Abstract
Background:
Female patients are more likely than male patients to experience various musculoskeletal (MSK) injuries. Because MSK tissues are sensitive to the female hormones relaxin, estrogen, and progesterone, studies have examined whether hormonal contraceptives, which change female hormone levels, can alter the female MSK injury risk. These studies have reached contradictory conclusions, leaving unclear the influence of hormonal contraception on female MSK injury risk.
Hypothesis:
Hormonal contraceptives act to decrease female soft tissue injury risk and soft tissue laxity.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
Reviewers searched for clinically relevant studies evaluating the relationship between hormonal contraceptive use and soft tissue injuries, soft tissue laxity, muscle injuries, and muscle strength in the PubMed, Cochrane, Scopus, CINAHL, and Embase databases. Studies meeting inclusion criteria were scored by 2 independent researchers for risk of bias, imprecision, inconsistency, and indirectness with a template designed using the British Medical Journal Clinical Evidence GRADE (Grades of Recommendation Assessment, Development and Evaluation) scoring system and GRADEPro guidelines. Scores were uploaded into the GRADEPro scoring system software, which calculated each study’s final GRADE score (very low, low, moderate, or high quality).
Results:
A total of 29 studies met inclusion criteria. Of the 7 studies evaluating oral contraceptive (OC) use and soft tissue injury risk, only 2 received a high quality-of-evidence score; all other studies received a very low score. The high-quality studies concluded that OC use decreases anterior cruciate ligament (ACL) injury risk. Only 1 of the 10 studies evaluating OC use and soft tissue laxity was found to have a high quality of evidence; this study determined that OC use decreases ACL laxity.
Conclusion:
Higher quality studies suggest that OCs decrease a female patient’s risk of ACL injuries and ACL laxity. The strength of these findings, however, is weak. Female patients are up to 8 times more likely to tear their ACLs than male patients. OCs may serve a therapeutic role in decreasing the sex disparity in ACL injury rates.
Keywords: anterior cruciate ligament, ACL, relaxin, muscle strength, ligament, tendon, injury, laxity, contraceptive, birth control, hormone, estrogen, progesterone
Female patients suffer from a far greater number of some musculoskeletal (MSK) injuries than do male patients.6,19,26,37,59 They are up to 8 times more likely to experience an anterior cruciate ligament (ACL) tear3,26 and twice as likely to experience an ankle sprain.19 Female patients are also more prone to patellofemoral syndrome, stress fractures, and injuries of the shoulder.6,37,59 While anatomic and biomechanical factors likely contribute to this sex disparity in MSK injuries, there is compelling evidence that female hormones, such as relaxin, estrogen, and progesterone, play a role as well.14,15,21,32,45 This has generated speculation that compounds such as hormonal contraceptives, which change relaxin, estrogen, and progesterone levels, may also influence MSK health.
The ACL is a prime example of a soft tissue susceptible to hormonal influences. Relaxin, best known for its role in loosening the pubic symphysis during pregnancy,60 increases knee laxity in female guinea pigs,16 exhibits collagenolytic effects in human female—but not male—ACL cells,38 and has greater receptor levels in female ACLs than in male ACLs.15,21 Female athletes with elevated relaxin levels are also more likely to tear their ACLs than female athletes with lower circulating levels.14 Estrogen, similar to relaxin, has been shown to promote catabolic processes in ACL cells,29,40,45 decrease ACL strength in a rabbit model,66 and increase ACL laxity in humans.57 In contrast, studies have found that progesterone decreases ACL laxity57 and decreases relaxin-induced collagen degradation.13,29,60
Additional research indicates that other MSK tissues are also susceptible to female hormones. Receptors for relaxin, estrogen, and progesterone have been found on a variety of MSK tissues including the lateral collateral ligament13 and patellar,13,28 Achilles,8 posterior tibial,7 and flexor digitorum longus62 tendons. As in the ACL, relaxin and estrogen have been shown to promote catabolic processes in other MSK tissues,28 with estrogen also up-regulating relaxin receptors in the rat patellar tendon and lateral collateral ligament.13 Because relaxin, estrogen, and progesterone all affect soft tissue and muscle health, different combinations and levels of these hormones could influence MSK injury risk.
Hormonal contraceptives dramatically change levels of relaxin, estrogen, and progesterone and therefore have been theorized to affect MSK function.14,28 Contraceptive formulations may be combined, containing both estrogen and progestin, or contain progestin only. Supplying exogenous hormones inhibits the hypothalamic-pituitary-gonadal axis.30 Thus, the endogenous production of estrogen and progesterone decreases significantly, the cyclic hormonal changes found throughout the normal menstrual cycle are eliminated, and the natural estrogen and progesterone peaks are lost.30 Similarly, hormonal contraceptives alter relaxin production. Relaxin levels change when the contraceptive-induced elimination of normal hormone cycling results in ovulation suppression, thus inhibiting corpus luteum formation.30 Because the corpus luteum is the primary site of relaxin production in nonpregnant female patients, relaxin levels decrease with hormonal contraceptive use.46
Various clinical studies have examined the effects of hormonal contraceptive regimens on MSK function.1,8,9,14,25,35,44,54,58 Yet, very little analysis has been conducted to determine the impact of specific contraceptive formulations on ligaments, tendons, and muscles in vitro and in vivo. Only 1 study has assessed this relationship in an animal model; the authors concluded that contraceptive administration decreases the average stiffness and increases the total energy absorbed before a rupture of the rat ACL.71 Although the available literature examining the effects of hormonal contraceptive use in patient populations is more extensive, very little agreement exists within this body of research. Several studies have argued each side of the debate, leaving it unclear whether hormonal contraceptives are protective, detrimental, or noninfluential with respect to MSK injury risk.1,14,25,35,44,54,58
In this systematic review, studies that evaluated the impact of hormonal contraceptives on soft tissue and muscle health were analyzed to better understand the literature’s conflicting findings. We aimed to provide clinically relevant and evidence-based recommendations on the use of hormonal contraceptives in female patients who are at a high risk of experiencing an MSK injury, such as competitive female athletes.
Methods
This systematic review was designed using the Cochrane Collaboration guidelines.34 Literature was only included if the study compared human soft tissue injuries, soft tissue laxity, muscle injuries, or muscle strength in hormonal contraceptive users versus nonusers. Hormonal contraceptives were defined as oral contraceptives (OCs) or hormonal intrauterine devices, implants, or patches. A soft tissue injury was defined as clinically relevant ligament, tendon, or cartilage damage. Soft tissue laxity was defined as the measurement of ligament or tendon laxity, stiffness, or strain under stress. A muscle injury was defined as clinically relevant muscle damage. Muscle strength was defined as the measurement of isometric strength, force output, endurance, peak torque, stretch reflex, or parameters of physical performance.‡
Two independent reviewers conducted searches in the PubMed, Cochrane, Scopus, CINAHL, and Embase databases. The search parameters included hormonal contraceptive terms combined using the “AND” function with either soft tissue injury/laxity terms or muscle injury/strength terms.
Hormonal contraceptive terms: hormonal contraceptive OR “birth control” OR oral contraceptive OR contraceptive agent OR steroidal contraceptive.
Soft tissue injury and laxity terms: ligament injury OR ligament laxity OR ligament rupture OR ligament stiffness OR ligament reconstruction OR ligament elasticity OR anterior tibial displacement OR tendon injury OR tendon laxity OR tendon rupture OR tendon reconstruction OR tendon stiffness OR tendon elasticity OR cartilage injury OR cartilage repair OR cartilage regeneration OR joint laxity OR joint stiffness OR joint dislocation OR musculotendinous stiffness OR musculoskeletal injury OR sports injury OR soft tissue injury OR tendinopathy OR sprain OR cartilage degradation.
Muscle injury and strength terms: muscle strength OR muscle stiffness OR muscle force production OR muscle injury OR muscle voluntary contraction.
