Abstract
Synthesis of a new phosphene-free nano-size formamidine-based palladium complex have been achieved. The molecular structure of novel palladium complex have been confirmed using spectroscopic methods of analysis as well as physical characterizations. The synthesized complex has been used as a catalyst for microwave assisted aqueous Suzuki-Miyaura Cross-coupling (SMC) of aryl bromides with phenylboronic acid. The formamidine-based Pd(II)-complex exhibited excellent catalytic activity to obtain biaryls using mild reaction conditions.
Keyword: Organic chemistry
1. Introduction
Cross-coupling reactions with transition-metal-catalysts are considerably useful synthetic process for C-C bonds formation [1, 2, 3]. The Suzuki-Miyaura cross-coupling (SMC) [4], has become charming standard procedure for the synthesis of biaryls, which have an assorted spectrum of implementations, extending from pharmaceuticals to materials science [5]. Traditionally, palladium intermediates were stabilized by using phosphine ligands [6]. The air-susceptibility of these kinds of ligands, however, can prevent their use in a variety of synthetic implementations [7]. Moreover, phosphine ligands are often expensive and their price can override that of the palladium salt. On the other hand, phosphorus-free palladium catalysts have many advantages such as high air stability, thermal stability, higher catalytic activity which permit the catalyzed reaction to occur under mild reaction conditions [8, 9]. Thus, the development of ligands free from phosphine has been increasingly inspected in recent times, with amines, carbenes, thioureas, etc. [10, 11]. Construction of appropriate ligands might be a good controlling factor to obtain catalysts that push the weak leaving group such as chloride (in SMC) to take its role in the reaction and easily displaced. Also, developed appropriate ligands will led to catalysts that exhibit improved lifetimes, higher TON, recyclability and are stable to run the reactions in aerobic conditions at lower temperatures [12, 13]. Formamidines are multilateral ligands, qualified to form flexible coordination modes which display transition metal complexes with new electronic properties and recently showed excellent ability for stabilization of metals high oxidation states [14, 15, 16, 17, 18, 19, 20]. On the other side, it is predestined that main source of the reaction chemical waste is due to the solvent [21]. From green chemistry, safety and economic considerations, utility of water as a medium for chemical reactions is a target, although defy and in several cases demanding longer times and higher temperatures. In the case of the SMC, using aqueous medium causes the fastness of boronic acids which make utility of water as reaction medium a feature over other solvents [22]. Moreover, the use of microwave radiation as heating source in chemical reactions is a milestone in the development of green chemistry which reduces heat losses, increase the homogenous heating and reduce the by-products and consider as clean heat source [23, 24, 25]. However, numerous homogenous or heterogeneous palladium catalyst have been designed for microwave assisted Suzuki reactions [26, 27, 28, 29, 30, 31, 32, 33, 34], while, the formamidine palladium(II)-complexes utilized in catalysis of SMC are much little. Derived from the excellent results obtained in our previous works on the use transition metal complexes in various applications especially palladium(II)-complexes [35, 36, 37, 38, 39], we report here our study on the synthesis of new pyrimidyl formamidine-based palladium(II)-complex 4 (Fig. 2) and study its catalytic activity in the microwave assisted SMC of aryl bromides with phenylboronic acid under aerobic conditions in water.
Fig. 2.
Synthesis of palladium complex 4 (precatalyst 4).
2. Results and discussion
2.1. Synthesis and characterization of the Pd (II)-complex 4 (precatalyst 4)
2.1.1. Synthesis of the Pd (II)-complex 4 (precatalyst 4)
Reaction of 2-amino-pyrimidine (1) with dimethylformamide dimethylacetal (2) (DMF DMA), using benzene as a solvent according to previously reported procedure [40] afforded the corresponding N,N-dimethyl-N'-(pyrimidin-2-yl)formimidamide ligand 3 as depicted in Fig. 1. The structure of ligand 3 was confirmed by its spectroscopic data. Thus, the 1H NMR spectrum of the ligand 3 showed two singlet signals at δ 3.01 and 3.11 ppm due to the two methyl groups of N,N-dimethylamino group protons, in addition to a triplet at δ 6.91 ppm with coupling constant J = 4.8 Hz due to the C5-H pyrimidine ring proton, a doublet signal at δ 8.47 ppm with coupling constant J = 4.8 Hz due to the C4-H and C6-H pyrimidine ring protons and a singlet signal at δ 8.60 ppm due to the formamidine moiety proton. In addition, the infra-red spectrum of the ligand 3 showed the C=N group characteristic band at 1634 cm−1.
Fig. 1.
Preparation of the N, N-dimethyl-N′-(pyrimidin-2-yl) formimidamide 3.
The formamidine-based palladium(II) complex 4 was prepared by dissolving N,N-dimethyl-N'-(pyrimidin-2-yl)formimidamide ligand 3 in MeOH with consequent dropwise addition of equivalent moles of Na2PdCl4 (dissolved in methanol), at room temperature as depicted in Fig. 2. The molecular structure of the formamidine-based palladium(II) complex 4 was confirmed on the basis of its spectroscopic data as well as physical characterizations. Examining the 1H NMR spectrum of the formamidine-based palladium(II) complex 4 indicates the presence of a singlet at δ 3.28 ppm due to protons of N-Me2 group, in addition to multiplet signal at δ 7.0–7.20 ppm due to C5-H pyrimidine ring proton, a doublet signal at δ 8.50 ppm with coupling constant J = 5.4 Hz due to the C4-H, a doublet signal at δ 8.60 ppm with coupling constant J = 5.1 Hz due to C6-H pyrimidine ring protons and a singlet at δ 8.78 ppm due to the proton of formamidine N=CH group. Nitrogen atom coordination of the NMe2 group of formamidine moiety with the Pd affected the value of chemical shift (δ) of the protons of the two methyl groups of NMe2 group as well as N=CH formamidine proton, Thus, by comparison of the value of the chemical shift δ of the same group of protons in the ligand 3, it was found that protons resonance occurs at down field region of the spectrum due to the electronic nature of Pd atom. In addition, IR spectrum of the formamidine-based palladium(II) complex 4 showed a vibrational band at 1624 cm−1 due to the presence of the C=N functional group and vibrational bands at 430 and 526 cm−1 due to the presence of Pd-N bond.
