Abstract
Background:
Bridge-enhanced anterior cruciate ligament repair (BEAR) combines suture repair of the anterior cruciate ligament (ACL) with a specific extracellular matrix scaffold (the BEAR scaffold) that is placed in the gap between the torn ends of the ACL to facilitate ligament healing.
Purpose/Hypothesis:
The purpose of this study was to report the 12- and 24-month outcomes of patients who underwent the BEAR procedure compared with a nonrandomized concurrent control group who underwent ACL reconstruction (ACLR) with an autograft. We hypothesized that the BEAR group would have physical examination findings, patient-reported outcomes, and adverse events that were similar to those of the ACLR group.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
Ten patients underwent BEAR, and 10 underwent ACLR with a 4-stranded hamstring autograft. At 24 months, 9 of the 10 BEAR patients and 7 of the 10 ACLR patients completed a study visit. Outcomes reported included International Knee Documentation Committee (IKDC) subjective and objective results, knee anteroposterior (AP) laxity findings via an arthrometer, and functional outcomes.
Results:
There were no graft or repair failures in the first 24 months after surgery. The IKDC subjective scores in both groups improved significantly from baseline (P < .0001) at 12 and 24 months, to 84.6 ± 17.2 in the ACLR group and to 91.7 ± 11.7 in the BEAR group. An IKDC objective grade of A (normal) was found in 44% of patients in the BEAR group and in 29% of patients in the ACLR group at 24 months; no patients in either group had C (abnormal) or D (severely abnormal) grades. Arthrometer testing demonstrated mean side-to-side differences in AP laxity that were similar in the 2 groups at 24 months (BEAR, 1.94 ± 2.08 mm; ACLR, 3.14 ± 2.66 mm). Functional hop testing results were similar in the 2 groups at 12 and 24 months after surgery. Hamstring strength indices were significantly higher in the BEAR group compared with the ACLR group (P = .0001).
Conclusion:
In this small, first-in-human study, BEAR produced similar outcomes to ACLR with a hamstring autograft. BEAR may result in knee stability and patient-reported outcomes at 2 years sufficient to warrant longer term studies of efficacy in larger groups of patients.
Keywords: anterior cruciate ligament, human, ACL reconstruction, ACL repair, bridge-enhanced ACL repair, BEAR
Injuries to the anterior cruciate ligament (ACL) in active patients are currently treated with ACL reconstruction (ACLR) using a tendon graft, typically an autologous hamstring or patellar tendon. The outcomes of ACLR are commonly evaluated via patient-reported outcome measures and a physical examination. The results of ACLR have been reported to be good, with over 60% of reconstructed patients able to return to their preinjury level of sports participation.6
The International Knee Documentation Committee (IKDC) subjective knee form27–29,53 and the Knee injury and Osteoarthritis Outcome Score (KOOS)34,45,52 are patient-reported outcome instruments designed for use in the athletic population. The IKDC subjective score has been found to increase from an average baseline (after injury) of 45 points to approximately 60 points 1 year after ACLR,33 and then to approximately 80 points by 2 years,13 in which uninjured patients aged 18 to 35 years have a score closer to 89 in men and 86 in women.4 The KOOS Sports subscore has been shown to increase from a preoperative average of 55 to an average of 80 at 1 and 2 years postoperatively,13,16 and the KOOS Knee-Related Quality of Life subscore increases from 35 to 70 at 1 year16 and 2 years13 (with noninjured controls averaging 98 points for both subscores).16 Thus, patients experience a significant improvement in patient-reported outcomes after ACLR with an autograft; however, knees do not return to normal even at 2 years after surgery.
After ACLR, approximately 90% of patients can expect to have an IKDC objective grade of A (normal) or B (nearly normal) after ACLR.12,16,18,38 However, the percentage of patients who have a “normal” result (IKDC grade A, <3° loss of extension, <5° loss of flexion, <2-mm side-to-side difference in anteroposterior [AP] laxity, pivot shift symmetric with the contralateral uninjured side, and no effusion) has been reported to be 51% at 6 months,18 43% to 70% at 2 to 3 years,12,16 and 20% at 5 years after ACLR.38
We recently completed a first-in-human safety study of a new ACL surgical alternative to ACLR in which suture repair is combined with a specific extracellular matrix scaffold (the BEAR scaffold), which is placed in the space between the 2 torn ends of the ACL and activated with the patient’s blood.41 This technique is called bridge-enhanced anterior cruciate ligament repair (BEAR). The scaffold is used to bridge the gap between the 2 torn ends of the ligament, so absolute reapproximation is not required for healing.30,37,40 In animal models, the repaired ACL was noted to gradually change from a relatively disorganized fibrovascular scar to a more highly aligned collagenous structure over the first year after surgery.8,30,47 The BEAR technique has shown comparable mechanical properties with ACLR as well as a lower incidence of posttraumatic osteoarthritis in preclinical models.40,59 In addition, the BEAR technique does not require the compromise of other healthy tissues around the knee, as is required with ACLR with an autograft. Prior studies of ACLR performed with a hamstring autograft have demonstrated hamstring strength losses of 10% to 50% at time points between 1 and 3 years after surgery.2,19,20,23 Thus, one possible benefit of the BEAR technique may be less loss of hamstring strength when compared with ACLR with a hamstring autograft.
We previously reported the initial 3-month safety outcomes of this procedure.41 The current study assesses these same patients (10 who underwent BEAR and 10 who underwent ACLR) at the 12- and 24-month postoperative time points. The objectives of this report were to present the patient-reported outcomes, physical examination findings, and functional outcomes for these 2 groups of patients. We hypothesized that the outcomes would be similar between the groups.
Methods
Trial Design
An investigational device exemption (G140151) from the Food and Drug Administration (FDA) and institutional review board approval from Boston Children’s Hospital were obtained before initiating the study. The trial was registered at ClinicalTrials.gov (NCT02292004). All patients provided informed consent before participating. The cohort study was designed under the guidance of the FDA as an interventional, parallel-assignment, nonrandomized, first-in-human trial for the BEAR technique. For all physical examinations and functional testing, the examiner was blinded to the group assignment and operative knee. All surgical procedures were performed at a single site (Boston Children’s Hospital) by a single surgeon (L.J.M.). Ten patients were enrolled in the interventional (BEAR) group and 10 in the control (ACLR) group. Enrollment was completed from February to October 2015. Patients were evaluated preoperatively, intraoperatively, and postoperatively at 6, 12, and 24 months after surgery. These 2 groups were previously reported at 2 weeks, 6 weeks, and 3 months,41 and those results justified this longer follow-up.
Participants and Inclusion Criteria
Patients aged 18 to 35 years with a complete ACL tear who were less than 1 month from injury and who had at least 50% of the length of the ACL attached to the tibia on preoperative magnetic resonance imaging (MRI) were eligible to enroll in the BEAR group.41 As the ACL remnant is commonly removed during ACLR, and thus resorption of the torn ACL over time was not as critical, patients with a complete ACL tear who were within 3 months of injury were eligible to enroll in the ACLR group. Patients with a partial ACL tear were not eligible. Patients were excluded from either group if they had a history of surgery on the knee, had a history of infections in the knee, or had risk factors that might adversely affect healing.41 Patients were excluded if they had a displaced bucket-handle tear of the medial meniscus that required repair; all other meniscal injuries were included. Patients were excluded if they had a full-thickness chondral injury, a grade III medial collateral ligament injury, a concurrent complete patellar dislocation, or an operative posterolateral corner injury. Patients in the BEAR group were also excluded at the time of surgery if they were found to have less than 50% of the length of the ACL still attached to the tibial footprint.
A total of 242 patients presenting with an ACL injury were screened for participation in this study. Of these patients, 22 were enrolled in the study, of whom 2 were excluded before surgery: 1 was excluded because of a history of corticosteroid use not discovered in the initial enrollment meeting, and the second patient elected to move out of the region for school. The primary reason for exclusion before enrollment was age (181 patients): 174 patients were younger than 18 years and 7 patients were older than 35 years at the time of their presentation to the sports medicine clinic at Boston Children’s Hospital.