After compiling search results from all databases, studies were excluded if they were duplicates, if their titles and abstracts did not meet inclusion criteria, or if they were not written in English. Subsequently, studies were excluded if, upon review of the full article, they failed to meet inclusion criteria. Each included study was scored by 2 independent reviewers using the GRADEPro scoring system software.20 A custom template was designed based on the British Medical Journal Clinical Evidence GRADE (Grades of Recommendation Assessment, Development and Evaluation) scoring system5 and the GRADEPro guidelines.63 Following these guidelines, each study was evaluated as having very serious, serious, or nonserious flaws in the risk of bias, imprecision, inconsistency, and indirectness categories. Additionally, studies were assessed for effect size, dose response, publication bias, and adjustment for confounders. The findings were entered into the GRADEPro scoring system software, which provided a final GRADE score (very low, low, moderate, or high) for each article based on these inputs (Table 1).
TABLE 1.
Scoring Results
| Study | Risk of Bias | Imprecision | Indirectness | Other Considerations | GRADE Score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Blinding | Incomplete Reporting or Analysis | Methodological Bias | Dose Response | Adjustment for Confounders Increases Effect Size | Large Effect Size | ||||
| Soft tissue injury | |||||||||
| Gray et al25 | Yes | No | Flawed measurement | Not serious | Serious | No | Yes | Very large | High |
| Rahr-Wagner et al54 | Yes | No | Flawed measurement | Not serious | Not serious | Yes | Yes | Very large | High |
| Agel et al1 | No | Yes | None | Very serious | Serious | No | No | No | Very low |
| Dragoo et al14 | No | No | None | Serious | Serious | No | No | No | Very low |
| Liederbach et al44 | No | Yes | None | Serious | Serious | No | No | No | Very low |
| Ruedl et al58 | Yes | Yes | None | Serious | Serious | No | No | No | Very low |
| Holmes and Lin35 | No | Yes | None | Very serious | Serious | No | No | Very large | Very low |
| Soft tissue laxity | |||||||||
| Martineau et al47 | Yes | No | None | — | Not serious | No | Yes | Large | High |
| Lee et al41 | No | No | None | — | Not serious | No | No | Large | Low |
| Pokorny et al53 | Yes | No | None | — | Not serious | No | No | No | Low |
| Shultz et al64 | No | No | Confounding risk | — | Not serious | No | Yes | No | Low |
| Casey et al10 | Yes | Yes | None | — | Not serious | No | No | No | Very low |
| Hicks-Little et al33 | No | No | Confounding risk | — | Not serious | No | No | No | Very low |
| Lee et al42 | No | No | None | — | Not serious | No | No | No | Very low |
| Cammarata and Dhaher9 | No | No | None | — | Not serious | No | No | Large | Low |
| Bryant et al8 | Yes | No | Confounding risk, flawed measurement | — | Not serious | No | No | No | Very low |
| Hansen et al27 | No | Yes | Flawed measurement | — | Not serious | No | No | No | Very low |
| Muscle strength | |||||||||
| Wirth and Lohman68 | No | No | Confounding risk | — | Not serious | No | No | Very large | Low |
| Sarwar et al61 | No | No | Confounding risk | — | Not serious | No | No | Large | Very low |
| Ekenros et al17 | No | Yes | Confounding risk, flawed measurement | — | Not serious | No | No | No | Very low |
| Petrofsky et al51 | No | No | Confounding risk | — | Not serious | No | No | No | Very low |
| Elliott et al18 | No | No | Confounding risk | — | Not serious | No | No | No | Very low |
| Phillips et al52 | No | No | Confounding risk | — | Not serious | No | No | No | Very low |
| Savage and Clarkson62 | No | No | Confounding risk | — | Not serious | No | No | Very large | Low |
| Gordon et al24 | No | No | Confounding risk | — | Not serious | No | No | No | Very low |
| Nichols et al50 | Yes | No | None | — | Not serious | No | No | No | Low |
| Minahan et al48 | No | No | None | — | Not serious | No | No | No | Very low |
| Casey et al10 | Yes | Yes | None | — | Not serious | No | No | No | Very low |
| Allali et al2 | No | No | None | — | Serious | No | Yes | Large | Low |
In the risk of bias category, blinding, incomplete reporting, and methodological bias were considered. Studies were marked “serious” for having weaknesses in 1 of these sections and “very serious” for weaknesses in ≥2 sections. Studies in which over 10% of participants withdrew or failed to follow up, or studies that did not report data for all patients for whom data was collected, were downgraded for incomplete reporting. In the methodological bias section, appropriate exclusion criteria and potential confounders were assessed (Table 2). Studies were also downgraded for methodological bias if the exposed and nonexposed groups were not evaluated for the same amount of time, were evaluated differently, or were from different populations.
TABLE 2.
Methodological Bias Considerationsa
| Study | Age, y | BMI,b kg/m2 | Past/Current Pregnancy | Menstrual History | Gynecological History | MSK Injury History | Time on OC | Nonuser Group’s History of OC Use | Type and Dose of OC | Connective Tissue Disease History | Activity Levelc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soft tissue injury | |||||||||||
| Gray et al25 | 15-19 | — | Not in past year | Regular | None | —d | ≥3 mo | — | Many types | —d | — |
| Rahr-Wagner et al54 | 23.7-24.0b | — | —d | — | — | None | 1-≥5 y | None | Low dose | — | —d |
| Agel et al1 | — | — | — | — | — | None | — | — | Monophasic/triphasic | — | Competitive |
| Dragoo et al14 | 19.56b | —d | None | —d | — | — | — | — | — | — | Competitive |
| Liederbach et al44 | 18-41 | 19.5-20.5 | — | — | — | — | — | — | — | — | Competitive |
| Ruedl et al58 | 14-56 | 23.2-24.1 | — | Regular | — | —d | — | — | — | — | Recreational |
| Holmes and Lin35 | 35-44 | — | — | — | — | — | — | — | — | — | — |
| Soft tissue laxity | |||||||||||
| Martineau et al47 | 20.4b | 22.1-23.1 | None | Regular | — | None | ≥3 mo | Not in past 3 mo | Low-dose monophasic/triphasic | None | Competitive |
| Lee et al41 | 25.1-25.2b | 21.9-22.3 | — | Regular | None | None | ≥1 y | Not in past 1 y | Medium- or high-dose monophasic | None | Recreational |
| Pokorny et al53 | 20-25 | — | None | Regular | — | None | ≥3 mo | Not in past 3 mo | Low-dose monophasic/triphasic | — | Sedentary to recreational |
| Shultz et al64 | 20.7-23.5b | 23.7-24.8 | — | Regular | None | — | ≥3 mo | — | Medium- or low-dose monophasic/triphasic | None | Recreational |
| Casey et al10 | 24.0-24.1b | 21.8-22.7 | None | Regular | None | None | ≥6 mo | Not in past 6 mo | Medium- or low-dose monophasic/triphasic | None | Sedentary to recreational |
| Hicks-Little et al33 | 18-23 | — | — | Regular | — | None | — | — | — | — | Competitive |
| Lee et al42 | 24.7-25.1b | 21.0-21.6 | None | Regular | — | None | ≥6 mo | — | Medium- or low-dose monophasic/triphasic | — | Sedentary |
| Cammarata and Dhaher9 | 25.0-26.3b | 22.3-22.4 | — | — | — | None | ≥3 mo | — | Low-dose monophasic/triphasic | — | Recreational |
| Bryant et al8 | 28.0-31.9b | 22.1-23.5 | — | Regular | — | None | ≥1 y | Not in past 1 y | Low-dose monophasic | — | Recreational |
| Hansen et al27 | 22-23b | 23-24 | None | Regular | None | None | 3-10 y | Not in past 5 y | Low-dose monophasic | None | Competitive |
| Muscle strength | |||||||||||
| Wirth and Lohman68 | 18-33 | — | — | Regular | — | — | ≥6 mo | Not in past 1 y | Many types | — | — |
| Sarwar et al61 | 20.5-20.7b | 21.2-21.3 | — | — | — | — | ≥6 mo | — | Medium- or low-dose monophasic | — | Sedentary |
| Ekenros et al17 | 26-27b | 23.1-23.7 | — | Regular | — | None | 1 cycle | Not in past 3 moe | Low-dose monophasic | — | Recreational |
| Petrofsky et al51 | 24-26b | 20.7-22.4 | Regular | — | — | ≥3 mo | — | Monophasic | — | — | |
| Elliott et al18 | 22-24b | 28.1-28.5 | — | Regular | — | None | ≥6 mo | — | Medium- or low-dose monophasic | — | Sedentary |
| Phillips et al52 | 21-26b | 22.1-23.8 | — | Regular | — | — | ≥3 mo | — | — | — | Competitive |
| Savage and Clarkson62 | 20.8-22.3b | 22.4-23.2 | — | Regular | — | — | ≥3 mo | Not in past 3 mo | — | — | — |
| Gordon et al24 | 20.3-20.7b | 21.3-22.5 | — | Regular | — | — | ≥3 mo | — | Monophasic | — | — |
| Nichols et al50 | 18.7-20.0b | 24.8-25.0 | — | Regular | — | None | ≥3 mo | Not in past 3 mo | — | — | Competitive |
| Minahan et al48 | 20-22b | 21.9-22.1 | None | Regular | — | — | ≥1 y | — | Monophasic | — | Recreational |
| Casey et al10 | 24.0-24.1b | 21.8-22.7 | None | Regular | None | None | ≥6 mo | Not in past 6 mo | Medium- or low-dose monophasic/triphasic | None | Sedentary to recreational |
| Allali et al2 | 54-57b | 28.0-28.5 | —d | — | None | None | ≥2 y | None | — | — | — |
aBMI, body mass index; MSK, musculoskeletal; OC, oral contraceptive.