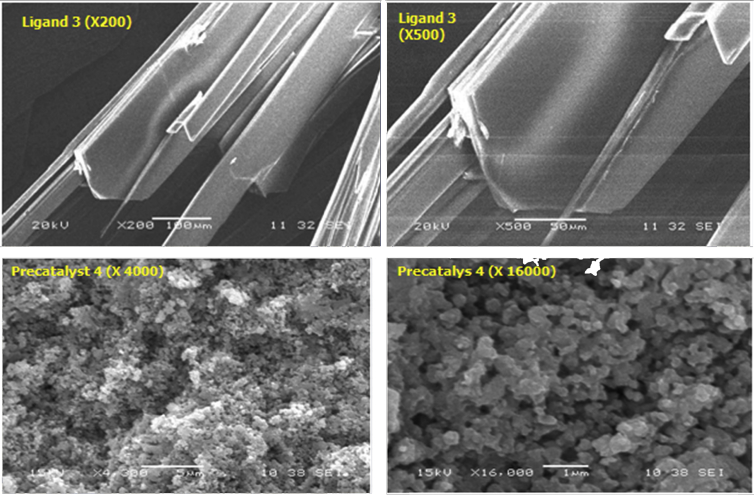
2.1.2. Scanning electron microscopy (SEM) and EDX of precatalyst 4
The SEM images of catalyst 4 and ligand 3 with different magnifications are presented in Fig. 3. As it can be seen, different morphologies of the catalyst are observed. The particles are agglomerated in different shapes and sizes compared with the ligand 3. Also, the spherical shape of the catalyst particles encourages their catalytic activity. The EDX study of the catalyst (Fig. 4) reveals that, the catalyst surface composed of Pd, Cl and carbon atoms with different concentrations.
Fig. 3.
The SEM images of ligand 3 and precatalyst 4 with different magnifications.
Fig. 4.
The EDX study of the precatalyst 4.
2.1.3. X-ray diffraction (XRD) analysis of precatalyst 4
The crystalline structure of the prepared formamidine-based palladium(II) complex 4 was examined and characterized by X-ray diffraction (XRD) analysis, the results are presented in Fig. 5. Careful inspection of Fig. 5 reveals that, the catalyst has well crystalline diffraction peaks at 2θ between 5° to 30°. Moreover, metallic palladium (2θ = 39.9o, 46.2o and 67.8o) (JCPDS-ICDD Card No. 46-1043) diffraction peaks can be identified [41]. The formation of Pd nanoparticles is originated during the catalyst preparation procedures. Also, no peaks characteristic to palladium oxide can be observed in the XRD analysis of the formamidine-based palladium(II) complex 4. The XRD results confirm the formation of metallic Pd nanoparticles which agree well with SEM images results.
Fig. 5.
X-ray diffraction (XRD) analysis of precatalyst 4.
Thus, Deby–Scherrer equation:
| B = 0.94 λ/(S Cos θ) |
S is the crystallite size.θ is the diffraction angle.B is the line width at half maximum height, Cu/Kα (λ) = 1.5406 A°.
The above equation was used to calculate the crystallite size, where the calculated size is present excellently in nanometer range which equal 8.763 nm.
2.1.4. Thermogravimetric analysis (TGA) of formamidine-based palladium(II) complex 4
Fig. 6 shows thermogravimetric analysis of (1:1) complex which formed between N,N-dimethyl-N'-(pyrimidin-2-yl)formimidamide ligand 3 and sodium tetrachloropalladate. The obtained results of TGA are listed in Table 1. As shown in the thermogram, the weight loss percent for the 1st step in the temperature range 47–131 °C was corresponding to the loss of one hygroscopic water molecule with weight loss 5.37% (Calc. 5.21). Whereas the second thermal degradation step in temperature range of 131–310 °C was attributed to the loss of two chloride ions with weight loss 18.9% (Calc. 21.3). The weight loss in the temperature range 310–891 °C was a result of the decomposition of organic part of complex. The final product is palladium oxide which has weight loss of 66.1% (theoretically 64.6%).
Fig. 6.
Thermogravimetric analysis (TGA) of precatalyst 4.
Table 1.
Results of thermogravimetric analysis of (1:1) (Pd:formamidine-based ligand).