Scaffold
The extracellular matrix scaffold (the BEAR scaffold; Boston Children’s Hospital) passed all biocompatibility and sterility testing.46,48,49 The scaffold was composed of extracellular matrix proteins, including collagen, that were obtained from bovine tissue. The DNA content of the scaffold was less than 50 ng per milligram of scaffold, and the scaffolds were not crosslinked. The scaffold measured 22 mm in diameter by 45 mm in length and was hydrophilic, able to absorb up to 5 times its weight in fluid. The scaffold softened when blood was added to it, making it conformable to the intra-articular notch and able to fill in the irregular contours of the gap between the torn ligament ends. The efficacy of the scaffold for stimulating ACL healing has previously been demonstrated in preclinical studies.30,36,40,42,59
Surgical Techniques
Bridge-Enhanced ACL Repair
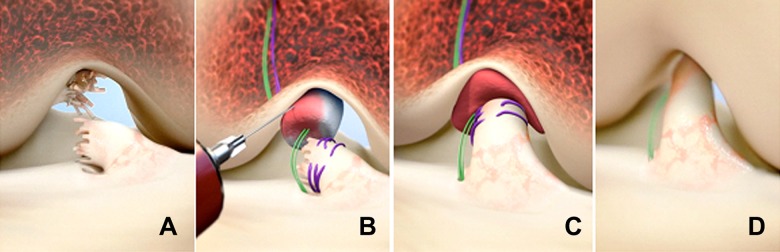
An examination under anesthesia was performed to verify that the ACL of the injured knee was deficient. The Lachman, range of motion, and pivot-shift tests were performed manually and results recorded for both knees. Knee arthroscopic surgery was performed and meniscal lesions addressed if necessary. A 2.4-mm guide pin was placed through the tibial footprint of the ACL using a tibial aimer (ACUFEX DIRECTOR drill guide; Smith & Nephew). A 4.5-mm tunnel was made by overreaming the pin (4.5-mm endoscopic drill; Smith & Nephew). A second 4.5-mm tunnel was made in the femur over a guide pin with the starting point in the femoral footprint. Also, 50-mm arthrotomy was performed at the medial border of the patellar tendon, through which a whipstitch of No. 2 absorbable suture (Vicryl; Ethicon) was placed into the tibial stump of the torn ACL (Figure 1). Two No. 2 nonabsorbable sutures (Ethibond; Ethicon) were looped through the 2 center holes of a cortical button (Endobutton; Smith & Nephew) and the free ends of the suture from the tibial stump passed through the cortical button. The button carrying the nonabsorbable and absorbable sutures was passed through the femoral tunnel and engaged on the lateral femoral cortex. The No. 2 nonabsorbable sutures were passed through the scaffold and then brought through the tibial tunnel. Ten milliliters of autologous blood was obtained from the patient’s antecubital vein and 5 to 10 mL added to the scaffold, which was then passed up along the sutures into the femoral notch. The nonabsorbable sutures were tensioned with the knee in full extension and tied over a second cortical button on the anterior tibial cortex. The absorbable sutures from the tibial stump were tied over the femoral cortical button to bring the tibial ACL stump into the scaffold and directed toward the location of the femoral insertion. The arthrotomy site was closed in layers.
Figure 1.
Stepwise demonstration of the bridge-enhanced anterior cruciate ligament repair (BEAR) technique using the scaffold. (A) The torn anterior cruciate ligament (ACL) tissue is preserved. A whipstitch of No. 2 absorbable suture (purple) is placed into the tibial stump of the ACL. Small tunnels (4 mm) are drilled in the femur and tibia, and a cortical button with two No. 2 nonabsorbable sutures (green sutures) and No. 2 absorbable sutures attached to it is passed through the femoral tunnel and engaged on the proximal femoral cortex. The nonabsorbable sutures are threaded through the BEAR scaffold and tibial tunnel and secured in place with an extracortical button. (B) The scaffold is then saturated with 5 to 10 mL of the patient’s blood, and (C) the tibial stump is pulled up into the saturated scaffold. (D) The ends of the torn ACL then grow into the scaffold, which is gradually replaced by healing ligament tissue. (From Murray et al.41)
ACL Reconstruction
A standard arthroscopically assisted hamstring autograft procedure was performed using a quadrupled semitendinosus-gracilis graft looped over a continuous-loop cortical button (Endobutton) for proximal fixation. The knees were placed into extension, tensioned under maximum manual tension, and fixed in place using a bioabsorbable interference screw (BioRCI HA; Smith & Nephew) for tibial fixation.
Postoperative Rehabilitation
For all patients, a locking hinged brace (T Scope; Breg) was applied to limit joint range of motion between 0° and 50° of knee flexion for the first 2 weeks postoperatively and from 0° to 90° for the next 4 weeks unless they underwent concomitant meniscal repair, in which case the brace range was restricted to 0° to 40° for the first 4 weeks postoperatively before opening the brace up to 0° to 90° of flexion. All patients were provided with a cold therapy unit (IceMan; DJO Global) for postoperative use. Both groups followed the same standardized physical therapy protocol, which included partial weightbearing for 2 weeks and then weightbearing as tolerated with crutches until 4 weeks postoperatively. The use of a functional ACL brace (CTi brace; Ossur) was recommended from 6 to 12 weeks postoperatively and then for cutting and pivoting sport activities for 2 years after surgery. Running was allowed at 3 months postoperatively, and a graded return to sports started at 6 months postoperatively.
Outcome Measures
Patient-Reported Outcome Measures
The IKDC subjective score and KOOS were used to assess patient-reported outcomes.28,29,34,45,52,53 The KOOS evaluates 5 domains: Pain, Symptoms, Activities of Daily Living, Sports/Recreation, and Knee-Related Quality of Life. Both questionnaires were administered preoperatively and at the 6-, 12-, and 24-month time points.
IKDC Physical Examination
Knee effusion, range of motion, and ligament stability measures (Lachman test and pivot-shift test) were performed and results recorded individually and then combined as specified by the IKDC form (http://www.sportsmed.org) to generate an overall grade (A = normal, B = nearly normal, C = abnormal, D = severely abnormal), which was defined as the worst of the effusion, range of motion, and ligament grades. For range of motion, the difference between the surgical and nonsurgical knees for passive range of motion was used for all time points, with the preoperative measurement (taken after the knee had undergone preoperative rehabilitation) used for the baseline grade. The overall range of motion grade was based on the worst of the extension and flexion grades. The Lachman and pivot-shift tests were performed under anesthesia for the baseline values and without anesthesia at follow-up. The differences between the surgical knee and contralateral knee were reported for all measures. The overall ligament grade was defined as the worst of the Lachman and pivot-shift grades. An independent examiner performed the tests, and knee sleeves were used to cover both knees. The examiner was blinded to the surgical side and study group when performing the physical examinations until the end, when effusion was assessed after removal of the sleeves.
Functional Outcome Measures
The following functional outcome measures were performed at 6, 12, and 24 months after surgery. All measures were performed in duplicate on each side, with the duplicate measurements averaged for further analysis. Examiners were blinded to the surgical procedure and operative leg with the use of knee sleeves for strength and arthrometer testing. The operative leg was unblinded for hop testing, as the patients used a brace on the operative side; however, the examiners remained blinded to the surgical procedure that the patient had undergone. Results were normalized by expressing the injured knee result as a percentage of the uninjured contralateral knee result or by subtracting the contralateral laxity measurement. Isokinetic strength testing (Biodex 3; Biodex Medical Systems) was performed at 60 deg/s for both extension and flexion torques.10,11 Hamstring, quadriceps, and hip abductor isometric muscle strengths were measured using a handheld dynamometer (MicroFET 2; Hoggan Scientific) that had specifically been validated as a reliable handheld dynamometer in multiple studies.9,31,39,50 Hamstring strength was measured with the patient prone and the knee in 90° of flexion. The dynamometer was placed on the posterior surface of the lower leg proximal to the ankle. The manufacturer states that either the “make” or “break” techniques57 can be utilized for isometric strength measurements; we chose to use the “make” technique in our study because of previous evidence of its superiority with intertester reliability.24
Hip abductor strength was tested with the patients lying on their side with the knee extended, placing the dynamometer over the midlateral thigh. Quadriceps strength was measured with the knee at 90° of flexion. Arthrometer testing (KT-1000 arthrometer; MEDmetric) was used to measure anterior displacement of the tibia with respect to the femur under 130 N of applied anterior force, as recommended by the manufacturer.14 Arthrometer testing was performed in duplicate on each leg, with both values recorded. The results were reported as a side-to-side difference (mean value on the surgical knee minus mean value on the contralateral knee). Patients performed a single hop, triple hop, 6-m timed hop, and crossover hop test as previously described.44
Magnetic Resonance Imaging
MRI, which included sagittal proton density (intermediate-weighted) images to evaluate tissue continuity, was performed for all operated knees at 12 and 24 months. Images were obtained using a 3-T scanner (Tim Trio; Siemens) and a 15-channel knee coil. The region of the ACL repair or graft was assessed for integrity, continuity of fibers from the femoral attachment/tunnel to the tibial attachment/tunnel, and surrounding fluid and inflammatory change. Implant or graft failure was classified as the absence of intact continuous fibers in the expected region of the repair or graft.
