unselected populations with bronchiectasis and chronic obstructive pulmonary disease (COPD) to date [20, 36–38].
In this study we examined questions for the design of future NE inhibitor trials. First, whether NE activity is stable over time, and therefore whether patients can be grouped into “high” and “low” groups with the aim of including only patients with high NE. We examined this by measuring longitudinal NE levels on five occasions over 14 days while clinically stable. This approach removed possible biases induced by antibiotic treatment, which is known to reduce NE, and the impact of new treatments by conducting measurements over a carefully controlled time period. Our results showed high day-to-day variability, with >200% variation over 14 days in some patients. Nevertheless, as an example, while a change in NE from 1152 to 2291 µg·mL−1 occurred in a subject with CF, which represents a high degree of variability, in practical terms this patient has sustained high levels of NE activity at every time-point. Examining the data in this way we showed high sensitivity and specificity for two active NE assays and one total NE ELISA to identify patients' longitudinal behaviour. This means that a single measurement of sputum NE in stable patients could reliably be used to stratify patients for trial inclusion. We identified no advantage of 24 h sputum collection over induced sputum sampling at a single time-point and so recommend the more straightforward method of collection [39]. We acknowledge the limitation of this study's small sample size, but our recent results are supported by repeatability data from our previous study in 381 patients and therefore we consider it highly unlikely a study with a larger sample size would find different results [16].
There may be practical reasons why using an NE assay at baseline to determine inclusion in a clinical trial may be challenging. For a phase 3 trial with large numbers of subjects in particular, deploying and standardising an assay across potentially hundreds of sites could be very difficult. We were therefore interested to test whether clinical parameters could be used as a proxy for a “high” NE population. We found that sputum colour, MRC dyspnoea score and presence of P. aeruginosa were the variables most strongly associated with NE activity. These are not surprising, as the green colour of sputum is generated by myeloperoxidase, a green protein which is co-released with NE from neutrophil azurophil granules during degranulation or neutrophil extracellular trap formation [40–42]. P. aeruginosa is known to be a potent neutrophil activator and acts as a stimulus for persistent neutrophil recruitment [43, 44]. Both have previously been shown to be associated with elevated NE [45–47]. What is novel in our study is that we can estimate using a large cohort of 381 patients, the likelihood of successfully targeting the right patient population in a future trial. It is not as simple as suggesting that investigators should use one or other of these criteria, but rather it would depend on the study design and study objectives.
For example, in a small proof-of-concept study such as those already conducted in bronchiectasis, it might be important to ensure all patients had elevated NE so that a signal could be observed in a small number of subjects. In this case, a high specificity would be important, and the investigators may wish to limit enrolment to patients with P. aeruginosa infection (92% specificity in our data) or even P. aeruginosa plus purulent sputum and a history of two or more exacerbations (98% specificity). However, for a large phase 3 trial with an NE inhibitor, a balance would be needed between specificity and the feasibility of enrolling large numbers of subjects, and therefore different criteria with higher sensitivity may be used.
Our study has limitations which we acknowledge. We chose to study repeatability of NE in both bronchiectasis and CF patients, but only focused the subsequent cohort analysis on bronchiectasis based on our prior observation of a third of subjects having undetectable NE. A similar analysis is required for CF. Mean NE activity is higher in adult CF than bronchiectasis and so the cut-offs used in our study should not be extrapolated to CF or other conditions such as COPD [48, 49]. We used NE >20 µg·mL−1 as a cut-off based on our prior publication [11], but we acknowledge that we cannot prove patients with lower levels of NE would not benefit from NE inhibitors. Our study was conducted in a single region in Scotland and so may not be generalisable, although we consider this unlikely given the high level of similarity between our patient population and others across Europe [50].
Conclusion
We have shown that patients with bronchiectasis and CF can be effectively divided into those with consistently elevated or lower levels of sputum NE using sputum NE assays or using clinical parameters. This provides evidence that NE inhibitors could be targeted using a “precision medicine approach” in patients with bronchiectasis and warrants further research in patients with CF.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Tables S1 and S2 00252-2018.supp_tables (133.3KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com.
Author contributions: H.R. Keir, M.L. Crichton, P. Barth, J. Zimmermann, P.L.B. Bruijnzeel, A.J. Dicker and J.D. Chalmers designed the study. H.R. Keir, M.L. Crichton, G. Brady and J.D. Chalmers recruited patients and collected the data. H.R. Keir, C.J. Fong, E. Chevalier, G. Kennedy, J. Zimmermann, A.J. Dicker and J.D. Chalmers designed and performed the laboratory analyses. H.R. Keir, C.J. Fong, E. Chevalier, P. Barth, P.L.B. Bruijnzeel, A.J. Dicker and J.D. Chalmers analysed and interpreted the data. H.R. Keir and J.D. Chalmers wrote the manuscript. All authors revised the manuscript and approved the final version of the manuscript.