| Complex chemical formula (M.wt) | Step order | Temperature range (°C) | Process | Weight loss % |
|
|---|---|---|---|---|---|
| Calc. | Found | ||||
| [PdPymCl2] H2O (345.4) | 1st | 47–131 | 1- Loss of a water molecule | 5.21 | 5.37 |
| [PdPymCl2] (327.4) | 2nd | 131–310 | 2- Loss of two chloride ions | 21.3 | 18.9 |
| [PdPym] (256.4) | 3rd | 310–891 | 3- Loss of organic part and PdO formation | 64.6 | 66.1 |
2.2. Catalytic study
2.2.1. Optimization and factors affecting the catalytic ability of formamidine-based palladium(II) complex 4 in SMC
2.2.1.1. Effect of catalyst concentration
In order to judge the catalytic activity of the developed catalyst we have started by the study of the effect of concentration of precatalyst 4 on the C-C cross-coupling of phenylboronic acid (5) with 4′-bromoacetophenone (6a) (model reaction), using water as a solvent, in the presence of potassium carbonate as a base and a co-catalyst ((tetrabutylammonium bromide) (TBAB)). Thus, under thermal reaction conditions heating the reaction mixture in the presence of different concentrations of the precatalyst 4 for three hours at 100 °C afforded the corresponding cross coupling product, 4-acetyl-1,1′-biphenyl (7a) as shown in Fig. 7 and Table 2. In the beginning, the reaction was carried out using one mol% of the precatalyst 4 with a molar ratio of 6a: 5: K2CO3: TBAB is 1:1.2:1:0.6, to give 100% conversion (based on GC-analysis) of the cross coupling product 7a (entry 1, Table 2). In entries 2-5, 0.75–0.125 mol% of the precatalyst 4 were used which gave also full GC-conversion under the same reaction conditions (Table 2). When PdCl2 is used in the presence of ligand 3 instead of precatalyst 4 we obtained lower conversion of the cross-coupling (entry 7, Table 2). From the data in Table 2, it can be concluded, that the precatalyst 4 showed excellent catalytic ability in the SMC even at very low concentrations. Expectedly, the starting 4′-bromoacetophenone (6a) was completely recovered when the reaction was carried out without the precatalyst 4 (entry 7, Table 2). The structure of the obtained product, 4-acetyl-1,1′-biphenyl (7a) was confirmed by spectroscopic data (see experimental part).
Fig. 7.
Effect of concentration of precatalyst 4 on the coupling of 4′-bromoacetophenone (6a) with phenylboronic acid (5) in water.
Table 2.
Effect of concentration of precatalyst 4 on the yield, TON, and TOF of the coupling of 4′-bromoacetophenone (6a) with phenylboronic acid (5) in water.
| Entry | Pd, mol% | Conversion %a,b | TON | TOF (h−1) |
|---|---|---|---|---|
| 1 | 1.00 | 100 | 100 | 33 |
| 2 | 0.75 | 100 (95) | 133 | 44 |
| 3 | 0.50 | 100 | 200 | 66 |
| 4 | 0.25 | 100 | 400 | 133 |
| 5 | 0.125 | 100 | 800 | 266 |
| 6 | 0.05 | 89 | 1780 | 593 |
| 7 | 1.00 (PdCl2/ligand 3)c | 87 | 87 | 26 |
| 8 | 0.00 | 0 | 0 | 0 |
Conditions: 4′-bromoacetophenone (6a)/phenylboronic acid (5)/base/TBAB/water: 1/1.2/1/0.6/10 mL, under thermal heating at 100 °C for 3 h.
Conversions were based on GC-analysis and the value between parenthesis refer to the highest isolated yield%.
Instead of precatalyst 4 1.00 mol% of PdCl2 in the presence of 1.00 mol% of ligand 3 was used.
In order to clarify the catalytic role of the precatalyst in the cross coupling, it should be noted that the palladium complex serves like “dormant species” that does not actually participate in the reaction catalytic cycle but it is considered as a main source of a catalytically active species related to palladium of unknown nature. In general, the Pd(0) species was reported in many literature to be the true active catalysts. Therefore, the precatalyst 4 may serve in this catalytic cycle as a reservoir that is indirectly involved in cross coupling but is main source of release of nano-sized Pd(0) which can show catalytic activity even at low concentrations [42]. Attempts for reusability of the precatalyst have been done using the same experimental conditions, unfortunately we could not get reasonable results even with using the higher mol% of the precatalyst 4.
2.2.1.2. Effect of solvent and base on SMC using precatalyst 4 under thermal conditions
The catalytic efficiency of the precatalyst 4 have been examined in order to get full conversions of the reactants and high products yields. Thus, different parameters that may affect such C-C cross-coupling have been optimized. The reaction media and type of base are among the controlling factors that affect the yield optimization. In general, there is no rule for selection of the base and the choice of it is still empirical, thus, the combination between different bases and a variety of solvents for the coupling reaction between 6a and phenylboronic acid (5) were investigated. As presented in Fig. 8 and Table 3, in all cases, the cross coupling was carried out using different media e.g. water, dimethylformamide (DMF), toluene, benzene, acetonitrile (AN), dioxane and tetrahydrofuran (THF) using 0.75 mol% concentration of the precatalyst 4 and in the presence of appropriate base such as potassium hydroxide (KOH), potassium carbonate (K2CO3) or triethylamine (TEA) under thermal conditions. The optimization also included the use of different types of co-catalyst such as tetrabutylammonium bromide (TBAB), tetrabutylammonium iodide (TBAI), tetrabutylammonium tetrafluoroborate (TBATFB), cetyl dimethyl benzyl ammonium bromide (CDBAB), cetyl trimethyl ammonium bromide (CTAB). The best results were obtained with water solvent using K2CO3 as a base in the presence of variety of the co-catalyst after refluxing at 100 °C (entries 1-4, Table 3). Also, in many cases we achieved 100% conversions by using nonpolar organic solvents as shown in Table 3 (entries 8, 10, and 15). When water was replaced with DMF, acetonitrile, dioxane, and THF in the presence of K2CO3, the reaction conversions were 77, 84, 100, and 88%, respectively (entries 6,7,9, and 11, Table 3). While the replacement of K2CO3 with KOH as a base improved the conversions when DMF and THF were reaction solvent, it is not suitable in case of water as a solvent (entries 13, 14, and 16, Table 3). Toluene was proved to be suitable solvent for the cross coupling regardless the type of the base used (entries 8, and 15, Table 3). Finally, Also TEA as an organic base using water afforded 83% conversion (entry 12, Table 3).
Fig. 8.