Methods to Minimize Potential, Actual, or Perceived Bias
This study underwent a comprehensive review by a panel of medical device experts at the FDA before investigational device exemption approval. These experts were appointed by the FDA without input from the investigative team, and the outcome measures for the study were approved by that panel before the start of the study. The defined outcome measures and study design were also registered at ClinicalTrials.gov (NCT02292004) before the start of the study. Patient recruiting and consent, as well as data collection and entry into the database and statistical analysis, were performed by investigators with no financial stake in any commercial interest that stood to gain from the results of this study. All physical examination and functional measurements were taken by licensed examiners independent of the surgical team, who were blinded to the procedure and surgical limb. Bilateral knee sleeves were placed by the research coordinators before the examiner meeting with the patient to perform the tests. This study was overseen by a data and safety monitoring board, with the members approved by both the institutional review board and the Boston Children’s Hospital’s Conflict of Interest Committee. A clinical research manager and study monitor, who were independent of the Department of Orthopedic Surgery, were appointed by the Institutional Centers for Clinical and Translational Research at Boston Children’s Hospital to oversee and monitor the study.
Statistical Analysis
Means and 95% CIs were calculated for continuous variables. Although power may have been limited, we compared the surgical groups on the patient characteristics and outcomes that might directly represent a difference between the 2 surgical approaches: length of the ACL tibial remnant (Cochran-Armitage trend test), time from injury to surgery, and hamstring strength as measured by a dynamometer and flexion torque (2-sample t test). We also present the 95% CIs for the treatment differences, representing a range of plausible magnitudes consistent with the data. Changes from baseline in IKDC and KOOS scores were also assessed within each surgical group using paired t tests. In post hoc power calculations using sample sizes ranging from 6 per group to 7 and 9 in the 2 groups (the range of numbers of patients in our data who had 24-month outcomes), there was 80% power to detect effect sizes of 1.52 to 1.80. For example, with 7 and 9 patients in the 2 groups, there was an 80% chance of finding a P value <.05 if the true difference between group means was about 1.5 times the within-group standard deviation. All P values are 2-sided.
Results
Baseline Characteristics and Intraoperative Findings
The baseline characteristics of both groups are shown in Table 1. In summary, the 2 groups were similar in age, sex, race, and body mass index. Most of the injuries in both groups were noncontact and occurred during sports participation. The mean time from injury to surgery was significantly longer in the ACLR group compared with the BEAR group (52.9 vs 20.8 days, respectively). One patient in the ACLR group was noted to have a medial collateral ligament tear on preoperative MRI, which was treated nonoperatively. All patients had at least 50% of the length of the ACL preserved as a tibial remnant (Table 2). The number of patients with concomitant meniscal tears were similar between groups, as was the degree of effusion at the time of surgery. Preoperative side-to-side differences in Lachman test results were similar in the 2 groups, and all patients had either a “glide” or “clunk” with pivot-shift testing under anesthesia (Table 2). The mean preoperative Marx activity level was 12.8 ± 3.7 in the BEAR group and 10.9 ± 5.9 in the ACLR group. Three of the patients in the BEAR group and 4 of the patients in the ACLR group played collegiate sports preoperatively (BEAR: lacrosse, field hockey, and tennis; ACLR: soccer, rugby, ice hockey, and baseball). All of the remaining patients participated in sports at a recreational level (BEAR: basketball [n = 2], volleyball, skiing, karate, and hiking/biking [n = 2]; ACLR: basketball, soccer, dance, hiking/biking [n = 2], and jogging [n = 1]). All patients in the BEAR group and 9 of 10 patients in the ACLR group were injured while playing sports; 9 of 10 patients in both groups had a noncontact injury.
TABLE 1.
Baseline Characteristicsa
| BEAR Group (n = 10) | ACLR Group (n = 10) | P | |
|---|---|---|---|
| Male sex, n | 4 | 2 | |
| White (non-Hispanic) ethnicity, n | 7 | 8 | |
| Age, y | 24.1 ± 4.9 (18.1-34.6) | 24.6 ± 5.5 (18.6-33.8) | |
| Body mass index, kg/m2 | 24.2 ± 2.0 (21.5-28.1) | 25.1 ± 2.9 (20.0-30.0) | |
| Time from injury to surgery, d | 20.8 ± 4.8 (11.0-28.0) | 52.9 ± 16.7 (24.0-80.0) | <.001 |
| Left knee injured, n | 5 | 6 | |
| Sports injury mechanism, n | 10 | 9 | |
| Noncontact injury, n | 9 | 9 | |
| MRI findings, n | |||
| Torn posterior cruciate ligament | 0 | 0 | |
| Torn medial collateral ligament | 0 | 1 |
aData are presented as mean ± SD (range) unless otherwise indicated. Previously published with 3-month data for this cohort.41 ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair; MRI, magnetic resonance imaging.
TABLE 2.
Intraoperative Findingsa
| BEAR (n = 10) | ACLR (n = 10) | P | |
|---|---|---|---|
| Length of ACL tibial remnant, n | .13 | ||
| 0%-24% | 0 | 0 | |
| 25%-49% | 0 | 0 | |
| 50%-74% | 9 | 6 | |
| ≥75% | 1 | 4 | |
| Meniscal tear (≥1),b n | 4 | 5 | |
| Medial (excised/repaired) | 2 (0/2) | 1 (0/1) | |
| Lateral (excised/repaired) | 2 (1/1) | 4 (0/4) | |
| Effusion grade (0-3)c | 1.3 ± 0.7 | 0.9 ± 0.8 | |
| Side-to-side difference in Lachman test result,c mm | 5.2 ± 1.4 | 5.0 ± 2.5 | |
| Pivot-shift test result, n | |||
| Glide | 2 | 3 | |
| Clunk | 8 | 7 |
aData are presented as mean ± SD unless otherwise indicated. ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair.
bBEAR group: 1 lateral tear in 1 patient, 2 lateral tears in 1 patient, and 1 medial tear in 2 patients. ACLR group: 1 lateral tear in 3 patients, 2 lateral tears in 1 patient, and 1 medial tear in 1 patient.
cn = 9 in ACLR group.
Adverse Events
No patient in either group required ACL revision surgery or had a superficial or deep infection involving the operative knee. Subsequent knee procedures were performed on patients in both groups within the first 12 months after surgery. No additional surgeries were performed between 12 and 24 months. One patient in each group underwent partial medial meniscectomy. In the BEAR group, 1 patient required removal of the tibial cortical button. In the ACLR group, 1 patient underwent arthroscopic surgery for arthrofibrosis to remove a cyclops lesion that caused a 10° loss of extension and pain with provocative extension. Additional adverse events between 3 and 12 months postoperatively included a nondisplaced medial femoral condyle fracture with no associated ACL injury after a fall on the ipsilateral knee (BEAR group), a concussion (BEAR group), formation of a cyclops lesion treated nonoperatively (ACLR group), and pain at the tibial screw that resolved without removal (ACLR group).
Patient-Reported Outcomes
In both groups, IKDC subjective scores improved approximately 35 to 55 points from baseline to 6, 12, and 24 months (all P < .0001) (Table 3). With 1 exception (KOOS Symptoms subscore at 6 months in the ACLR group, P = .11), all 5 KOOS subscores also improved significantly from baseline in both groups, typically by about 25 to 60 points (all P ≤ .02). There was no significant difference between groups at 2 years for the IKDC or KOOS scores (P > .05 for all comparisons).
