Abstract
Optical coherence tomography is designed to evaluate in vivo qualitative and quantitative changes of the anterior segment, optic nerve and the retina. Initial applications of this technology were confined mainly to ophthalmic diseases. However recently, numerous studies have evaluated its use in systemic conditions and in therapeutics where, optic nerve and retinal architecture can be assessed to monitor progression of systemic conditions and its response to treatment. This is a narrative review aimed at evaluating the debate surrounding the role of spectral domain optical coherence tomography, in systemic conditions where optic nerve affection can be measured and be used in the diagnosis, monitoring and assessment of treatment effect as a non-invasive, quick, novel technique.
Keywords: nutrition, optical coherence tomography, retinal nerve fibre layer, systemic disease, therapeutics
Introduction
Optical coherence tomography (OCT) is a technique designed to evaluate in vivo qualitative and quantitative changes of the anterior segment, optic nerve and the retina. Since its first use in 1991, with advancing technology, the newer generation spectral domain optical coherence tomography (SD-OCT) has become an integral part of ophthalmic assessment. SD-OCT has faster axial scan velocities, higher axial resolution and better reproducibility than older generation time domain (TD) OCT technology.1 In recent years, the advent of swept source OCT has allowed better evaluation of diseases affecting the optic nerve head (ONH), as it uses longer wavelengths which allow better penetration.
Initial applications of this technology were confined mainly to ophthalmic diseases. However recently, numerous studies have evaluated its use in systemic conditions and in therapeutics where optic nerve and retinal architecture can be assessed to monitor disease progression and treatment effects. This is a narrative review aimed at evaluating the debate surrounding the role of SD-OCT, in systemic conditions where optic nerve affection can be measured and be used in the diagnosis, monitoring and assessment of treatment effect as a non-invasive, quick, novel technique.
Methods
We included all the relevant published evidence, in English language, on the role of OCT in pathological processes affecting the optic nerve and retina in the following subcategories: neurological disorders; respiratory disorders; autoimmune disease; intracranial trauma; inflammatory conditions; psychiatric disease; nutrition; changes due to medication, malignancy and infectious diseases; and, finally, miscellaneous conditions. This sub-classification was reached after the initial analyses of all the articles published in relation to the topic were retrieved.
A PubMed and Medline search was conducted between 12 August 2016 and 5 July 2017, using the following terms within the categories defined above:
1. Neurological disorder
((“Nervous System Diseases”[Mesh]) AND ((((retina or retinal)) AND (“nerve fibre layer” or “nerve fibre layer” or rnfl or “stratum opticum”)) AND thickness)) AND “Tomography, Optical Coherence”[Mesh].
2. Malignancy
(malignant or malignancy or cancer or cancers or neoplasm or neoplasms or neoplasia or carcinoma or carcinomas) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
3. Nutritional disorders
((nutrition or nutritional or malnutrition or vitamin or vitamins)) AND ((optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness).
4. Respiratory disorders (COPD, sleep apnoea)
(“Respiratory Tract Diseases” [Mesh] AND ((retina or retinal) AND (“nerve fibre layer” or “nerve fibre layer” or rnfl or “stratum opticum”) AND thickness)) AND “Tomography, Optical Coherence”[Mesh].
(optical coherence tomography or optical coherence tomographic or OCT) AND (nerve fibre layer or nerve fibre layer or rnfl) AND thickness AND (COPD or chronic obstructive pulmonary disease or emphysema or sleep apnoea or sleep apnoea or sleep disordered breathing).
(optical coherence tomography or optical coherence tomographic or Oct) AND (nerve fibre layer or nerve fibre layer or rnfl) AND thickness AND (bronchitis or bronchial or pulmonary or bronchopulmonary or respiratory or respiration).
5. Psychiatric disorders
(“Mental Disorders”[Mesh] or depression or schizophrenia) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
6. Autoimmune disease and Susac syndrome
(“Autoimmune Diseases”[Mesh] OR “Susac Syndrome”[Mesh]) AND “Tomography, Optical Coherence”[Mesh] AND ((nerve fibre layer or nerve fibre layer or rnfl) AND thickness).
susac AND ((nerve fibre layer or nerve fibre layer or rnfl) AND thickness).
7. Inflammatory conditions
(vasculitis OR arteritis OR wegner OR wegner’s OR polyarteritis OR sarcoid OR sarcoidosis OR takayasu OR churg strauss OR inflammatory) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
8. Infectious diseases
(“Bacterial Infections and Mycoses”[Mesh]) OR “Parasitic Diseases”[Mesh]) OR “Virus Diseases”[Mesh]) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
((Infection or infections or infectious or tuberculosis or TB or HIV or human immunodeficiency virus)) AND ((optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness).
9. Miscellaneous
(optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness AND systemic.
10. Therapeutics
(optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND ((((retina or retinal)) AND thickness AND (“Nerve Fibres/drug effects”[Mesh:NoExp] OR “Drug-Related Side Effects and Adverse Reactions”[Mesh] OR “Steroids/adverse effects”[Mesh] OR “Pharmaceutical Preparations/adverse effects”[Mesh]).
(optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND ((((retina or retinal)) AND thickness) AND (steroids or corticosteroids or steroid or corticosteroid or glucocorticoid or glucocorticoids or ethambutol or vigabatrin or chloroquine or isotretinoin).
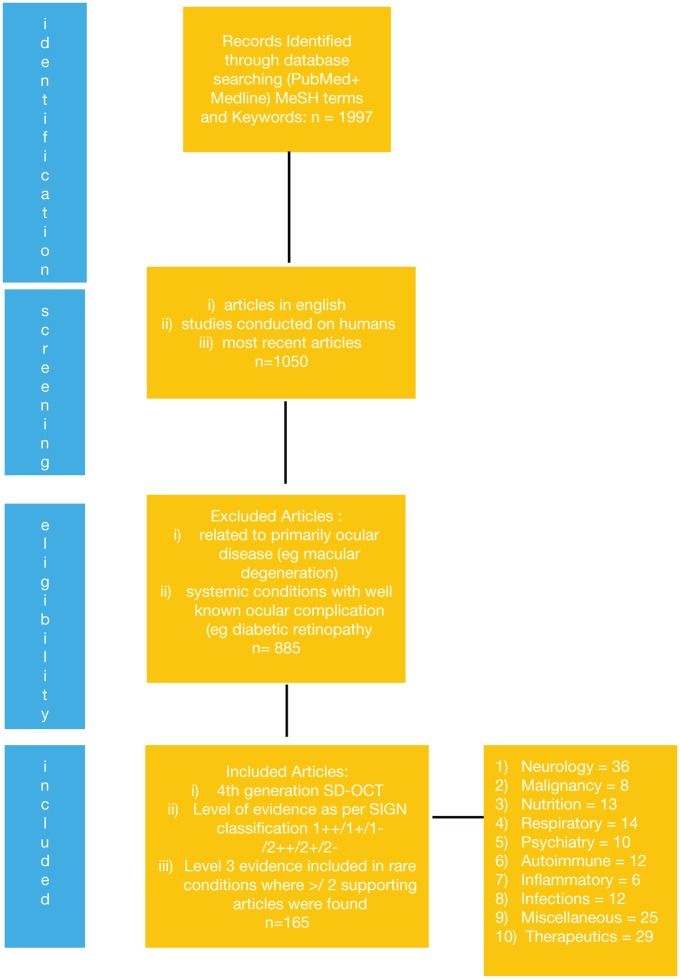
The search generated 1997 articles over 15 years (October 2001–July 2016), as shown in Figure 1. Articles that were not in English were excluded. The articles were then manually reviewed to exclude those relating primarily to ocular disease (such as macular degeneration), paediatric cases and studies in healthy subjects. We also excluded articles on the SD-OCT use in systemic diseases with widespread retinal complications such as vascular conditions like diabetic retinopathy and hypertensive retinopathy, as these were not the aim of this review. Thereafter, the articles were further evaluated to include studies, which used fourth-generation SD-OCT. These articles were then categorised according to the level of evidence as per the Scottish Intercollegiate Guidelines Network (SIGN) classification. For the purpose of this review, evidence from meta-analyses, systematic reviews, randomised controlled trials, case control and cohort studies was included. Non-analytical studies such as case reports or case series were included only if there were two or more articles with supporting evidence in order to incorporate rare diseases as an indicator of future areas of research. We excluded articles reflecting expert opinion and letters to the editor.
Figure 1.
Flow diagram of search steps.