bMean value (if reported as a range, the range is bounded by user and nonuser mean values).
c“Competitive” includes collegiate athletes, elite dancers, competitive rowers, and semiprofessional handball players.
dAssessed as a covariate but no additional information presented.
eThis study was a crossover design; “not in past 3 mo” refers to the group of participants who began the study with a non–OC use cycle.
The imprecision category was only scored for studies evaluating the relationship between hormonal contraceptive use and soft tissue injuries. Adequately powered trials were designated as having met the optimal information size. Following guidelines in the GRADE handbook,63 articles were listed as having serious imprecision issues if they had fewer than 2000 participants and did not meet optimal information size calculations or if they met optimal information size calculations but their 95% CI included both “no effect” and a 25% difference in relative risk. As recommended in the British Medical Journal Clinical Evidence GRADE scoring system, studies were listed as having very serious imprecision issues if they also examined fewer than 200 injury events in addition to the aforementioned criteria.5 In the soft tissue laxity and muscle strength categories, it was discovered that all articles failed to meet the optimal information size. Therefore, all studies in these 2 categories scored “very low” overall according to the GRADEPro software. To distinguish between articles and to score them in a fashion that was useful for quality comparisons, imprecision was not evaluated in the soft tissue laxity and muscle injury/strength categories. Thus, in the GRADEPro software, all studies in the soft tissue laxity and muscle injury/strength categories were scored as having no problems with imprecision.
In the indirectness category, studies were evaluated for applicability to the general population. This review defined the general population as adult premenopausal women with a body mass index (BMI) near the female national average (28.5 kg/m2) 22 and with sedentary to low recreational activity levels. For the soft tissue injury category, studies were downgraded if their participants had a BMI considerably above the national average (>34 kg/m2) or had more than a low recreational activity level because increased BMI and activity level increase one’s risk of a soft tissue injury.36,39 None of the soft tissue laxity studies were graded down for indirectness, as little information exists on the impact of BMI on soft tissue laxity, and studies have reached contradictory conclusions on the impact of activity level on soft tissue laxity.23,65,67,69 Muscle injury/strength studies were graded down if the outcome measured was not generalizable to the other muscle strength study outcomes or if participants had more than a low recreational activity level because training can influence muscle strength.23,69 In the soft tissue injury section, studies that were downgraded in 1 area were scored as “serious,” and those that were downgraded in 2 areas were scored as “very serious.” In the muscle injury/strength section, to distinguish quality-of-evidence scores among this body of literature, studies downgraded in both areas were scored as “serious,” and no studies were scored as “very serious.”
Studies were then evaluated for inconsistency. Within a category (soft tissue injury, laxity, or muscle injury/strength), the overlap of studies’ 95% CIs was assessed. An article for which the 95% CI did not overlap with the 95% CIs of the other studies in its category was considered to have serious inconsistencies. However, it was determined that this analysis could only be performed in the soft tissue injury category because the units of the measured outcomes in the soft tissue laxity and muscle injury/strength sections varied greatly between studies. This made it unfeasible to fairly assess for consistency using the 95% CI overlap. Therefore, all studies in the soft tissue laxity and muscle injury/strength sections were listed as having no problems with inconsistency in the GRADEPro software. Additionally, it was found that the 95% CIs for studies in the soft tissue injury category were all overlapping; therefore, all studies were scored “not serious” for inconsistency. Because of this lack of differentiation, the inconsistency scores are not included in Table 1.
In the effect size category, studies were graded up 1 level if they had a P value <.01 and 2 levels for a P value <.001. Furthermore, studies were upgraded 1 level for exhibiting a dose response or showing that an adjustment for confounders increased the effect size. Too few articles were included in this review to evaluate for publication bias.
If authors stratified results or assessed outcomes under multiple conditions, the results presented in this review correspond to the conditions that produced the largest effect size. Additionally, when studies did not present a P value, a P value was calculated from the provided data using 1-way analysis of variance (see P values marked with a citation to endnote a). If data were collected at multiple points across the menstrual cycle, to appropriately compare studies that assessed different menstrual cycle phases with those that did not, the reviewers averaged these multiple measurements and presented the calculated means (see P values marked with a citation to endnote b).
Results
Search Results
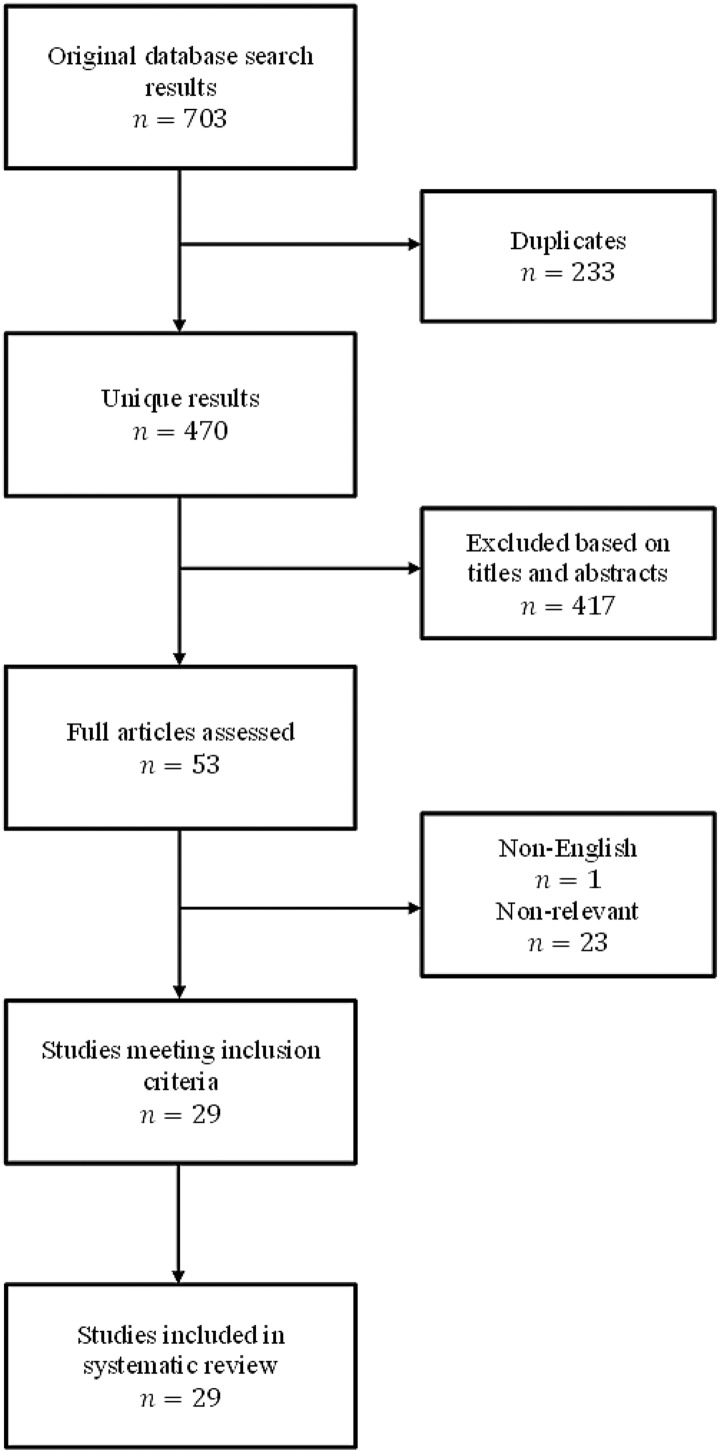
The database searches revealed 703 potentially relevant studies, but ultimately, only 29 studies met inclusion criteria for this review (Figure 1). Study characteristics for the included articles can be found in Table 3.