Conflict of interest: H.R. Keir has nothing to disclose.
Conflict of interest: C.J. Fong has nothing to disclose.
Conflict of interest: M.L. Crichton has nothing to disclose.
Conflict of interest: P. Barth is an employee of Polyphor.
Conflict of interest: E. Chevalier is an employee of Polyphor.
Conflict of interest: G. Brady has nothing to disclose.
Conflict of interest: G. Kennedy has nothing to disclose.
Conflict of interest: J. Zimmermann is an employee of Polyphor.
Conflict of interest: P.L.B. Bruijnzeel is an employee of Polyphor.
Conflict of interest: A.J. Dicker has nothing to disclose.
Conflict of interest: J.D. Chalmers reports research grants for COPD and personal fees from GSK, Boehringer Ingelheim and Pfizer, research grants for COPD from AstraZeneca, grants and personal fees for research into bronchiectasis from Bayer Healthcare and Grifols, consulting fees from Napp and Aradigm Corporation, and grants and personal fees from Insmed, outside the submitted work.
Support statement: J.D. Chalmers is supported by the GSK/British Lung Foundation Chair of Respiratory Research. The repeatability study was funded by a grant from Polyphor AG (Basel, Switzerland). The TAYBRIDGE study was funded by Tenovus Scotland and the European Respiratory Society through the clinical research collaboration EMBARC. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Quint JK, Millett ERC, Joshi M, et al. . Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018; 6: 715–726. [DOI] [PubMed] [Google Scholar]
- 4.Aliberti S, Lonni S, Dore S, et al. . Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016; 47: 1112–1122. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery ST, Mall MA, Kicic A, et al. . Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur Respir J 2017; 49: 1600903. [DOI] [PubMed] [Google Scholar]
- 6.Aliberti S, Masefield S, Polverino E, et al. . Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. Eur Respir J 2016; 48: 632–647. [DOI] [PubMed] [Google Scholar]
- 7.Ringshausen FC, de Roux A, Pletz MW, et al. . Bronchiectasis-associated hospitalizations in Germany, 2005–2011: a population-based study of disease burden and trends. PLoS One 2013; 8: e71109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksamit T, De Soyza A, Bandel T-J, et al. . RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702053. [DOI] [PubMed] [Google Scholar]
- 9.De Soyza A, Aksamit T, Bandel T-J, et al. . RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702052. [DOI] [PubMed] [Google Scholar]
- 10.Barker AF, O'Donnell AE, Flume P, et al. . Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014; 2: 738–749. [DOI] [PubMed] [Google Scholar]
- 11.De Soyza A, Pavord I, Elborn JS, et al. . A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J 2015; 46: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 12.Smallman LA, Hill SL, Stockley RA. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax 1984; 39: 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized Pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest 1990; 86: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratjen F, Waters V, Klingel M, et al. . Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J 2016; 47: 829–836. [DOI] [PubMed] [Google Scholar]
- 15.Gramegna A, Amati F, Terranova L, et al. . Neutrophil elastase in bronchiectasis. Respir Res 2017; 18: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. . Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med 2017; 195: 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai N, Kondo M, Izumo T, et al. . Inhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in mice. Eur Respir J 2010; 35: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 18.Sjo P. Neutrophil elastase inhibitors: recent advances in the development of mechanism-based and nonelectrophilic inhibitors. Future Med Chem 2012; 4: 651–660. [DOI] [PubMed] [Google Scholar]
- 19.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest 2008; 133: 489–495. [DOI] [PubMed] [Google Scholar]
- 20.Stockley R, De Soyza A, Gunawardena K, et al. . Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir Med 2013; 107: 524–533. [DOI] [PubMed] [Google Scholar]
- 21.Watz H, Pedersen F, Kirsten A, et al. . Safety and tolerability of the NE inhibitor BAY 85-8501 in patients with non-CF bronchiectasis. Eur Respir J 2016; 48: Suppl. 60, PA4088. [Google Scholar]
- 22.Pavord ID, Korn S, Howarth P, et al. . Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FJ, Calverley PMA, Goehring U-M, et al. . Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015; 385: 857–866. [DOI] [PubMed] [Google Scholar]
- 24.Korkmaz B, Attucci S, Epinette C, et al. . Measurement of neutrophil elastase, proteinase 3, and cathepsin G activities using intramolecularly quenched fluorogenic substrates. Methods Mol Biol 2012; 844: 125–138. [DOI] [PubMed] [Google Scholar]