Effect of base and solvent Suzuki cross coupling of 4′-bromoacetophenone (6a) with phenylboronic acid (5) thermally.
Table 3.
Effect of base and solvent Suzuki cross coupling of 4′-bromoacetophenone (6a) with phenylboronic acid (5) under thermal conditions.
| Entry | Base | Solvent/co-catalyst | Yield%a |
|---|---|---|---|
| 1 | K2CO3 | H2O/TBAB | 100 (95) |
| 2 | K2CO3 | H2O/TBAI | 100 (90) |
| 3 | K2CO3 | H2O/TBATFB | 100 (92) |
| 4 | K2CO3 | H2O/CDBAB | 100 (90) |
| 5 | K2CO3 | H2O/CTAB | 80 (78) |
| 6 | K2CO3 | DMF | 77 (75) |
| 7 | K2CO3 | Acetonitrile | 84 (83) |
| 8 | K2CO3 | Toluene | 100 (83) |
| 9 | K2CO3 | Dioxane | 100 (95) |
| 10 | K2CO3 | Benzene | 84 (80) |
| 11 | K2CO3 | THF | 88 (87) |
| 12 | TEA | H2O/TBAB | 83 (80) |
| 13 | KOH | H2O/TBAB | 90 (87) |
| 14 | KOH | DMF | 100 (93) |
| 15 | KOH | Toluene | 100 (91) |
| 16 | KOH | THF | 100 (81) |
Conversion by GC-analysis and the value between parenthesis indicates the product isolated yield%. Conditions: 4′-Bromoacetophenone/phenylboronic acid/co-catalyst (if used)/base/solvents: 1/1.2/0.6/1/10 mL, under thermal heating at 100 °C for 3 h.
The decision of choice of a solvent for palladium-catalysts is of the most important controlling parameters, specifically its complexing properties. From environmental point of view, H2O as an eco-friendly solvent and K2CO3 as a cheap base and considered as the common base for carrying out all the SMC of aryl bromides in the presence of TBAB as a co catalyst that were used as optimized conditions here this work.
2.2.2. SMC using precatalyst 4 under microwave irradiation (MW)
Using the optimized conditions, the model cross-coupling reaction in water was carried out under microwave conditions (MW) at different MW power as shown in Fig. 9 and Table 4. The cross coupling reaction 6a and phenylboronic acid (5) was carried out using the precatalyst 4 (0.75 mol%) with a molar ratio of 6a/5/TBAB/K2CO3: 1/1.2/0.6/1 to give full conversion and 98% yield of 4-acetyl-1,1′-biphenyl (7a) after only 10 min of microwaves irradiation.
Fig. 9.
Cross-coupling of 4′-bromoacetophenone (6a) with phenylboronic acid (5) under microwaves conditions.
Table 4.
Cross-coupling of 4′-bromoacetophenone (6a) with phenylboronic acid (5) under microwaves conditions using different conditions.
| Entry | Temp. (°C) | Time (min) | μW power (W) | Conversion %a |
|---|---|---|---|---|
| 1 | 100 | 10 | 100 | 92 |
| 2 | 95 | 10 | 100 | 92 |
| 3 | 95 | 10 | 200 | 96 |
| 4 | 95 | 10 | 300 | 97 |
| 5 | 95 | 12 | 300 | 97 |
| 6 | 90 | 10 | 300 | 85 |
| 7 | 95 | 10 | 300 | 100(98)b |
| 8 | 95 | 10 | 400 | 100 |
| 9 | 95 | 10 | 500 | 100 |
Conditions: p-bromoacetophenone/phenylboronic acid/TBAB/K2CO3/water: 1/1.2/0.6/1/10 mL.
Isolated yield.
2.2.3. SMC of different aryl bromides with phenylboronic acid using precatalyst 4 under microwaves irradiation conditions
Applying the optimized conditions (water/K2CO3/TBAB) under microwaves irradiation, SMC between aryl bromides 6b-j and phenylboronic acid (5), using 0.75 mol% of the precatalyst 4, resulted in the corresponding biaryl derivatives in moderate to excellent yields as shown in Fig. 10 and Table 5. Cross coupling of 1-bromonaphthalene 6i with the phenylboronic acid (5) afforded the corresponding 1-phenyl naphthalene in lower yield than the other aryl bromides (Table 5). The obtained results showed the reasonable catalytic efficiency of the precatalyst 4 towards Suzuki cross-coupling of aryl bromides 6b-j with phenylboronic acid (5).
Fig. 10.
Suzuki cross-coupling of aryl bromides 6b-j with phenylboronic acid (5) using the prcatalyst 4 under microwaves conditions.
Table 5.
Yield % of Suzuki cross-coupling of aryl bromides 6b-j with phenylboronic acid (5) using the prcatalyst 4 under microwaves conditions.
| Compd. No. | Ar | Isolated yield% |
|---|---|---|
| 6b, 7b | -C6H4-2-COCH3 | 80 |
| 6c, 7c | -C6H4-4-OCH3 | 84 |
| 6d, 7d | -C6H4-2-OH | 61 |
| 6e, 7e | -C6H4-4-OH | 98 |
| 6f, 7f | -C6H4-4-CF3 | 60 |
| 6g, 7g | -C6H4-2-CHO | 80 |
| 6h, 7h | -C6H4-4-CHO | 96 |
| 6i, 7i | -1-Naphyl | 40 |
| 6j, 7j | -6-Naphyl-2-OH | 92 |
Conditions: Aryl bromide/phenylboronic acid/TBAB/K2CO3/water: 1/1.2/0.6/1/10 mL, microwave heating (300 W) at 95 °C for 10 min.
