Abstract
Purpose: To report the 1-year results of a multicenter study of peripheral artery disease (PAD) treatment with a variety of endovascular treatment strategies employed in routine practice. Materials and Methods: The LIBERTY trial (ClinicalTrials.gov identifier NCT01855412) is a prospective, observational, core laboratory–assessed, multicenter study of endovascular device intervention in 1204 subjects (mean age 69.8±10.7 years; 770 men) stratified by Rutherford category (RC): claudicants (RC2,3; n=501) and critical limb ischemia (CLI) with no/minimal tissue loss (RC4,5; n=603) or significant tissue loss (RC6; n=100). Key outcomes included quality of life (QoL) measures (VascuQol and EuroQol) and freedom from major adverse events (MAE), defined as death (within 30 days), major amputation, and target vessel revascularization based on Kaplan-Meier analysis. Results: Successful revascularization was beneficial, with RC improvement noted across all groups. Thirty-day freedom from MAE estimates were high across all groups: 99.2% in RC2,3, 96.1% in RC4,5, and 90.8% in RC6. At 12 months, the freedom from MAE was 82.6% in RC2,3, 73.2% in RC4,5, and 59.3% in RC6 patients. Estimates for freedom from major amputation at 12 months were 99.3%, 96.0%, and 81.7%, respectively. QoL scores improved significantly across all domains in all groups with 12-month VascuQol total scores of 5.3, 5.0, and 4.8 for RC2,3, RC4,5, and RC6, respectively. Conclusion: The results indicate that peripheral endovascular intervention is a viable treatment option for RC2,3, RC4,5, and RC6 patients as evidenced by the high freedom from major amputation, as well as the improvement in QoL and the RC at 12 months. Furthermore, primary unplanned amputation is often not necessary in RC6.
Keywords: atherectomy, balloon angioplasty, claudication, critical limb ischemia, endovascular intervention, femoropopliteal segment, peripheral artery disease, quality of life, stent
Introduction
The global prevalence of peripheral artery disease (PAD) increased by 23.5% from 2000 to 2010 (164 million to 202 million), indicating a global pandemic of PAD.1,2 In addition, only about a one-third of symptomatic PAD patients adhere to all guideline-recommended therapies, including aspirin, statin medications, angiotensin-converting enzyme (ACE) inhibitors, and smoking abstention.3 That low compliance with disease prevention measures, in combination with the aging population, will continue to accelerate the incidence and prevalence of PAD, as well as the subsequent burden of PAD on health care systems and society.
Lower extremity PAD has varied manifestations4,5 that can be categorized according to the Rutherford category (RC1–6)6: mild to severe claudication (RC1–3) and critical limb ischemia (CLI), classified as ischemic rest pain (RC4), minor tissue loss (RC5), and major tissue loss extending cephalad to the transmetatarsal level (RC6). Lower extremity PAD and CLI are highly prevalent in older patients with diabetes mellitus and/or chronic kidney disease7 and are associated with high risk of amputation and mortality.8 The 1-year mortality of patients with CLI is as high as 45%.9-11 An estimated 150,000 amputations due to CLI occur annually in the United States,2,12 and primary amputation continues to be first-line therapy for CLI at some institutions (average rate >20%).8,13,14 In addition, of the patients receiving primary amputation, 73%15 had no diagnostic angiography and 54%16 received no revascularization procedure prior to the amputation.
The 2016 American College of Cardiology/American Heart Association (AHA/ACC) PAD guidelines recommend that an evaluation for revascularization options should be performed by an interdisciplinary care team before amputation in patients with CLI (Class I).4 In addition, revascularization is a reasonable treatment option for patients with lifestyle-limiting claudication and an inadequate response to medical management and exercise (Class IIa).4 Several reviews9,17-24 have recently described the epidemiology and pathology of PAD and CLI, but there is a paucity of data on the optimal revascularization strategies, especially for CLI.25-28 The LIBERTY study was designed to fill that knowledge gap.
Materials and Methods
Study Design
LIBERTY is a prospective, observational, multicenter study examining predictors of clinical outcomes in symptomatic PAD patients undergoing lower extremity endovascular device intervention. Approximately 1200 subjects were to be enrolled and stratified according to their Rutherford category. The LIBERTY study design, endpoints, and data analysis plan were previously described.29 Briefly, sites were selected according to the following criteria: (1) experience in and use of multiple endovascular technologies to treat lower extremity PAD, (2) research team with an adequate number of qualified personnel, (3) adequate patient population, and (4) familiarity with and accessibility to electronic data capture. Clinical follow-up was performed at 30 days, 6 months, and 12 months; subjects will be followed for up to 5 years. The trial was registered on the National Institutes of Health website (ClinicalTrials.gov; identifier NCT01855412).
A steering committee, composed of LIBERTY principal investigators, representatives from the study core laboratories, and the sponsor (Cardiovascular Systems, Inc) were responsible for the development of the protocol; the sponsor was responsible for approval and oversight of the protocol, which was approved by the institutional review board at each site. A list of sites, principal investigators, and steering committee members is provided in Supplementary Appendix 1 (available in the online version of the article). Adjudication of angiographic data was performed by SynvaCor/Prairie Educational and Research Cooperative (PERC; Springfield, IL, USA); VasCore (Boston, MA, USA) acted as the duplex ultrasound core laboratory. Statistical analysis was performed by NAMSA (Northwood, OH, USA), with input from the sponsor. All authors reviewed the manuscript and vouch for the accuracy and completeness of the data and analysis and for the fidelity of the trial protocol.