Figure 1.
Workflow of compiled database searches.
TABLE 3.
Study Characteristicsa
| Study | Outcome | Methodology: Design (LOE) or Instrumentation | Group 1’s Population | Group 2’s Population | Effect Size | P Value | Conclusion: OC Use on Outcome | GRADE Score |
|---|---|---|---|---|---|---|---|---|
| Soft tissue injury | ||||||||
| Gray et al25 | ACL injury rate | Cohort (3) | 5857 injured | 17,571 noninjured | OR = 0.82 | <.0001 | Beneficial | High |
| Rahr-Wagner et al54 | ACL injury rate | Cohort (3) | 785 injured | 8858 noninjured | OR = 0.75 | .0001b | Beneficial | High |
| Agel et al1 | ACL injury rate | Case-control (3) | 1124 OC users | 2026 nonusers | RR = 0.99 | .49b | No effect | Very low |
| Dragoo et al14 | ACL injury rate | Case-control (3) | 63 OC users | 65 nonusers | RR = 1.03 | >.05 | No effect | Very low |
| Liederbach et al44 | ACL injury rate | Case-control (3) | 136 OC users | 47 nonusers | RR = 0.35 | .13 | No effect | Very low |
| Ruedl et al58 | ACL injury rate | Cohort (3) | 93 injured | 93 noninjured | OR = 0.98 | >.05 | No effect | Very low |
| Holmes and Lin35 | Instance of Achilles tendinopathy | Case-control (3) | 13 injured | National database | —c | <.001 | Detrimental | Very low |
| Soft tissue laxity | ||||||||
| Martineau et al47 | Anterior tibial translation | KT-1000 arthrometer | 42 OC users | 36 nonusers | MD = –0.86 mm | .008 | Beneficial | High |
| Lee et al41 | Anterior tibial translation | KT-2000 arthrometer | 15 OC users | 25 nonusers | MD = 0.79 mm | .01 | Beneficial | Low |
| Pokorny et al53 | Anterior tibial translation | KT-1000 arthrometer | 30 OC users | 25 nonusers | MD = 0.20 mm | >.05 | No effect | Low |
| Shultz et al64 | Anterior tibial translation | KT-2000 arthrometer | 10 OC users | 10 nonusers | MD = 0.15 mmb ,d | .83b | No effect | Low |
| Casey et al10 | Anterior tibial translation | KT-1000 arthrometer | 11 OC users | 8 nonusers | MD = 0.10 mmb ,d | .88b | No effect | Very low |
| Hicks-Little et al33 | Anterior tibial translation | KT-1000 arthrometer | 25 OC users | 28 nonusers | MD = 0.17 mm | >.05 | No effect | Very low |
| Lee et al42 | Anterior tibial translation | KT-2000 arthrometer | 9 OC users | 10 nonusers | MD = –1.1 mm | <.05 | Beneficial | Very low |
| Cammarata and Dhaher9 | Frontal plane knee stiffness | Dynamometer chair | 9 OC users | 11 nonusers | MD = 0.012 N-m/deg | <.05 | Detrimental | Low |
| Bryant et al8 | Achilles tendon strain (% of resting length) | Ultrasound | 20 OC users | 20 nonusers | MD = –1.20% | <.05 | Beneficial | Very low |
| Hansen et al27 | Patellar tendon laxity | Ultrasound | 15 OC users | 15 nonusers | MD = 0.30 mm | .33 | No effect | Very low |
| Muscle strength | ||||||||
| Wirth and Lohman68 | Handgrip (maximum force output) | Transducer reading | 5 OC users | 10 nonusers | MD = –2955 N-s | <.05 | Detrimental | Low |
| Sarwar et al61 | Handgrip (maximum isometric strength) | Dynamometer | 10 OC users | 10 nonusers | MD = –57 Nb ,d | .002b | Detrimental | Very low |
| Ekenros et al17 | Handgrip (maximum isometric strength) | Dynamometer | 8 OC users | 9 nonusers | MD = –0.30 kg | .76 | No effect | Very low |
| Petrofsky et al51 | Handgrip (strength endurance) | Dynamometer | 2 OC users | 3 nonusers | MD = –68 sd | .064b | No effect | Very low |
| Elliott et al18 | First dorsal interosseous isometric strength | Dynamometer | 14 OC users | 7 nonusers | MD = 7.0 N | <.05 | Beneficial | Very low |
| Phillips et al52 | Adductor pollicis maximum voluntary contraction | Transducer reading | 5 OC users | 10 nonusers | MD = 12.89 N | .27b | No effect | Very low |
| Savage and Clarkson62 | Elbow flexor maximum isometric strength | Modified preacher curl bar | 8 OC users | 14 nonusers | MD = 75.9 N | <.05 | Beneficial | Low |
| Gordon et al24 | Knee flexor peak torque | Dynamometer chair | 6 OC users | 11 nonusers | MD = 2.15 N-md | .64b | No effect | Very low |
| Nichols et al50 | Knee extensor 10-repetition maximum strength | Leg extension machine | 13 OC users | 18 nonusers | MD = 1.0 kgb | .50b | No effect | Low |
| Minahan et al48 | Quadriceps peak isometric torque | Dynamometer chair | 8 OC users | 8 nonusers | MD = 6.4 N-m | .26 | No effect | Very low |
| Casey et al10 | Rectus femoris muscle stretch reflex | Electromyography recording | 11 OC users | 8 nonusers | Median = –0.67 1/Nb ,d,e | >.05b | No effect | Very low |
| Allali et al2 | Physical performance testing | Get Up and Go Test | 210 OC users | 200 nonusers | MD = –1.84 s | <.005 | Beneficial | Low |
aReported P values and effect sizes reflect the relationship exhibiting the most significance. ACL, anterior cruciate ligament; LOE, level of evidence; MD, mean difference; OC, oral contraceptive; OR, odds ratio; RR, relative risk.
bEffect size or P value was not reported; the effect size or P value presented in this table was supplied by the authors through email communications or was calculated by the reviewers based on the available figures or raw data.
cCould not calculate or was an estimate, given the data provided in the text.
dMeasurements were taken at several different points across the menstrual cycle; the reviewers averaged these multiple measurements.
eMedian is the difference in group median values.
