Abstract
Soy consumption in human diet has been linked to decreased incidence of a variety of cancers, suggesting a potential role of soy products in cancer prevention and control. Furthermore, a substantial body of evidence in the literature suggests that soy supplementation may improve the efficacy and prevent the adverse effects of cancer chemotherapy and radiation therapy. Isoflavones constitute the predominant anticancer bioactive compounds in soy. Genistein, which is the most abundant and active isoflavone in soy, has a multitude of effects on cancer cells, including inhibition of NF-κB activation and DNA methylation, enhancement of histone acetylation, inhibition of cell growth and metastasis, and antiangiogenic, anti-inflammatory, and anti-oxidant effects. Isoflavones are orally bioavailable, easily metabolized, and usually considered safe. In this article, we review in vitro and in vivo evidence as well as the results of clinical and epidemiological studies on the effects of soy isoflavones, with a focus on sensitization of cancer cells to chemotherapy and radiation while at the same time protecting normal cells from the harmful effects of these treatments.
Keywords: prostate cancer, genistein, chemotherapy, radiotherapy
Introduction
Many studies have been conducted to investigate the interaction of dietary constituents and antineoplastic therapies in both animal models and clinical trials.1 In particular, soy isoflavones have been suggested to have cancer preventive effects.2 Epidemiological studies have shown a significant difference in cancer incidence among different ethnic groups, which could be attributed in part to dietary habits. For example, the incidence of breast cancer and prostate cancer (PCa) are much higher in the United States and Europe where dietary soy intake is low as compared with countries such as China and Japan, where dietary consumption of soy products is much higher. Genistein, a predominant isoflavone in soy, has been shown to inhibit cancer development, growth, and metastasis in animal models. It may act by modulating the genes related to cell cycle control and apoptosis.3
Chemotherapy is a common form of treatment for many types of cancer. Chemotherapeutic drugs are designed to interfere with rapidly dividing cells such as cancer cells. This means that they may also harm normal cells that divide normally—that is, the cells in the bone marrow, mouth, gastrointestinal tract, nose, nails, vagina, and hair cells, which undergo constant division making them vulnerable to the toxic effects of chemotherapy as well as radiotherapy. Therefore, major research efforts have been aimed at discovering ways of protecting normal proliferating cells from cancer therapy. Many chemotherapeutic agents work by producing free radicals or reactive oxygen species (ROS) which, if not quenched, can damage the cell and its various constituents. However, these species also affect the normal cells. Detoxification of free radicals and ROS is, therefore, important and opens up avenues for the use of natural products, most of which have antioxidant properties, useful in the prevention and control of cancer.4-6 Inflammation is another hallmark of cancer, and it has been reported in various studies that its inhibition could be beneficial in cancer. In this review, we discuss the effect of soy isoflavones, well known for their antioxidant and anti-inflammatory effects, as potential supplements during conventional cancer therapy, with a special focus on PCa therapy.
Soy Isoflavones: Structure and Metabolism
Isoflavones are polyphenolic plant-derived compounds that have both estrogenic (estrogen agonist) and antiestrogenic (estrogen antagonist) activity. They are the major flavonoids in legumes, particularly soybean, where they are present bound to sugars (glycosides). Soy isoflavones include genistein, daidzein, and glycitein. These compounds are structurally characterized by their 3-phenylchromen-4-one backbone, which consists of 2 benzene rings linked by a heterocyclic pyran ring. In addition to the heterocyclic core, genistein and its related isoflavones are polyphenols (contain several hydroxyl groups attached to core phenyl rings). Genistein, or 4′,5,7-trihydroxyisoflavone and daidzein are the most prominent isoflavones in soy products. The structure of genistein is shown in Figure 1. Soybeans and soy products constitute the richest source of isoflavones in human diet.
Figure 1.
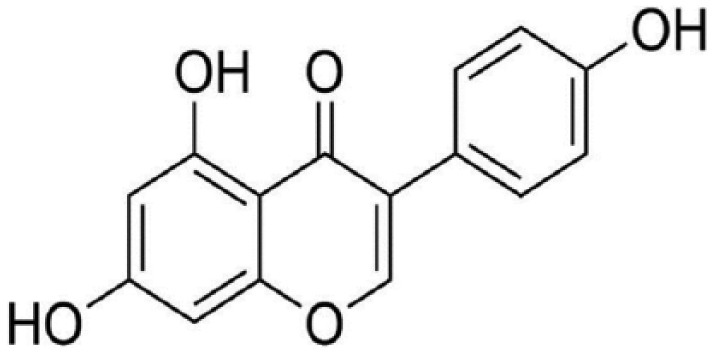
Genistein, 4′,5,7-trihydroxyisoflavone.
Genistein can also be synthesized, but humans obtain it from the diet. In soy and soy products, the primary chemical form of genistein exists in the conjugated form known as genistin.7 Therefore, people are exposed to genistin far more than genistein when consuming soy and soy products in their diet. Genistein undergoes first-pass metabolism in the liver as well as enterohepatic circulation after absorption from the intestine. The byproducts of isoflavone metabolism may produce different effects on the body.8 Studies showed that 90% of genistein in the blood undergoes extensive liver metabolism and exists in either the glucuronidated or sulfated form, whereas only 10% exists in the nonconjugated or free form.9
Soy Isoflavones in Cancer Therapy: Effects and Mechanisms
Soy, soybean, or soya bean, is a native East Asian legume, which is widely grown for its edible beans. The beans and its products have been reported to have beneficial effects in cancer, which is largely attributed to the presence of isoflavones.10-14 In a recent meta-analysis of 30 articles, a clear statistically significant association between soy consumption and decreased PCa risk was reported.14 Populations in countries like China and Japan, which consume soy foods as part of the regular diet, showed low incidence of PCa. In the analysis of the potential impacts of soy food and isoflavone intake and that of the circulating isoflavones, in both primary and advanced PCa, total soy food (P < .001), genistein (P = .008), daidzein (P = .018), and unfermented soy food (P < .001) intakes were found to be significantly associated with decreased risk of PCa. Neither soy food intake, nor circulating isoflavones were associated with advanced PCa risk.14 The meta-analysis provided a comprehensive updated analysis of previously published data demonstrating that soy foods/isoflavones were associated with reduced PCa risk.