Subject Eligibility
Eligible patients were aged ≥18 years and presented with RC2 to RC6 PAD with an indication for revascularization of lesions extending from 10 cm above the medial epicondyle to the digital arteries. Eligible subjects were further required to have at least 1 lesion in the native vessel to be successfully crossed with a guidewire and treated with a Food and Drug Administration (FDA)–approved endovascular device. Subjects were excluded under the following conditions: conversion from endovascular intervention to surgical revascularization was required, in-stent restenosis in all lesions in the target area, a life span <1 year, and standard requirements regarding unwillingness to sign the informed consent, inability to understand the study protocol requirements, participation in other investigational studies, and females pregnant or planning on becoming pregnant during the study. No further criteria were specified. Written informed consent was obtained from every participant prior to enrollment
Study Endpoints
As an observational study, LIBERTY was not designed to test any specific hypothesis; there were no primary endpoints defined, thus, no power calculations were performed to determine sample size. There were multiple prespecified outcome measures, including (1) procedure and lesion success defined as <50% residual stenosis for treated lesions without significant angiographic complications (flow-limiting dissections, perforation, distal embolization, or acute vessel closure) per patient (procedure success) or lesion (lesion success) as assessed by the angiographic core laboratory; (2) rate of major adverse events (MAE) defined as death within 30 days of the index procedure, unplanned major amputation of the target limb, and clinically-driven target vessel revascularization as assessed by the angiographic core laboratory when angiographic images were available; (3) patency in RC2,3 lesions as determined using duplex ultrasonography (peak systolic velocity ratio ≤2.4), with oversight by the ultrasound core laboratory (duplex was not required per protocol on RC4–6 subjects); and (4) change in self-reported quality of life (QoL) measures per the EuroQol EQ-5D-5L and the Vascular Quality of Life Questionnaire (VascuQol-25), a PAD-specific health-related QoL instrument.
Patient Enrollment
From May 2013 to February 2016, 1204 subjects (mean age 69.8±10.7 years; 770 men) were enrolled at 51 hospitals and office-based laboratories in the United States: 501 in the RC2,3 cohort, 603 in the RC4,5 cohort, and 100 in the RC6 cohort (Supplementary Table 1; available in the online version of the article). Patient demographics stratified by RC group are summarized in Table 1. The majority of subjects were Caucasian; however, there were proportionally more Hispanic/Latino and Black/African American subjects in the RC6 cohort. Elderly subjects were prevalent across all RCs, and there was a higher percentage of men in RC6 compared with RC4,5.
Table 1.
Baseline Characteristics of the Participants.a
| Baseline RC |
p |
|||||
|---|---|---|---|---|---|---|
| Characteristics | RC2,3 (n=500)b | RC4,5 (n=589)b | RC6 (n=100)b | RC2,3 vs RC4,5 | RC2,3 vs RC6 | RC4,5 vs RC6 |
| Age, y | 69.7±10.0 [69.0, IQR 64.0, 77.0] | 70.3±10.9 [71.0, IQR 63.0, 79.0] | 68.0±13.0 (n=99) [67.0, IQR 62.0, 78.0] | 0.42 | 0.13 | 0.06 |
| Men | 333 (66.6) | 364 (61.8) | 73 (73.0) | 0.11 | 0.24 | 0.03 |
| Race | ||||||
| American Indian or Alaska native | 1 (0.2) | 4 (0.7) | 0 (0.0) | 0.38 | >0.99 | >0.99 |
| Asian | 5 (1.0) | 1 (0.2) | 1 (1.0) | 0.10 | >0.99 | 0.27 |
| Black or African American | 68 (13.6) | 89 (15.1) | 21 (21.0) | 0.49 | 0.06 | 0.14 |
| Native Hawaiian or other Pacific Islander | 1 (0.2) | 1 (0.2) | 0 (0.0) | >0.99 | >0.99 | >0.99 |
| White | 411 (82.2) | 483 (82.0) | 78 (78.0) | 0.94 | 0.33 | 0.33 |
| Multiple or not specified | 14 (2.8) | 11 (1.9) | 0 (0.0) | 0.32 | 0.14 | 0.38 |
| Hispanic or Latino ethnicity | 77 (15.4) | 63 (10.7) | 29 (29.0) | 0.02 | 0.002 | <0.001 |
| Body mass index, kg/m2 | 28.8±5.3 [28.3, IQR 25.1, 31.7] | 29.1±6.2 [28.2, IQR 24.7, 32.7] | 29.1±7.6 [27.3, IQR 23.8, 33.0] | 0.29 | 0.58 | 0.97 |
| eGFR, mL/min/1.73 m2 | 68.5±28.0 [66.9, IQR 51.0, 83.3] | 59.7±27.6 [60.7, IQR 40.4, 77.0] | 56.7±37.1 (n=99) [57.7, IQR 24.2, 80.9] | <0.001 | <0.001 | 0.34 |
| Current/former smoker | 370 (74.0) | 378 (64.2) | 61 (61.0) | <0.001 | 0.01 | 0.57 |
| Diabetes | 241 (48.2) | 407 (69.1) | 79 (79.0) | <0.001 | <0.001 | 0.04 |
| Hyperlipidemia | 454 (90.8) | 510 (86.6) | 69 (69.0) | 0.04 | <0.001 | <0.001 |
| Hypertension | 468 (93.6) | 549 (93.2) | 93 (93.0) | 0.81 | 0.82 | >0.99 |
| Kidney disease | 137 (27.4) | 232 (39.4) | 43 (43.0) | <0.001 | 0.003 | 0.51 |
| Coronary artery disease | 298 (59.6) | 375 (63.7) | 54 (54.0) | 0.17 | 0.32 | 0.07 |