3. Experimental
All melting points were measured on a Gallenkamp melting point apparatus. The infrared spectra were recorded in potassium bromide discs on a Pye Unicam SP 3–300 and Shimadzu FT IR 8101 PC infrared spectrophotometers. The NMR spectra were recorded in dimethyl sulfoxide (DMSO-d6) or CDCl3.on a Varian Mercury VXR-300 NMR spectrometer. Chemical shifts were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer at 70 eV. The Microwave irradiation was carried out on a CEM mars machine. CEM has several vessel types that are designed for their ovens: closed-system vessels including the HP-500 (500 psig material design pressure and 260 °C), have liners are composed of PFA and are ideal for many types of samples. HP-500 Plus vessels are ideal for routine digestion applications. Process up to 14 high-pressure vessels per run with temperatures up to 260 °C or pressures up to 500 psi.
3.1. Synthesis
3.1.1. Preparation of N,N-dimethyl-N'-(pyrimidin-2-yl)formimidamide 3
A mixture of 2-aminopyrimidine (1) (5 mmol) and DMF-DMA (2) (10 mmol) in benzene (30 mL) was refluxed for 6 hours. After the reaction was complete, the mixture was cooled in room temperature and the white product of low melting was separated. Purification of the product have been achieved by flash column chromatography using chloroform as eluent to give 3 (75 % yield). IR (KBr) 1634, 1585, 1570, 1397, 1283 cm−1. 1H NMR (CDCl3) δ 3.01 (s, 3H, CH3), 3.11 (s, 3H, CH3), 6.91 (t, 1H, J = 4.8 Hz, C5-H pyrimidine), 8.47 (d, 2H, J = 4.8 Hz, C4-H and C6-H pyrimidine), 8.60 (s, 1H, CH-formamidine); MS m/z (%) 150 (M+).
3.1.2. Synthesis of the palladium (II)-complex 4
A methanolic solution of sodium tetrachloropalladate (1 mmol) was added dropwise to a stirred solution of the formamidine 3 (1 mmol) in methanol (10 mL). After stirring for 1 h at room temperature, the green precipitate was filtered off, washed with methanol and dried. The complex 4 was obtained as green powder (80%). Mp. 239 °C; IR (KBr) 1624, 1550, 1534, 1490, 1386, 1302, 819, 526, 430 cm−1. 1H NMR (DMSO-d6) δ 3.28 (s, 6H, 2-CH3), 7.0–7.20 (m, 1H, C5-H pyrimidine), 8.50 (d, 1H, J = 5.4 Hz, C4-H pyrimidine), 8.60 (d, 2H, J = 5.1 Hz, C6-H pyrimidine), 8.78 (s, 1H, CH-formamidine).
3.1.3. Suzuki coupling of aryl bromides
3.1.3.1. Effect of concentration of the Pd-complex 4 on the Suzuki coupling of 4-bromoacetophenone (6a) with phenylboronic acid (5) in water under thermal conditions
A mixture of 4′-bromoacetophenone (6a) (199 mg, 1 mmol) and phenylboronic acid (5) (146 mg, 1.2 mmol), tetrabutylammonium bromide (TBAB) (194 mg, 0.6 mmol), palladium complex 4 (4.03 mg, 1 mol%), K2CO3 (138 mg, 1 mmol) and water (10 mL) was refluxed for 3 h at 100 °C to give 4-acetyl-1,1′-biphenyl (7a). The same experiment was repeated using palladium complex 4 in (0.75, 0.5, 0.25, 0.125, 0.05 and 0.00) mol%. The molar ratio of the reaction components were in all cases as follows; 4-bromoacetophenone, phenylboronic acid, TBAB, K2CO3, water: 1/1.2/0.6/1/10 mL water. The yield% versus concentration of palladium complex 4 is outlined in Table 2.
4-Acetyl-1,1′-biphenyl (7a): Colorless solid; mp. 118–120 °C (Lit. [43] mp. 119–120 °C). 1H NMR (CDCl3): δ = 2.65 (s, 3H, COCH3), 7.27–7.51 (m, 3H), 7.63–7.65 (d, 2H), 7.68–7.71 (d, 2H), 8.03–8.06 (d, 2H). MS (EI, 70 eV): m/z (%) = 196 [M+].
3.1.3.2. Effect of base and solvent on Suzuki cross-coupling of 4-bromoacetophenone (6a) with phenylboronic acid (5) under thermal heating
A mixture of 4-bromoacetophenone (6a) (199 mg, 1 mmol) and phenylboronic acid (5) (146 mg, 1.2 mmol), TBAB (194 mg, 0.6 mmol) (in case of using water as a solvent), Pd-complex 4 (0.75 mol%), a base (1 mmol) and solvent (10 mL) was stirred under reflux in open air for 3 hour to give acetyl-1,1′-biphenyl (7a). The molar ratio of the reaction components were, in all cases, as follows; 4-bromoacetophenone, phenylboronic acid, tetrabutylammonium bromide (in case of water), base, solvent: 1/1.2/0.6/1/10 ml. The yield % versus different solvents and bases is outlined in Table 3.
3.1.3.3. Suzuki cross-coupling of aryl bromides with phenylboronic acid in water under microwave irradiation
3.1.3.3.1. General procedure
A mixture of the appropriate aryl bromides 6b-j (1 mmol), and phenylboronic acid (5) (146 mg, 1.2 mmol), tetrabutylammonium bromide (194 mg, 0.6 mmol), Pd-complex 4 (0.75 mol %), K2CO3 (138 mg, 1 mmol), and distilled water (10 mL) were mixed in the specified CEM reaction vessel HP-500. The mixture was heated under microwave irradiating conditions at 95 °C and 300 Watt for 10 min. After the reaction was complete (monitored by TLC), the reaction mixture was extracted with ethyl acetate (3 × 20 mL). The combined organic extracts were dried over anhydrous Na2SO4 then filtered and the solvent was evaporated under reduced pressure. The products 7b-j were purified by flash column chromatography as described above. The yields% are outlined in Table 5.