TABLE 3.
IKDC Subjective and KOOS Scoresa
| ACLR Group | BEAR Group | Difference, Mean (95% CI) | |
|---|---|---|---|
| IKDC subjective | |||
| Baseline | 39.0 ± 9.3 (n = 10) | 35.1 ± 11.4 (n = 10) | –3.9 (–13.7 to 5.9) |
| 6 mo | 80.0 ± 9.1 (n = 9) | 67.9 ± 11.3 (n = 10) | –12.0 (–22.0 to –2.0) |
| 12 mo | 85.4 ± 14.0 (n = 9) | 83.4 ± 12.3 (n = 10) | –2.0 (–14.7 to 10.7) |
| 24 mo | 84.6 ± 17.2 (n = 7) | 91.7 ± 11.7 (n = 9) | 7.1 (–8.4 to 22.6) |
| KOOS Pain | |||
| Baseline | 63.6 ± 16.8 (n = 10) | 58.1 ± 15.6 (n = 10) | –5.6 (–20.8 to 9.7) |
| 6 mo | 90.6 ± 5.3 (n = 8) | 88.3 ± 14.5 (n = 9) | –2.4 (–13.9 to 9.2) |
| 12 mo | 92.4 ± 9.1 (n = 8) | 96.3 ± 3.1 (n = 9) | 3.9 (–2.9 to 10.8) |
| 24 mo | 90.5 ± 13.5 (n = 7) | 94.8 ± 8.6 (n = 9) | 4.3 (–7.6 to 16.1) |
| KOOS Symptoms | |||
| Baseline | 55.7 ± 13.7 (n = 10) | 56.1 ± 15.4 (n = 10) | 0.4 (–13.4 to 14.1) |
| 6 mo | 74.6 ± 22.3 (n = 8) | 81.3 ± 15.9 (n = 9) | 6.8 (–13.1 to 26.7) |
| 12 mo | 81.3 ± 12.3 (n = 8) | 89.3 ± 9.9 (n = 9) | 8.0 (–3.5 to 19.6) |
| 24 mo | 85.2 ± 15.8 (n = 7) | 93.1 ± 9.4 (n = 9) | 7.9 (–5.6 to 21.4) |
| KOOS Activities of Daily Living | |||
| Baseline | 68.2 ± 19.5 (n = 10) | 66.0 ± 16.7 (n = 10) | –2.2 (–19.2 to 14.8) |
| 6 mo | 98.5 ± 3.0 (n = 8) | 95.1 ± 9.4 (n = 9) | –3.4 (–10.9 to 4.0) |
| 12 mo | 98.0 ± 3.1 (n = 8) | 98.5 ± 2.4 (n = 9) | 0.6 (–2.3 to 3.4) |
| 24 mo | 98.3 ± 2.5 (n = 7) | 97.7 ± 5.8 (n = 9) | –0.6 (–5.7 to 4.5) |
| KOOS Sports/Recreation | |||
| Baseline | 24.0 ± 32.0 (n = 10) | 11.5 ± 15.5 (n = 10) | –12.5 (–36.1 to 11.1) |
| 6 mo | 72.5 ± 19.5 (n = 8) | 69.4 ± 12.4 (n = 9) | –3.1 (–19.7 to 13.6) |
| 12 mo | 86.3 ± 15.1 (n = 8) | 85.0 ± 12.7 (n = 9) | –1.3 (–15.6 to 13.1) |
| 24 mo | 85.7 ± 16.9 (n = 7) | 91.7 ± 14.4 (n = 9) | 6.0 (–10.8 to 22.7) |
| KOOS Knee-Related Quality of Life | |||
| Baseline | 28.8 ± 18.9 (n = 10) | 26.9 ± 12.2 (n = 10) | –1.9 (–16.8 to 13.1) |
| 6 mo | 57.0 ± 24.4 (n = 8) | 58.3 ± 14.0 (n = 9) | 1.3 (–19.0 to 21.6) |
| 12 mo | 69.5 ± 24.4 (n = 8) | 70.1 ± 16.2 (n = 9) | 0.6 (–20.6 to 21.8) |
| 24 mo | 70.5 ± 22.2 (n = 7) | 84.0 ± 15.7 (n = 9) | 13.5 (–6.7 to 33.7) |
aData are presented as mean ± SD unless otherwise indicated. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score.
Physical Examination Findings
Manual Lachman and Pivot-Shift Tests
At 2 years postoperatively, 8 of 9 patients in the BEAR group and 6 of 7 patients in the ACLR group had Lachman grade A. One patient in each group had Lachman grade B. All patients had a firm endpoint. Two patients in each group had a pivot shift that was 1 grade higher on the operative side than on the contralateral side, and the remaining patients in each group had a symmetric pivot shift in the 2 knees.
Instrumented AP Laxity of the Knee
Arthrometer testing results were similar in the 2 groups at all time points (Table 4). Mean differences between the groups were within 1.58 mm at all time points.
TABLE 4.
Side-to-Side Differences in Anteroposterior Laxity (mm)a
| ACLR Group | BEAR Group | Difference,b Mean (95% CI) | |
|---|---|---|---|
| 6 mo | 0.78 ± 1.97 (n = 9) | 2.36 ± 1.81 (n = 10) | 1.58 (–0.25 to 3.40) |
| 12 mo | 0.91 ± 3.17 (n = 8) | 1.20 ± 1.88 (n = 10) | 0.29 (–2.25 to 2.84) |
| 24 mo | 3.14 ± 2.66 (n = 7) | 1.94 ± 2.08 (n = 8) | –1.21 (–3.85 to 1.44) |
aData are presented as mean ± SD unless otherwise indicated. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair.
bPositive difference favors ACLR, and negative difference favors BEAR.
IKDC Physical Examination
Preoperatively, all patients in the BEAR and ACLR groups had IKDC grades of C (abnormal) or D (grossly abnormal). All patients in both groups had improved to either A (normal) or B (nearly normal) by 24 months postoperatively (Table 5). At 24 months, 44% of patients in the BEAR group and 29% of patients in the ACLR group had a grade of A (normal).
TABLE 5.
IKDC Objective Gradesa
| A | B | C | D | Total | |
|---|---|---|---|---|---|
| Preoperative | |||||
| BEAR | 0 (0) | 0 (0) | 5 (50) | 5 (50) | 10 (100) |
| ACLR | 0 (0) | 0 (0) | 6 (60) | 4 (40) | 10 (100) |
| 6 mo | |||||
| BEAR | 1 (10) | 8 (80) | 1 (10) | 0 (0) | 10 (100) |
| ACLR | 3 (33) | 6 (67) | 0 (0) | 0 (0) | 9 (100) |
| 12 mo | |||||
| BEAR | 6 (60) | 4 (40) | 0 (0) | 0 (0) | 10 (100) |
| ACLR | 2 (25) | 5 (62.5) | 1 (12.5) | 0 (0) | 8 (100) |
| 24 mo | |||||
| BEAR | 4 (44) | 5 (56) | 0 (0) | 0 (0) | 9 (100) |
| ACLR | 2 (29) | 5 (71) | 0 (0) | 0 (0) | 7 (100) |
aData are presented as n (%). ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair; IKDC, International Knee Documentation Committee.
Functional Outcomes
At 6, 12, and 24 months after surgery, the only difference between the 2 groups with a 95% CI at all time points that excluded zero was isometric hamstring strength as measured by a handheld dynamometer (Table 6). Patients in the BEAR group had a significantly greater return of strength than patients in the ACLR group (P = .001 at 6 months, P = .006 at 12 months, and P = .0001 at 24 months) (Table 6). Patients in the BEAR group recovered a mean of approximately 90% to 99% strength relative to their contralateral side, in contrast to only 56% to 64% strength recovery for patients in the ACLR group. However, the mean peak flexion torque, another measure of hamstring strength, was similar in the 2 groups at all time points. Quadriceps strength and hop testing results were similar in the 2 groups at 6, 12, and 24 months after surgery.
TABLE 6.