Genistein is one of the most predominant and active flavonoids in soybeans. It can alter a variety of biological processes in estrogen-related malignancies, which include PCa, and has been found to act mainly by altering apoptosis, the cell cycle, and angiogenesis and inhibiting metastasis. The molecular mechanisms of the anticancer and therapeutic effects of genistein has been suggested to involve caspases, B cell lymphoma 2 (Bcl2)-associated X protein, Bcl-2, kinesin-like protein 20A, extracellular signal-regulated kinase 1/2, nuclear transcription factor κB (NF-κB), mitogen-activated protein kinase, inhibitor of NF-κB, Wingless and integration 1 β-catenin, and phosphoinositide 3 kinase/Akt signaling pathways.2,15 Its inhibitory effect on NF-κB is particularly important because the inhibition suppresses inflammation.16 The NF-κB and the serine/threonine-specific protein kinase Akt both maintain a homeostatic balance between cell survival and apoptosis. Interestingly, genistein has also been reported to be a potent angiogenesis and metastasis inhibitor, which is promising in cancer prevention, control, and treatment. Genistein also shows synergistic behavior with anticancer drugs, such as doxorubicin, docetaxel, and tamoxifen, suggesting a potential role in combination therapy.15
In colon cancer in humans, the soy isoflavones, especially genistein, inhibit cell growth and facilitate apoptosis and cell cycle arrest in the G2/M phase.3 The cell cycle arrest in the G2/M phase is accompanied by the activation of ATM/p53, p21waf1/cip1 and GADD45α as well as downregulation of cdc2 and cdc25A, as demonstrated by quantitative polymerase chain reaction and immunoblotting.3 Interestingly, genistein induced G2/M cell cycle arrest in a p53-dependent manner and suggested the crucial role of the ATM/p53-p21 cross-regulatory network in mediating the anticarcinogenic effect of genistein. In another study, genistein was found to cooperate with the histone deacetylase inhibitor vorinostat to induce cell death in PCa cells.17 Recently, this important isoflavonoid has been reported to synergistically increase radiosensitivity of PCa cells. Earlier, the compound was reported to enhance the efficacy of cancer therapy by sensitizing cancer cells to chemotherapy18-20 and also radiotherapy.21-25 At the same time, the antioxidant26-28 and anti-inflammatory29-33 properties of isoflavones add to their beneficial effects in cancer.
Soy isoflavones, mainly genistein, have been reported to have a number of biological effects other than their effects in cancer. These have been summarized in Table 1.
Table 1.
Miscellaneous Effects of Soy Isoflavones.
| Property | Effect | Reference |
|---|---|---|
| Free radical scavenging effect | Protects cells in central nervous system | Wei et al61 |
| Protection against oxidative modification of LDL | Tikkanen et al62 | |
| Protection of human cortical neuronal HCN1-A and HCN2 cells | Ho et al63 | |
| Protection of primary cortical neurons from iron-induced free radical reaction and lipid peroxidation | Sonee et al64 | |
| Protection of dopaminergic neurons from lipopolysaccharide-induced injury by inhibiting microglia activation | Wang et al65 | |
| Rescue human amniotic fluid mesenchymal stem cells and Schwann cells from apoptosis by suppressing the macrophage deposits, associated inflammatory cytokines, and fibrin deposits | Pan et al66 | |
| Prevention of endoplasmic reticulum stress-mediated neurotoxicity by inhibiting tau hyperphosphorylation in SH-SY5Y cells | Park et al67 | |
| Alleviation of the endoplasmic reticulum stress-mediated and DNA damage–mediated neurodegeneration caused by homocysteine | Park et al68 | |
| Anti-inflammatory | Suppression of lipopolysaccharide-induced inflammation in rat liver | Zhao et al69 |
| Effect on immune system | Immunomodulation | Sakai and Kogiso70 |
| Antiviral | Antiviral properties in vitro and in vivo against a wide range of viruses | Andres et al71 |
| Tyrosine kinase inhibitor | Tyrosine kinase inhibition | Akiyama et al72 |
| Effect on vascular system | Prevention of atherosclerosis and related vascular events | Holzer et al73 |
| Antihypertensive | Attenuation of hypertension, targeting the kidney to increase renal blood flow, sodium excretion | Martin et al74 |
| Osteopenia | Positive effect in cyclosporin A–induced osteopenia only in sites with high turnover and improvement of the osteoprotective effect of l-arginine | Clementi et al75 |
| Epidermal hyperplasia | Reduction in epidermal hyperplasia caused by topical retinoid treatment | Rittie et al76 |
| Hepatoprotective effect | Effect via suppression of necrosis of hepatocytes and the cellular infiltration in liver parenchyma and prevention of the development of fatty and protein dystrophy in the liver and normalization of the activity of aminotransferases | Saratikov et al77 |
| Drug toxicity | Protection of post–neural tube closure defects of rodents induced by cyclophosphamide | Zhao et al78 |
| Protection of normal and cancer cells against genotoxic potential of tamoxifen | Wozniak et al79 | |
| Radiation injury | Protection against acute radiation injury | Landauer et al80 |
Abbreviation: LDL, low-density lipoprotein.