2-Acetyl-1,1′-biphenyl (7b): mp. 54–57 °C (lit [44] mp. 54–57 °C); 1H NMR (CDCl3) δ 2.65 (s, 3H, COCH3); 7.37–7.66 (m, 7H); 7.83 (d, 1H); 8.5 (d, 1H). MS (EI, 70 eV): m/z (%) = 196 [M+].
4-Methoxy-1,1′-biphenyl (7c): mp. 87–88 °C (lit [45] mp. 87–88 °C); 1H NMR (CDCl3) δ 3.87 (s, 3H, -OCH3), 6.99 (d, 2H), 7.27–7.45 (m, 3H), 7.54 (d, 2H), 8.3 (d, 2H).
2-Hydroxy-1,1′-biphenyl (7d): mp. 57–59 °C (lit. [46] mp. 59 °C); 1H NMR (CDCl3)δ 5.4 (s, 1H, OH), 7.02 (d, 1H), 7.26 (d, 1H), 7.40–7.43 (m, 6H), 7.50 (d, 1H). MS (EI, 70 eV): m/z (%) = 170 [M+].
4-Hydroxy-1,1′-biphenyl (7e): mp. 164–166 °C (lit. [47] mp. 164-165); 1H NMR (CDCl3) δ 5.1 (s, 1H, OH), 6.91 (d, 2H), 7.27–7.58 (m, 7H). MS (EI, 70 eV): m/z (%) = 170 [M+].
4-(trifluoromethyl)-1,1′-biphenyl (7f): mp.70–72 °C (lit. [48] mp. 70–72 °C); 1H NMR (CDCl3) δ: 7.27–7.63 (m, 7H), 8.25 (d, 2H). MS (EI, 70 eV): m/z (%) = 222 [M+].
2-phenyl benzaldehyde (7g): Colorless oil lit [49]; 1H NMR (CDCl3) δ: 7.38–8.06 (m, 9H), 10.0 (s, 1H, CHO). MS (EI, 70 eV): m/z (%) = 182(49.3) [M+].
4-phenyl benzaldehyde (7h): mp. 57–59 °C (lit. [49] mp. 57–59 °C); 1H NMR (CDCl3) δ: 7.43–7.66 (m, 5H), 7.67 (d, 2H), 7.86 (d, 2H), 10.07 (s, 1H, CHO). MS (EI, 70 eV): m/z (%) = 182(49.3) [M+].
1-Phenylnaphthalene (7i): Colorless oil lit [50]; 1H NMR (CDCl3) δ 7.43–7.94 (m, 12H). MS (EI, 70 eV): m/z (%) = 204 [M+].
6-Phenyl-2-naphthol (7j): yellow oil lit [51]; 1H NMR (CDCl3) δ 5.3 (s, 1H, OH), 7.1 4–7.98 (m, 11H). MS (EI, 70 eV): m/z (%) = 220 [M+].
4. Conclusions
We developed a new formamidine-based palladium(II) complex as robust, stable efficient nano-sized catalyst for aqueous Suzuki-Miyaura cross-coupling of aryl bromides with phenylboronic acids under microwave irradiation. The pyrimidyl formamidine-based palladium complex showed a high catalytic activity, high even at low mol% concentrations, in mild reaction conditions.
Declarations
Author contribution statement
Mohamed R. Shaaban: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Thoraya. A. Farghaly: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Afaf Y. Khormi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.de Mejiere A., Diederich F. second ed. John Wiley & Sons; New York: 2008. Metal Catalyzed Cross-Coupling Reactions. [Google Scholar]
- 2.Espinet P., Echavarren A.M. The mechanisms of the Stille reaction. Angew. Chem. Int. Ed. 2004;43:4704–4734. doi: 10.1002/anie.200300638. [DOI] [PubMed] [Google Scholar]
- 3.Cardenas D.J. Advances in functional-group-tolerant metal-catalyzed alkyl–alkyl cross-coupling reactions. Angew. Chem. Int. Ed. 2003;42:384–387. doi: 10.1002/anie.200390123. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002;653:83–90. [Google Scholar]
- 5.Maluenda I., Navarro O. Recent developments in the Suzuki-Miyaura reaction: 2010–2014. Molecules. 2015;20:7528–7557. doi: 10.3390/molecules20057528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibasaki M., Boden C.D., Kojima A. The asymmetric Heck reaction. Tetrahedron. 1997;53:7371–7395. [Google Scholar]
- 7.Laughlin S.T., Bertozzi C.R. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation. Nat. Protoc. 2007;2:2930–2944. doi: 10.1038/nprot.2007.422. [DOI] [PubMed] [Google Scholar]
- 8.Zim D., Gruber A.S., Ebeling G., Dupont J., Monteiro A.L. Sulfur-containing palladacycles: efficient phosphine-free catalyst precursors for the Suzuki cross-coupling reaction at room temperature. Org. Lett. 2000;2:2881–2884. doi: 10.1021/ol0063048. [DOI] [PubMed] [Google Scholar]
- 9.Botella L., Nájera C.A. A convenient oxime-carbapalladacycle-catalyzed Suzuki cross-coupling of aryl chlorides in water. Angew. Chem. Int. Ed. 2002;41:179–181. doi: 10.1002/1521-3773(20020104)41:1<179::aid-anie179>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Yang D., Chen Y.C., Zhu N.-Y. Sterically bulky thioureas as air- and moisture-stable ligands for Pd-catalyzed Heck reactions of aryl halides. Org. Lett. 2004;6:1577–1580. doi: 10.1021/ol049697+. [DOI] [PubMed] [Google Scholar]
- 11.Page P.C.B., Buckley B.R., Christie S.D., Edgar M., Poulton A.M., Elsegood M.R., A McKee V. A new paradigm in N-heterocyclic carbenoid ligands. Organomet. Chem. 2005;690:6210–6216. [Google Scholar]
- 12.Miura M. Rational ligand design in constructing efficient catalyst systems for Suzuki–Miyaura coupling. Angew. Chem. Int. Ed. 2004;43:2201–2203. doi: 10.1002/anie.200301753. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann W.A. N-heterocyclic carbenes: a new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002;41:1290–1309. doi: 10.1002/1521-3773(20020415)41:8<1290::aid-anie1290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Barker J., Kilner M. The coordination chemistry of the amidine ligand. Coord. Chem. Rev. 1994;133:219–300. [Google Scholar]