- 25.Keir HR, Fong CJ, Dicker AJ, et al. . Profile of the ProAxsis active neutrophil elastase immunoassay for precision medicine in chronic respiratory disease. Expert Rev Mol Diagn 2017; 17: 875–884. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell MJ, Aliberti S, Goeminne PC, et al. . Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016; 71: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers JD, Goeminne P, Aliberti S, et al. . The bronchiectasis severity index an international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finch S, McDonnell MJ, Abo-Leyah H, et al. . A Comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12: 1602–1611. [DOI] [PubMed] [Google Scholar]
- 29.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010; 65: 577. [DOI] [PubMed] [Google Scholar]
- 30.Hill AT, Haworth CS, Aliberti S, et al. . Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. [DOI] [PubMed] [Google Scholar]
- 31.Agustí A, Bafadhel M, Beasley R, et al. . Precision medicine in airway diseases: moving to clinical practice. Eur Respir J 2017; 50: 1701655. [DOI] [PubMed] [Google Scholar]
- 32.Burgener EB, Moss RB. Cystic fibrosis transmembrane conductance regulator modulators: precision medicine in cystic fibrosis. Curr Opin Pediatr 2018; 30: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polverino E, Dimakou K, Hurst J, et al. . The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J 2018; 52: 1800328. [DOI] [PubMed] [Google Scholar]
- 34.Boaventura R, Sibila O, Agusti A, et al. . Treatable traits in bronchiectasis. Eur Respir J 2018; 52: 1801269. [DOI] [PubMed] [Google Scholar]
- 35.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008; 372: 1107–1119. [DOI] [PubMed] [Google Scholar]
- 36.Vogelmeier C, Aquino TO, O'Brien CD, et al. . A randomised, placebo-controlled, dose-finding study of AZD9668, an oral inhibitor of neutrophil elastase, in patients with chronic obstructive pulmonary disease treated with tiotropium. COPD 2012; 9: 111–120. [DOI] [PubMed] [Google Scholar]
- 37.Elborn JS, Perrett J, Forsman-Semb K, et al. . Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur Respir J 2012; 40: 969–976. [DOI] [PubMed] [Google Scholar]
- 38.Kuna P, Jenkins M, O'Brien CD, et al. . AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir Med 2012; 106: 531–539. [DOI] [PubMed] [Google Scholar]
- 39.Tsang KW, Chan K, Ho P, et al. . Sputum elastase in steady-state bronchiectasis. Chest 2000; 117: 420–426. [DOI] [PubMed] [Google Scholar]
- 40.Goeminne PC, Vandooren J, Moelants EA, et al. . The Sputum Colour Chart as a predictor of lung inflammation, proteolysis and damage in non-cystic fibrosis bronchiectasis: a case-control analysis. Respirology 2014; 19: 203–210. [DOI] [PubMed] [Google Scholar]
- 41.Dickerhof N, Pearson JF, Hoskin TS, et al. . Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency. Free Radic Biol Med 2017; 113: 236–243. [DOI] [PubMed] [Google Scholar]
- 42.Dicker AJ, Crichton ML, Pumphrey EG, et al. . Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 141: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilliam Y, Moore MP, Lamont IL, et al. . Pseudomonas aeruginosa adaptation and diversification in the non-cystic fibrosis bronchiectasis lung. Eur Respir J 2017; 49: 1602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pattison SH, Gibson DS, Johnston E, et al. . Proteomic profile of cystic fibrosis sputum cells in adults chronically infected with Pseudomonas aeruginosa. Eur Respir J 2017; 50: 1601569. [DOI] [PubMed] [Google Scholar]
- 45.Yoo D, Floyd M, Winn M, et al. . NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase–DNA and neutrophil elastase–DNA complexes. Immunol Lett 2014; 160: 186–194. [DOI] [PubMed] [Google Scholar]
- 46.Chalmers JD, Smith MP, McHugh BJ, et al. . Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012; 186: 657–665. [DOI] [PubMed] [Google Scholar]
- 47.Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol 2008; 40: 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esther CRJ, Turkovic L, Rosenow T, et al. . Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J 2016; 48: 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartoli ML, Costa F, Malagrino L, et al. . Sputum inflammatory cells in COPD patients classified according to GOLD 2011 guidelines. Eur Respir J 2016; 47: 978–980. [DOI] [PubMed] [Google Scholar]
- 50.Araujo D, Shteinberg M, Aliberti S, et al. . Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur Respir J 2017; 50: 1701289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Tables S1 and S2 00252-2018.supp_tables (133.3KB, pdf)
Data Availability Statement
Access to data held by the European Bronchiectasis Registry can be accessed through: www.bronchiectasis.eu/dataaccess.