Isoflavones as Potential Candidates in the Prevention and Control of PCa
Epidemiological studies have shown the beneficial effects of soy isoflavones in breast, prostate, and colon cancers in countries such as China and Japan, where the dietary consumption of soy isoflavones is high.34 A recent randomized, double-blind phase 2 clinical study that aimed to investigate the effect of synthetic genistein concentrations and its safety in patients with localized PCa showed that genistein at a dose that can be easily obtained from a diet rich in soy reduced the level of serum prostate-specific antigen (PSA) with no adverse effects of clinical significance.35 A comprehensive meta-analysis on the extent of the possible association between soy-based food consumption and the risk of PCa indicated an inverse relationship between dietary soy isoflavone (genistein and daidzein) consumption and PCa incidence and mortality.36 Nutritionally relevant levels of genistein may modulate the expression of prostate tissue biomarkers associated with cancer prediction and progression.37 In various studies, of all the isoflavones investigated, genistein appears to be the most potent in the prevention and control of cancers, which makes it a promising molecule in PCa.
The use of soy isoflavones has also been considered in combination with other natural products, such as lycopene.38 Dietary intake of lycopene and soy lowers the PCa risk. In vitro studies with lycopene and genistein showed an increase in apoptosis and inhibition of cell growth in androgen-sensitive (LNCaP) and androgen-independent (PC3 and VeCaP) PCa cell lines. PSA stabilization has been observed with soy isoflavone intake in PCa patients.10 Vaishampayan et al38 enrolled 71 patients who had rising PSA levels after failing radical prostatectomy and/or radiation therapy with or without hormone therapy. Participants were randomly assigned to receive a tomato extract capsule containing 15 mg of lycopene alone (n = 38) or together with a capsule containing 40 mg of a soy isoflavone mixture (n = 33) twice daily orally for a maximum of 6 months. There was no decline in serum PSA in either group qualifying for a partial or complete response. However, 35 of 37 (95%) evaluable patients in the lycopene group and 22 of 33 (67%) evaluable patients in the lycopene plus soy isoflavone group achieved stable disease, described as stabilization in serum PSA level. Data suggested that lycopene and soy isoflavones had activity in PCa patients and may delay progression of both hormone-refractory and hormone-sensitive PCa. However, the effect was not additive. Importantly, a recent phase 2 dose-escalating study in PCa patients examined plasma, prostate, and urine biomarkers of carotenoid and isoflavone exposure and described foundation for tomato-soy juice in future human clinical trials.39 Investigating the efficacy of more compounds of natural origin might be a fruitful area of research in PCa control and treatment. Clinical trials with a mechanistic approach could elucidate potential clinical uses of natural compounds.
Soy Isoflavones in Chemotherapy Toxicity
Chemotherapy often results in the generation of ROS in excess, and thus the oxidative stress, which is evidenced by increased lipid peroxidation and decrease in the levels of total radical-trapping capacity of the tissue and body fluids.40-42 The anthracyclines generate by far the highest levels of oxidative stress. The cytochrome P450 monooxygenase system is the primary site of the generation of ROS during cancer chemotherapy.43 It is important to highlight that the drugs that do not depend on the generation of ROS in their mechanism of action can only mediate their anticancer effects on cancer cells that exhibit unrestricted progression through the cell cycle and have intact apoptotic pathways.44 However, oxidative stress can interfere with chemotherapy-induced apoptosis and with cell cycle progression by inhibiting the transition of cells from the G0 to G1 phase, slowing the progression through the S phase by inhibition of DNA synthesis, inhibiting cell cycle progression of the G1 to S phase, and checkpoint arrest. By reducing oxidative stress, antioxidants may counteract the effects of chemotherapy-induced oxidative stress on cell cycle and apoptosis. Thus, antioxidants may enhance the cytotoxicity of antineoplastic drugs.5 In contrast, antioxidants might also protect cancer cells against the oxidative damage induced by chemotherapy, which suggests a harmful effect as a result of their use.1 Antioxidants may decrease chemotherapy-induced damage of normal cells by reducing lipid peroxidation or halting cancer cell proliferation.6 In their study, Block et al45,46 systematically reviewed randomized controlled clinical trials evaluating the effects of concurrent use of antioxidants in chemotherapy on toxic side effects. The review suggested that antioxidant supplementation may reduce the toxic effects of ROS-generating chemotherapies. Additionally, analysis suggested that concurrent use of supplements and chemotherapy might produce better tumor response rates and increased chances of survival, although small sample size and low quality of studies precluded firm conclusions.
Chemotherapeutic drugs are designed to interfere with rapidly dividing cells and kill cancer cells as well as normal cells, such as healthy intestinal mucosal cells, because of their rapid rate of division. Methotrexate (MTX) is one of the common antineoplastic agents that destroys mucosal cells and may result in mucositis, stomatitis, diarrhea, decreased nutrient absorption, translocation of gastrointestinal bacteria, and anorexia.47-49 Various soy products have been shown to provide dramatic protection against MTX toxicity in animal models48,50-52 Funk and Baker52 investigated isolated soybean components in a semipurified diet and showed that they alter MTX toxicity and that soybean meal and soybean concentrate offered the greatest protection, completely alleviating MTX-induced anorexia and diarrhea when included as sole protein source and fed 14 days prior to and 7 days following intraperitoneal MTX injection at 20 mg/kg body weight. In addition, although the rats fed casein-based semipurified diet had necrotic intestine, those fed the soybean-containing complex diet or semipurified diet containing soybean concentrate or soybean isolate showed no signs of necrosis in any part of the small intestine. In a different study,51 the same group aimed to determine whether MTX toxicity associated with a casein-based semipurified diet could be ameliorated or prevented by adding fiber or by replacing casein with other protein source. Results of the study showed that addition of amorphous cellulose to semipurified casein-based diet slightly reduced toxicity symptoms. Addition of crystalline cellulose, hemicellulose, and pectin did not lessen toxicity symptoms, whereas replacing casein with soybean concentrate totally alleviated the toxicity symptoms. These findings suggested that it may be possible to develop soybean-based enteral products that would minimize gastrointestinal toxicity experienced by cancer patients undergoing MTX chemotherapy.