Review of diseases
Neurology
Idiopathic intracranial hypertension
Idiopathic intracranial hypertension (IIH) is a disorder of raised intracranial pressure (ICP) that mainly affects overweight women of childbearing age.2,3 The natural history of the disease is variable. Some degree of vision loss occurs in 86% of patients, and 10% can develop severe visual symptoms.3 Early diagnosis, appropriate treatment and careful follow-up with combined assessment of optic disc morphology and visual field (VF) are vital for preserving vision. In practice, Frisen4 scale has been the standard method for evaluating papilloedema and monitoring the alterations in the ONH. It is a non-continuous ordinal grading based on descriptive features described in fundus photographs or on ophthalmoscopy.4 However, although the scale is useful clinically, it lacks sensitivity to small changes in the degree of disc oedema, and grading can vary among observers.5,6 Determining progression or regression can be obscured by ischaemia, gliosis and dilated venules.5,6 In contrast, SD-OCT with three-dimensional (3D) segmentation of the retinal nerve fibre layer (RNFL), provides reliable, reproducible measurements that reflect the effects of intracellular and extracellular oedema and axonal loss and thinning.5,7,8 These measurements can give a continuous quantitative assessment of papilloedema that correlates with Frisen grading and are an objective indication of disease severity.5,7,8 The additional advantage of measuring several parameters such as alterations in the ONH and total retinal thickness (TRT) and retinal ganglion cell layer (RGL) allows for in-depth interpretation of changes. This is aided by 3D segmentation of SD-OCT images.9 Severe papilloedema and multiple disc haemorrhages can make it difficult to ascertain the posterior border of the RNFL with the OCT due to technical challenges, and in such situations, it has been hypothesised that TRT may be useful and superior to RNFL thickness alone.5,10 However, this needs further research and evolving technology to improve OCT-based algorithms to assess severe papilloedema.5,9,10
Quantification of optic disc changes related to resolution of papilloedema during regulated medical therapy is also possible with SD-OCT. The papilloedema outcomes from the OCT sub study of the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) showed that OCT assessments of papilloedema improved after weight loss with or without acetazolamide therapy.11 This was supported by the findings of Skau and colleagues,10 where 1.5 g/day of acetazolamide tended to normalise OCT within 2–3 months and lead to symptomatic relief in 94% of patients. In this study, improvement in OCT was accompanied by corresponding improvement in VF mean deviation.
It is important to bear in mind that OCT and automated perimetry offer different but complimentary physiological and anatomical information and therefore are best used in combination to monitor IIH patients.
In conclusion, 3D segmentation of SD-OCT can be used in grading mild to moderate papilloedema. More research is needed to evaluate the role of OCT in assessing severe papilloedema, where current OCT programmes pose technical challenges. Response to treatment can be quantified using OCT to measure RNFL and ONH thickness and when used in conjunction with VFs can be useful in monitoring patients’ response to treatment.
Multiple sclerosis
Multiple sclerosis (MS) is a common neurological disease, characterised by recurrent inflammatory episodes within the central nervous system (CNS), causing demyelination, axonal loss and neuronal degeneration, leading to permanent functional disability. Interestingly, within the eye, the RNFL consists of axons that are unmyelinated, with myelination starting after the optic nerve. This is a unique advantage, whereby RNFL consists of easily visible neurones which makes it an ideal structure for studying the process of neurodegeneration and neurorepair.12
Parisi and colleagues13 reported the earliest application of OCT in MS. The study revealed that RNFL thickness was not only reduced in MS patients who suffered an acute episode of optic neuritis (ON) but also in ‘unaffected’ eyes although not to the same extent. Further longitudinal studies have confirmed this finding, indicating that a more subtle RNFL loss can occur in MS due to presumed retrograde trans-synaptic retinal ganglion cell (RGC) degeneration.14–17
Progressive thinning of the RNFL can start early in MS with or without a prior history of ON, and the maximal loss was seen in the papillomacular bundle in all MS subtypes, and this can be reduced by 10–60% of normal, depending on disease severity, duration and history of ON.18,19 It is now recognised that macular volume is also reduced in MS. Approximately, 34% of the macular volume is made up of ganglion cells and their axons, so it may be expected that macular volume loss would follow RNFL loss.20 However, OCT evidence of macular thinning has recently been demonstrated even in the absence of RNFL thinning. There is also evidence of damage to retinal layers with inner and outer retinal atrophy in the macular area.21 Reduction in macular volume is characteristic in relapsing-remitting multiple sclerosis (RRMS) and can be found at baseline in many patients within this subtype.22
Most MS patients present with an RRMS that may transform into secondary progressive multiple sclerosis (SPMS), whereas a smaller percentage of patients present with primary progressive multiple sclerosis (PPMS) from the start.23
Currently, there is extensive research in evaluating RNFL and macula thickness in MS subtypes. Although this has shown that between subtypes, overall RNFL values and predominantly the temporal quadrant thickness are significantly reduced in SPMS patients compared with RRMS,24 there is no consistent evidence at present; some have shown good correlation25 while others have failed to achieve statistical significance.26,27 However, results suggest that retinal axonal damage can occur without clinical ON as a part of MS progression, and OCT can be used as a potential biomarker.
Retinal architecture has also been shown to correlate with visual function. Costello and colleagues25 identified an injury threshold where overall RNFL thickness below the threshold level led to a corresponding decline in automated perimetry. A similar relationship was seen between RNFL decline and low contrast letter acuity.28 Both amplitude and latency of visual evoked potentials (VEPs) have been shown to deteriorate with corresponding decline in RNFL thickness.29,30 Fisher and colleagues28 quantified this functional decline by measuring increasing neurological impairment as evaluated by the expanded disability status scale and showed that it is directly proportional to the average overall RNFL thickness.
There is also a good correlation between RNFL thickness and brain atrophy, evaluated using magnetic resonance imaging (MRI).31–33 Interestingly, Grazioli and colleagues32 found that RNFL thickness in MS non-ON patients was associated with a reduction of brain parenchyma fraction and percentage grey matter. A similar association was not present in MS patients with ON. The authors pointed out that axonal loss after ON occurs independently of brain atrophy, whereas in non-ON cases, RNFL degeneration was associated with the degenerative process in the brain.
In summary, the application of OCT as a biomarker of MS is vast. First, there is a clear pathological correlation with axonal loss. Second, the analytical reproducibility is excellent, and finally, OCT is of high clinical relevance, correlating with clinical measures such as visual function and disability.
Neuromyelitis optica
Neuromyelitis optica (NMO) is an autoimmune condition, which causes simultaneous inflammation and demyelination of the optic nerve and spinal cord. It is known to have a remitting relapsing course similar to MS; however, in NMO, a specific immune target, namely the aquaporin 4 on the surface of astrocytes has been identified. No specific immune target has yet been identified in MS. OCT has rapidly gained importance as a tool to quantify optic nerve damage in this condition. Greater loss of RNFL and subclinical damage are notable features of NMO.34–37 This can cause significant reduction in visual acuity and field, when compared with MS patients.34,38
Recently, Monteiro and colleagues34 compared structure–function differences between MS and NMO with and without history of ON and found that the two conditions differed significantly in their relationship between retinal axonal loss and VF defect. It was noted in patients without prior history of ON the correlation between RNFL thickness and corresponding VF defects was stronger in NMO due to greater axonal loss. However, the correlation between VF defect and macular thickness was greater in MS patients, where loss of macular thickness can be independent of RGC loss and can be accounted by atrophy of other retinal layers. This has highlighted differences in retinal pathology between the two diseases in the absence of ON. Thus, it has been suggested that OCT features along with brain and spinal cord MRI can help differentiate MS from NMO.
Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disease, which often starts as a mild cognitive impairment (MCI).39 It is difficult to diagnose especially in its early stages due to the lack of physical and neurological findings that are specific to the disease. RNFL thinning may be the earliest finding of AD: even before memory impairment,40 OCT data from meta-analyses have shown a significant decline in RNFL thickness in all four quadrants of the retina as well as reduced macular volume in AD and MCI patients than age-matched controls, suggesting that the pathology affects the entire retinal layer and cannot be attributed to ageing alone.41 A reduction in RNFL thickness has been shown to be directly proportional to abnormalities in pattern electroretinogram (pERG), suggesting that neuronal degeneration occurs in the RGL.42,43 However, these were not related to changes in cortical VEP and therefore unlikely to be a result of retrograde degeneration.44
Clinical correlation between reduction of total macular volume and the degree of cognitive impairment as assessed by the Mini-Mental State Examination (MMSE) have produced positive results.45,46 They have also produced negative results.47–50
These findings suggest that RNFL thickness can be used to distinguish AD patients from normal ageing. Moreover, OCT can be useful to detect early RNFL abnormalities in MCI patients. However, its role as a measure of disease severity and an aid to identifying MCI patients with higher predisposition to convert to AD remains to be determined.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a hereditary small-vessel disease caused by Notch3 mutations.51 Histologically, it is characterised by accumulation of granular osmiophillic material in systemic, specifically intracranial, vasculature.52 Clinically, it manifests as migraines, transient ischemic attacks and strokes leading to dementia and physical and visual disabilities.53–56 The wide spectrum of signs and symptoms and the technical challenge of examining brain micro vessels in vivo make diagnosis difficult. Although MRI findings show characteristic white matter subcortical involvement particularly in the anterior temporal lobe and internal capsule in early stages of the disease, they are by no means specific to CADASIL.57 In vivo imaging of retinal vessels SD-OCT is evolving as a new diagnostic approach. Alten and colleagues58 reported the first study exploring retinal findings and retinal vessel measurements in CADASIL patients using high-resolution SD-OCT. They found that the RNFL was thicker globally and hypothesised that this may be caused by pathological enlargement of retinal vessel diameter which runs through the RNFL. They also visualised and measured increased retinal venous lumina, thickened vessel walls and reduced arterial lumina representing ischaemia. In the future, retinal imaging may be used as a complementary tool along with MRI, genetic diagnostics and immunohistology, to diagnose and follow-up CADASIL patients and other cerebral small-vessel diseases.