Functional Outcomesa
| ACLR Group | BEAR Group | Difference,b Mean (95% CI) | |
|---|---|---|---|
| Prone hamstring strengthc | |||
| 6 mo | 64.3 ± 14.5 (n = 9) | 89.5 ± 13.8 (n = 10) | 25.1 (11.4 to 38.9) |
| 12 mo | 59.8 ± 23.9 (n = 8) | 92.7 ± 20.4 (n = 10) | 32.9 (10.8 to 55.0) |
| 24 mo | 56.3 ± 19.0 (n = 7) | 98.6 ± 10.5 (n = 8) | 42.3 (25.5 to 59.1) |
| Seated quadriceps strength | |||
| 6 mo | 90.1 ± 15.4 (n = 9) | 87.4 ± 26.5 (n = 10) | 2.7 (–24.0 to 18.6) |
| 12 mo | 96.4 ± 26.6 (n = 8) | 83.2 ± 22.0 (n = 10) | –13.2 (–37.4 to 11.1) |
| 24 mo | 103.1 ± 13.3 (n = 7) | 98.5 ± 11.2 (n = 8) | –4.6 (–18.3 to 9.1) |
| Lying hip abductor strength | |||
| 6 mo | 101.2 ± 11.4 (n = 9) | 97.7 ± 8.2 (n = 10) | –3.5 (–13.0 to 6.1) |
| 12 mo | 96.9 ± 18.0 (n = 8) | 105.4 ± 6.6 (n = 10) | 8.5 (–4.5 to 21.4) |
| 24 mo | 91.2 ± 26.1 (n = 7) | 106.3 ± 15.3 (n = 7) | 15.1 (–9.8 to 40.0) |
| Peak flexor torque at 60 deg/s | |||
| 6 mo | 79.7 ± 16.7 (n = 9) | 89.5 ± 18.3 (n = 9) | 9.8 (–7.7 to 27.4) |
| 12 mo | 85.0 ± 10.2 (n = 8) | 84.3 ± 19.2 (n = 10) | –0.7 (–16.8 to 15.3) |
| 24 mo | 80.9 ± 21.0 (n = 6) | 96.3 ± 12.2 (n = 7) | 15.4 (–5.1 to 36.0) |
| Single hop | |||
| 6 mo | 84.2 ± 14.2 (n = 8) | 64.5 ± 21.8 (n = 9) | –19.7 (–39.0 to –0.4) |
| 12 mo | 93.4 ± 12.0 (n = 4) | 77.4 ± 19.0 (n = 9) | –16.0 (–39.0 to 7.0) |
| 24 mo | 83.9 ± 8.3 (n = 6) | 88.8 ± 10.7 (n = 6) | 4.9 (–7.4 to 17.2) |
| Triple hop | |||
| 6 mo | 85.5 ± 10.8 (n = 8) | 73.8 ± 18.9 (n = 6) | –11.7 (–29.1 to 5.6) |
| 12 mo | 92.0 ± 8.8 (n = 4) | 82.1 ± 14.0 (n = 8) | –9.9 (–27.2 to 7.4) |
| 24 mo | 93.8 ± 9.9 (n = 6) | 94.2 ± 6.4 (n = 6) | 0.5 (–10.2 to 11.2) |
| 6-m timed single hop | |||
| 6 mo | 113.7 ± 9.0 (n = 8) | 119.1 ± 15.7 (n = 7) | 5.5 (–8.6 to 19.5) |
| 12 mo | 101.2 ± 11.0 (n = 4) | 118.4 ± 24.7 (n = 9) | 17.1 (–11.7 to 46.0) |
| 24 mo | 102.2 ± 12.0 (n = 6) | 112.4 ± 13.3 (n = 6) | 10.2 (–6.2 to 26.5) |
| Crossover single-leg hop | |||
| 6 mo | 85.9 ± 9.7 (n = 8) | 81.6 ± 18.8 (n = 5) | –4.3 (–21.5 to 12.9) |
| 12 mo | 94.4 ± 11.6 (n = 4) | 85.7 ± 9.9 (n = 6) | –8.7 (–24.4 to 7.1) |
| 24 mo | 95.0 ± 2.9 (n = 6) | 94.2 ± 5.7 (n = 6) | –0.8 (–6.6 to 5.0) |
| Single-leg squat >60° (operative side), n (%) | |||
| 6 mo | 8/9 (88.9) | 6/10 (60.0) | –28.9 (–67.6 to 14.1) |
| 12 mo | 7/8 (87.5) | 10/10 (100.0) | 12.5 (–34.9 to 56.1) |
| 24 mo | 6/7 (85.7) | 8/9 (88.9) | 3.2 (–43.5 to 49.8) |
aData are presented as mean ± SD unless otherwise indicated. Strength and hop testing results are presented as percentages of the contralateral leg. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair.
bPositive difference favors BEAR, and negative difference favors ACLR, for all outcomes except the 6-m timed single hop and single-leg squat >60°.
cHamstring strength was significantly better in the BEAR group than in the ACLR group at all time points (P < .05 for comparison between groups at all time points).
MRI Findings
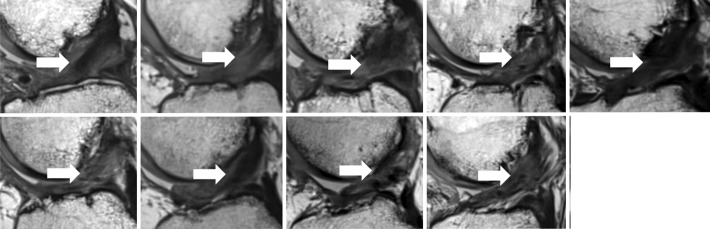
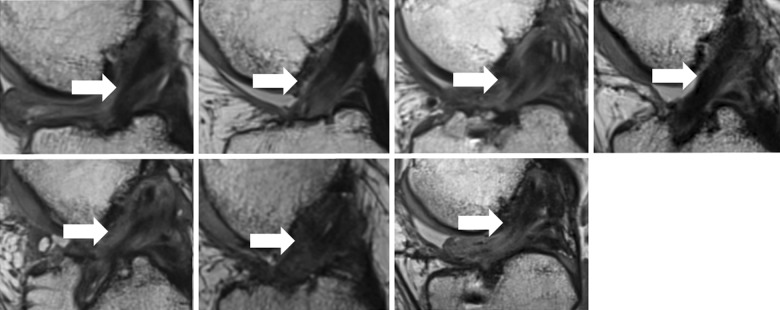
All 9 patients in the BEAR group who underwent MRI at follow-up exhibited continuous tissue in the region of the ACL at 24 months (Figure 2). This was not always a uniformly low signal structure, and approximately half had areas of higher signal consistent with higher water content in the midsubstance of the ACL (Figure 2). The ACL grafts were intact in all 7 patients in the ACLR group who returned for follow-up, with no gap between the proximal and distal limbs at 24 months (Figure 3). The grafts were not always a uniformly low signal structure, and approximately half had areas of higher signal consistent with higher water content in the midsubstance of the graft (Figure 3).
Figure 2.
Magnetic resonance imaging from the 9 patients in the bridge-enhanced anterior cruciate ligament repair (BEAR) group at 24 months shows intact anterior cruciate ligament (ACL) fibers from the femoral to tibial attachment sites (arrows). The intact fibers have low signal intensity (black), reflecting highly organized tissue with little free water. The peripheral higher signal intensity (lighter gray) indicates increased higher water content in the tissues surrounding the repaired ACL.
Figure 3.
Magnetic resonance imaging from the 7 patients in the anterior cruciate ligament reconstruction (ACLR) group at 24 months shows an intact graft between the femoral and tibial tunnels (arrows). The signal intensity within the graft is variable. The homogeneous low signal intensity (black) in some patients (eg, top row [first from left] and bottom row [second from left]) is typical of the normal in situ hamstring tendon because of highly organized connective tissue with little free water. A more heterogeneous appearance is present in several patients (eg, top row [third from left]) with central low signal intensity and peripheral high signal intensity (lighter gray), indicating surrounding edema. Other patients showed higher signal intensity within the graft itself (eg, bottom row [third from left]), reflecting increased fluid within the graft.