Included studies evaluated the effect of OCs on ligament/tendon injuries, ligament/tendon laxity, or muscle strength. None of the included studies examined hormonal intrauterine devices, patches, or implants or other types of soft tissue such as cartilage. Only 2 studies evaluated muscle injuries, making it unfeasible to analyze.48,62 Muscle injury results were therefore not evaluated, although the studies themselves were included in the review because they also examined muscle strength.
Oral Contraceptive Use and Soft Tissue Injuries
Seven studies examined the effects of OCs on the incidence of soft tissue injuries: 2 concluded that OCs decrease the risk of ACL injuries (P < .000125 and P = .0001a,54), 4 found OCs to have no effect on ACL injury rates (P = .49,a,1 P > .05,14 P = .13,44 and P > .0558), and 1 determined that OCs increase the risk of developing Achilles tendinopathy (a chronic injury) (P < .001)35 (Table 3).
Gray et al25 stratified their results based on age and only obtained significant results in the 15- to 19-year age range (P < .0001); they did not find significant results in their other age groups. Rahr-Wagner et al54 stratified the user group based on the length of time that users had been on OCs, and the largest effect was observed when comparing nonusers to OC users who had been taking OCs for at least 3 years (P = .0001a). Similarly, Holmes and Lin,35 who found OCs to increase the risk of developing Achilles tendinopathy, discovered that this relationship was most significant in the 35- to 44-year age range (P < .001), with OC users in other age brackets having less significant or nonsignificant differences in tendinopathy occurrence compared with nonusers.
The 2 studies that found OCs to decrease the risk of ACL injuries (Gray et al25 and Rahr-Wagner et al54) had high quality-of-evidence scores according to the GRADE scoring system. The 5 remaining studies had very low quality-of-evidence scores (see Table 1).1,14,35,44,58 Furthermore, 2 of the studies that evaluated the soft tissue injury risk had features that made it challenging to assess their findings. In Agel et al,1 there were discrepancies between the tables and the text regarding the total number of basketball players and the number of basketball players who were on contraceptives. In addition, Holmes and Lin35 compared their 13 Achilles tendinopathy cases with an unexplained national database, which served as the control representing nontendinopathy cases. The number of patients in the national database was not stated, nor was it established that the database had been evaluated to confirm that it excluded patients who suffered from Achilles tendinopathy.35
Overall, the highest quality evidence currently available suggests that OC use decreases the risk of sustaining an ACL injury.25,54 Notably, this was found to be the case when participants had been on OCs for at least 3 months in 1 study25 and for 1 to 5 years in the other54 (see Table 2). It was not possible to determine any effect of OC dosing, as only 1 of these studies specified that the user group was taking only low-dose formulations,54 while the other included participants using a variety of doses.25 Most lower quality studies did not examine the type of OCs used or the length of time that patients were on contraceptives (see Table 2).14,35,44,58
Oral Contraceptive Use and Soft Tissue Laxity
Ten studies evaluated the relationship between OC use and soft tissue laxity. Of these studies, 4 found OCs to be associated with decreased soft tissue laxity: 3 found OCs to decrease anterior tibial translation (P = .008,47 P = .01,41 and P < .0542), and 1 determined that OCs decrease Achilles tendon strain (P < .05)8 (Table 3). In contrast, 1 study discovered that OCs increase frontal plane knee laxity (P < .05).9 The remaining 5 studies found no effect of OCs on soft tissue laxity10,27,33,53,64: 4 concluded that OCs do not affect anterior tibial translation (P > .05,53 P = .83,b,64 P = .88,b,10 and P > .0533), and 1 concluded that OCs do not affect patellar tendon laxity (P = .33)27 (Table 3).
Three articles assessed the effects of OCs under multiple conditions. Cammarata and Dhaher9 compared frontal plane knee laxity in users of monophasic OCs, users of triphasic OCs, and nonusers at multiple levels of varus and valgus torque. The most significant detrimental effect of OC use was demonstrated in the comparison of triphasic OC users with nonusers at 90% maximum varus torque, with results normalized for body weight and height (P < .05).9 Pokorny et al53 assessed anterior tibial translation at multiple loading levels and found the most significant difference between users and nonusers when evaluating the nondominant knee at a loading level of 89 N (P > .05). Hansen et al27 compared patellar tendon laxity in both the dominant and nondominant knees of OC users and nonusers. The most substantial difference between groups was found in the nondominant knees; however, the effect was still not significant (P = .33).27
Of all of the studies examining the effects of OC use on soft tissue laxity, only 1 was given a high quality-of-evidence score. That study concluded that OC use decreased anterior tibial translation.47 Four studies, concluding that OCs decrease frontal plane knee stiffness,9 decrease anterior tibial translation,41 or have no effect on anterior tibial translation,53,64 were graded as low quality. The other 5 studies were scored as very low quality; they concluded that OCs have no effect on soft tissue laxity10,27,33 or decrease laxity8,42 (see Table 1).
The data presentation in 3 studies from the soft tissue laxity category presented difficulties with analysis in this review. In Shultz et al64 and Casey et al,10 raw data were only presented in figures; no means, SDs, or 95% CIs were provided in the text. This is likely because both articles focused on assessing changes in knee laxity over the menstrual cycle, while this review was concerned with average laxity measurements across the menstrual cycle.
Ultimately, the only study in the soft tissue laxity category that earned a high quality-of-evidence score found that OCs decrease ACL laxity.47 Of note, the study participants had been on low-dose combined OCs for at least 3 months (see Table 2).47 The remaining studies received low or very low quality-of-evidence scores and reached a variety of conclusions. In summary, the highest quality research suggested that OC use decreases ACL laxity; however, this finding is limited by the lack of complementary high-quality studies.47
Oral Contraceptive Use and Muscle Strength
Twelve studies evaluated the impact of OC use on muscle strength: 3 concluded that OCs increase muscle strength, as measured by first dorsal interosseous isometric strength (P < .05),18 elbow flexor maximum isometric strength (P < .05),62 and physical performance testing (P < .005)2; 2 concluded that OCs decrease muscle strength, as measured by handgrip maximum force output (P < .05)68 and handgrip maximum isometric strength (P = .002b)61; and 7 found OCs to have no effect on muscle strength, as measured by handgrip maximum isometric strength (P = .76),17 handgrip strength endurance (P = .064b),51 adductor pollicis maximum voluntary contraction (P = .27a),52 knee extensor 10-repetition maximum strength (P = .50a),50 knee flexor peak torque (P = .64b),24 quadriceps peak isometric torque (P = .26),48 and rectus femoris muscle stretch reflex (P > .05)b,10 (Table 3).
Gordon et al24 assessed both knee flexors and extensors and stratified their study to measure peak torque at various muscle contraction speeds. The difference between OC users and nonusers was largest for the performance of knee flexors measured at a rotational speed of 240 deg/s; however, this difference was nonsignificant (P = .64b).24 Nichols et al50 evaluated several outcomes, including maximum 1-repetition bench press and maximum 10-repetition knee extension strength. This study also evaluated changes in strength parameters over the course of a specialized training program.50 We only considered the baseline measurements in the study of Nichols et al50 because the patients’ subsequent participation in the training program made their later performances less comparable with those of the participants in other studies. The largest difference between OC users and nonusers was in the assessment of 10-repetition knee extension strength.50 However, this difference was still nonsignificant (P = .50a).50 Elliott et al18 examined the maximum voluntary contraction strength of patients’ quadriceps, hamstring, and first dorsal interosseous muscles. The most significant discrepancies between OC users and nonusers were apparent in the first dorsal interosseous strength outcome: users were found to be significantly stronger than their nonuser peers (P < .05).18
Of the 12 studies assessing the influence of OCs on muscle strength, 4 were given low quality-of-evidence scores, and 8 were given very low quality-of-evidence scores. Two of the 4 studies with low scores found that OCs were beneficial for muscle strength (P < .0052 and P < .0562), 1 found that OCs were detrimental (P < .05),68 and 1 found that OCs have no effect (P = .50a).50 The remaining 8 studies, all of which were of very low quality, determined that OCs were beneficial (P < .05),18 that OCs were detrimental (P = .002b),61 or that there was no association between OC use and muscle strength outcomes (P = .76,17 P = .064,b,51 P = .27,a,52 P = .64,b,24 P = .26,48 and P > .05b,10).