| Myocardial infarction | 115 (23.0) | 155 (26.3) | 15 (15.0) | 0.23 | 0.08 | 0.02 |
| Stroke/TIA | 77 (15.4) | 92 (15.6) | 9 (9.0) | 0.93 | 0.12 | 0.09 |
| ABI, target limbs | ||||||
| ≤0.90 | 339/482 (70.3) | 284/534 (53.2) | 42/90 (46.7) | <0.001 | <0.001 | 0.26 |
| >0.90 to <1.00 | 46/482 (9.5) | 53/534 (9.9) | 4/90 (4.4) | 0.92 | 0.15 | 0.11 |
| ≥1.00 to ≤1.40 | 67/482 (13.9) | 125/534 (23.4) | 19/90 (21.1) | <0.001 | 0.11 | 0.69 |
| >1.40 or noncompressible | 30/482 (6.2) | 72/534 (13.5) | 25/90 (27.8) | <0.001 | <0.001 | 0.001 |
| Previous EVT for lower limb PAD | ||||||
| None | 236 (47.2) | 285 (48.4) | 58 (58.0) | 0.72 | 0.06 | 0.08 |
| Target limb only | 61 (12.2) | 90 (15.3) | 20 (20.0) | 0.16 | 0.05 | 0.24 |
| Contralateral limb only | 112 (22.4) | 98 (16.6) | 14 (14.0) | 0.02 | 0.06 | 0.56 |
| Both limbs | 89 (17.8) | 115 (19.5) | 8 (8.0) | 0.48 | 0.02 | 0.004 |
| Unknown | 2 (0.4) | 1 (0.2) | 0 (0.0) | 0.60 | >0.99 | >0.99 |
| Target limb procedures in the last 3 years | 0.4±1.1 [0.0, IQR 0.0, 1.0] | 0.7±1.9 [0.0, IQR 0.0, 1.0] | 0.6±1.5 [0.0, IQR 0.0, 1.0] | 0.01 | 0.01 | 0.28 |
| Previous amputations | ||||||
| None | 477 (95.4) | 489 (83.0) | 55 (55.0) | <0.001 | <0.001 | <0.001 |
| Both limbs | 4 (0.8) | 17 (2.9) | 9 (9.0) | 0.01 | <0.001 | 0.007 |
| Target limb only | 8 (1.6) | 35 (5.9) | 17 (17.0) | <0.001 | <0.001 | <0.001 |
| Highest levelc | ||||||
| Toe(s) only | 11 (91.7) | 52 (100.0) | 22 (84.6) | 0.19 | >0.99 | 0.01 |
| Foot only | 1 (8.3) | 0 (0.0) | 4 (15.4) | 0.19 | >0.99 | 0.01 |
| Contralateral limb only | 11 (2.2) | 48 (8.1) | 19 (19.0) | <0.001 | <0.001 | 0.002 |
| Highest leveld | ||||||
| Toe(s) only | 8 (53.3) | 35 (53.8) | 11 (39.3) | >0.99 | 0.52 | 0.26 |
| Foot only | 2 (13.3) | 1 (1.5) | 2 (7.1) | 0.09 | 0.60 | 0.21 |
| Below knee/above ankle | 3 (20.0) | 22 (33.8) | 10 (35.7) | 0.37 | 0.49 | >0.99 |
| Above the knee | 2 (13.3) | 7 (10.8) | 5 (17.9) | 0.67 | >0.99 | 0.50 |
Abbreviations: ABI, ankle-brachial index; eGFR, estimated glomerular filtration rate; EVT, endovascular treatment; IQR, interquartile range; PAD, peripheral artery disease; RC, Rutherford category; TIA, transient ischemic attack.
Continuous data are presented as the mean ± standard deviation [median, IQR Q1, Q3]; categorical data are given as the number (percentage).
Number of subjects unless otherwise noted.
Percentages are based on the number of subjects with previous target limb amputation.
Percentages are based on the number of subjects with previous contralateral limb amputation.
Comorbidities associated with PAD were prevalent across all RCs. The prevalence of diabetes increased significantly with the level of ischemia and was highest (79%) in RC6. In addition, kidney disease was significantly more common in RC4,5 and RC6 as compared to RC2,3. The history of a previous amputation increased with RC, indicating the severity of the disease state prior to the intervention in the LIBERTY study.
Target lesion characteristics are summarized in Table 2. There were more target lesions per subject with increasing severity of RC. In addition, patients with CLI presented with more distal, isolated tibial artery below-the-knee lesions with smaller distal reference vessel diameters and greater severity of TransAtlantic Inter-Society Consensus (TASC) lesion type compared to claudicants (RC2,3). RC4,5 had longer lesions as compared with RC2,3 and RC6 and more chronic total occlusions (CTO) as compared to RC2,3. The majority (58.5%) of lesions were calcified, with most classified as moderate or severe by the PARC definition.30 Across all RC groups, all lesions were highly stenotic (median percent stenosis >85%).
Table 2.
Lesion Characteristics.a
| Baseline RC |
p |
|||||
|---|---|---|---|---|---|---|
| Characteristics | RC2,3 (n=605)b | RC4,5 (n=775)b | RC6 (n=148)b | RC2,3 vs RC4,5 | RC2,3 vs RC6 | RC4,5 vs RC6 |
| Lesion location | ||||||
| ATK only | 293 (48.4) | 207 (26.7) | 40 (27.0) | <0.001 | <0.001 | 0.92 |
| SFA only | 78 (12.9) | 23 (3.0) | 6 (4.1) | <0.001 | 0.001 | 0.45 |
| SFA to popliteal | 99 (16.4) | 86 (11.1) | 13 (8.8) | 0.005 | 0.02 | 0.47 |
| POP only | 116 (19.2) | 98 (12.6) | 21 (14.2) | 0.001 | 0.19 | 0.59 |
| ATK and BTK | 80 (13.2) | 103 (13.3) | 18 (12.2) | >0.99 | 0.79 | 0.79 |
| SFA to BTK | 18 (3.0) | 26 (3.4) | 3 (2.0) | 0.76 | 0.78 | 0.61 |
| POP to BTK | 62 (10.2) | 77 (9.9) | 15 (10.1) | 0.86 | >0.99 | 0.88 |
| BTK only | 232 (38.3) | 464 (59.9) | 89 (60.1) | <0.001 | <0.001 | >0.99 |
| Unknown | 0 (0.0) | 1 (0.1) | 1 (0.7) | >0.99 | 0.20 | 0.30 |
| Target lesion length, mm | 87.9±86.9 (n=562) [59.1, IQR 25.9, 115.0] | 131.3±117.8 (n=727) [86.3, IQR 38.3, 200.4] | 105.8±93.7 (n=137) [82.8, IQR 30.1, 147.6] | <0.001 | 0.03 | 0.02 |
| <40 | 206 (36.7) | 190 (26.1) | 45 (32.8) | <0.001 | 0.43 | 0.12 |