Parkinson’s disease
Parkinson’s disease (PD) is a commonly encountered neurodegenerative disease among the ageing population. The accuracy of clinical diagnosis is limited especially in early stages when signs are inconclusive and neuroimaging fails to provide characteristic features.59 OCT data from meta-analyses have shown a reduction in average circumpapillary RNFL thickness comparing PD to age-matched controls specifically in the temporal quadrant.60 This may be due to a greater susceptibility of the temporal fibres to neurodegeneration or as a result of involvement of the papillomacular bundle. The pathophysiology of retinal thinning is believed to be due depletion of dopaminergic amacrine cells, which is a well-known consequence of PD. Retinal dopamine (DA) deficiency can alter visual processing by modifying ganglion cells.60 It has been hypothesised that diminished dopaminergic input to ganglion cells can lead to atrophy.61 OCT data from several studies have confirmed these findings, where mean peripapillary RNFL thickness as well as the total macular volume have been reduced in patients with PD.62,63
Visual hallucinations (VH) are a specific feature similar to Charles Bonnet syndrome and thought to be a result of deteriorating visual acuity, contrast sensitivity and defective visual information processing from both the central and peripheral pathways, as the disease progresses.64 RNFL thinning in PD has been reported to be strongly associated with VHs and correlated with PD duration and severity.63 Thus, it is possible that the thinning of the retinal layers can be an indication of disease progression and risk of VHs in PD.63
In view of these results, OCT could be a useful tool for evaluating structural changes in the early diagnosis and progression of the neurodegenerative disorders such as PD.
Chiasmal compression
Pathologies causing compression of the chiasm can lead to loss of visual acuity, colour vision as well as VF defects. Although surgical excision helps with visual recovery, this recovery can be variable and unpredictable.65–67 Traditionally, these conditions have been diagnosed and monitored by neuroimaging and VFs; however, recent studies have shown that RNFL and RGC thinning as measured by OCT, correlate closely with VF loss68,69 and can also be used to detect early changes before VF loss occurs.68,69 Tieger and colleagues69 found that bi-nasal RGC loss could be seen in patients with compression with minimal (or before detectable) VF loss and before RNFL was affected. Patients with less RGC loss had better visual recovery postoperatively. Reversibility of visual dysfunction has also been shown to be related to the loss of RNFL thickness. In patients with normal RNFL thickness, visual function shows large improvements in the presence of advanced pre-operative VF or acuity loss, whereas patients with thin RNFL and advanced VF defect demonstrate significantly less improvement.70,71 Loo and colleagues72 specifically demonstrated that RNFL thickness below an injury threshold pre
dicts a lesser probability of visual improvement after decompression surgery or radiation therapy for anterior pathway meningiomas and thus may be useful in predicting posttreatment visual recovery. Unlike other modalities, which cannot differentiate between reversible and irreversible factors, OCT can quantitatively measure axons by measuring RNFL and RGC thickness and can therefore be used to monitor and provide a prognostic indicator in chiasmal compression. However, it is important to note that OCT cannot distinguish normal density from swollen but reduced cell density so these results need to be interpreted with caution.
Haematological malignancy
RNFL loss in patients with haematological malignancy can lead to VFs defects.73 The pathophysiology may be chronic vascular insufficiency, chronic ischaemic injury due to anaemia or damage from retinal haemorrhages, which can be a result of platelet count changes.74–76 Han and colleagues73 found patients with haematological malignancies had reduced RNFL thickness in the 11 o’clock sector of the optic nerve compared with healthy controls, and reduction was proportional to the severity of anaemia. The authors found lower haemoglobin (Hb) levels were associated with RNFL thinning and RGC loss. Further studies are required to evaluate whether these defects and VF defects are progressive and whether treatment can reverse changes or prevent progression.
Nutrition
Vitamin B12 deficiency
Vitamin B12 deficiency is a common nutritional disorder associated with megaloblastic anaemia, gastrointestinal disorders and neuropsychiatric disorders as well disorders of the visual pathways.77–79 The neurological pathophysiology starts with demyelination followed by axonal degeneration and irreversible cell damage.80 Increasing knowledge and awareness of this at an early stage enable detection and treatment with supplements. Diagnosis can be difficult in patients with subclinical vitamin B12 deficiency without anaemia. However, if treatment is delayed in these patients, there may be permanent neurological sequelae. In all patients with unexplained neuropsychiatric symptoms, vitamin B12 deficiency should be suspected. OCT can be used to obtain cross-sectional images of the peripapillary RNFL, as this has been shown to be decreased especially in the temporal quadrant with involvement of the papillomacular bundle.79,81,82 This reduction has been attributed to a decrease in myelinisation, since vitamin B12 is a major co-factor in the synthesis of myelin. Interestingly, thinning of the RNFL can be seen before patients have manifest VF defects.81 Therefore, OCT can be used to detect early subclinical structural changes.
Vitamin D deficiency
Traditionally, vitamin D is known to play an important role in bone formation and mineral homeostasis. Recent studies have shown that it can affect immune and CNS development and function and has immune regulatory capacity.83,84 There is accumulating evidence that vitamin D has a protective role in the development and progression in various systemic conditions that affect the nervous system. Evaluation of the retinal architecture with OCT scans in patients with vitamin D deficiency has shown reduced macular thickness specifically the macular ganglion cell layer (GCL).85,86 Uro and colleagues86 also demonstrated that in patients with early vitamin D deficiency, thickness of the GCL was reduced without affecting the RNFL. Additional thinning of the RNFL occurred at a later stage. Thus, these findings indicate that OCT may be used to detect subclinical changes in vitamin D deficiency and may also have a role in monitoring progression. Further studies are required to evaluate whether these defects are reversible with treatment and whether OCT can be used as a quantitative measure.
Respiratory disorders
Obstructive sleep apnoea hypopnea syndrome
This is a condition characterised by brief episodes of partial or complete obstruction of the upper airway during sleep with resultant hypoxia.87
Different ophthalmological conditions have been associated with this condition including optic neuropathy as well as a presumed increased incidence of glaucoma.87
OCT as a non-invasive, reproducible, imaging technique has been used to study RNFL, ONH, macular thickness and volume to assist in diagnosis and monitor progression.
Several studies evaluating OCT assessment of the peripapillary RNFL in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) have reported thinning of this layer, nevertheless age-related thinning of this layer and increased incidence of glaucoma in these patients implies cautious interpretation of these findings.
Sun and colleagues88 and Zhao and colleagues89 suggested that OSAHS patients tend to have thinner RNFL. The overall mean difference was higher in moderate and severe OSAHS cases as indicated by the Apnoea Hypopnoea Index (AHI). Mild cases did not show significant difference from healthy controls. The inferior quadrant of the RNFL followed by the superior quadrant were reported to suffer the most; however, the temporal quadrant may to be spared and some studies have reported increased thickness secondary to hypoxia which can cause raised ICP with secondary swelling of the optic nerve.90
Although the correlation between the severity of the condition and the degree of thinning was not universal,91 a recent meta-analysis by Wang and colleagues60 showed the pooled weighted mean difference (WMD) for the average RNFL thickness and any of the quadrants was significantly lower in patients with OSAHS, compared with healthy subjects, and this difference increased with increasing severity of the condition.
In summary, OCT of the RNFL can be a useful ancillary test to monitor and assess severity of the condition; however, further longitudinal studies are required to establish specific changes.
Psychiatry
Schizophrenia
In spite of the lack of characteristic neuropathological abnormalities in schozophrenia, the neurodegenerative nature of this condition has been confirmed by MRI and neuropathological studies.92 Reduction in grey matter volume and decreased size and density of neurons in the hippocampus and thalamic area have been demonstrated.92
In vivo imaging of the unmyelinated retinal nerve fibres by OCT provides an opportunity to study neurodegenerative disorders of the CNS.93
Studies, which investigated the use of OCT to measure different retinal parameters in schizophrenic patients, reported heterogeneous results.5,94 Some reported overall reduction of the RNFL thickness, others showed reduction of specific quadrants,5,94 whereas others have failed to show any co-relation at all.93
Lee and colleagues95 showed significant reduction of the overall thickness of the peripapillary RNFL in schizophrenic patients with macular thinning and macular volume loss, which were more prominent in patients with chronic disease.
Yılmaz and colleagues96 and Celik and colleagues97 reported that the volume of the GCL and the internal plexiform layer is also reduced in these patients. Furthermore changes in these two layers are strongly related to the severity of the disease more so than changes in the RNFL.
Although the existing evidence does not allow for a definite evaluation for the role of OCT as a biomarker in schizophrenia, it confirms the neurodegenerative nature of the disease, and future research is needed to define the extent of correlation between changes in retinal parameters and the disease severity and duration as well assessing the sensitivity and specificity of the test.
Major depression and bipolar disorder
Major depression is a chronic or recurrent mood disorder with underlying neurodegenerative processes affecting certain areas of the cerebral cortex as confirmed by neuroimaging studies, postmortem and animal studies. Schönfeldt-Lecuona and colleagues98 suggested that OCT may be used as a diagnostic tool to evaluate the neurodegenerative process in psychiatric disorders including depression. In their study, RNFL thickness in patients with major depression did not differ from healthy controls, but a negative correlation was noticed between the duration of the latest depressive episode and the thickness of the ganglion cell inner plexiform layer (GCL) and nasal RNFL. Yıldiz and colleagues99 found the total RNFL thickness to be positively correlated with the quick inventory of depressive symptomatology (QIPDS) score. However, the small sample size and the mild symptoms in the study patients imply cautious interpretation of the results. Thinning of the RNFL has also been reported in patients with bipolar disorder. Mehraban and colleagues100 found thinning of RNFL on OCT in all quadrants except the temporal quadrant when compared with healthy controls, and the extent of thinning was significantly correlated to the duration of the illness. However, in these studies, although the controls were age and sex matched, body mass index (BMI) was not considered. BMI and nutrition status is an important contributing factor in RNFL thickness and overall functioning of the visual pathway. This is significant in studying a population with major depression or bipolar disorder, as these patients often suffer from nutritional deficiencies due to self neglect. Quantifying OCT parameters as an indicator of neurodegeneration in patients with psychiatric disorders need further evaluation with larger studies, which accounts for nutritional status, BMI and the toxic effects of smoking and other recreational drugs on RNFL thickness.