Discussion
This first-in-human study of the BEAR technique found that the patient-reported outcomes, physical examination findings, and functional outcomes were similar to those for ACLR in this small number of patients. As the BEAR technique does not require compromising previously healthy tissues to obtain a graft, these results suggest that a randomized controlled trial comparing these 2 procedures is warranted. Within 2 years after surgery, the quantity and severity of the adverse events in both groups were similar, including 1 patient in each group sustaining a medial meniscal tear. Patient-reported outcomes were similar in the 2 groups, as both attained significant gains over baseline values for the IKDC subjective score and all KOOS subscores. The percentage of patients with a normal IKDC grade in the BEAR group was 50% higher than in the ACLR group (44% vs 29%, respectively). Functional testing showed no differences between groups, with the exception of isometric hamstring strength recovery as measured by a handheld dynamometer, which was more complete in patients in the BEAR group.
In the first year after surgery, 1 of 10 patients in each group required subsequent medial meniscal surgery. The risk of needing meniscal surgery in the first few years after ACLR has been reported to range from 4% to 24%, with younger cohorts generally having a higher rate of postoperative meniscal injuries.22,51,60,61 Our observation that 1 in 10 of the patients in each group required additional meniscal surgery is thus within the range previously reported for ACLR.
The patient-reported outcomes were similar in the 2 groups, with both groups attaining significant gains over baseline preoperative values. The IKDC and KOOS scores were similar in the ACLR group to what has been previously reported in the literature.13,16 In this study, the IKDC subjective score averaged about 85 points in the ACLR group at both the 12- and 24-month follow-ups, which were similar to the 24-month scores reported by the Multicenter Orthopaedic Outcomes Network (MOON) Group (81 points) after ACLR.13 Patients in the BEAR group had a similar mean score at 12 months (∼83 points), with a subsequent mean score of approximately 92 points at 24 months after surgery, and similar to that previously reported for men and women without knee injuries (∼93 points).4
The KOOS score has been previously reported for patients after ACLR in cohort studies. In the Knee Anterior Cruciate Ligament Nonoperative versus Operative Treatment (KANON) trial, which compared outcomes in patients undergoing structured rehabilitation with optional delayed ACLR versus those undergoing early reconstruction,17 the investigators reported that in 18- to 35-year-old active adults with acute ACL tears, a strategy of rehabilitation with optional delayed reconstruction was just as effective as acute ACLR. Patients in the ACLR group in our study had scores that were similar to the KANON trial values for KOOS Pain and Symptoms. In contrast, patients in the BEAR group in our trial had mean values that were higher than those of the rehabilitation group in the KANON trial, by a clinically relevant margin for KOOS Pain, Symptoms, Sports/Recreation, and Knee-Related Quality of Life (Table 7). In addition, the mean values in the KANON trial were below the previously established values suggestive of problematic knees for KOOS Symptoms and Sports/Recreation,35 while mean values for the BEAR group were well above those values in both domains. Although the small number of patients in this study prevents reliable calculations of significance for these differences, it does suggest that the BEAR technique is worthy of further study.
TABLE 7.
KOOS Scores in the BEAR Group Versus Patients in the KANON Trial17 at 2 Yearsa
| KOOS Domain | Mean Score in KANON Trial | Mean Score in BEAR Group | Cutoff Score for Problematic Knee in Athletes With ACL Injury35 | Difference Between Mean Scores in KANON Trial and BEAR Group | Minimal Clinically Important Difference in Athletes After ACLR54 |
|---|---|---|---|---|---|
| Pain | 87.7 | 94.8 | ≤86.1 | 6.1 | 6.1 |
| Symptoms | 83.0 | 93.1 | ≤85.7 | 10.1 | 8.5 |
| Activities of Daily Living | 94.7 | 97.7 | ≤86.8 | 3.0 | 8.0 |
| Sports/Recreation | 71.2 | 91.7 | ≤85.0 | 20.5 | 5.8 |
| Knee-Related Quality of Life | 63.0 | 84.0 | ≤87.5 | 21.0 | 7.2 |
aThe Kinetically Activated Nerve Organ Normalization (KANON) trial consisted of patients undergoing rehabilitation with optional delayed ACLR. ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair; KOOS, Knee injury and Osteoarthritis Outcome Score.
The mean side-to-side difference in instrumented AP laxity for both groups at 24-month follow-up (3.14 mm for ACLR and 1.94 mm for BEAR) were similar to those that have been reported for ACLR with an autograft. Evidence-based reviews and meta-analyses have reported that autografts stretch out between 1 and 4 mm postoperatively.7,56,64 The increase in the mean side-to-side difference in AP laxity between 6 months and 2 years of the current study for the ACLR group has also been previously reported for autografts.3,15 In a recent meta-analysis of studies with a minimum 2-year follow-up, it was determined that 32% of patients with a hamstring autograft have a side-to-side difference in knee laxity greater than 3 mm.62
For the IKDC objective grade, all patients in both groups had grade A (normal) or B (nearly normal) at 24 months. These findings are consistent with prior studies of patients undergoing ACLR that have reported normal or nearly normal grades in 80% to 95% of patients.16,26,32,58 The BEAR group had 50% more patients with normal knees (IKDC grade A); however, the percentage in both groups remained well below those reported for uninjured control knees, in which grade A is found in over 95% of examined knees.16 Thus, improvements in both the surgical technique and rehabilitation after BEAR may be necessary if we are to increase the number of patients having normal knee examination parameters restored after ACL surgery.
We utilized 2 measures of hamstring strength postoperatively: isometric and isokinetic muscle strength. Both measures are important. Isokinetic muscle deficits are associated with altered knee mechanics during sprinting,5 while isometric deficits are associated with slower walking speeds and altered knee mechanics during walking and running gait.1 While isokinetic measures of flexor torque incorporate contributions from all of the flexor muscles, isometric measurements with a handheld dynamometer at higher knee flexion angles better isolate the contributions of the gracilis and semitendinosis.21,43,63 This difference in muscle recruitment may be one reason why there was a significant difference in strength when measured isometrically (40%), and a smaller difference between the means of each group (15%) when measured isokinetically, which was not statistically significant possibly because of the limited power of this study. Hop testing results in the ACLR and BEAR groups were also similar to what has been previously published for ACLR, in which patients were able to achieve approximately 90% of the contralateral side in the single-leg hop for distance.3,16
The major limitations of this study were the small number of patients and the nonrandomized allocation of treatment groups, which precludes making any definitive conclusions about efficacy or performance. When translating a new scaffold to human patients, the first-in-human study is commonly a small study to determine if significant problems will occur before the initiation of larger studies. The previously observed failure rates of 16% to 100% within the early postoperative period for other extracellular matrix–based implants22,35,55 justified the need for this small first-in-human study before proceeding to a larger controlled trial. For example, in prior studies of extracellular matrix patches,25 a serious inflammatory response was seen in 20% of patients. Assuming this is the true rate of inflammatory responses, our study would have had an 89% chance of observing ≥1 such responses in our BEAR group of 10 patients. For most of the measurements reported here, the means between the groups were similar, suggesting that a larger study with greater power would be necessary to determine if differences between groups were statistically significant.
In light of the required small size of this study, we have also discussed the results from the BEAR group as compared with both the results in our ACLR group and other larger ACLR cohorts (Tension study,16 MOON Group study,13 and KANON trial17). In addition, the BEAR procedure was performed through arthrotomy, while ACLR was performed arthroscopically. This may be a variable that contributes to efficacy differences in longer term and larger studies that may have the power to detect such differences. The BEAR procedure may have other potential advantages and disadvantages when compared with traditional ACLR with an autograft. Because healthy tendons about the knee are not compromised to create a graft, the BEAR procedure may result in less loss of strength in the postoperative period. If the proprioceptive fibers contained in the native ACL can be preserved, this may lead to improved proprioceptive function of the joint and possibly less posttraumatic osteoarthritis, as was observed in preclinical studies.40 However, the BEAR procedure was performed within 1 month of injury, which may be difficult to achieve for all patients, given the current typical time for the identification of these injuries and obtaining a consultation with an orthopaedic surgeon. In addition, in the current study, we required 50% length of the tibial stump for a patient to be a candidate, which led to screening out 2 of 220 patients. Last, although the BEAR procedure may save operative time because of the elimination of graft harvest, and it uses simpler implants (2 cortical buttons) rather than more advanced implants such as interference screws or cortical buttons with fixed or adjustable loops, whether the BEAR procedure will be a cost-effective alternative to ACLR remains to be determined. Future work, including a longer follow-up of this initial cohort and performance of larger randomized trials, will be geared to demonstrating the efficacy, applicability, and cost-effectiveness of the BEAR procedure, one of which is currently underway (NCT02664545).