| 40–99 | 185 (32.9) | 190 (26.1) | 31 (22.6) | 0.01 | 0.02 | 0.46 |
| ≥100 | 171 (30.4) | 347 (47.7) | 61 (44.5) | <0.001 | 0.002 | 0.51 |
| Distal RVD, mm | 3.8±1.2 (n=578) [3.7, IQR 2.8, 4.6] | 3.2±1.2 (n=745) [2.9, IQR 2.3, 4.0] | 3.0±1.1 (n=140) [2.7, IQR 2.2, 3.6] | <0.001 | <0.001 | 0.14 |
| Preprocedure MLD, mm | 0.7±0.8 (n=589) [0.5, IQR 0.0, 1.2] | 0.6±0.8 (n=750) [0.2, IQR 0.0, 0.9] | 0.6±0.8 (n=144) [0.5, IQR 0.0, 1.1] | 0.001 | 0.19 | 0.43 |
| Preprocedure stenosis, % | 80.7±19.2 (n=590) [85.0, IQR 66.0, 100.0] | 83.3±19.7 (n=753) [91.0, IQR 70.0, 100.0] | 80.5±20.1 (n=145) [85.0, IQR 65.0, 100.0] | 0.01 | 0.91 | 0.11 |
| Chronic total occlusions | 195/590 (33.1) | 331/753 (44.0) | 57/145 (39.3) | <0.001 | 0.17 | 0.32 |
| TASC lesion type | <0.001 | 0.005 | 0.19 | |||
| A | 366/581 (63.0) | 348/744 (46.8) | 68/142 (47.9) | <0.001 | 0.001 | 0.85 |
| B | 105/581 (18.1) | 129/744 (17.3) | 34/142 (23.9) | 0.77 | 0.12 | 0.08 |
| C | 65/581 (11.2) | 141/744 (19.0) | 20/142 (14.1) | <0.001 | 0.38 | 0.19 |
| D | 45/581 (7.7) | 126/744 (16.9) | 20/142 (14.1) | <0.001 | 0.02 | 0.46 |
| Predominantly calcified plaque | 334/560 (59.6) | 411/717 (57.3) | 85/141 (60.3) | 0.42 | 0.92 | 0.58 |
| PARC category | 0.04 | 0.003 | 0.11 | |||
| Focal | 37/304 (12.2) | 51/366 (13.9) | 5/74 (6.8) | 0.57 | 0.22 | 0.12 |
| Mild | 71/304 (23.4) | 54/366 (14.8) | 6/74 (8.1) | 0.005 | 0.003 | 0.14 |
| Moderate | 78/304 (25.7) | 102/366 (27.9) | 26/74 (35.1) | 0.54 | 0.11 | 0.21 |
| Severe | 118/304 (38.8) | 159/366 (43.4) | 37/74 (50.0) | 0.24 | 0.09 | 0.31 |
| Access site | ||||||
| Femoral | 618/654 (94.5) | 796/852 (93.4) | 149/158 (94.3) | 0.45 | 0.85 | 0.86 |
| Popliteal | 5/654 (0.8) | 4/852 (0.5) | 1/158 (0.6) | 0.51 | >0.99 | 0.57 |
| Tibial | 33/654 (5.0) | 63/852 (7.4) | 5/158 (3.2) | 0.07 | 0.40 | 0.06 |
| Pedal | 8/654 (1.2) | 50/852 (5.9) | 8/158 (5.1) | <0.001 | 0.005 | 0.85 |
| Brachial | 2/654 (0.3) | 1/852 (0.1) | 0/158 (0.0) | 0.58 | >0.99 | >0.99 |
| Device data available (per lesion) | 597 (98.7) | 766 (98.8) | 142 (95.9) | 0.81 | 0.04 | 0.02 |
| Balloons | 578 (96.8) | 740 (96.6) | 141 (99.3) | 0.88 | 0.15 | 0.10 |
| Atherectomy | 437 (73.2) | 494 (64.5) | 107 (75.4) | <0.001 | 0.67 | 0.01 |
| Stent | 120 (20.1) | 111 (14.5) | 25 (17.6) | 0.007 | 0.56 | 0.37 |
| Bailout stent | 27 (4.5) | 34 (4.4) | 0 (0.0) | >0.99 | 0.005 | 0.006 |
| Postprocedure MLD, mm | 2.9±1.2 (n=575) [2.8, IQR 2.0, 3.7] | 2.3±1.2 (n=723) [2.1, IQR 1.5, 3.1] | 2.2±1.2 (n=142) [2.0, IQR 1.4, 2.9] | <0.001 | <0.001 | 0.29 |
| Postprocedure stenosis, % | 29.5±15.9 (n=577) [28.0, IQR 19.0, 37.0] | 33.9±20.8 (n=724) [31.0, IQR 21.0, 41.0] | 35.0±23.5 (n=142) [33.0, IQR 21.0, 43.0] | <0.001 | <0.001 | 0.58 |
Abbreviations: ATK, above the knee; BTK, below the knee; IQR, interquartile range; MLD, minimum lumen diameter; PARC, Peripheral Academic Research Consortium; RC, Rutherford category; RVD, reference vessel diameter; SFA, superficial femoral artery; TASC, Trans-Atlantic Inter-Society Consensus.
Continuous data are presented as the mean ± standard deviation [median, IQR Q1, Q3]; categorical data are given as the number (percentage).
Number of lesions unless otherwise noted.
Statistical Analysis
Categorical data are presented as number (percentage); cohorts for comparison were generated from a Monte Carlo approximation of the Fisher exact test. Groups of discrete data were compared with the Kruskal-Wallis test or Wilcoxon signed rank test for paired data. Numeric data are presented as mean ± standard deviation and median [interquartile range (IQR) Q1, Q3]; continuous variables were compared using ANOVA or a paired t test as appropriate.
Predictors of 12-month MAE were analyzed using Cox proportional hazard regression modeling. Covariates were chosen based on traditional predictors of negative outcomes, clinically relevant demographics, and components of procedure success. Covariates found significant (p<0.1) in a univariable model were placed into a multivariable model. The final multivariable model was created using stepwise selection with an entry criterion of 0.15 and a stay criterion of 0.05. Imputation of significant angiographic complications for procedure and lesion success of core laboratory–identified lesions was performed by using site data when the core laboratory was unable to perform angiographic assessment. The Kaplan-Meier time-to-event method was used to estimate event rates through each time point; curves were compared with the log-rank test. No additional imputation methods were used to manage missing data, as such the denominators may change based on available data. P-values were considered significant at an alpha of 0.05. Data analysis conventions were published previously29 and performed with the SAS Software System (SAS Institute, Inc, Cary, NC, USA).