Anorexia nervosa
Anorexia nervosa (AN) is a multisystem disease. Ocular effects may involve corneal ulceration, cataracts, rod dysfunction and vein occlusion. Moschos and colleagues101 reported that patients with this disorder and normal vision have a statistically significant lower overall RNFL and foveal thickness as well as altered electrical activity of the macula when compared with normal controls. The multifocal ERG patterns of these patients go against nutritional deficiencies such as vitamin A deficiency, so the authors have hypothesised that the changes may be related to impairment of dopaminergic neurotransmission as a result of food restriction or related to the underlying pathophysiology of AN. DA is an important neurotransmitter in the visual pathway and reduced dopaminergic input can cause atrophy of selected optic nerve fibres. These findings were also found to be related to BMI at presentation and duration of disease, RNFL thickness being negatively correlated to both.102 In addition, patients with different subtypes of AN, namely restrictive AN and purge type AN, exhibited differences in overall retinal thickness of the macula, values being higher in the restrictive type which indicates that the anatomical impairment of the fovea maybe greater in the purge type AN. These findings suggest that OCT can help us understand the pathophysiology and ocular effects of AN, a subject that is currently poorly understood.
Inflammatory and autoimmune disorders
Rheumatoid arthritis
SD-OCT has been used to study the choroid, foveal thickness and RNFL in patients with rheumatoid arthritis (RA). Tetikoglu and colleagues103 reported that the mean sub foveal, nasal and temporal choroidal thickness was significantly higher in patients with RA. However, RNFL and foveal thickness did not show significant difference in RA patients when compared with healthy controls.103 On the contrary, Duru and colleagues104 reported lower mean sub-foveal choroidal thickness as well as thinner para-foveal choroid in patients with RA. No correlation was seen between the choroidal thickness and disease activity as measured by Disease Activity Score-28 (DAS-28).104
The contradictory results of these studies may be explained by the difference in sample size and the heterogeneity of the study sample with regard to disease activity. However, it is interesting to note both pointed to changes in the choroidal layer as part of the systemic vasculature. These changes were also shown to vary with the disease activity. Further studies evaluating OCT assessments in such patients could help us understand the disease process and its implications better.
Systemic lupus erythematosus
SD-OCT has been used to assess macular thickness and RNFL in patients with this multisystem autoimmune disorder. It was reported that the mean sub foveal, nasal and temporal choroidal thickness was lower in these patients.105 Although RNFL thickness was reported to be lower in patients with systemic lupus erythematosus (SLE), the presence of neuropsychiatric complications did not correlate with the degree of RNFL loss.106 It was also noted that the loss of the temporal RNFL was positively correlated to cognitive function.106 This raises the possibility that OCT measurement of the macular thickness and RNFL can be a useful marker for early involvement of the retina and the CNS in patients with SLE.
Autoimmune retinopathy
This involves a spectrum of rare but visually devastating immune-mediated retinal degenerations characterised by acute or subacute visual loss, abnormal ERG and the presence of antibodies to retinal antigens. Autoimmune retinopathy (AR) includes carcinoma-associated retinopathy (CAR), melanoma-associated retinopathy (MAR) and non-paraneoplastic-associated retinopathy (NPAR). The role of OCT in this condition was clearly highlighted in two retrospective case series by Abazari and colleagues107 and Pepple and colleagues.108 In addition to establishing the diagnosis in patients with unexplained visual loss, OCT can give an insight into the cause of this and help in monitoring as well as assessing the response to treatment.
Both studies showed that the damage is mainly targeting the outer retinal complex, namely the ellipsoid zone (EZ), external limiting membrane (ELM) and outer nuclear layer (ONL). The RNFL and GCL were reported to be spared in these two studies with reduced foveal and central macular thickness being noticed as well.
Nevertheless, Sepah and colleagues109 with SD-OCT confirmed that the loss involves all retinal layers including the RNFL and GCL.
Behcet disease
Both peripapillary RNFL and macular thickness were reported to be lower in patients with neuro-Behcet than in healthy age-matched controls, and the temporal quadrant of the RNFL seems to be spared.110 Furthermore, localised defects in the RNFL not associated with scarring were detected on OCT examination, and these may give an indication of posterior segment involvement in Behcet disease (BD), which carries poor visual prognosis.111 The use of enhanced depth imaging (EDI)-OCT to study choroidal thickness in both the active and quiescent phase of the disease showed higher thickness in the active phase and was greater in patients with BD than healthy controls.112
Susac syndrome
This is an autoimmune microvasculopathy with predilection to the brain, retina and inner ear, the most challenging differential diagnosis being MS.
Brandt and colleagues’113 cross-sectional multicentre observational study reported that patients with Susac Syndrome (SS) have lower RNFL thickness and macular measurements than healthy controls and patients with RRMS irrespective of a history of ON. The majority of patients with SS showed a distinct severe and patchy thinning of the RNFL, which was also reported by Bernard and colleagues.114 The loss was more on the nasal quadrant with an abnormal foveal contour, and the extent and degree of OCT abnormalities were correlated with the stage and severity of the disease. Apart from the RNFL, other layers namely GCL, inner plexiform, inner nuclear and outer plexiform were reported to be thinner in patients with SS as well. However, the ONL and the retinal pigment epithelium are not affected suggesting retinal rather than choroidal damage.115
OCT clearly has a distinct pattern in patients with SS which can help to differentiate this condition from RRMS even in the absence of the characteristic branch retinal artery occlusion (BRAO) and can be used as a non-invasive tool to monitor patients with BRAO instead of fluorescein angiography.
Infectious diseases
HIV and AIDS syndrome
Since its discovery in the early 1980s, HIV has presented itself as a constant challenge for healthcare professionals worldwide with regards to management of the long-term health effects in people living with HIV. Thankfully, the introduction of highly active antiretroviral therapy (HAART) was the stepping stone in improving the long-term outlook in terms of morbidity and mortality in this patient group.116 The incidence of opportunistic infections has significantly been reduced since the further development and better patient access to HAART. However, a new spectrum is emerging in the form of metabolic and neurologic degenerative phenomena, which may have a long-term impact on the ageing process. The term HIV-associated ‘neuroretinal disorder’ has been described in the literature but remains poorly understood.117
It appears that the maculae of HIV-infected individuals tended to be thicker than age- and gender-matched HIV negative individuals. This could be explained by inner retinal oedema secondary to HIV-related retino-vascular disease.118
On quantification of the periapapillary RNFL thickness of the four quadrants, there was a direct relationship between RNFL thickness and duration of HIV treatment. It was found that the longer the patients were on HAART, the more likely they were to have thinner RNFLs, especially in the inferior and nasal quadrants.119
In addition, HIV patients who had low CD4 counts (<100 cells/mm3 for at least 6 months) had significantly thinner average RNFLs than patients who had healthier CD4 counts (>100 cells/mm3) since diagnosis.120
In conclusion, OCT may be useful to diagnose early subclinical HIV-associated visual functional loss, with more prospective, larger-scale research needed to analyse the various correlations noted above.
Therapeutics
Vigabatrin
Damage to vision from vigabatrin toxicity is an important safety concern.121,122 Currently, both VF testing and electroretinography are used to monitor patients.123,124 Vigabatrin-attributed field loss can exist in the presence of an apparently normal ONH and retina or can be associated with ONH pallor with or without a variety of accompanying subtle retinal abnormalities. These abnormalities include surface wrinkling retinopathy, peripheral retinal arterial narrowing, abnormal pigmentation and thinning of either the peripapillary or peripheral RNFL.125 OCT can be used to provide a more objective means of assessing retinal damage. Studies have shown that in vigabatrin toxicity, peripapillary RNFL thinning occurs first in the nasal quadrant with sparing of the temporal retina with or without involvement of the superior and inferior retina.126–128 Peripapillary nasal RNFL thinning can be one of the earliest signs of toxicity even before VF loss and has been seen in patients receiving cumulative doses of 1000 g or less in the presence of normal VFs.129 The time to occurrence of an attenuated RNFL thickness resulting from vigabatrin therapy is unknown, neither is the extent of any further attenuation over time.125 However, with higher cumulative doses of vigabatrin exposure, additional RNFL thinning can occur which is proportional to VF loss.129 Vigabatrin is often used as an adjunctive anti-epileptic medication for partial onset epilepsy, which is not satisfactorily controlled by conventional therapy, and as monotherapy for infantile spasm, particularly secondary to tuberous sclerosis.129
Unfortunately children on vigabatrin often are not able to cooperate with VF or ERG testing and often require general anaesthesia for drug monitoring. Given these challenges, OCT may be an option for patients who can tolerate imaging while awake or perhaps decrease anaesthesia time. In conclusion, OCT can be a useful diagnostic tool and serves as an adjunct to VF testing or can even be used in isolation for patients who do not tolerate other forms of assessments in monitoring vigabatrin-induced retinal toxicity. However, the C pattern or ‘inverse atrophy’ often seen in these cases can be confounded by the presence optic nerve hypoplasia and other coexisting pathologies, and OCT findings need to be interpreted in caution when used alone.130
Antituberculous medication
Antituberculous medication, especially isoniazid and ethambutol commonly cause drug-related optic neuropathy.131 Prediction of onset and consequent drug withdrawal is essential to stop visual loss. OCT evaluation of peripapillary RNFL thickness has shown significant reduction in all quadrants with maximal involvement in the temporal quadrant and with marked involvement of the papillomacular bundle.131–133 These changes have also been seen in patients on isoniazid.134 Furthermore, structural change has been shown to correlate well with severity of visual impairment and visual recovery after discontinuation of treatment where patients with thicker papillomacular bundles have a better prognosis.132 Wei and colleagues135 also demonstrated that ethambutol toxicity could specifically cause reduction of macular thickness. In this study, reduction in macular thickness was proportional to reduction in visual sensitivity and the authors suggested that macular thickness maybe the optimal structural marker to detect early ethambutol toxicity. Thus the OCT can be used as an objective measure of detecting early structural damage in ethambutol toxicity as well as help predict visual recovery on cessation of the drug.