In 2 studies that evaluated the influence of OCs on muscle strength, various features of the data presentation complicated the interpretation of the results. Gordon et al24 examined peak torque generated by knee flexors and knee extensors; however, the extensor data reported for the OC user group were identical to the extensor data reported for the nonuser group. Sarwar et al61 did not provide any form of raw numerical data or statistical analysis; a graph displaying handgrip strength versus menstrual cycle phase was the only quantitative information provided. As with the soft tissue laxity section, Sarwar et al61 focused on examining other outcomes in addition to this review’s outcomes of interest, possibly explaining the presence of data presentation features that imparted challenges for this review.
In summary, the available research concerning the relationship between OC use and muscle strength is of low or very low quality on the GRADE scoring system. This body of literature presents contradictory conclusions; thus, it remains unclear whether OCs have beneficial,2,18,62 detrimental,61,68 or insignificant effects on muscle strength.10,17,24,48,50–52 Ultimately, no conclusions regarding the influence of OC use on muscle strength can be made because of the lack of high-quality literature on the topic.
Discussion
This review analyzed studies evaluating the impact of OC use on soft tissue injuries, soft tissue laxity, and muscle strength. Two conclusions were reached: (1) OC use may act to decrease the rate of ACL injuries, and (2) OC use may act to decrease anterior tibial translation.
The 2 studies that determined that OC use decreases ACL injury rates25,54 earned high quality-of-evidence scores. These studies required their participants to have been on OCs for at least 3 months25 or 1 to 5 years,54 and in 1 study, all of the OC users had been taking low-dose formulations.54 In contrast, all other studies evaluating OC use and ACL injuries had very low quality-of-evidence scores and found OC use to have no effect.1,14,44,58
Another systematic review of the literature by Herzberg et al31 analyzed the relationship between OC use and ACL injuries. Although a less extensive scoring system that only examined the risk of bias on a scale of good, fair, or poor was used and methodological biases were not dissected (see Table 2), the authors reached similar conclusions to the present review. Their results support the findings from the higher quality studies of Rahr-Wagner et al54 and Gray et al25 that OC use may act to decrease ACL injury rates.31 Notably, the authors did not analyze the studies of Dragoo et al14 and Liederbach et al,44 although 3 other studies were alternatively reviewed.4,43,70 These 3 studies examined the influence of OC use on ACL injuries at a particular phase in the menstrual cycle but were found to be of poor quality, making their results difficult to interpret.31
One method by which OCs may reduce the ACL injury risk is through decreasing the production of relaxin. OCs inhibit ovulation, thereby inhibiting the formation of a corpus luteum,18,30 which is the main site of relaxin production.12 Relaxin is a hormone with catabolic properties at the ACL21,38; elevated levels have been associated with increased ACL injury rates in female athletes.12,14 Therefore, in theory, OCs that better inhibit ovulation, such as combined pills compared with progestin-only pills,56 may be better at decreasing the ACL injury risk because increased ovulation suppression should better decrease relaxin levels. However, the only study that compared progestin-only pills with combined pills found no statistically significant difference in ACL injury rates between groups.25
Another method by which OCs may influence ACL injury risk is by inhibiting the hypothalamic-pituitary-gonadal axis, decreasing cyclic fluctuations of estrogen.30 With cycling suppressed, estrogen levels never peak, and endogenous estrogen production decreases.30 Estrogen exhibits catabolic properties at the ACL72 and acts synergistically with relaxin.60 Thus, a decreased ACL injury risk in female patients after OC use is likely the product of a combined decrease in both relaxin and estrogen, and OC regimens that include both estrogen and progestin should theoretically decrease the ACL injury risk more substantially if the estrogen dosage is lower. Of the 2 high-quality studies examining OC use and ACL injuries, 1 study did in fact find that OC use decreased ACL injuries with all patients on low-dose estrogen.54 Although the estrogen dose was not compared, the other study compared monophasic to triphasic users and found monophasic users to have a decreased risk of ACL injuries.25 Triphasic contraceptives contain substantially lower progesterone levels for the earlier parts of the 28-day cycle than monophasic formulas.55 It is possible that the monophasic users had a lower rate of ACL injuries than the triphasic users because they were exposed to more progesterone. Progesterone has in fact been shown to act opposite to estrogen and relaxin at the ACL, decreasing ACL laxity57 and decreasing relaxin-induced collagen degradation.13,29,60 Another study included OC users on monophasic or triphasic OCs but, because of inadequate power, was unable to compare these types of formulations or estrogen dosage and was notably graded as very low quality in this review.1 No other studies examined the influence of OC dosage on ACL injuries (see Table 2).
A similar pattern of conclusions was found in the soft tissue laxity group to the soft tissue injury group. The only study in the soft tissue laxity group that was given a high quality-of-evidence score found that OCs were beneficial.47 Analyzing participants who had taken low-dose combined OCs for at least 3 months, this study concluded that OC use decreased anterior tibial translation.47 In contrast, the other 6 studies evaluating OC use and anterior tibial translation received either low or very low quality-of-evidence scores and concluded that OCs had either beneficial or insignificant effects.10,33,41,42,53,64
As with the high-quality ACL injury studies,25,54 it is likely that the high-quality anterior tibial translation study47 found OCs to decrease anterior tibial translation because of a similar physiological mechanism. Anterior tibial translation is commonly used as a proxy for ACL laxity.10,33 Because of the aforementioned hormonal mechanisms, shifting the metabolic balance away from catabolism with OCs would result in an increase in ACL collagen content. As collagen is the main structural component of the ACL,38 this shift would result in a ligament with greater structural integrity that is less prone to both damage and laxity. Reduced ACL laxity would manifest as decreased anterior tibial translation.
Approximately 35% of American women of reproductive age already use hormonal contraception, with OCs being the most popular nationwide.11 Prescribing OCs would thus be an efficient and easily implementable strategy for reducing the ACL injury prevalence in high-risk female patients.49 This is especially important, given that female patients are up to 8 times more likely than male patients to sustain an ACL tear.3,26 However, it remains poorly understood whether there are particular female patients who may be most responsive to the effects of OCs. Perhaps OCs can significantly decrease the ACL injury risk in nonpregnant female patients with higher than average relaxin levels but not in those with low relaxin levels. Moreover, it is unclear whether different contraceptive formulations may affect ACL injuries differently depending on the route of administration, estrogen or progestin dose, estrogen or progestin type, or administration schedule. The duration of a patient’s hormonal contraceptive use may also alter her predisposition to ACL injuries.
Furthermore, this review examined the influence of OC use compared with nonusers, irrespective of the menstrual cycle phase in nonusers. There is currently debate in the literature regarding the influence of menstrual cycle phase on ACL injuries and laxity. Some of the studies included in this review primarily examined OC use compared with controls at certain menstrual cycle phases and found statistically significant differences when looking at menstrual cycle phase but not when looking at the results in aggregate across the entire menstrual cycle.51,64 Furthermore, a 2017 meta-analysis found increased ACL laxity during the ovulatory phase of the menstrual cycle, raising the possibility that the results in this review may be confounded by menstrual cycle phase.31 However, none of the studies included in this meta-analysis were of high quality; all studies were graded as fair quality on a scale of good, fair, or poor.31 This study also systematically reviewed the influence of OC use compared with nonusers on ACL injuries, examining menstrual cycle phase in nonusers, but found all studies examining menstrual cycle phase to be of poor quality.31 Additional higher quality studies are needed to better understand the influence of menstrual cycle phase on soft tissue health and its role as a potential confounder in the literature.