Chevreau and Funk-Archuleta,50 indicated that soy concentrate was superior in alleviating MTX toxicity compared with commercial enteral products. Rats fed soy concentrate maintained food intake above 90% of preinjection levels, which was greater than all other groups at day 3 and those receiving hydrolyzed or intact casein without fiber on day 4 (P < .05). The soy concentrate group also gained more weight when compared with other groups fed hydrolyzed or intact casein without fiber (P < .05) and had no diarrhea. The same study showed that crypt necrosis occurred in all groups except those consuming the soy concentrate diet or enteral product containing soy fiber. Funk (52) investigated the ability of a soy-derived antiapoptotic fraction that could inhibit apoptosis in an in vitro assay to inhibit MTX-induced gastrointestinal toxicity in rats. Rats fed high doses of the soy-derived antiapoptotic fraction–supplemented diets experienced significantly less weight loss and diarrhea and better food intake (P < .05). They also performed mouse embryonic C3H10T1/2 cell apoptosis assay and demonstrated that soy-derived antiapoptotic fraction was a potent inhibitor of apoptosis. It is well known that many chemotherapeutic agents, including MTX, increase the incidence of apoptosis, particularly in the gastrointestinal tract.53 Thus, the studies suggested that the mechanism behind the protection of undesirable gastrointestinal MTX toxicity by soy-derived antiapoptotic fraction was likely to be a result of reduction of apoptotic cell death.
Similarly, despite the beneficial chemotherapeutic effects of bleomycin on cancer cells, cytotoxicity and genotoxicity of bleomycin on normal cells persists as a major problem in chemotherapy. Lee et al12 demonstrated a number of effects of genistein pretreatment on the toxicity of bleomycin in normal lymphocytes and human leukemia (HL-60) cells. It increased the frequencies of micronuclei in HL-60 cells, decreased the frequencies of micronuclei in human lymphocytes during G0 and G2, increased DNA damage in HL-60 cells, and reduced DNA damage in blood lymphocytes. As a result, dual antagonistic effects of genistein were observed from this study—genistein enhanced the bleomycin-induced cytotoxicity in HL-60 cells, whereas it protected normal blood lymphocytes. In another study, cisplatin was tested in vitro in human lymphocytes for its toxicity. This study showed that cisplatin when combined with genistein considerably reduced the genotoxicity, possibly because of the free radical scavenging activity of genistein.54
The results of the in vitro and in vivo studies supported a novel chemotherapy strategy for treating cancer patients by concurrent administration of chemotherapy with soy isoflavones. Results of a clinical trial conducted by Tacyildiz et al55 demonstrated that genistein reduced the adverse effects of chemotherapy in pediatric cancer patients. In this study, 9 cycles of chemotherapy were administered without genistein supplementation, whereas 57 cycles were administered with genistein supplementation. Patients served as their own controls, and the clinical-laboratory data from the first cycle were compared with the data from subsequent cycles. Chemotherapy doses and schedules between first and subsequent cycles remained the same. Genistein levels were 2 to 6 times higher (range = 0.215-0.411 mg/L; median = 0.258 mg/L) during genistein supplementation compared with the no supplementation period (range = 0.058-0.111 mg/L; median = 0.061 mg/L). The results of the study showed that there was less myelosuppression, oral mucositis, infections, and requirement of blood products during the cycles given with genistein supplementation. In addition, 3 children receiving genistein during abdominal radiotherapy experienced less pain and no diarrhea, which is a common side effect of abdominal radiation. Studies with soy products and chemotherapy toxicities are summarized in Table 2.
Table 2.
Studies With Soy Products and Chemotherapy Toxicities.