Chloroquine and hydroxychloroquine
The antimalarial drugs, chloroquine (CQ) and hydroxychloroquine (HCQ) have been used in the treatment of various rheumatologic diseases such as RA, SLE and other inflammatory and dermatological conditions. Retinal toxicity is a serious complication of both drugs and appears to be dose related, with HCQ being considered a safer option.136 The effects may be reversible on discontinuation of the drug at the preclinical stage if changes are detected early.137,138 In the preclinical stage, patients may be asymptomatic, and the fundus may remain normal with no detectable of maculopathy.138 Hence, screening for early detection in the preclinical maculopathy stage is recommended.138,139 Screening can be challenging due to lack of fundal findings at early stages and variability of functional tests. Recent studies have shown that changes in retinal thickness and loss of inner retinal layers can be detected by SD-OCT in patients with early CQ and HCQ toxicity, even in areas that appeared normal on fundoscopy and perimetry.140–142 Patients exhibit thinning of the foveal and parafoveal areas in early stages and thinning of the inner retinal layers at the posterior pole can be seen before the appearance of retinal lesion and peripapillary RNFL thinning.142 Given the toxic nature of both drugs and of CQ in particular, SD-OCT could be a helpful screening tool for the detection of preclinical macular toxicity in conjunction with other functional tests (such as VF perimetry) early in the course of CQ treatment.
Chemotherapy
The use of chemotherapeutic agents can induce adverse side effects in the eye.143 Bakbak and colleagues144 looked at the effects of cisplatin and paclitaxel on RNFL, two chemotherapeutic agents that are known to cause neurotoxicity.145–147 The study demonstrated a reduction in the peripapillary RNFL thickness and correlated with VF defects on frequency-doubled perimetry, indicating that these agents damage the RGC axons.
Moschos and colleagues148 evaluated the effects of aromatase inhibitors (AIs) used in patients with breast cancer on peripapillary RNFL thickness, optic nerve and macular function. The study showed that AIs significantly decreased RNFL thickness (overall average, superior and inferior). Structural changes correlated with functional tests with evident decrease on visual acuity and delayed VEPs (both amplitude and latency of P100).
Although there may be difficulties in differentiating drug-related changes on the retina from effects of the malignancy itself, given the widespread systemic effects of these disease processes, OCT could potentially be an essential adjunct to functional tests such as visual acuity and perimetry in screening for the ocular toxicity of these agents.
Conclusion
Systemic disease affecting the choroid, retinal layers and optic nerve are better understood and evaluated due to the advent of high definition OCT imaging.
The technology uses low-coherence interferometry to generate images. A low-coherence near-infrared light beam is directed towards the target tissue, and the magnitude and relative location of the backscattered light from the different layers of the tissue are interpreted by the OCT to generate an image.149 Staurenghi and colleagues150 have developed a nomenclature consensus for the classification of the retinal and choroidal layers. The innermost retinal layers identified by OCT are the RNFL, which corresponds to the axons of the ganglion cell and, the ganglion cell inner plexiform layer composed of ganglion cell bodies.150 Although they represent parts of the same cell, the measurement of their thicknesses is taken at different locations by OCT. The GCL thickness is measured at the macula, where the concentration of cell bodies is higher, whereas the RNFL thickness is measured at the centre of the optic disc.151 Scan position can often affect the readings of the RNFL, and in such instances, it is important to interpret these readings in context of the GCL measurements, as these are not influenced by positioning of the scanning diameter.151 Unfortunately, GCL measurements are not available in all OCT machines; however, new generation machines are looking to integrate of all these parameters.
The inner retinal layers are a key marker in neurodegeneration and retinal vascular pathology. The outer retinal layers are often affected in isolation as is seen in AR or, secondary to choroidal disease and extensive damage to inner retinal layers.
The measurements of these different layers are normally stored within the OCT system, which are then available for analysis using different protocols designed for different applications.149 In most studies considered in this review, integrated custom analysis was used which gives an overall gross estimate of the measurements taken. Manual segmentation of the layers can help eliminate inaccuracies, which can be dependent on scan position and misaligned signals in unhealthy tissue.152 Many researchers are now focusing on segmentation of retina in OCT images to produce the retinal thickness maps and to find a correlation between the quantitative and morphological features of the map and different retinopathies.153
The implications of this evolving imaging technique in non-ophthalmic disease are widespread. Knowledge of the pathophysiology of these conditions and the ability to interpret OCT findings is fast becoming essential for both ophthalmologists and non-ophthalmic clinicians. There is opportunity for further research in this area as well as collaborative work. There are many challenges to overcome before SD-OCT becomes part of the routine diagnostic tests. This review highlights its use as an adjunct to other modalities in diagnosing and monitoring systemic conditions and its response to treatment.
At present, there are still a lot of variations in the measurements of the different parameters, such that the changes seem rather ubiquitous and may become of limited value. This may be refined further when combining structural assessment with functional assessment (such as visual acuity or VF perimetry). With more OCT machines looking to incorporate structural and functional assessment in one device and to combine this in smaller, portable devices, the future incorporation of this technology in a variety of clinical settings is likely to open numerous new frontiers.
From a proactive and practical perspective, the immediate action, that may be both possible and beneficial, is for physicians of different levels of experience to consider training in the interpretation of OCT images, since its use is now becoming common practice.
Acknowledgments
The authors would like to thank Anna Brown, CEBIS Specialist – University Hospital Coventry and Warwickshire for her contribution to the study.
Appendix 1
Literature review search strategy
Database used:
PubMed search;
Medline.
Timeline:
From 1 October 2001 to 31 July 2016 = 15 years
Search Strategy
1. ((“Nervous System Diseases”[Mesh]) AND ((((retina or retinal)) AND (“nerve fibre layer” or “nerve fibre layer” or rnfl or “stratum opticum”)) AND thickness)) AND “Tomography, Optical Coherence”[Mesh].
2. (malignant or malignancy or cancer or cancers or neoplasm or neoplasms or neoplasia or carcinoma or carcinomas) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
3. ((nutrition or nutritional or malnutrition or vitamin or vitamins)) AND ((optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness).
4. (“Respiratory Tract Diseases”[Mesh] AND ((retina or retinal) AND (“nerve fibre layer” or “nerve fibre layer” or rnfl or “stratum opticum”) AND thickness)) AND “Tomography, Optical Coherence”[Mesh].
(optical coherence tomography or optical coherence tomographic or OCT) AND (nerve fibre layer or nerve fibre layer or rnfl) AND thickness AND (COPD or chronic obstructive pulmonary disease or emphysema or sleep apnoea or sleep apnoea or sleep disordered breathing).
(optical coherence tomography or optical coherence tomographic or Oct) AND (nerve fibre layer or nerve fibre layer or rnfl) AND thickness AND (bronchitis or bronchial or pulmonary or bronchopulmonary or respiratory or respiration).
5. (“Mental Disorders”[Mesh] or depression or schizophrenia) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
6. (“Autoimmune Diseases”[Mesh] OR “Susac Syndrome”[Mesh]) AND “Tomography, Optical Coherence”[Mesh] AND ((nerve fibre layer or nerve fibre layer or rnfl) AND thickness).
susac AND ((nerve fibre layer or nerve fibre layer or rnfl) AND thickness)
7. (vasculitis OR arteritis OR wegner OR wegner’s OR polyarteritis OR sarcoid OR sarcoidosis OR takayasu OR churg strauss OR inflammatory) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
8. (“Bacterial Infections and Mycoses”[Mesh]) OR “Parasitic Diseases”[Mesh]) OR “Virus Diseases”[Mesh]) AND (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness.
((Infection or infections or infectious or tuberculosis or TB or HIV or human immunodeficiency virus)) AND ((optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness).
9. (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND thickness AND systemic.
10. (optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND ((((retina or retinal))AND thickness AND (“Nerve Fibres/drug effects”[Mesh:NoExp] OR “Drug-Related Side Effects and Adverse Reactions”[Mesh] OR “Steroids/adverse effects”[Mesh] OR “Pharmaceutical Preparations/adverse effects”[Mesh])(optical coherence tomography OR optical coherence tomographic OR oct) AND (nerve fibre layer OR nerve fibre layer OR rnfl) AND ((((retina or retinal)) AND thickness) AND (steroids or corticosteroids or steroid or corticosteroid or glucocorticoid or glucocorticoids or ethambutol or vigabatrin or chloroquine or isotretinoin).
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: C. Mukherjee  https://orcid.org/0000-0002-5408-3302
https://orcid.org/0000-0002-5408-3302
Contributor Information
Chandoshi Mukherjee, Birmingham Midland Eye Centre, Birmingham, UK.
Qusay Al-Fahad, Birmingham Midland Eye Centre, Birmingham, UK; Machen Eye Unit, South Warwickshire Foundation Trust, Warwick, UK.
Samer Elsherbiny, Birmingham Midland Eye Centre, Birmingham, UK; Machen Eye Unit, South Warwickshire Foundation Trust, Warwick, UK.