Results
As shown in Table 3, balloon and/or atherectomy were the preferred devices. Procedure and postprocedure characteristics are summarized in Supplementary Table 2 (available in the online version of the article). As shown in Table 4, a high percentage of subjects in all RC groups attained a final residual stenosis <50% while maintaining a low significant angiographic complication rate. In the group most at risk for amputation (RC6), a <50% residual stenosis in all lesions treated was achieved in 72 (76.6%) of the 94 subjects evaluated for this outcome; significant angiographic complications in this subgroup occurred in 14 (14.7%) of 95 subjects. There were significant differences in procedure and lesion success (Table 4) and postprocedure minimum lumen diameter (MLD) and percent stenosis (Table 2) between RC2,3 as compared with RC4,5 as well as RC2,3 vs RC6. Postprocedure hospitalization for additional therapy occurred in all Rutherford categories (0.8% RC2,3, 1.7% RC4,5, and 2.0% RC6); 78% of the 100 RC6 patients were discharged to home (Supplementary Table 2; available in the online version of the article).
Table 3.
Target Lesion Device Use.a
| Baseline RC |
p |
|||||
|---|---|---|---|---|---|---|
| RC2,3 (n=605)b | RC4,5 (n=775)b | RC6 (n=148)b | RC2,3 vs RC4,5 | RC2,3 vs RC6 | RC4,5 vs RC6 | |
| Balloons | ||||||
| Angioplasty | 494/597 (82.7) | 635/766 (82.9) | 96/142 (67.6) | 0.94 | <0.001 | <0.001 |
| DCB | 74/597 (12.4) | 54/766 (7.0) | 11/142 (7.7) | 0.001 | 0.14 | 0.72 |
| Cutting | 42/597 (7.0) | 58/766 (7.6) | 26/142 (18.3) | 0.75 | <0.001 | <0.001 |
| Focal Force | 73/597 (12.2) | 97/766 (12.7) | 31/142 (21.8) | 0.87 | 0.005 | 0.006 |
| Scoring | 3/597 (0.5) | 7/766 (0.9) | 4/142 (2.8) | 0.53 | 0.03 | 0.08 |
| Atherectomy devices | ||||||
| Diamondback, Stealth | 274/437 (45.9) | 347/494 (45.3) | 88/107 (62.0) | 0.83 | 0.001 | <0.001 |
| Jetstream | 19/437 (3.2) | 12/494 (1.6) | 1/107 (0.7) | 0.07 | 0.15 | 0.70 |
| Excimer laser | 35/437 (5.9) | 39/494 (5.1) | 15/107 (10.6) | 0.55 | 0.06 | 0.02 |
| Rotablator | 7/437 (1.2) | 7/494 (0.9) | 0/107 (0.0) | 0.79 | 0.36 | 0.60 |
| Turbohawk, Silverhawk, Hawk One | 87/437 (14.6) | 82/494 (10.7) | 5/107 (3.5) | 0.04 | <0.001 | 0.005 |
| Phoenix | 6/437 (1.0) | 14/494 (1.8) | 0/107 (0.0) | 0.26 | 0.60 | 0.14 |
| Crosser | 14/437 (2.3) | 8/494 (1.0) | 0/107 (0.0) | 0.08 | 0.08 | 0.62 |
| Stents | ||||||
| DES | 34/120 (5.7) | 37/111 (4.8) | 12/25 (8.5) | 0.54 | 0.25 | 0.10 |
| BMS | 88/120 (14.7) | 72/111 (9.4) | 16/25 (11.3) | 0.003 | 0.35 | 0.54 |
| Covered | 5/120 (0.8) | 6/111 (0.8) | 0/25 (0.0) | >0.99 | 0.59 | 0.60 |
Abbreviations: BMS, bare metal stent; DCB, drug-coated balloon; DES, drug-eluting stent; RC, Rutherford category.
Categorical data are given as the number (percentage).
Number of lesions unless otherwise noted.
Table 4.
Procedure and Lesion Success Outcome Measures.a
| Baseline RC |
p |
|||||
|---|---|---|---|---|---|---|
| RC2,3; 486 subjects, 605 lesionsb | RC4,5; 571 subjects, 775 lesionsb | RC6; 98 subjects, 148 lesionsb | RC2,3 vs RC4,5 | RC2,3 vs RC6 | RC4,5 vs RC6 | |
| Procedure success | 392/469 (83.6) [79.9 to 86.8] | 413/545 (75.8) [72.0 to 79.3] | 63/92 (68.5) [58.0 to 77.8] | 0.002 | 0.001 | 0.15 |
| Final residual stenosis <50% in all lesions | 425/469 (90.6) [87.6 to 93.1] | 456/546 (83.5) [80.1 to 86.5] | 72/94 (76.6) [66.7 to 84.7] | 0.001 | <0.001 | 0.11 |
| Significant angiographic complications | 41/483 (8.5) [6.2 to 11.3] | 66/566 (11.7) [9.1 to 14.6] | 14/95 (14.7) [8.3 to 23.5] | 0.10 | 0.08 | 0.40 |
| Severe dissection (C-F) | 13/485 (2.7) [1.4 to 4.5] | 17/569 (3.0) [1.7 to 4.7] | 6/98 (6.1) [2.3 to 12.9] | 0.85 | 0.11 | 0.13 |
| Perforation | 6/485 (1.2) [0.5 to 2.7] | 4/569 (2.5) [1.4 to 4.1] | 2/98 (2.0) [0.2 to 7.2] | 0.18 | 0.63 | >0.99 |
| Abrupt closure | 4/485 (0.8) [0.2 to 2.1] | 13/569 (2.3) [1.2 to 3.9] | 3/98 (3.1) [0.6 to 8.7] | 0.08 | 0.10 | 0.72 |
| Distal embolization | 21/482 (4.4) [2.7 to 6.6] | 31/566 (5.5) [3.8 to 7.7] | 5/95 (5.3) [1.7 to 11.9] | 0.48 | 0.60 | >0.99 |
| Lesion success | 491/578 (84.9) [81.8 to 87.8] | 580/733 (79.1) [76.0 to 82.0] | 106/141 (75.2) [67.2 to 82.1] | 0.007 | 0.01 | 0.36 |
| Final residual stenosis <50% | 533/580 (91.9) [89.4 to 94.0] | 636/733 (86.8) [84.1 to 89.1] | 119/143 (83.2) [76.1 to 88.9] | 0.003 | 0.003 | 0.32 |
| Significant angiographic complications | 47/600 (7.8) [5.8 to 10.3] | 78/771 (10.1) [8.1 to 12.5] | 18/144 (12.5) [7.6 to 19.0] | 0.15 | 0.07 | 0.33 |
| Severe dissection (C-F) | 13/604 (2.2) [1.2 to 3.7] | 18/773 (2.3) [1.4 to 3.7] | 7/148 (4.7) [1.9 to 9.5] | 0.78 | 0.11 | 0.14 |
| Perforation | 6/604 (1.0) [0.4 to 2.1] | 14/773 (1.8) [1.0 to 3.0] | 2/148 (1.4) [0.2 to 4.8] | 0.21 | 0.71 | 0.69 |
| Abrupt closure | 4/604 (0.7) [0.2 to 1.7] | 13/773 (1.7) [0.9 to 2.9] | 3/148 (2.0) [0.4 to 5.8] | 0.10 | 0.14 | 0.77 |
| Distal embolization | 27/599 (4.5) [3.0 to 6.5] | 41/775 (5.3) [3.8 to 7.1] | 8/144 (5.6) [2.4 to 10.7] | 0.60 | 0.72 | 0.96 |
Abbreviation: RC, Rutherford category.