| Agents | Toxicities | Treatment | Results | Reference |
|---|---|---|---|---|
| Methotrexate | Gastrointestinal toxicity | 34 SD rats; groups; control group (casein), casein + soy fraction (0.164% of diet) group, casein + soy fraction (0.493% of diet), casein + soy fraction (1.643% of diet) | Improved food intake P < .05, weight gain P < .05, decreasing incidence of diarrhea P < .05 | Funk-Archuleta et al48 |
| Bleomycin | Cytotoxicity and genotoxicity | Human blood lymphocytes obtained from healthy women and human leukemia cell line HL-60 cells (KCLB 10240) pretreated with genistein followed by bleomycin | Enhanced bleomycin-induced cytotoxicity in human leukemia (HL-60) while protecting normal blood lymphocytes | Lee et al12 |
| Cisplatin | Genotoxicity | Human lymphocyte culture of 2 healthy donors treated with cisplatin only and in combination with genistein and gingerol separately in the presence of a metabolic activation system | Reduced genotoxicity because of the free radical scavenging activity of genistein | Beg et al54 |
| Various combination chemotherapy regimens + radiotherapy | Various toxicities | 8 Pediatric patients with cancer (served as their own controls); 9 cycles of chemotherapy without soy isoflavone, 57 cycles of chemotherapy with soy isoflavones; chemotherapy doses and schedules same | Genistein levels were 2 to 6 times higher (range = 0.215-0.411 mg/L; median = 0.258 mg/L) during genistein supplementation compared with the no supplementation period (range = 0.058-0.111 mg/L; median = 0.061 mg/L); genistein supplementation: less myelosuppression (shorter duration of neutropenia), oral mucositis, infections (shorter duration of antibiotic use), blood product requirements; no diarrhea during abdominal radiotherapy | Tacyildiz et al55 |
| Etoposide | Alopecia | 10 SD rats; etoposide injected daily in 11-day-old SD rats at 1.2 mg/kg ip for 3 days, 5 days before the first injection of etoposide, soymetide-4 orally for 8 days concomitantly with indomethacin, AH23848B, pyrilamine, cimetidine, and PDTC | Oral administration soymetide-4: suppression of alopecia induced by etoposide in neonatal rat models | Tsuruki et al81 |
| Methotrexate | Gastrointestinal toxicities | Rats; 5 enteral products containing casein or soy isolate in various forms to 10 rats for 7 days before injection and 7 days after injection of MTX (20 mg/kg) | Soy concentrate diet consumption; maintained food intake above 90% of preinjection levels, which was greater than all other groups at day 3 and those receiving hydrolyzed or intact casein without fiber on day 4 (P < .05), no diarrhea, weight gain when compared with other groups fed hydrolyzed or intact casein without fiber (P < .05), crypt necrosis (in intestine) occurred in all groups except those consuming the soy concentrate diet | Chevreau and Funk-Archuleta50 |
| Methotrexate | Gastrointestinal toxicities | Male SD rats; 5 different experiments; products tested: soybean meal, soybean concentrate, soybean isolate and soybean fiber; 14 days prior to and 7 days following intraperitoneal MTX injection | Soybean meal and soybean concentrate offered the greatest protection, completely alleviating MTX-induced anorexia and diarrhea; soybean concentrate and soybean isolate prevented the necrosis (in the small intestine of MTX-injected animals) observed in animals fed the casein-based semipurified diet | Funk and Baker51 |
| Methotrexate | Gastrointestinal toxicities | Male SD rats; 6 different experiments; products tested: (1) fiber sources, including crystalline cellulose, amorphous cellulose, hemicellulose, and pectin; (2) protein sources, including casein, soybean concentrate, whey isolate, egg albumen, com gluten meal, and hamburger; in experiments 1 to 4, diets for 14 days before MTX injection, experiments 5 and 6 to evaluate time periods prior to or after MTX dosing on toxicity development | Toxicity was lower when 25% of the protein normally supplied by casein was replaced with soybean concentrate, and no toxicity symptoms were present when 50% or more of the protein was provided by soybean concentrate | Funk and Baker52 |
| Cisplatin | Nephrotoxicity | Mice; control (n = 10), genistein (10 mg/kg; n = 10), cisplatin (20 mg/kg; n = 10), and cisplatin plus genistein (n = 10) | Genistein; significantly reduced cisplatin-induced renal injury, ameliorated the cisplatin-induced upregulation of ICAM-1 and MCP-1 expression, resulting in decreased infiltration of macrophages into the kidney, significantly reduced cisplatin-induced generation of ROS and NF-×B activation in HK-2 cells, reduced cisplatin-induced apoptosis in kidney through downregulation of p53 induction | Sung et al82 |
Abbreviations: AH23848B, an antagonist of the PGE2 receptor EP4; HK-2, human kidney; HL-60, human leukemia cells; ICAM-1, immunostaining for intercellular adhesion molecule-1; MCP-1, immunostaining for monocyte chemoattractant protein-1; MTX, methotrexate; NF-κB, nuclear factor-κB; PDTC, pyrrolidine dithiocarbamate ammonium; ROS, reactive oxygen species; SD, Sprague-Dawley.
Bioavailability of Isoflavonoids
Pharmacokinetic studies in healthy adults compared plasma kinetics of pure daidzein, genistein, and their β-glycosides administered as a single-bolus dose to 19 healthy women.56 Results demonstrated differences in the pharmacokinetics of isoflavone glycosides compared with their respective β-glycosides. Even though all isoflavones were efficiently absorbed from the intestine, there were striking differences in the fate of aglycones and β-glycosides. Mean times to attain peak plasma concentrations, tmax for the aglycones genistein and daidzein were 5.2 and 6.6 hours, respectively, whereas for the corresponding glycosides, it was delayed to 9.3 and 9.0 hours, respectively, which was consistent with the residence time needed for hydrolytic cleavage of the glycoside moiety. The apparent volume of distribution of isoflavones confirmed extensive tissue distribution. In this study, the plasma genistein concentration was consistently higher than that of daidzein when