References
- 1. Warner CV, Syc SB, Stankiewicz AM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS ONE 2011; 6: e22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Am J Ophthalmol 1982; 94: 830. [DOI] [PubMed] [Google Scholar]
- 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain 1991; 114: 155–180. [PubMed] [Google Scholar]
- 4. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 1982; 45: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott C. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol 2010; 128: 705. [DOI] [PubMed] [Google Scholar]
- 6. Sinclair A, Burdon M, Nightingale P, et al. Rating papilloedema: an evaluation of the Frisén classification in idiopathic intracranial hypertension. J Neurol 2012; 259: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 7. Echegaray S, Zamora G, Yu H, et al. Automated analysis of optic nerve images for detection and staging of papilledema. Invest Ophthalmol Vis Sci 2011; 52: 7470. [DOI] [PubMed] [Google Scholar]
- 8. Tang L, Kardon R, Wang J, et al. Quantitative evaluation of papilledema from stereoscopic color fundus photographs. Invest Ophthalmol Vis Sci 2012; 53: 4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baseline OCT. Measurements in the Idiopathic Intracranial Hypertension Treatment Trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci 2014; 55: 8180–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skau M, Sander B, Milea D, et al. Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Neurol 2010; 258: 277–283. [DOI] [PubMed] [Google Scholar]
- 11. Auinger P, Durbin M, Feldon S, et al. Papilledema outcomes from the optical coherence tomography substudy of the Idiopathic Intracranial Hypertension Treatment Trial. Ophthalmology 2015; 122: 1939.e2–1945.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galetta K, Calabresi P, Frohman E, et al. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics 2011; 8: 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 1999; 40: 2520–2527. [PubMed] [Google Scholar]
- 14. Albrecht P, Frohlich R, Hartung HP, et al. Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol 2007; 254: 1595–1596. [DOI] [PubMed] [Google Scholar]
- 15. Gundogan F, Demirkaya S, Sobaci G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis? – a structural and functional evaluation. Invest Ophthalmol Vis Sci 2007; 48: 5773. [DOI] [PubMed] [Google Scholar]
- 16. Pueyo V, Martin J, Fernandez J, et al. Axonal loss in the retinal nerve fiber layer in patients with multiple sclerosis. Mult Scler 2008; 14: 609–614. [DOI] [PubMed] [Google Scholar]
- 17. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 921–932. [DOI] [PubMed] [Google Scholar]
- 18. Gelfand J, Goodin D, Boscardin W, et al. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS ONE 2012; 7: e36847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khanifar AA, Parlitsis GJ, Ehrlich JR, et al. Retinal nerve fiber layer evaluation in multiple sclerosis with spectral domain optical coherence tomography. Clin Ophthalmol 2010; 4: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paunescu L, Schuman J, Price L, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci 2004; 45: 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saidha S, Syc S, Frohman E, et al. Reply: primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain 2011; 134: e194. [DOI] [PubMed] [Google Scholar]
- 22. Winges K, Werner J, Harvey D, et al. Baseline retinal nerve fiber layer thickness and macular volume quantified by OCT in the North American Phase 3 Fingolimod Trial for relapsing – remitting multiple sclerosis. J Neuroophthalmol 2013; 33: 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 24. Zamzam D, Gaafar A, Ismail A, et al. Retinal nerve fiber layer thickness in multiple sclerosis subtypes. Egypt J Neurol Psychiat Neurosurg 2015; 52: 216. [Google Scholar]
- 25. Costello F, Hodge W, Pan Y, et al. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci 2009; 281: 74–79. [DOI] [PubMed] [Google Scholar]
- 26. Henderson A, Trip S, Schlottmann P, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 2008; 131: 277–287. [DOI] [PubMed] [Google Scholar]
- 27. Pulicken M, Gordon-Lipkin E, Balcer L, et al. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 2007; 69: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 28. Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006; 113: 324–332. [DOI] [PubMed] [Google Scholar]
- 29. Klistorner A, Arvind H, Nguyen T, et al. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol 2008; 64: 325–331. [DOI] [PubMed] [Google Scholar]
- 30. Trip S, Schlottmann P, Jones S, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 2005; 58: 383–391. [DOI] [PubMed] [Google Scholar]
- 31. Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007; 68: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 32. Grazioli E, Zivadinov R, Weinstock-Guttman B, et al. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci 2008; 268: 12–17. [DOI] [PubMed] [Google Scholar]
- 33. Siger M, Dziegielewski K, Jasek L, et al. Optical coherence tomography in multiple sclerosis: thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol 2008; 255: 1555–1560. [DOI] [PubMed] [Google Scholar]
- 34. Monteiro M, Fernandes D, Apostolos-Pereira SL, et al. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012; 53: 3959. [DOI] [PubMed] [Google Scholar]
- 35. De Seze J, Blanc F, Jeanjean L, et al. Optical coherence tomography in neuromyelitis optica. Arch Neurol 2008; 65: 920–923. [DOI] [PubMed] [Google Scholar]
- 36. Merle H, Olindo S, Donnio A, et al. Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci 2008; 49: 4412. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura M, Nakazawa T, Doi H, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol 2010; 248: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 38. Fernandes D, Ramos Rde I, Falcochio C, et al. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol 2012; 32: 102–106. [DOI] [PubMed] [Google Scholar]
- 39. Morris J, Storandt M, Miller J, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 2001; 58: 397–405. [DOI] [PubMed] [Google Scholar]
- 40. Günes A, Demirci S, Tök L, et al. Evaluation of retinal nerve fiber layer thickness in Alzheimer disease usingspectral-domain optical coherence tomography. Turk J Med Sci 2015; 45: 1094–1097. [PubMed] [Google Scholar]
- 41. Coppola G, Di Renzo A, Ziccardi L, et al. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS ONE 2015; 10: e0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parisi V, Restuccia R, Fattapposta F, et al. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol 2001; 112: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 43. Parisi V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin Ophthalmol 2003; 18: 50–57. [DOI] [PubMed] [Google Scholar]
- 44. Yu J, Feng Y, Xiang Y, et al. retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS ONE 2014; 9: e85718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iseri P, Altinas O, Tokay T, et al. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 2006; 26: 18–24. [DOI] [PubMed] [Google Scholar]
- 46. Moreno-Ramos T, Benito-León J, Villarejo A, et al. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J Alzheimers Dis 2013; 34: 659–664. [DOI] [PubMed] [Google Scholar]
- 47. Paquet C, Boissonnot M, Roger F, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 2007; 420: 97–99. [DOI] [PubMed] [Google Scholar]
- 48. Kirbas S, Turkyilmaz K, Anlar O, et al. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol 2013; 33: 58–61. [DOI] [PubMed] [Google Scholar]
- 49. Kesler A, Vakhapova V, Korczyn A, et al. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg 2011; 113: 523–526. [DOI] [PubMed] [Google Scholar]
- 50. Kromer R, Serbecic N, Hausner L, et al. Detection of retinal nerve fiber layer defects in Alzheimer’s disease using SD-OCT. Front Psychiatry 2014; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996; 383: 707–710. [DOI] [PubMed] [Google Scholar]
- 52. Tikka S, Mykkanen K, Ruchoux M, et al. Congruence between NOTCH3 mutations and GOM in 131 CADASIL patients. Brain 2008; 132: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dichgans M, Mayer M, Uttner I, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol 1998; 44: 731–739. [DOI] [PubMed] [Google Scholar]
- 54. Haritoglou C, Rudolph G, Hoops J, et al. Retinal vascular abnormalities in CADASIL. Neurology 2004; 62: 1202–1205. [DOI] [PubMed] [Google Scholar]
- 55. Haritoglou C, Hoops J, Stefani F, et al. Histopathological abnormalities in ocular blood vessels of CADASIL patients. Am J Ophthalmol 2004; 138: 302–305. [DOI] [PubMed] [Google Scholar]
- 56. Pretegiani E, Rosini F, Dotti M, et al. Visual system involvement in CADASIL. J Stroke Cerebrovasc Dis 2013; 22: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 57. Rufa A, Pretegiani E, Frezzotti P, et al. Retinal nerve fiber layer thinning in CADASIL: an optical coherence tomography and MRI study. Cerebrovasc Dis 2011; 31: 77–82. [DOI] [PubMed] [Google Scholar]
- 58. Alten F, Motte J, Ewering C, et al. Multimodal retinal vessel analysis in CADASIL patients. PLoS ONE 2014; 9: e112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gaenslen A, Berg D. Early diagnosis of Parkinson’s disease. Int Rev Neurobiol 2010; 90: 81–92. [DOI] [PubMed] [Google Scholar]