Data are presented as the number/sample (percentage) [95% confidence interval].
Number of subjects/lesions with data available for core laboratory analysis, unless otherwise noted.
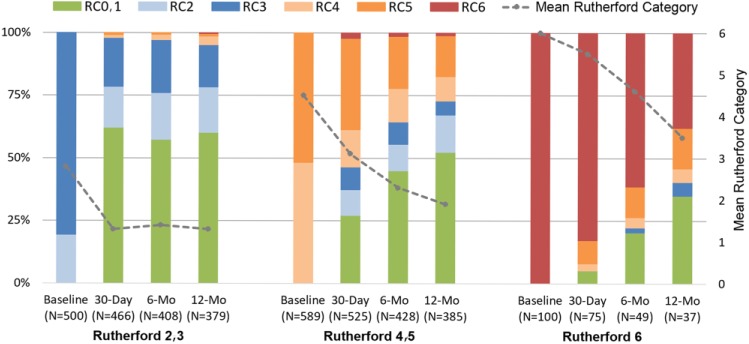
In all, 831 subjects completed a 12-month follow-up visit (388 RC2,3, 402 RC4,5, and 41 RC6). The RC improved from 30 days through 12 months in RC4,5 and RC6; in RC2,3 subjects, the RC improved to 30 days and sustained that rate through 12 months (Figure 1).
Figure 1.
Change in the distribution of Rutherford categories (RC) through 12 months (mo). The dotted line tracks the means at each time point.
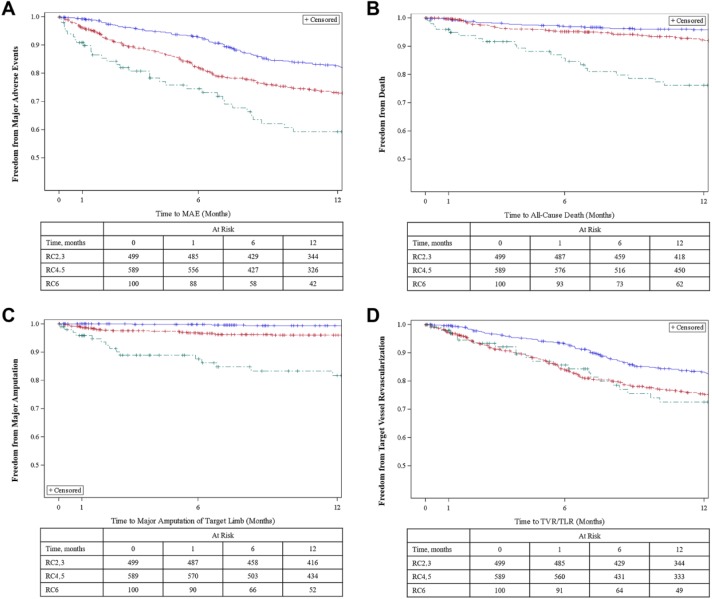
As shown in Figure 2A and Supplementary Table 3 (available in the online version of the article), freedom from MAE estimates at 30 days and 12 months were 99.2% and 82.6%, respectively, in RC2,3; 96.1% and 73.2% in RC4,5; and 90.8% and 59.3% in RC6. Freedom from major amputation estimates at 30 days and 12 months were, respectively, 100% and 99.3% in RC2,3; 98.8% and 96.0% in RC4,5; and 95.8% and 81.7% in RC6 (Figure 2C and Supplementary Table 3).
Figure 2.
Kaplan-Meier curves for (A) freedom from major adverse events, (B) death, (C) major target limb amputation, and (D) target vessel revascularization. The standard error did not exceed 10% for any group. MAE, major adverse events; RC, Rutherford category; TLR, target lesion revascularization; TVR, target vessel revascularization. The blue line represents the RC2,3 subgroup, the red line is the RC4,5 subgroup, and the green line denotes the RC6 subgroup.
As listed in Table 5, a multivariate analysis of the outcomes demonstrated statistically significant predictors of 12-month MAE, specifically: history of previous lower limb endovascular treatment, number of target limb procedures in the last 3 years, treatment of CTO, number of wounds on the target limb at baseline, history of coronary artery disease, distal region treated, and RC.
Table 5.