- 15.Cotton F.A., Daniels L.M., Murillo C.A., Schooler P. Chromium(II) complexes bearing 2,6-substituted N,N′-diarylformamidinate ligands. J. Chem. Soc. Dalton Trans. 2000;0:2001–2005. [Google Scholar]
- 16.Patai S. Wiley; New York: 1975. Chemistry of Amidines and Imidates. [Google Scholar]
- 17.Cotton F.A., Daniels L.M., Matonic J.H., Murillo C.A. Highly distorted diiron (II, II) complexes containing four amidinate ligands. A long and short metal-metal distance. Inorg. Chim. Acta. 1997;256:277–282. [Google Scholar]
- 18.Clerac R., Cotton F.A., Dunbar K.R., Murillo C.A., Wang X. Dinuclear and heteropolynuclear complexes containing Mo24+ units. Inorg. Chem. 2001;40:420–426. doi: 10.1021/ic001002n. [DOI] [PubMed] [Google Scholar]
- 19.Arnold D.I., Cotton F.A., Matonic J.H., Murillo C.A. Bis(N,N′-diphenylformamidine)silver(I) triflate: a three-coordinate silver formamidine compound stabilized by intramolecular hydrogen bonds. Polyhedron. 1997;16:1837–1841. [Google Scholar]
- 20.Mitzi D., Liang K. Synthesis, resistivity, and thermal properties of the cubic perovskite NH2CH=NH2SnI3and related systems. J. Solid State Chem. 1997;134:376–381. [Google Scholar]
- 21.Shaughnessy K.H., DeVasher R.B. Palladium-catalyzed cross-coupling in aqueous media: recent progress and current applications. Curr. Org. Chem. 2005;9:585–604. [Google Scholar]
- 22.Li C.J. Organic reactions in aqueous media with a focus on carbon–carbon bond formations: a decade update. Chem. Rev. 2005;105:3095–3166. doi: 10.1021/cr030009u. [DOI] [PubMed] [Google Scholar]
- 23.Lidström P., Tierney J., Watheyb B., Westmana J. Microwave assisted organic synthesis-a review. Tetrahedron. 2001;57:9225–9283. [Google Scholar]
- 24.Kappe C.O., Stadler A. Wiley-VCH; Weinheim: 2005. Microwaves in Organic and Medicinal Chemistry. [Google Scholar]
- 25.de la Hoz A., Diaz-Ortiz A., Moreno A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005;34:164–178. doi: 10.1039/b411438h. [DOI] [PubMed] [Google Scholar]
- 26.Baran T. Highly recoverable, reusable, cost-effective, and Schiff base functionalized pectin supported Pd (II) catalyst for microwave-accelerated Suzuki cross-coupling reactions. Int. J. Biol. Macromol. 2019;127:232–239. doi: 10.1016/j.ijbiomac.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 27.Baran T. Pd(0) nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki-Miyaura cross coupling reaction and reduction of o-nitroaniline. Carbohydr. Polym. 2018;195:45–52. doi: 10.1016/j.carbpol.2018.04.064. [DOI] [PubMed] [Google Scholar]
- 28.Baran T. Ultrasound-accelerated synthesis of biphenyl compounds using novel Pd(0) nanoparticles immobilized on bio-composite. Ultrason. Sonochem. 2018;45:231–237. doi: 10.1016/j.ultsonch.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Baran N.Y., Baran T., Mentes A. Fabrication and application of cellulose Schiff base supported Pd(II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl. Catal. A. 2017;531:36–44. [Google Scholar]
- 30.Baran T., Sargin I., Kaya M., Mentes A. Green heterogeneous Pd(II) catalyst produced from chitosan-cellulose micro beads for green synthesis of biaryls. Carbohydr. Polym. 2016;152:181–188. doi: 10.1016/j.carbpol.2016.06.103. [DOI] [PubMed] [Google Scholar]
- 31.Baran T. A new chitosan Schiff base supported Pd(II) complex for microwave-assisted synthesis of biaryls compounds. J. Mol. Struct. 2017;1141:535–541. [Google Scholar]
- 32.Baran N.Y., Baran T., Menteş A. Production of novel palladium nanocatalyst stabilized with sustainable chitosan/cellulose composite and its catalytic performance in Suzuki-Miyaura coupling reactions. Carbohydr. Polym. 2018;181:596–604. doi: 10.1016/j.carbpol.2017.11.107. [DOI] [PubMed] [Google Scholar]
- 33.Baran T., Menteş A. Polymeric material prepared from Schiff base based on O-carboxymethyl chitosan and its Cu(II) and Pd(II) complexes. J. Mol. Struct. 2016;1115:220–227. [Google Scholar]
- 34.Baran T., Sargin I., Kaya M., Mentes A., Ceter T. Design and application of sporopollenin microcapsule supported palladium catalyst: remarkably high turnover frequency and reusability in catalysis of biaryls. J. Coll. Interface Sci. 2017;486:194–203. doi: 10.1016/j.jcis.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 35.Darweesh A.F., Shaaban M.R., Farag A.M., Metz P., Dawood K.M. Facile access to biaryls and 2-acetyl-5-arylbenzofurans via Suzuki coupling in water under thermal and microwave conditions. Synthesis. 2010;2010:3163–3173. [Google Scholar]