- 60. Wang W, He M, Huang W. Changes of retinal nerve fiber layer thickness in obstructive sleep apnea syndrome: a systematic review and meta-analysis. Curr Eye Res 2017; 42: 796–802. [DOI] [PubMed] [Google Scholar]
- 61. Inzelberg R, Ramirez J, Nisipeanu P, et al. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 2004; 44: 2793–2797. [DOI] [PubMed] [Google Scholar]
- 62. Altintaş Işeri ÖP, Özkan B, Çağlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Document Ophthalmol 2007; 116: 137–146. [DOI] [PubMed] [Google Scholar]
- 63. Satue M, Garcia-Martin E, Fuertes I, et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye 2013; 27: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Biousse V, Skibell B, Watts R, et al. Ophthalmologic features of Parkinson’s disease. Neurology 2004; 62: 177–180. [DOI] [PubMed] [Google Scholar]
- 65. Kerrison J, Lynn M, Baer C, et al. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol 2000; 130: 813–820. [DOI] [PubMed] [Google Scholar]
- 66. Laws ER, Jr, Trautmann JC, Hollenhorst RW., Jr. Transsphenoidal decompression of the optic nerve and chiasm. J Neurosurg 1977; 46: 717–722. [DOI] [PubMed] [Google Scholar]
- 67. Powell M. Recovery of vision following transsphenoidal surgery for pituitary adenomas. Br J Neurosurg 1995; 9: 367–373. [DOI] [PubMed] [Google Scholar]
- 68. Cameron J, Tatham A. A window to beyond the orbit: the value of optical coherence tomography in non-ocular disease. Acta Ophthalmol 2016; 94: 533–539. [DOI] [PubMed] [Google Scholar]
- 69. Tieger M, Hedges TR, 3rd, Ho J, et al. Ganglion cell complex loss in chiasmal compression by brain tumors. J Neuroophthalmol 2017; 37: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Danesh-Meyer H, Papchenko T, Savino P, et al. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 2008; 49: 1879. [DOI] [PubMed] [Google Scholar]
- 71. Park H, Oh M, Kim E, et al. Use of optical coherence tomography to predict visual outcome in parachiasmal meningioma. J Neurosurg 2015; 123: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 72. Loo J, Tian J, Miller N, et al. Use of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomas. Br J Ophthalmol 2013; 97: 1455–1458. [DOI] [PubMed] [Google Scholar]
- 73. Han J, Kim J, Yoo H, et al. Retinal nerve fiber layer thickness is decreased in patients with hematologic malignancy. J Glaucoma 2016; 25: e175–e181. [DOI] [PubMed] [Google Scholar]
- 74. Brouzas D, Charakidas A, Ladas I, et al. Nonarteritic anterior ischemic optic neuropathy associated with chronic anemia: a case series of myelodysplastic syndrome patients. Clin Ophthalmol 2009; 3: 133–137. [PMC free article] [PubMed] [Google Scholar]
- 75. Eze B, Ibegbulam G, Ocheni S. Ophthalmic manifestations of leukemia in a tertiary hospital population of adult Nigerian Africans. Middle East Afr J Ophthalmol 2010; 17: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carraro M, Rossetti L, Gerli G. Prevalence of retinopathy in patients with anemia or thrombocytopenia. Eur J Haematol 2001; 67: 238–244. [DOI] [PubMed] [Google Scholar]
- 77. Perez L, Heim L, Sherzai A, et al. Nutrition and vascular dementia. J Nutr Health Aging 2012; 16: 319–324. [DOI] [PubMed] [Google Scholar]
- 78. Kumar N. Acute and subacute encephalopathies: deficiency states (nutritional). Semin Neurol 2011; 31: 169–183. [DOI] [PubMed] [Google Scholar]
- 79. Amemiya T. The eye and nutrition. Jpn J Ophthalmol 2000; 44: 320. [DOI] [PubMed] [Google Scholar]
- 80. Miller A, Korem M, Almog R, et al. Vitamin B12, demyelination, remyelination and repair in multiple sclerosis. J Neurol Sci 2005; 233: 93–97. [DOI] [PubMed] [Google Scholar]
- 81. Türkyılmaz K, Oner V, Türkyılmaz AK, et al. Evaluation of peripapillary retinal nerve fiber layer thickness in patients with vitamin B12 deficiency using spectral domain optical coherence tomography. Curr Eye Res 2013; 38: 680–684. [DOI] [PubMed] [Google Scholar]
- 82. Chester E, Agamanolis D, Harris J, et al. Optic atrophy in experimental vitamin B12 deficiency in monkeys. Acta Neurol Scand 2009; 61: 9–26. [DOI] [PubMed] [Google Scholar]
- 83. Kalueff A, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care 2007; 10: 12–19. [DOI] [PubMed] [Google Scholar]
- 84. Annweiler C, Schott A, Berrut G, et al. Vitamin D and ageing: neurological issues. Neuropsychobiology 2010; 62: 139–150. [DOI] [PubMed] [Google Scholar]
- 85. Graffe A, Beauchet O, Fantino B, et al. Vitamin D and macular thickness in the elderly: an optical coherence tomography study. Invest Ophthalmol Vis Sci 2014; 55: 5298. [DOI] [PubMed] [Google Scholar]
- 86. Uro M, Beauchet O, Cherif M, et al. Age-related vitamin D deficiency is associated with reduced macular ganglion cell complex: a cross-sectional high-definition optical coherence tomography study. PLoS ONE 2015; 10: e0130879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Casas P, Ascaso FJ, Vicente E, et al. Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea–hypopnea syndrome (OSAHS). Graefes Arch Clin Exp Ophthalmol 2013; 251: 1625–1634. [DOI] [PubMed] [Google Scholar]
- 88. Sun CL, Zhou LX, Dang Y, et al. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea syndrome: a meta-analysis. Medicine 2016; 95: e4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao XJ, Yang CC, Zhang JC, et al. Obstructive sleep apnea and retinal nerve fiber layer thickness: a meta-analysis. J Glaucoma 2016; 25: e413–e418. [DOI] [PubMed] [Google Scholar]
- 90. Xin C, Wang J, Zhang W, et al. Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS). Eye 2014; 28: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sagiv O, Fishelson-Arev T, Buckman G, et al. Retinal nerve fibre layer thickness measurements by optical coherence tomography in patients with sleep apnoea syndrome. Clin Exp Ophthalmol 2014; 42: 132–138. [DOI] [PubMed] [Google Scholar]
- 92. Schönfeldt-Lecuona C, Kregel T, Schmidt A, et al. From imaging the brain to imaging the retina: optical coherence tomography (OCT) in schizophrenia. Schizophr Bull 2016; 42: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chu EM, Kolappan M, Barnes TR, et al. A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res 2012; 203: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ascaso FJ, Rodriguez-Jimenez R, Cabezón L, et al. Retinal nerve fiber layer and macular thickness in patients with schizophrenia: influence of recent illness episodes. Psychiatry Res 2015; 229: 230–236. [DOI] [PubMed] [Google Scholar]
- 95. Lee WW, Tajunisah I, Sharmilla K, et al. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: evidence from optical coherence tomography. Invest Ophthalmol Vis Sci 2013; 54: 7785–7792. [DOI] [PubMed] [Google Scholar]
- 96. Yılmaz U, Küçük E, Ülgen A, et al. Retinal nerve fiber layer and macular thickness measurement in patients with schizophrenia. Eur J Ophthalmol 2016; 26: 375–378. [DOI] [PubMed] [Google Scholar]
- 97. Celik M, Kalenderoglu A, Sevgi Karadag A, et al. Decreases in ganglion cell layer and inner plexiform layer volumes correlate better with disease severity in schizophrenia patients than retinal nerve fiber layer thickness: findings from spectral optic coherence tomography. Eur Psychiatry 2016; 32: 9–15. [DOI] [PubMed] [Google Scholar]
- 98. Schönfeldt-Lecuona C, Schmidt A, Pinkhardt EH, et al. Optical coherence tomography (OCT)–a new diagnostic tool in psychiatry? Fortschr Neural Psychiatr 2014; 82: 566–571. [DOI] [PubMed] [Google Scholar]
- 99. Yıldız M, Alim S, Batmaz S, et al. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: optical coherence tomography findings in major depression. Psychiatry Res Neuroimaging 2016; 251: 60–66. [DOI] [PubMed] [Google Scholar]
- 100. Mehraban A, Samimi SM, Entezari M, et al. Peripapillary retinal nerve fiber layer thickness in bipolar disorder. Graefes Arch Clin Exp Ophthalmol 2016; 254: 365–371. [DOI] [PubMed] [Google Scholar]
- 101. Moschos MM, Gonidakis F, Varsou E, et al. Anatomical and functional impairment of the retina and optic nerve in patients with anorexia nervosa without vision loss. Br J Ophthalmol 2010; 95: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 102. Moschos MM, Moustafa GA, Gonidakis F, et al. Retinal and choroidal alterations in patients with anorexia nervosa without vision loss. Int J Eat Disord 2016; 49: 386–390. [DOI] [PubMed] [Google Scholar]
- 103. Tetikoglu M, Temizturk F, Sagdik HM, et al. Evaluation of the choroid, fovea, and retinal nerve fiber layer in patients with rheumatoid arthritis. Ocul Immunol Inflamm 2017; 25: 210–214. [DOI] [PubMed] [Google Scholar]
- 104. Duru N, Altinkaynak H, Erten Ş, et al. Thinning of choroidal thickness in patients with rheumatoid arthritis unrelated to disease activity. Ocul Immunol Inflamm 2016; 24: 246–253. [DOI] [PubMed] [Google Scholar]
- 105. Altinkaynak H, Duru N, Uysal BS, et al. Choroidal thickness in patients with systemic lupus erythematosus analyzed by spectral-domain optical coherence tomography. Ocul Immunol Inflamm 2016; 24: 254–260. [DOI] [PubMed] [Google Scholar]