Conclusion
The results of this first-in-human study demonstrate that the use of the BEAR scaffold resulted in similar clinical, functional, and patient-reported outcomes compared with patients undergoing ACLR with an autograft 24 months after surgery. The procedure did not result in any patients having an infection or severe inflammatory reaction, arthrofibrosis, or a reaction that required scaffold removal. In addition, manual and instrumented measures suggested that stability of the knee after both procedures may be comparable at this early time point. Combined, these findings suggest that the BEAR procedure is a method deserving further study.
Acknowledgment
The authors acknowledge the significant contributions of the clinical trial team, including Bethany Trainor, Andrea Hale, and Shanika Coney. They also acknowledge the contributions of the medical safety monitoring team of Joseph DeAngelis and Peter Nigrovic, data monitors Maggie Malsch and Megan Fitzgerald, and the clinical care team for the trial patients, including Kathryn Ackerman, Alyssa Aguiar, Judd Allen, Samuel Barnett, Michael Beasley, Jennifer Beck, Dennis Borg, Nicole Bottino, Jeff Brodeur, Stephanie Burgess, Melissa Christino, Andrea Cianci, Sarah Collins, Gianmichel Corrado, Sara Cline, Corey Dawkins, Pierre D’Hemecourt, Peter Fabricant, Jon Ferguson, Michele Flannery, Joseph Founds, Casey Gavin, Ellen Geminiani, Stacey Gigante, Annie Griffin, Emily Hanson, Elspeth Hart, Jackie Hastings, Pamela Horne-Goffigan, Christine Gonzalez, Meghan Keating, Ata Kiapour, Elizabeth KillKelly, Elizabeth Kramer, Pamela Lang, Hayley Lough, Chaimae Martin, Michael McClincy, William Meehan, Ariana Moccia, Jen Morse, Mariah Mullen, Stacey Murphy, Emily Niu, Michael O’Brien, Nikolas Paschos, Katrina Plavetsky, Bridget Quinn, Lauren Redler, Nicholas Sant, Shannon Savage, Edward Schleyer, Gabriella Scippa, Benjamin Shore, Cynthia Stein, Andrea Stracciolini, Dai Sugimoto, Dylan Taylor, Ashleigh Thorogood, Natasha Trentacosta, Patrick Vavken, Lisa Vopat, and Lenise Young. The authors thank the perioperative and operating room staff and the members of the Department of Anesthesia at Boston Children's Hospital, who were extremely helpful in developing the perioperative and intraoperative protocols. They furthermore acknowledge the efforts of the scaffold manufacturing team, including Benedikt Proffen, Gabriel Perrone, Gordon Roberts, Doris Peterkin, Jakob Sieker, Cameron Crowley, and David Thomas. They gratefully acknowledge the study design guidance provided by the Division of Orthopedic Devices, Center for Devices and Radiological Health, FDA, under the guidance of Mark Melkerson and Laurence Coyne and particularly the efforts of Casey Hanley, Peter Hudson, Jemin Dedania, Pooja Panigrahi, and Neil Barkin. M.M.M. is also grateful to John Feagin and Kurt Spindler for their encouragement during this project. The authors are especially grateful to the 20 patients and their families who participated in this study; their willingness to participate in research that may help others in the future inspires everyone.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was funded by the Translational Research Program at Boston Children’s Hospital, the Children’s Orthopaedic Surgery Foundation, the Children’s Sports Medicine Foundation, and the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant Nos. AR065462 and R01AR056834). This research was also conducted with support from the Football Players Health Study at Harvard University. The Football Players Health Study is funded by a grant from the National Football League (NFL) Players Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Medical School, Harvard University or its affiliated academic health care centers, the NFL Players Association, Boston Children’s Hospital, or the NIH. M.M.M. has equity interests in and is a consultant for MIACH Orthopaedics, a company that has licensed the BEAR scaffolding technology from Boston Children’s Hospital, which is also an equity holder in MIACH Orthopaedics; has patents/patents pending for the BEAR technology from Boston Children’s Hospital and Rhode Island Hospital; has received payment for grant review from the Musculoskeletal Transplant Foundation; receives royalties from Springer; and has research grants from the NIH, the Department of Defense, and the NFL Players Association through the Football Players Health Study. B.C.F. has patents/patents pending for the BEAR technology from Boston Children’s Hospital and Rhode Island Hospital, is a paid associate editor for The American Journal of Sports Medicine, has received royalties from Springer, and has received research grants from the NIH and the Department of Defense. B.L.P. has equity interests in and is a consultant for MIACH Orthopaedics. D.E.K. has received educational support and hospitality payments from Kairos Surgical. Y.-M.Y. has received educational funding from Kairos Surgical and hospitality payments from Smith & Nephew and Kairos Surgical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Boston Children’s Hospital’s Office of Clinical Investigation (P0012985).
References
- 1. Abourezk MN, Ithurburn MP, McNally MP, et al. Hamstring strength asymmetry at 3 years after anterior cruciate ligament reconstruction alters knee mechanics during gait and jogging. Am J Sports Med. 2017;45(1):97–105. [DOI] [PubMed] [Google Scholar]
- 2. Ageberg E, Roos HP, Silbernagel KG, Thomee R, Roos EM. Knee extension and flexion muscle power after anterior cruciate ligament reconstruction with patellar tendon graft or hamstring tendons graft: a cross-sectional comparison 3 years post surgery. Knee Surg Sports Traumatol Arthrosc. 2009;17(2):162–169. [DOI] [PubMed] [Google Scholar]
- 3. Akelman MR, Fadale PD, Hulstyn MJ, et al. Effect of matching or overconstraining knee laxity during anterior cruciate ligament reconstruction on knee osteoarthritis and clinical outcomes: a randomized controlled trial with 84-month follow-up. Am J Sports Med. 2016;44(7):1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ; International Knee Documentation Committee. The International Knee Documentation Committee subjective knee evaluation form: normative data. Am J Sports Med. 2006;34(1):128–135. [DOI] [PubMed] [Google Scholar]
- 5. Anderson MA, Gieck JH, Perrin DH, Weltman A, Rutt RA, Denegar CR. The relationships among isometric, isotonic, and isokinetic concentric and eccentric quadriceps and hamstring force and three components of athletic performance. J Orthop Sports Phys Ther. 1991;14(3):114–120. [DOI] [PubMed] [Google Scholar]
- 6. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. [DOI] [PubMed] [Google Scholar]
- 7. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biercevicz AM, Proffen BL, Murray MM, Walsh EG, Fleming BC. T2* relaxometry and volume predict semi-quantitative histological scoring of an ACL bridge-enhanced primary repair in a porcine model. J Orthop Res. 2015;33(8):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bohannon R. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26–32. [DOI] [PubMed] [Google Scholar]
- 10. Brosky JA, Jr, Nitz AJ, Malone TR, Caborn DN, Rayens MK. Intrarater reliability of selected clinical outcome measures following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1999;29(1):39–48. [DOI] [PubMed] [Google Scholar]
- 11. Bush-Joseph CA, Hurwitz DE, Patel RR, et al. Dynamic function after anterior cruciate ligament reconstruction with autologous patellar tendon. Am J Sports Med. 2001;29(1):36–41. [DOI] [PubMed] [Google Scholar]
- 12. Conteduca F, Caperna L, Ferretti A, Iorio R, Civitenga C, Ponzo A. Knee stability after anterior cruciate ligament reconstruction in patients older than forty years: comparison between different age groups. Int Orthop. 2013;37(11):2265–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox CL, Huston LJ, Dunn WR, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42(5):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniel DM. Reference, Maintenance and User’s Guide for the Knee Ligament Arthrometer. San Diego, California: MEDmetric Corp; 1993. [Google Scholar]
- 15. Fleming BC, Brattbakk B, Peura GD, Badger GJ, Beynnon BD. Measurement of anterior-posterior knee laxity: a comparison of three techniques. J Orthop Res. 2002;20(3):421–426. [DOI] [PubMed] [Google Scholar]
- 16. Fleming BC, Fadale PD, Hulstyn MJ, et al. The effect of initial graft tension after anterior cruciate ligament reconstruction: a randomized clinical trial with 36-month follow-up. Am J Sports Med. 2013;41(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363(4):331–342. [DOI] [PubMed] [Google Scholar]