- 36.Shaaban M.R., Darweesh A.F., Dawood K.M., Farag A.M. Mizoroki-Heck cross-couplings of 2-acetyl-5-bromobenzofuran and aryl halides under microwave irradiation. Arkovic. 2010;2010:208–225. [Google Scholar]
- 37.Althagafi I., El-Metwaly N.M., Elghalban M.G., Farghaly T.A., Khedr A.M. Synthesis of pyrazolone derivatives and their nanometer Ag(I) complexes and physicochemical, DNA binding, antitumor, and theoretical implementations. Bioinorg. Chem. Appl. 2018;2010:1–15. doi: 10.1155/2018/2727619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gouda A.M., El-Ghamry H.A., Bawazeer T.M., Farghaly T.A., Abdalla A.N., Aslam A. Antitumor activity of pyrrolizines and their Cu(II) complexes: design, synthesis and cytotoxic screening with potential apoptosis-inducing activity. Eur. J. Med. Chem. 2018;145:350–359. doi: 10.1016/j.ejmech.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Dawood K.M., Darweesh A.F., Shaaban M.R., Farag A.M. Microwave-promoted Heck and Suzuki coupling reactions of new 3-(5- bromobenzofuranyl)pyrazole in aqueous media. Arkivoc. 2018:348–358. [Google Scholar]
- 40.Podergajs S., Stanovnik B., Tisler M. A new approach for the synthesis of fused imidazoles: the synthesisof 3-acyl-substituted imidazo[1,2-x]azines. Synthesis. 1984;3:263–265. [Google Scholar]
- 41.Huang Y., Guo Y., Wang Y. Ethylene glycol electrooxidation on core–shell PdCuBi nanoparticles fabricated via substitution and self-adsorption processes. J. Power Sources. 2014;249:9–12. [Google Scholar]
- 42.(a) Mingos D.M.P., Baghurst D.R. Tilden lecture. Applications of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev. 1991;20:1–47. [Google Scholar]; (b) Strauss C.R., Trainor R.W. Developments in microwave-assisted organic chemistry. Aust. J. Chem. 1995;48:1665–1692. [Google Scholar]; (c) Gabriel C., Gabriel S., Grant E.H., Halstead B.S., Mingos D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998;27:213–224. [Google Scholar]; (d) Farina V. High turnover palladium catalysts in cross-coupling and Heck chemistry: a critical overview. Adv. Synth. Catal. 2004;346:1553–1582. [Google Scholar]
- 43.Paizs C., Toşa M., Majdik C., Moldovan P., Novák L., Kolonits P., Marcovici A., Irimie F.-D., Poppe L. Optically active 1-(benzofuran-2-yl)ethanols and ethane-1,2-diols by enantiotopic selective bioreductions. Tetrahedron Asymmetry. 2003;14:1495–1501. [Google Scholar]
- 44.Huber W.F., Renoll M., Rossow A.G., Mowry D.T. Vinyl aromatic compounds. II. o-, m- and p-vinylbiphenyls. J. Am. Chem. Soc. 1946;68:1109–1112. [Google Scholar]
- 45.Zhang G. Ligand-free Suzuki-Miyaura reaction catalysed by Pd/C at room temperature. J. Chem. Res. 2004;2004:593–595. [Google Scholar]
- 46.Duan S., Xu Y., Zhang X., Fan X. Synthesis of 2,2′-biphenols through direct C(sp2)–H hydroxylation of [1,1′-biphenyl]-2-ols. Chem. Commun. 2016;52:10529–10532. doi: 10.1039/c6cc04756d. [DOI] [PubMed] [Google Scholar]
- 47.Minabe M. Hydrogenation of 4-substituted biphenyls. J. Org. Chem. 1987;52:1745–1748. [Google Scholar]
- 48.Fodor A., Magyar A., Barczikai D., Pirault-Roy L., Hell Z. Study of the structure–activity relationship in a heterogeneous copper–palladium catalysed Suzuki–Miyaura coupling. Cat. Lett. 2015;145:834–839. [Google Scholar]
- 49.Peng H., Chen Y.-Q., Mao S.-L., Pi Y.-X., Chen Y., Lian Z.-Y., Meng T., Liu S.-H., Yu G.-A. A general catalyst for Suzuki–Miyaura and Sonogashira reactions of aryl and heteroaryl chlorides in water. Org. Biomol. Chem. 2014;12:6944–6952. doi: 10.1039/c4ob00846d. [DOI] [PubMed] [Google Scholar]
- 50.Kataoka N., Shelby Q., Stambuli J.P., Hartwig J.F. Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C−C, C−N, and C–O bond-forming cross-couplings. J. Org. Chem. 2002;67:5553–5566. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 51.Marchais-Oberwinkler S., Kruchten P., Frotscher M., Ziegler E., Bhoga A., Neugebauer U., Bey E., Müller-Vieira U., Messinger J., Thole H. Substituted 6-phenyl-2-naphthols. Potent and selective nonsteroidal inhibitors of 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1): design, synthesis, biological evaluation, and pharmacokinetics. J. Med. Chem. 2008;51:4685–4698. doi: 10.1021/jm800367k. [DOI] [PubMed] [Google Scholar]