- 106. Liu GY, Utset TO, Bernard JT. Retinal nerve fiber layer and macular thinning in systemic lupus erythematosus: an optical coherence tomography study comparing SLE and neuropsychiatric SLE. Lupus 2015; 24: 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Abazari A, Allam SS, Adamus G, et al. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol 2012; 153: 750.e1–756.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pepple KL, Cusick M, Jaffe GJ, et al. SD-OCT and autofluorescence characteristics of autoimmune retinopathy. Br J Ophthalmol 2013; 97: 139–144. [DOI] [PubMed] [Google Scholar]
- 109. Sepah YJ, Sadiq MA, Hassan M, et al. Assessment of retinal structural and functional characteristics in eyes with autoimmune retinopathy. Curr Mol Med 2015; 15: 578–586. [DOI] [PubMed] [Google Scholar]
- 110. Ucar D, Uygunoglu U, Dikkaya F, et al. Retinal nerve fiber layer structure abnormalities in patients with Neuro-Behcet’s disease. Graefes Arch Clin Exp Ophthalmol 2015; 253: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 111. Oray M, Onal S, Bayraktar S, et al. Nonglaucomatous localized retinal nerve fiber layer defects in Behçet uveitis. Am J Ophthalmol 2015; 159: 475–481. [DOI] [PubMed] [Google Scholar]
- 112. Kim M, Kim H, Kwon HJ, et al. Choroidal thickness in Behcet’s uveitis: an enhanced depth imaging-optical coherence tomography and its association with angiographic changes. Invest Ophthalmol Vis Sci 2013; 54: 6033–6039. [DOI] [PubMed] [Google Scholar]
- 113. Brandt AU, Zimmermann H, Kaufhold F, et al. Patterns of retinal damage facilitate differential diagnosis between Susac syndrome and MS. PLoS ONE 2012; 7: e38741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bernard JT, Romero R, Agrawal K, et al. Optical coherence tomography in Susac’s syndrome. Mult Scler Relat Disord 2014; 3: 110–116. [DOI] [PubMed] [Google Scholar]
- 115. Ringelstein M, Albrecht P, Kleffner I, et al. Retinal pathology in Susac syndrome detected by spectral-domain optical coherence tomography. Neurology 2015; 85: 610–618. [DOI] [PubMed] [Google Scholar]
- 116. Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS update 2016. Geneva: UNAIDS, 2016. [PubMed] [Google Scholar]
- 117. Arcinue CA, Bartsch DU, El-Emam SY, et al. Retinal thickening and photoreceptor loss in HIV eyes without retinitis. PLoS ONE 2015; 10: e0132996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pathai S, Lawn SD, Weiss HA, et al. Retinal nerve fibre layer thickness and contrast sensitivity in HIV-infected individuals in South Africa: a case-control study. PLoS ONE 2013; 8: e73694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Faria E, Arantes TE, Garcia CR, Mello PA, et al. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. Am J Ophthalmol 2010; 149: 571.e2–576.e2. [DOI] [PubMed] [Google Scholar]
- 120. Peng PH, Lin HS, Lin S. Nerve fibre layer thinning in patients with preclinical retinopathy. Can J Ophthalmol 2009; 44: 417–422. [DOI] [PubMed] [Google Scholar]
- 121. Maguire M, Hemming K, Wild J, et al. Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review. Epilepsia 2010; 51: 2423–2431. [DOI] [PubMed] [Google Scholar]
- 122. Frisen L, Malmgren K. Characterization of vigabatrin-associated optic atrophy. Acta Ophthalmol Scand 2003; 81: 466–473. [DOI] [PubMed] [Google Scholar]
- 123. Miller N, Johnson M, Paul S, et al. Visual dysfunction in patients receiving vigabatrin: clinical and electrophysiologic findings. Neurology 1999; 53: 2082–2087. [DOI] [PubMed] [Google Scholar]
- 124. Harding G, Wild J, Robertson K, et al. Electro-oculography, electroretinography, visual evoked potentials, and multifocal electroretinography in patients with vigabatrin-attributed visual field constriction. Epilepsia 2000; 41: 1420–1431. [DOI] [PubMed] [Google Scholar]
- 125. Wild J, Robson C, Jones A, et al. Detecting vigabatrin toxicity by imaging of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci 2006; 47: 917. [DOI] [PubMed] [Google Scholar]
- 126. Kjellström U, Andréasson S, Ponjavic V. Attenuation of the retinal nerve fibre layer and reduced retinal function assessed by optical coherence tomography and full-field electroretinography in patients exposed to vigabatrin medication. Acta Ophthalmol 2013; 92: 149–157. [DOI] [PubMed] [Google Scholar]
- 127. Akcakaya AA, Gokceer S, Erbil HH, et al. Detecting retinal vigabatrin toxicity in patients with partial symptomatic or cryptogenic epilepsy. Eur J Ophthalmol 2010; 20: 763–769. [DOI] [PubMed] [Google Scholar]
- 128. Moseng L, Saeter M, Morch-Johnsen GH, et al. Retinal nerve fibre layer attenuation: clinical indicator for vigabatrin toxicity. Acta Ophthalmol 2011; 89: 452–458. [DOI] [PubMed] [Google Scholar]
- 129. Clayton L, Devile M, Punte T, et al. Patterns of peripapillary retinal nerve fiber layer thinning in vigabatrin-exposed individuals. Ophthalmology 2012; 119: 2152–2160. [DOI] [PubMed] [Google Scholar]
- 130. Lawthom C, Smith P, Wild J. Nasal retinal nerve fiber layer attenuation: a biomarker for vigabatrin toxicity. Ophthalmology 2009; 116: 565–571. [DOI] [PubMed] [Google Scholar]
- 131. Kim Y, Hwang J. Serial retinal nerve fiber layer changes in patients with toxic optic neuropathy associated with antituberculosis pharmacotherapy. J Ocul Pharmacol Ther 2009; 25: 531–535. [DOI] [PubMed] [Google Scholar]
- 132. Zoumalan C, Agarwal M, Sadun A. Optical coherence tomography can measure axonal loss in patients with ethambutol-induced optic neuropathy. Graefes Arch Clin Exp Ophthalmol 2004; 243: 410–416. [DOI] [PubMed] [Google Scholar]
- 133. Chai S, Foroozan R. Decreased retinal nerve fibre layer thickness detected by optical coherence tomography in patients with ethambutol-induced optic neuropathy. Br J Ophthalmol 2007; 91: 895–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Neriyanuri S, Pardhan S, Gella L, et al. Retinal sensitivity changes associated with diabetic neuropathy in the absence of diabetic retinopathy. Br J Ophthalmol 2017; 101: 1174–1178.Wei S,| Peng C,| Zhang A,| Chen B,| Yang [DOI] [PubMed] [Google Scholar]
- 135. Wei S, Peng C, Zhang A, et al. Macular thickness as a predictor of loss of visual sensitivity in ethambutol-induced optic neuropathy. Neural Regen Res 2016; 11: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Block J. Hydroxychloroquine and retinal safety. Lancet 1998; 351: 771. [DOI] [PubMed] [Google Scholar]
- 137. Maturi R. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol 2004; 122: 973–981. [DOI] [PubMed] [Google Scholar]
- 138. Lyons J, Severns M. Using multifocal ERG ring ratios to detect and follow Plaquenil retinal toxicity: a review. Document Ophthalmol 2008; 118: 29–36. [DOI] [PubMed] [Google Scholar]
- 139. Michaelides M. Retinal toxicity associated with hydroxychloroquine and chloroquine. Arch Ophthalmol 2011; 129: 30. [DOI] [PubMed] [Google Scholar]
- 140. Ma X, Yan L, He L, et al. Ocular fundus manifestation of two patients following long-term chloroquine therapy: a case report. Diagn Pathol 2010; 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chen E, Brown DM, Benz MS, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the ‘flying saucer’ sign). Clin Ophthalmol 2010; 4: 1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pasadhika S, Fishman G. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye 2009; 24: 340–346. [DOI] [PubMed] [Google Scholar]
- 143. Gokulgandhi M, Vadlapudi A, Mitra A. Ocular toxicity from systemically administered xenobiotics. Expert Opin Drug Metab Toxicol 2012; 8: 1277–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bakbak B, Gedik S, Koktekir B, et al. Assessment of ocular neurotoxicity in patients treated with systemic cancer chemotherapeutics. Cutan Ocul Toxicol 2013; 33: 7–10. [DOI] [PubMed] [Google Scholar]
- 145. Schmid K, Kornek G, Scheithauer W, et al. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol 2006; 51: 19–40. [DOI] [PubMed] [Google Scholar]
- 146. Walsh T, Clark A, Parhad I, et al. Neurotoxic effects of cisplatin therapy. Arch Neurol 1982; 39: 719–720. [DOI] [PubMed] [Google Scholar]
- 147. Urba S, Forastiere A. Retrobulbar neuritis in a patient treated with intraarterial cisplatin for head and neck cancer. Cancer 1988; 62: 2094–2097. [DOI] [PubMed] [Google Scholar]
- 148. Moschos M, Chatziralli I, Sergentanis T, et al. Electroretinographic and optical coherence tomography findings in breast cancer patients using aromatase inhibitors. Cutan Ocul Toxicol 2015; 35: 13–20. [DOI] [PubMed] [Google Scholar]
- 149. Arévalo J, Krivoy D, Fernández C. How does optical coherence tomography work? Berlin: Springer, 2009. [Google Scholar]
- 150. Staurenghi G, Sadda S, Chakravarthy U, et al. International nomenclature for optical coherence tomography, P. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology 2014; 121: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 151. Forrester J, Dick A, McMenamin P, et al. Anatomy of the eye and orbit. In: Forrester J, Dick A, Mcmenamin P, et al. (eds) The eye: basic sciences in practice. Philadelphia. PA: Saunders Elsevier, 2008, pp. 1–108. [Google Scholar]
- 152. Gama R, Relha C, Gomes Costa J, et al. Measurement of inner retinal layers of megalopapilla by optical coherhence tomography. Med Hypothesis Discov Innov Ophthalmol 2017; 6: 82–88. [PMC free article] [PubMed] [Google Scholar]
- 153. Kafieh R, Rabbani H, Hajizadeh F, et al. Thickness mapping of eleven retinal layers segmented using the diffusion maps method in normal eyes. J Ophthalmol 2015; 2015: 259123. [DOI] [PMC free article] [PubMed] [Google Scholar]