equal amounts of the 2 isoflavones were administered, and this was accounted for by more extensive distribution of daidzein (236 L) compared with genistein (161 L). The systemic bioavailability of genistein (mean area under the curve [AUC] = 4.54 µg/[mL h]) was much higher than that of daidzein (mean AUC = 2.94 µg/[mL h]). Bioavail-ability was greater when ingested as β-glycosides rather than as aglycones. The pharmacokinetics of methoxylated isoflavones showed distinct differences depending on the position of the methoxyl group in the molecule. Glycitin, found in 2 phytoestrogen supplements, underwent hydrolysis of the β-glycoside moiety and little further biotransformation, leading to high plasma glycitein concentrations. Biochanin A and formononetin, 2 isoflavones found in 1 phytoestrogen supplement, were rapidly and efficiently demethylated, resulting in high plasma genistein and daidzein concentrations typically observed after the ingestion of soy-containing foods. These differences in pharmacokinetics and metabolism have implications in clinical studies because it cannot be assumed that all isoflavones were comparable in their pharmacokinetics and bioavailability. An analysis of 33 phytoestrogen supplements and extracts revealed considerable differences in the isoflavone content from that claimed by the manufacturers. Plasma concentrations of isoflavones show marked qualitative and quantitative differences depending on the type of supplement ingested.56
Various studies, including in vivo studies, have shown that genistein from soy extracts, its free form, and its glycoside genistin are readily bioavailable.15 Extensive metabolism of genistein in the intestine, and postabsorption, has been documented both in humans and experimental animals. Among the several metabolites identified in the blood and excreta are dihydrogenistein, dihydrodaidzein, 6′-hydroxy-O-desmethylangolensin, 4-ethylphenol, glucuronide and sulfate conjugates of genistein and its metabolites, and 4-hydroxyphenyl-2-propionic acid. The gut microflora cleaves the C-ring of the isoflavonoid skeleton to give 4-hydroxyphenyl-2-propionic acid and dihydrogenistein.57 The metabolism in the gut wall and liver is also known to yield glucuronide and sulfated products.58 Few reports suggest that conjugation plays a role in rapid elimination by biliary and urinary excretion.8
Adverse Effects of Soy Isoflavones
In an analysis of data from PubMed and EMBASE from 1975 to 2015 (articles selected with the search terms isoflavone, phytoestrogen, soy, genistin, and PCa), isoflavones are reported not to play an important role in reducing PSA in PCa patients or healthy men, but the intake of various types of phytoestrogens with lower concentrations in the daily diet was reported to produce synergistic effects against PCa. The analysis suggests that the prostate tissue may concentrate isoflavones to potentially anticarcinogenic levels but cautioned that the isoflavones may also act as an agonist in PCa.59
Phytoestrogens are structurally and functionally analogous to estrogens. Phytoestrogens and their active metabolites such as equol can remain in food/meat and influence the hormonal balance of the consumers. In animals, intake of phytoestrogens may affect fertility, sexual development, and behavior.60
Consumption of soy as dietary supplement may cause mild stomach and intestinal side effects such as constipation, bloating, and nausea and may also cause allergic reactions involving rash, itching, and anaphylaxis in some people.
Conclusion
Chemotherapeutic agents and radiation induce oxidative stress and inflammation, producing side effects in cancer patients. Soy isoflavones, owing to their multiple mechanisms of effects, including the antioxidant and anti-inflammatory effects, may be used as dietary supplements to ameliorate the adverse reactions to anticancer drugs and radiation. At the same time, they may increase the efficacy of cancer chemotherapy and radiation, especially in PCa. The effect of soy isoflavones, particularly genistein, in the prevention and control of PCa has been supported by preclinical studies, meta-analyses, and clinical trials, but larger placebo-controlled clinical trials are needed to investigate the potential use of genistein for amelioration of the adverse effects of anticancer drugs and radiation therapy.
Acknowledgments
SA acknowledges the Indian Council of Medical Research (ICMR) for providing ICMR International Fellowship for Senior Biomedical Scientists 20l8-2019.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. D’Incalci M, Steward WP, Gescher AJ. Modulation of response to cancer chemotherapeutic agents by diet constituents: is the available evidence sufficiently robust for rational advice for patients? Cancer Treat Rev. 2007;33:223-229. [DOI] [PubMed] [Google Scholar]
- 2. Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744-757. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Z, Wang CZ, Du GJ, et al. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013;43:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tabassum A, Bristow RG, Venkateswaran V. Ingestion of selenium and other antioxidants during prostate cancer radiotherapy: a good thing? Cancer Treat Rev. 2010;36:230-234. [DOI] [PubMed] [Google Scholar]
- 5. Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294-300. [DOI] [PubMed] [Google Scholar]
- 6. Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773-783. [DOI] [PubMed] [Google Scholar]
- 7. Fukutake M, Takahashi M, Ishida K, Kawamura H, Sugimura T, Wakabayashi K. Quantification of genistein and genistin in soybeans and soybean products. Food Chem Toxicol. 1996;34:457-461. [DOI] [PubMed] [Google Scholar]
- 8. Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127:1260-1268. [DOI] [PubMed] [Google Scholar]
- 9. Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209-1210. [DOI] [PubMed] [Google Scholar]
- 10. Hussain M, Banerjee M, Sarkar FH, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111-117. [DOI] [PubMed] [Google Scholar]