Of the studies evaluating the effects of OC use on muscle strength, 3 found OC use to increase muscle strength,2,18,62 2 found OC use to decrease muscle strength,61,68 and the remaining 7 studies found OC use to have no effect on muscle strength.10,17,24,48,50–52 Because all 12 studies in the muscle strength group received either low or very low quality-of-evidence scores and examined a disparate assortment of muscle types, it was not possible to make definitive conclusions regarding the effects of OCs on muscle strength.
Conclusion
This review ultimately found an association between OC use and a decrease in both ACL injury rates and anterior tibial translation. The clinical applicability of these findings lies in the possibility that OCs could act to decrease female patients’ risk of ACL injuries. The literature is, however, limited by the lack of any multicenter randomized controlled trials and also by the low number of high-quality studies. The lack of high-quality studies is particularly evident in the evaluation of OC use as it pertains to muscle strength. Further research is also required to verify the OC-ACL relationship and to better understand the mechanism by which OCs may decrease ACL injury risk.
Notes
The P value was not reported; the reviewers calculated this P value based on the available data.
The outcome was assessed at various points of the menstrual cycle; the reviewers took the means of these multiple measurements and calculated P values using these means.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: J.L.D. is a consultant for DePuy/Medical Device Business Services, Exactech, RTI Surgical, and Zimmer Biomet. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Agel J, Bershadsky B, Arendt EA. Hormonal therapy: ACL and ankle injury. Med Sci Sports Exerc. 2006;38(1):7–12. [DOI] [PubMed] [Google Scholar]
- 2. Allali F, El Mansouri L, Abourazzak F, et al. The effect of past use of oral contraceptive on bone mineral density, bone biochemical markers and muscle strength in healthy pre and post menopausal women. BMC Womens Health. 2009;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23(6):694–701. [DOI] [PubMed] [Google Scholar]
- 4. Arendt EA, Bershadsky B, Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. J Gend Specif Med. 2002;5(2):19–26. [PubMed] [Google Scholar]
- 5. BMJ Best Practice. What is GRADE? Available at: https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade/. Accessed January 24, 2019.
- 6. Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2010;20(5):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bridgeman JT, Zhang Y, Donahue H, Wade AM, Juliano PJ. Estrogen receptor expression in posterior tibial tendon dysfunction: a pilot study. Foot Ankle Int. 2010;31(12):1081–1084. [DOI] [PubMed] [Google Scholar]
- 8. Bryant AL, Clark RA, Bartold S, et al. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol. 2008;105(4):1035–1043. [DOI] [PubMed] [Google Scholar]
- 9. Cammarata ML, Dhaher YY. The differential effects of gender, anthropometry, and prior hormonal state on frontal plane knee joint stiffness. Clin Biomech (Bristol, Avon). 2008;23(7):937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casey E, Hameed F, Dhaher YY. The muscle stretch reflex throughout the menstrual cycle. Med Sci Sports Exerc. 2014;46(3):600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015;86:1–14. [PubMed] [Google Scholar]
- 12. Dehghan F, Haerian BS, Muniandy S, Yusof A, Dragoo JL, Salleh N. The effect of relaxin on the musculoskeletal system. Scand J Med Sci Sports. 2014;24(4):e220–e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dehghan F, Muniandy S, Yusof A, Salleh N. Sex-steroid regulation of relaxin receptor isoforms (RXFP1 & RXFP2) expression in the patellar tendon and lateral collateral ligament of female WKY rats. Int J Med Sci. 2014;11(2):180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dragoo JL, Castillo TN, Braun HJ, Ridley BA, Kennedy AC, Golish SR. Prospective correlation between serum relaxin concentration and anterior cruciate ligament tears among elite collegiate female athletes. Am J Sports Med. 2011;39(10):2175–2180. [DOI] [PubMed] [Google Scholar]
- 15. Dragoo JL, Lee RS, Benhaim P, Finerman GA, Hame SL. Relaxin receptors in the human female anterior cruciate ligament. Am J Sports Med. 2003;31(4):577–584. [DOI] [PubMed] [Google Scholar]
- 16. Dragoo JL, Padrez K, Workman R, Lindsey DP. The effect of relaxin on the female anterior cruciate ligament: analysis of mechanical properties in an animal model. Knee. 2009;16(1):69–72. [DOI] [PubMed] [Google Scholar]
- 17. Ekenros L, Hirschberg AL, Heijne A, Friden C. Oral contraceptives do not affect muscle strength and hop performance in active women. Clin J Sport Med. 2013;23(3):202–207. [DOI] [PubMed] [Google Scholar]
- 18. Elliott KJ, Cable NT, Reilly T. Does oral contraceptive use affect maximum force production in women? Br J Sports Med. 2005;39(1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engstrom B, Johansson C, Tornkvist H. Soccer injuries among elite female players. Am J Sports Med. 1991;19(4):372–375. [DOI] [PubMed] [Google Scholar]
- 20. Evidence Prime. GRADEpro GDT: GRADEpro Guideline Development Tool [computer program]. Hamilton, Canada: McMaster University; 2015. [Google Scholar]
- 21. Faryniarz DA, Bhargava M, Lajam C, Attia ET, Hannafin JA. Quantitation of estrogen receptors and relaxin binding in human anterior cruciate ligament fibroblasts. In Vitro Cell Dev Biol Anim. 2006;42(7):176–181. [DOI] [PubMed] [Google Scholar]
- 22. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007-2010. Vital Health Stat 11. 2012;252:1–48. [PubMed] [Google Scholar]
- 23. Gentil P, de Lira CAB, Filho SGC, et al. High intensity interval training does not impair strength gains in response to resistance training in premenopausal women. Eur J Appl Physiol. 2017;117(6):1257–1265. [DOI] [PubMed] [Google Scholar]
- 24. Gordon D, Hughes F, Young K, et al. The effects of menstrual cycle phase on the development of peak torque under isokinetic conditions. Isokinet Exerc Sci. 2013;21(4):285–291. [Google Scholar]
- 25. Gray AM, Gugala Z, Baillargeon JG. Effects of oral contraceptive use on anterior cruciate ligament injury epidemiology. Med Sci Sports Exerc. 2016;48(4):648–654. [DOI] [PubMed] [Google Scholar]
- 26. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98–102. [DOI] [PubMed] [Google Scholar]
- 27. Hansen M, Couppe C, Hansen CSE, et al. Impact of oral contraceptive use and menstrual phases on patellar tendon morphology, biochemical composition, and biomechanical properties in female athletes. J Appl Physiol (1985). 2013;114(8):998–1008. [DOI] [PubMed] [Google Scholar]
- 28. Hansen M, Miller BF, Holm L, et al. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol (1985). 2009;106(4):1435–1443. [DOI] [PubMed] [Google Scholar]
- 29. Hashem G, Zhang Q, Hayami T, Chen J, Wang W, Kapila S. Relaxin and beta-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: progesterone prevents matrix loss. Arthritis Res Ther. 2006;8(4):R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hatcher RA, Nelson AL. Contraceptive Technology. New York: Ardent Media; 2007. [Google Scholar]
- 31. Herzberg SD, Motu’apuaka ML, Lambert W, Fu R, Brady J, Guise JM. The effect of menstrual cycle and contraceptives on ACL injuries and laxity: a systematic review and meta-analysis. Orthop J Sports Med. 2017;5(7):2325967117718781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hewett TE. Neuromuscular and hormonal factors associated with knee injuries in female athletes: strategies for intervention. Sports Med. 2000;29(5):313–327. [DOI] [PubMed] [Google Scholar]