Analysis of Independent Predictors of 12-Month Major Adverse Events.
| Unadjusted Hazard Ratioa | p | Adjusted Hazard Ratioa | p | |
|---|---|---|---|---|
| Number of wounds on target limb at baseline (1-unit increase) | 1.38 [1.24 to 1.53] | <0.001 | 1.30 [1.14 to 1.48] | <0.001 |
| Chronic total occlusions (at least 1 vs 0) | 2.09 [1.62 to 2.69] | <0.001 | 1.89 [1.46 to 2.46] | <0.001 |
| History of previous lower limb EVT | 1.76 [1.36 to 2.27] | <0.001 | 1.77 [1.34 to 2.33] | <0.001 |
| Number of target limb procedures in the past 3 years (1-procedure increase) | 1.12 [1.08 to 1.16] | <0.001 | 1.07 [1.02 to 1.13] | 0.008 |
| Distal region treatedb | NA | <0.001 | NA | 0.008 |
| Rutherford categoryb | NA | <0.001 | NA | 0.02 |
| 2,3 vs 4,5c | 0.58 [0.44 to 0.77] | <0.001 | 0.97 [0.70 to 1.34] | 0.85 |
| 2,3 vs 6c | 0.34 [0.23 to 0.50] | <0.001 | 0.56 [0.35 to 0.90] | 0.02 |
| 4,5 vs 6c | 0.58 [0.40 to 0.84] | 0.004 | 0.58 [0.39 to 0.87] | 0.008 |
| Most severe TASC lesion typeb | NA | <0.001 | ||
| Total treated lesion length (1-cm increase) | 1.02 [1.01 to 1.03] | <0.001 | ||
| Target lesions treated (1-lesion increase) | 1.41 [1.17 to 1.69] | <0.001 | ||
| History of diabetes | 1.47 [1.13 to 1.92] | 0.004 | ||
| History of coronary artery disease | 1.46 [1.12 to 1.90] | 0.006 | 1.34 [1.02 to 1.77] | 0.03 |
| Previous major (above ankle) amputation on contralateral limb | 1.79 [1.08 to 2.96] | 0.02 | ||
| History of renal disease | 1.32 [1.02 to 1.69] | 0.03 | ||
| Smoker (current/former vs never) | 0.81 [0.63 to 1.04] | 0.10 | ||
| History of hypertension | 1.58 [0.87 to 2.90] | 0.14 | ||
| Residual stenosis <50% for all target lesions per subject | 0.78 [0.55 to 1.10] | 0.15 | ||
| History of myocardial infarction | 1.20 [0.91 to 1.59] | 0.19 | ||
| Most severe PARC calcification gradeb | NA | 0.25 | ||
| Race (non-white vs white) | 1.21 [0.90 to 1.65] | 0.21 | ||
| Age (1-year increase) | 0.99 [0.98 to 1.01] | 0.28 | ||
| Significant angiographic complication | 0.83 [0.51 to 1.34] | 0.44 | ||
| Bailout stent used | 1.20 [0.71 to 2.02] | 0.50 | ||
| History of stroke/TIA | 0.91 [0.63 to 1.31] | 0.61 | ||
| Gender | 1.04 [0.80 to 1.34] | 0.78 | ||
| BMI (1-unit increase) | 1.00 [0.98 to 1.02] | 0.87 | ||
| Ethnicity (Hispanic vs non-Hispanic) | 0.97 [0.69 to 1.39] | 0.89 | ||
| Presence of any lesion with predominantly calcified plaque | 1.00 [0.77 to 1.30] | 0.99 | ||
| History of hyperlipidemia | 1.00 [0.69 to 1.44] | 0.99 |
Abbreviations: BMI, body mass index; EVT, endovascular treatment; NA, not applicable; PARC, Peripheral Academic Research Consortium; TASC, TransAtlantic Inter-Society Consensus; TIA, transient ischemic attack.
Hazard ratios are presented with the 95% confidence interval in brackets.
Type III p value displayed.
Contrast statement used to estimate hazard ratio between 2 levels of Rutherford category.
Early target lesion patency as determined by duplex was 95.5% in RC2,3 subjects (n=355) at 30 days, while patency at 6 months (n=248) was 81.5%. Patency was maintained at 12 months (n=206, 82.0%). Duplex was not required on RC4–6 subjects.
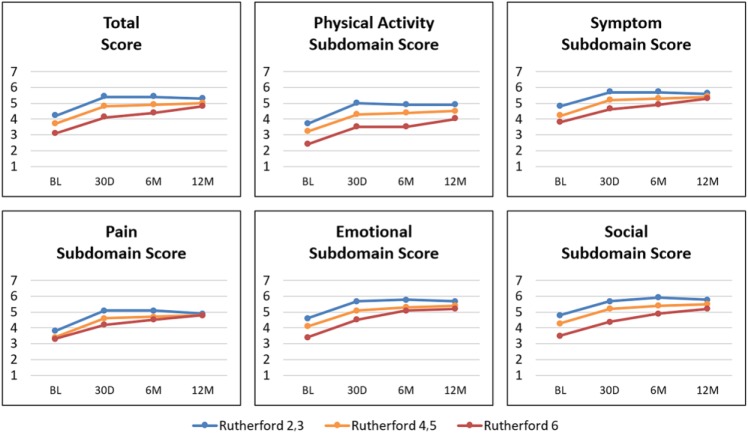
QoL as measured by EQ-5D (Supplementary Table 4; available in the online version of the article) and VascuQol (Figure 3 and Supplementary Table 5) improved from baseline to 30 days across all RCs and was maintained at 12 months in RC2,3 and RC4,5. RC6 patients continued QoL improvement through 12 months. One-year VascuQol total scores (7 maximum) were 5.3, 5.0, and 4.8 for RC2,3, RC4,5, and RC6 patients, respectively.
Figure 3.
Change in mean Vascular Quality of Life scores through 12 months (mo). Higher scores indicate a better rating of health. BL, baseline; D, day; M, month.
Discussion
Evidence from the LIBERTY study supports peripheral endovascular interventions in both claudicants and CLI patients as demonstrated by marked improvement in all QoL measures and most importantly, amputation prevention. PAD and CLI are underrecognized by patients and physicians alike, resulting in delayed or misdiagnosis, undertreatment, and a dearth of comparative PAD and CLI endovascular studies.9,27 LIBERTY demographic data reflect the well-known hallmark indicators of PAD: advanced age (≥64 years old), diabetes mellitus, renal insufficiency, and hypertension.1 Implications of racial disparities can also be seen in this dataset. For example, the RC6 group had proportionally more black Americans and Hispanics/Latinos than the other groups, possibly indicating disparities in access to care (or other unidentified disparities) until the disease is in the most advanced/critical stages. This finding is similar to that shown in a recent national health care database analysis.31
Patients with intermittent claudication suffer from significant functional limitations in their daily activities; over a 5-year period ~5% of these patients progress to lower extremity amputation.27 The LIBERTY study demonstrated high freedom from amputation (99.3%) in the RC2,3 group and improvement in the RC as well as patient-reported pain on the QoL questionnaires. These data suggest that it is reasonable to intervene early on claudicants with intractable pain who have failed medical management and supervised exercise therapy.