- 18. Guglielmetti LG, Cury Rde P, de Oliveira VM, de Camargo OP, Severino NR, Fucs PM. Anterior cruciate ligament reconstruction: a new cortical suspension device for femoral fixation with transtibial and transportal techniques. J Orthop Surg Res. 2014;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heijne A, Hagstromer M, Werner S. A two- and five-year follow-up of clinical outcome after ACL reconstruction using BPTB or hamstring tendon grafts: a prospective intervention outcome study. Knee Surg Sports Traumatol Arthrosc. 2015;23(3):799–807. [DOI] [PubMed] [Google Scholar]
- 20. Heijne A, Werner S. A 2-year follow-up of rehabilitation after ACL reconstruction using patellar tendon or hamstring tendon grafts: a prospective randomised outcome study. Knee Surg Sports Traumatol Arthrosc. 2010;18(6):805–813. [DOI] [PubMed] [Google Scholar]
- 21. Herzog W, Read LJ. Lines of action and moment arms of the major force-carrying structures crossing the human knee joint. J Anat. 1993;182(pt 2):213–230. [PMC free article] [PubMed] [Google Scholar]
- 22. Hettrich CM, Dunn WR, Reinke EK, MOON Group. Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Knee strength deficits after hamstring tendon and patellar tendon anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2000;32(8):1472–1479. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh C, Phillips R. Reliability of manual muscle testing with a computerized dynamometer. J Manipul Physiol Ther. 1990;13:72–82. [PubMed] [Google Scholar]
- 25. Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears: a randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238–1244. [DOI] [PubMed] [Google Scholar]
- 26. Ibrahim SA, Shohdy EM, Marwan Y, et al. Anatomic reconstruction of the anterior cruciate ligament of the knee with or without reconstruction of the anterolateral ligament: a randomized clinical trial. Am J Sports Med. 2017;45(7):1558–1566. [DOI] [PubMed] [Google Scholar]
- 27. Irrgang JJ, Anderson AF. Development and validation of health-related quality of life measures for the knee. Clin Orthop Relat Res. 2002;402:95–109. [DOI] [PubMed] [Google Scholar]
- 28. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 29. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2006;34(10):1567–1573. [DOI] [PubMed] [Google Scholar]
- 30. Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37(12):2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelln B, McKeon P, Gontkof L, Hertel J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil. 2008;17:160–170. [DOI] [PubMed] [Google Scholar]
- 32. Kondo E, Yasuda K, Kitamura N, et al. Effects of initial graft tension on clinical outcome after anatomic double-bundle anterior cruciate ligament reconstruction: comparison of two graft tension protocols. BMC Musculoskelet Disord. 2016;17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Chen J, Li H, Wu Z, Chen S. MRI-based ACL graft maturity does not predict clinical and functional outcomes during the first year after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3171–3178. [DOI] [PubMed] [Google Scholar]
- 34. Lingard EA, Katz JN, Wright RJ, Wright EA, Sledge CB; Kinemax Outcomes Group. Validity and responsiveness of the Knee Society Clinical Rating System in comparison with the SF-36 and WOMAC. J Bone Joint Surg Am. 2001;83(12):1856–1864. [DOI] [PubMed] [Google Scholar]
- 35. Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. [DOI] [PubMed] [Google Scholar]
- 36. Magarian EM, Vavken P, Connolly SA, Mastrangelo AN, Murray MM. Safety of intra-articular use of atelocollagen for enhanced tissue repair. Open Orthop J. 2012;6:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29(7):1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayr HO, Bruder S, Hube R, Bernstein A, Suedkamp NP, Stoehr A. Single-bundle versus double-bundle anterior cruciate ligament reconstruction: 5-year results. Arthroscopy. 2018;34(9):2647–2653. [DOI] [PubMed] [Google Scholar]
- 39. Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. 2015;10(10):e0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;26(suppl 9):S49–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nomura Y, Kuramochi R, Fukubayashi T. Evaluation of hamstring muscle strength and morphology after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2015;25(3):301–307. [DOI] [PubMed] [Google Scholar]
- 44. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513–518. [DOI] [PubMed] [Google Scholar]
- 45. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskelet Disord. 2006;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Proffen B, Perrone G, Roberts G, Murray M. Bridge-enhanced ACL repair: a review of the science and the pathway through FDA investigational device approval. Ann Biomed Eng. 2015;43(3):805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Proffen BL, Fleming BC, Murray MM. Histological predictors of maximum failure loads differ between the healing ACL and ACL grafts after 6 and 12 months in vivo. Orthop J Sports Med. 2013;1(6):2325967113512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Proffen BL, Perrone GS, Fleming BC, et al. Electron beam sterilization does not have a detrimental effect on the ability of extracellular matrix scaffolds to support in vivo ligament healing. J Orthop Res. 2015;33(7):1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Proffen BL, Perrone GS, Fleming BC, et al. Effect of low-temperature ethylene oxide and electron beam sterilization on the in vitro and in vivo function of reconstituted extracellular matrix-derived scaffolds. J Biomater Appl. 2015;30(4):435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reurink G, Goudswaard GJ, Moen MH, Tol JL, Verhaar JA, Weir A. Strength measurements in acute hamstring injuries: intertester reliability and prognostic value of handheld dynamometry. J Orthop Sports Phys Ther. 2016;46(8):689–696. [DOI] [PubMed] [Google Scholar]
- 51. Rochcongar G, Cucurulo T, Ameline T, et al. Meniscal survival rate after anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res. 2015;101(suppl 8):S323–S326. [DOI] [PubMed] [Google Scholar]
- 52. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rossi MJ, Lubowitz JH, Guttmann D. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2002;30(1):152. [DOI] [PubMed] [Google Scholar]
- 54. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406–410. [DOI] [PubMed] [Google Scholar]
- 55. Soler JA, Gidwani S, Curtis MJ. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears: a report of 4 cases. Acta Orthop Belg. 2007;73(4):432–436. [PubMed] [Google Scholar]
- 56. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE., Jr Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring. Does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986–1995. [DOI] [PubMed] [Google Scholar]
- 57. Stratford P, Balsor B. A comparison of make and break tests using a hand-held dynamometer and the Kin-Com. J Orthop Sports Phys Ther. 1994;19(1):28–32. [DOI] [PubMed] [Google Scholar]
- 58. Taylor DC, DeBerardino TM, Nelson BJ, et al. Patellar tendon versus hamstring tendon autografts for anterior cruciate ligament reconstruction: a randomized controlled trial using similar femoral and tibial fixation methods. Am J Sports Med. 2009;37(10):1946–1957. [DOI] [PubMed] [Google Scholar]
- 59. Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28(5):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wall EJ, Ghattas PJ, Eismann EA, Myer GD, Carr P. Outcomes and complications after all-epiphyseal anterior cruciate ligament reconstruction in skeletally immature patients. Orthop J Sports Med. 2017;5(3):2325967117693604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Willimon SC, Jones CR, Herzog MM, May KH, Leake MJ, Busch MT. Micheli anterior cruciate ligament reconstruction in skeletally immature youths: a retrospective case series with a mean 3-year follow-up. Am J Sports Med. 2015;43(12):2974–2981. [DOI] [PubMed] [Google Scholar]
- 62. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100–110. [DOI] [PubMed] [Google Scholar]
- 63. Yucesoy CA, Ates F, Akgun U, Karahan M. Measurement of human gracilis muscle isometric forces as a function of knee angle, intraoperatively. J Biomech. 2010;43(14):2665–2671. [DOI] [PubMed] [Google Scholar]
- 64. Zeng C, Gao SG, Li H, et al. Autograft versus allograft in anterior cruciate ligament reconstruction: a meta-analysis of randomized controlled trials and systematic review of overlapping systematic reviews. Arthroscopy. 2016;32(1):153–163. [DOI] [PubMed] [Google Scholar]