- 33. Hicks-Little CA, Thatcher JR, Hauth JM, Goldfuss AJ, Cordova ML. Menstrual cycle stage and oral contraceptive effects on anterior tibial displacement in collegiate female athletes. J Sports Med Phys Fitness. 2007;47(2):255–260. [PubMed] [Google Scholar]
- 34. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane Collaboration; 2011. [Google Scholar]
- 35. Holmes GB, Lin J. Etiologic factors associated with symptomatic Achilles tendinopathy. Foot Ankle Int. 2006;27(11):952–959. [DOI] [PubMed] [Google Scholar]
- 36. Hootman JM, Macera CA, Ainsworth BE, Martin M, Addy CL, Blair SN. Association among physical activity level, cardiorespiratory fitness, and risk of musculoskeletal injury. Am J Epidemiol. 2001;154(3):251–258. [DOI] [PubMed] [Google Scholar]
- 37. Hulkko A, Orava S. Stress fractures in athletes. Int J Sports Med. 1987;8(3):221–226. [DOI] [PubMed] [Google Scholar]
- 38. Konopka JA, DeBaun MR, Chang W, Dragoo JL. The intracellular effect of relaxin on female anterior cruciate ligament cells. Am J Sports Med. 2016;44(9):2384–2392. [DOI] [PubMed] [Google Scholar]
- 39. Kortt M, Baldry J. The association between musculoskeletal disorders and obesity. Aust Health Rev. 2002;25(6):207–214. [DOI] [PubMed] [Google Scholar]
- 40. Lee CY, Liu X, Smith CL, et al. The combined regulation of estrogen and cyclic tension on fibroblast biosynthesis derived from anterior cruciate ligament. Matrix Biol. 2004;23(5):323–329. [DOI] [PubMed] [Google Scholar]
- 41. Lee H, Petrofsky JS, Daher N, Berk L, Laymon M. Differences in anterior cruciate ligament elasticity and force for knee flexion in women: oral contraceptive users versus non-oral contraceptive users. Eur J Appl Physiol. 2014;114(2):285–294. [DOI] [PubMed] [Google Scholar]
- 42. Lee H, Petrofsky JS, Yim J. Do oral contraceptives alter knee ligament damage with heavy exercise? Tohoku J Exp Med. 2015;237(1):51–56. [DOI] [PubMed] [Google Scholar]
- 43. Lefevre N, Bohu Y, Klouche S, Lecocq J, Herman S. Anterior cruciate ligament tear during the menstrual cycle in female recreational skiers. Orthop Traumatol Surg Res. 2013;99(5):571–575. [DOI] [PubMed] [Google Scholar]
- 44. Liederbach M, Dilgen FE, Rose DJ. Incidence of anterior cruciate ligament injuries among elite ballet and modern dancers: a 5-year prospective study. Am J Sports Med. 2008;36(9):1779–1788. [DOI] [PubMed] [Google Scholar]
- 45. Liu SH, al-Shaikh R, Panossian V, et al. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14(4):526–533. [DOI] [PubMed] [Google Scholar]
- 46. MacLennan AH. Relaxin: a review. Aust N Z J Obstet Gynaecol. 1981;21(4):195–202. [DOI] [PubMed] [Google Scholar]
- 47. Martineau P, Al-Jassir F, Lenczner E, Burman M. Effect of the oral contraceptive pill on ligamentous laxity. Clin J Sport Med. 2004;14(5):281–286. [DOI] [PubMed] [Google Scholar]
- 48. Minahan C, Joyce S, Bulmer AC, Cronin N, Sabapathy S. The influence of estradiol on muscle damage and leg strength after intense eccentric exercise. Eur J Appl Physiol. 2015;115(7):1493–1500. [DOI] [PubMed] [Google Scholar]
- 49. Nappi RE, Kaunitz AM, Bitzer J. Extended regimen combined oral contraception: a review of evolving concepts and acceptance by women and clinicians. Eur J Contracept Reprod Health Care. 2016;21(2):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nichols AW, Hetzler RK, Villanueva RJ, Stickley CD, Kimura IF. Effects of combination oral contraceptives on strength development in women athletes. J Strength Cond Res. 2008;22(5):1625–1632. [DOI] [PubMed] [Google Scholar]
- 51. Petrofsky JS, LeDonne DM, Rinehart JS, Lind AR. Isometric strength and endurance during the menstrual cycle. Eur J Appl Physiol Occup Physiol. 1976;35(1):1–10. [DOI] [PubMed] [Google Scholar]
- 52. Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol. 1996;496(2):551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pokorny MJ, Smith TD, Calus SA, Dennison EA. Self-reported oral contraceptive use and peripheral joint laxity. J Orthop Sports Phys Ther. 2000;30(11):683–692. [DOI] [PubMed] [Google Scholar]
- 54. Rahr-Wagner L, Thillemann TM, Mehnert F, Pedersen AB, Lind M. Is the use of oral contraceptives associated with operatively treated anterior cruciate ligament injury? A case-control study from the Danish Knee Ligament Reconstruction Registry. Am J Sports Med. 2014;42(12):2897–2905. [DOI] [PubMed] [Google Scholar]
- 55. Rechichi C, Dawson B, Goodman C. Athletic performance and the oral contraceptive. Int J Sports Physiol Perform. 2009;4(2):151–162. [DOI] [PubMed] [Google Scholar]
- 56. Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5 pt 1):1263–1269. [DOI] [PubMed] [Google Scholar]
- 57. Romani W, Patrie J, Curl LA, Flaws JA. The correlations between estradiol, estrone, estriol, progesterone, and sex hormone-binding globulin and anterior cruciate ligament stiffness in healthy, active females. J Womens Health (Larchmt). 2003;12(3):287–298. [DOI] [PubMed] [Google Scholar]
- 58. Ruedl G, Ploner P, Linortner I, et al. Are oral contraceptive use and menstrual cycle phase related to anterior cruciate ligament injury risk in female recreational skiers? Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1065–1069. [DOI] [PubMed] [Google Scholar]
- 59. Sallis RE, Jones K, Sunshine S, Smith G, Simon L. Comparing sports injuries in men and women. Int J Sports Med. 2001;22(6):420–423. [DOI] [PubMed] [Google Scholar]
- 60. Samuel CS, Butkus A, Coghlan JP, Bateman JF. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137(9):3884–3890. [DOI] [PubMed] [Google Scholar]
- 61. Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(1):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Savage KJ, Clarkson PM. Oral contraceptive use and exercise-induced muscle damage and recovery. Contraception. 2002;66(1):67–71. [DOI] [PubMed] [Google Scholar]
- 63. Schunemann H, Brozek J, Guyatt G, Oxman A, ed. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Hamilton, Canada: GRADE Working Group; 2013. [Google Scholar]
- 64. Shultz SJ, Wideman L, Montgomery MM, Beasley KN, Nindl BC. Changes in serum collagen markers, IGF-I, and knee joint laxity across the menstrual cycle. J Orthop Res. 2012;30(9):1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Skinner HB, Wyatt MP, Stone ML, Hodgdon JA, Barrack RL. Exercise-related knee joint laxity. Am J Sports Med. 1986;14(1):30–34. [DOI] [PubMed] [Google Scholar]
- 66. Slauterbeck J, Clevenger C, Lundberg W, Burchfield DM. Estrogen level alters the failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17(3):405–408. [DOI] [PubMed] [Google Scholar]
- 67. Steiner ME, Grana WA, Chillag K, Schelberg-Karnes E. The effect of exercise on anterior-posterior knee laxity. Am J Sports Med. 1986;14(1):24–29. [DOI] [PubMed] [Google Scholar]
- 68. Wirth JC, Lohman TG. The relationship of static muscle function to use of oral contraceptives. Med Sci Sports Exerc. 1982;14(1):16–20. [DOI] [PubMed] [Google Scholar]
- 69. Wirth K, Keiner M, Hartmann H, Sander A, Mickel C. Effect of 8 weeks of free-weight and machine-based strength training on strength and power performance. J Hum Kinet. 2016;53:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wojtys EM, Huston LJ, Boynton MD, Spindler KP, Lindenfeld TN. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30(2):182–188. [DOI] [PubMed] [Google Scholar]
- 71. Woodhouse E, Schmale GA, Simonian P, Tencer A, Huber P, Seidel K. Reproductive hormone effects on strength of the rat anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):453–460. [DOI] [PubMed] [Google Scholar]
- 72. Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001;383:268–281. [DOI] [PubMed] [Google Scholar]