- 11. Sarkar FH, Li Y. The role of isoflavones in cancer chemoprevention. Front Biosci. 2004;9:2714-2724. [DOI] [PubMed] [Google Scholar]
- 12. Lee R, Kim YJ, Lee YJ, Chung HW. The selective effect of genistein on the toxicity of bleomycin in normal lymphocytes and HL-60 cells. Toxicology. 2004;195:87-95. [DOI] [PubMed] [Google Scholar]
- 13. Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628-1635. [DOI] [PubMed] [Google Scholar]
- 14. Applegate CC, Rowles JL, Ranard KM, Jeon S, Erdman JW. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients. 2018;10:E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spagnuolo C, Russo GL, Orhan IE, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67-79. [DOI] [PubMed] [Google Scholar]
- 17. Phillip CJ, Giardina CK, Bilir B, et al. Genistein cooperates with the histone deacetylase inhibitor vorinostat to induce cell death in prostate cancer cells. BMC Cancer. 2012;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934-6942. [DOI] [PubMed] [Google Scholar]
- 19. Khoshyomn S, Manske GC, Lew SM, Wald SL, Penar PL. Synergistic action of genistein and cisplatin on growth inhibition and cytotoxicity of human medulloblastoma cells. Pediatr Neurosurg. 2000;33:123-131. [DOI] [PubMed] [Google Scholar]
- 20. Sahin K, Tuzcu M, Basak N, et al. Sensitization of cervical cancer cells to cisplatin by genistein: the role of NFkappaB and Akt/mTOR signaling pathways. J Oncol. 2012;2012:461562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hillman GG, Forman JD, Kucuk O, et al. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin Cancer Res. 2001;7:382-390. [PubMed] [Google Scholar]
- 22. Hillman GG, Wang Y, Kucuk O, et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271-1279. [PubMed] [Google Scholar]
- 23. Raffoul JJ, Banerjee S, Che M, et al. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120:2491-2498. [DOI] [PubMed] [Google Scholar]
- 24. Yashar CM, Spanos WJ, Taylor DD, Gercel-Taylor C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol Oncol. 2005;99:199-205. [DOI] [PubMed] [Google Scholar]
- 25. Tang Q, Ma J, Sun J, et al. Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol Rep. 2018;40:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001;172:1-6. [DOI] [PubMed] [Google Scholar]
- 27. Kapiotis S, Hermann M, Held I, Seelos C, Ehringer H, Gmeiner BM. Genistein, the dietary-derived angiogenesis inhibitor, prevents LDL oxidation and protects endothelial cells from damage by atherogenic LDL. Arterioscler Thromb Vasc Biol. 1997;17:2868-2874. [DOI] [PubMed] [Google Scholar]
- 28. Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63-70. [DOI] [PubMed] [Google Scholar]
- 29. Blay M, Espinel AE, Delgado MA, et al. Isoflavone effect on gene expression profile and biomarkers of inflammation. J Pharm Biomed Anal. 2010;51:382-390. [DOI] [PubMed] [Google Scholar]
- 30. Duan W, Kuo IC, Selvarajan S, Chua KY, Bay BH, Wong WS. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am J Respir Crit Care Med. 2003;167:185-192. [DOI] [PubMed] [Google Scholar]
- 31. Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702-4709. [DOI] [PubMed] [Google Scholar]
- 32. Hall WL, Vafeiadou K, Hallund J, et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr. 2005;82:1260-1268. [DOI] [PubMed] [Google Scholar]
- 33. Kang JS, Yoon YD, Han MH, et al. Estrogen receptor-independent inhibition of tumor necrosis factor-alpha gene expression by phytoestrogen equol is mediated by blocking nuclear factor-kappaB activation in mouse macrophages. Biochem Pharmacol. 2005;71:136-143. [DOI] [PubMed] [Google Scholar]
- 34. Gescher A, Pastorino U, Plummer SM, Manson MM. Suppression of tumour development by substances derived from the diet—mechanisms and clinical implications. Br J Clin Pharmacol. 1998;45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lazarevic B, Boezelijn G, Diep LM, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr Cancer. 2011;63:889-898. [DOI] [PubMed] [Google Scholar]
- 36. Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598-606. [DOI] [PubMed] [Google Scholar]
- 37. Lazarevic B, Hammarstrom C, Yang J, et al. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br J Nutr. 2012;108:2138-2147. [DOI] [PubMed] [Google Scholar]
- 38. Vaishampayan U, Hussain M, Banerjee M, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1-7. [DOI] [PubMed] [Google Scholar]
- 39. Grainger EM, Moran NE, Francis DM, et al. A novel tomato-soy juice induces a dose-response increase in urinary and plasma phytochemical biomarkers in men with prostate cancer. J Nutr. 2019;149:23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faber M, Coudray C, Hida H, Mousseau M, Favier A. Lipid peroxidation products, and vitamin and trace element status in patients with cancer before and after chemotherapy, including adriamycin: a preliminary study. Biol Trace Elem Res. 1995;47:117-123. [DOI] [PubMed] [Google Scholar]
- 41. Sangeetha P, Das UN, Koratkar R, Suryaprabha P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radic Biol Med. 1990;8:15-19. [DOI] [PubMed] [Google Scholar]
- 42. Weijl NI, Hopman GD, Wipkink-Bakker A, et al. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann Oncol. 1998;9:1331-1337. [DOI] [PubMed] [Google Scholar]
- 43. Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37:1-18. [DOI] [PubMed] [Google Scholar]
- 44. Nicolson GL. Lipid replacement therapy: a nutraceutical approach for reducing cancer-associated fatigue and the adverse effects of cancer therapy while restoring mitochondrial function. Cancer Metastasis Rev. 2010;29:543-552. [DOI] [PubMed] [Google Scholar]
- 45. Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007;33:407-418. [DOI] [PubMed] [Google Scholar]
- 46. Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Cancer. 2008;123:1227-1239. [DOI] [PubMed] [Google Scholar]
- 47. Cunningham D, Morgan RJ, Mills PR, et al. Functional and structural changes of the human proximal small intestine after cytotoxic therapy. J Clin Pathol. 1985;38:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Funk-Archuleta MA, Foehr MW, Tomei LD, Hennebold KL, Bathurst IC. A soy-derived antiapoptotic fraction decreases methotrexate toxicity in the gastrointestinal tract of the rat. Nutr Cancer. 1997;29:217-221. [DOI] [PubMed] [Google Scholar]
- 49. Pinkerton CR, Milla PJ. Methotrexate enterotoxicity: influence of drug dose and timing in the rat. Br J Cancer. 1984;49:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chevreau N, Funk-Archuleta M. Effect of enteral formulas on methotrexate toxicity. Nutr Cancer. 1995;23:185-204. [DOI] [PubMed] [Google Scholar]
- 51. Funk MA, Baker DH. Effect of soy products on methotrexate toxicity in rats. J Nutr. 1991;121:1684-1692. [DOI] [PubMed] [Google Scholar]
- 52. Funk MA, Baker DH. Effect of fiber, protein source and time of feeding on methotrexate toxicity in rats. J Nutr. 1991;121:1673-1683. [DOI] [PubMed] [Google Scholar]
- 53. Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353-2362. [DOI] [PubMed] [Google Scholar]
- 54. Beg T, Siddique Y, Afzal M. Protective action of flavonoids genistein and gingerol against cisplatin toxicity in vitro. J Young Pharm. 2012;4:124-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tacyildiz N, Ozyoruk D, Yavuz G, et al. Soy isoflavones ameliorate the adverse effects of chemotherapy in children. Nutr Cancer. 2010;62:1001-1005. [DOI] [PubMed] [Google Scholar]
- 56. Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4, suppl):1362S-1375S. [DOI] [PubMed] [Google Scholar]
- 57. Tamura M, Hori S, Nakagawa H. Dihydrodaidzein-producing Clostridium-like intestinal bacterium, strain TM-40, affects in vitro metabolism of daidzein by fecal microbiota of human male equol producer and non-producers. Biosci Microflora. 2011;30:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588-594. [DOI] [PubMed] [Google Scholar]
- 59. Zhang HY, Cui J, Zhang Y, Wang ZL, Chong T, Wang ZM. Isoflavones and prostate cancer: a review of some critical issues. Chin Med J (Engl). 2016;129:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jargin SV. Soy and phytoestrogens: possible side effects. Ger Med Sci. 2014;12:Doc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20:1-12. [DOI] [PubMed] [Google Scholar]
- 62. Tikkanen MJ, Wähälä K, Ojala S, Vihma V, Adlercreutz H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc Natl Acad Sci U S A. 1998;95:3106-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ho KP, Li L, Zhao L, Qian ZM. Genistein protects primary cortical neurons from iron-induced lipid peroxidation. Mol Cell Biochem. 2003;247:219-222. [DOI] [PubMed] [Google Scholar]
- 64. Sonee M, Sum T, Wang C, Mukherjee SK. The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. Neurotoxicology. 2004;25:885-891. [DOI] [PubMed] [Google Scholar]
- 65. Wang X, Chen S, Ma G, Ye M, Lu G. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport. 2005;16:267-270. [DOI] [PubMed] [Google Scholar]
- 66. Pan HC, Yang DY, Ho SP, et al. Escalated regeneration in sciatic nerve crush injury by the combined therapy of human amniotic fluid mesenchymal stem cells and fermented soybean extracts, Natto. J Biomed Sci. 2009;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Park YJ, Jang YM, Kwon YH. Isoflavones prevent endoplasmic reticulum stress-mediated neuronal degeneration by inhibiting tau hyperphosphorylation in SH-SY5Y cells. J Med Food. 2009;12:528-535. [DOI] [PubMed] [Google Scholar]
- 68. Park YJ, Jang Y, Kwon YH. Protective effect of isoflavones against homocysteine-mediated neuronal degeneration in SH-SY5Y cells. Amino Acids. 2010;39:785-794. [DOI] [PubMed] [Google Scholar]
- 69. Zhao JH, Arao Y, Sun SJ, Kikuchi A, Kayama F. Oral administration of soy-derived genistin suppresses lipopolysaccharide-induced acute liver inflammation but does not induce thymic atrophy in the rat. Life Sci. 2006;78:812-819. [DOI] [PubMed] [Google Scholar]
- 70. Sakai T, Kogiso M. Soy isoflavones and immunity. J Med Invest. 2008;55:167-173. [DOI] [PubMed] [Google Scholar]
- 71. Andres A, Donovan SM, Kuhlenschmidt MS. Soy isoflavones and virus infections. J Nutr Biochem. 2009;20:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592-5595. [PubMed] [Google Scholar]
- 73. Holzer G, Esterbauer H, Kronke G, et al. The dietary soy flavonoid genistein abrogates tissue factor induction in endothelial cells induced by the atherogenic oxidized phospholipid oxPAPC. Thromb Res. 2007;120:71-79. [DOI] [PubMed] [Google Scholar]
- 74. Martin D, Song J, Mark C, Eyster K. Understanding the cardiovascular actions of soy isoflavones: potential novel targets for antihypertensive drug development. Cardiovasc Hematol Disord Drug Targets. 2008;8:297-312. [DOI] [PubMed] [Google Scholar]
- 75. Clementi G, Fiore CE, Mangano NG, et al. Role of soy diet and L-arginine in cyclosporin-A-induced osteopenia in rats. Pharmacol Toxicol. 2001;88:16-19. [DOI] [PubMed] [Google Scholar]
- 76. Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739. [DOI] [PubMed] [Google Scholar]
- 77. Saratikov AS, Chuchalin VS, Rat’kin AV, Rat’kin EV, Fedoreev SA, Bulgakov VP. Hepatoprotector properties of polyphenolic complexes from wood and cell culture of Maackia amurensis [in Russian]. Eksp Klin Farmakol. 2005;68:51-54. [PubMed] [Google Scholar]
- 78. Zhao H, Liang J, Li X, Yu H, Li X, Xiao R. Folic acid and soybean isoflavone combined supplementation protects the post-neural tube closure defects of rodents induced by cyclophosphamide in vivo and in vitro. Neurotoxicology. 2010;31:180-187. [DOI] [PubMed] [Google Scholar]
- 79. Wozniak K, Kolacinska A, Blasinska-Morawiec M, et al. The DNA-damaging potential of tamoxifen in breast cancer and normal cells. Arch Toxicol. 2007;81:519-527. [DOI] [PubMed] [Google Scholar]
- 80. Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23:379-385. [DOI] [PubMed] [Google Scholar]
- 81. Tsuruki T, Takahata K, Yoshikawa M. A soy-derived immunostimulating peptide inhibits etoposide-induced alopecia in neonatal rats. J Invest Dermatol. 2004;122:848-850. [DOI] [PubMed] [Google Scholar]
- 82. Sung MJ, Kim DH, Jung YJ, et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008;74:1538-1547. [DOI] [PubMed] [Google Scholar]