Delay in treatment may result in advanced PAD in patients who have multilevel disease, which may require complex treatment strategies.32 CLI, the most severe manifestation of PAD, is associated with a 1-year mortality of 20% and a 1-year limb loss rate of 20%.27 The LIBERTY observational evidence indicates high freedom from 12-month death and major amputation in this population. There was also marked improvement in the RC and QoL scores through 12 months, suggesting continued improvement even in difficult to treat RC6 subjects.
The LIBERTY study also offered a unique opportunity to assess predictors of long-term outcomes on a large varied population. Interestingly, while traditional variables, such as diabetes, renal disease, age, lesion TASC type, and lesion length, were predictors of 12-month MAE in the unadjusted (univariable) model, other related variables better predicted 12-month MAE in the adjusted model. Of note, <50% residual stenosis and significant angiographic complications were not identified as predictors of 12-month MAE in the unadjusted model. This provides evidence that endovascular therapy can be successful and even mitigate traditional risks associated with comorbidities in these patients. Additionally, variables such as history of a previous endovascular therapy, CTO, and number of wounds at enrollment were significant predictors of 12-month MAE and should be considered when planning treatment strategies for PAD patients. These may indicate the need for earlier screening, improved devices for treatment (such as those for CTOs), as well as the advancement of wound healing therapies, algorithms, and follow-up.
These real-world observational results indicate that endovascular revascularization is an effective PAD therapy for both inflow and outflow disease when it is tailored to the patient and not dictated by predefined inclusion, exclusion, and protocol algorithms that often limit enrollment of the most advanced PAD patients in controlled clinical trials of PAD devices. The current state of evaluating endovascular devices to treat patients with PAD is costly and difficult due to the heterogeneity of the disease and the multiple specialties that perform endovascular therapy on these patients.33 This has spurred initiatives by the FDA to support peripheral endovascular therapy registry efforts and potential use of real-world evidence in FDA regulatory submissions and postmarket surveilance.33,34 The LIBERTY registry provides a framework and an example for these initiatives going forward.
Limitations
LIBERTY was an observational nonrandomized study of endovascular therapies, excluding surgery. Extensive mandatory testing requirements may have resulted in site and patient participation bias. As this study was sponsored by a company whose principal endovascular strategy is atherectomy, bias may be attributed to physician selection of orbital atherectomy in a high number of cases. Bias in outcomes may also be attributed to preferred physician treatment algorithms and device availability. Finally, possible over- or underreporting of outcomes is possible due to subject withdrawal prior to 12 months. However, LIBERTY was a prospective, multicenter, core laboratory–adjudicated study including patient populations that are typically excluded from other PAD/CLI clinical trials; thus, the results of LIBERTY likely have greater external validity.
Conclusion
LIBERTY 12-month results provide not only supporting evidence related to recently updated ACC/AHA lower extremity PAD guidelines but also a framework for the endovascular treatment decisions for these complex patients. LIBERTY represents the largest, most modern real-world experience with various endovascular strategies across the full range of RC patients, including many PAD patients for whom controlled longitudinal data are sparse, particularly RC6 patients. The results of this novel all-comers PAD study continue to suggest that endovascular therapy is a viable treatment option for RC2,3, RC4,5, and RC6 patients. Furthermore, primary unplanned amputation is often not necessary in RC6 patients as endovascular therapy is successful in this subgroup.
Supplemental Material
Supplemental material, 18-0395_supplementary_tables for One-Year Results of the LIBERTY 360 Study: Evaluation of Acute and Midterm Clinical Outcomes of Peripheral Endovascular Device Interventions by Jihad Mustapha, William Gray, Brad J. Martinsen, Ryan W. Bolduan, George L. Adams, Gary Ansel and Michael R. Jaff in Journal of Endovascular Therapy
Acknowledgments
The authors thank Ann Behrens, BS, of Cardiovascular Systems, Inc, for editing and critical review of this manuscript.
Footnotes
Authors’ Note: This study was presented at the Amputation Prevention Symposium (August 10, 2017; Chicago, IL, USA) and the Leipzig Interventional Course (January 31, 2018; Leipzig, Germany).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jihad Mustapha receives consulting fees from Abbott Vascular, Bard Peripheral Vascular, Boston Scientific, Cagent Vascular, Cardiovascular Systems, Inc., Cook Medical, Medtronic, PQ Bypass, Spectranetics, and Terumo Medical. William Gray receives personal fees and institutional research support from Boston Scientific, Medtronic, and Spectranetics. Brad J. Martinsen and Ryan W. Bolduan are employees of Cardiovascular Systems, Inc. George L. Adams receives consultant fees from Bard Peripheral Vascular, Terumo Interventional Systems, Medtronic, Boston Scientific, Spectranetics, and Cardiovascular Systems, Inc. Gary Ansel receives consulting fees from Medtronic, Boston Scientific, Cook Medical, W.L. Gore & Associates, Abbott Vascular, CR Bard, and Phillips; he receives royalties from Cook Medical. Michael R. Jaff is a noncompensated advisor to Abbott Vascular, Boston Scientific, Medtronic Vascular, and Cordis, a Cardinal Health company; he is a consultant to Philips/Volcano, Silk Road Medical, Micell, Vactronix, and Venarum; and he has equity investments in PQ Bypass, Gemini, Vascular Therapies, Sano V, and Primacea.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Cardiovascular Systems, Inc.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Jihad Mustapha  https://orcid.org/0000-0002-6351-8080
https://orcid.org/0000-0002-6351-8080
Gary Ansel  https://orcid.org/0000-0003-0612-4269
https://orcid.org/0000-0003-0612-4269
References
- 1. Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet. 2013;382:1312–1314. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong EJ, Chen DC, Westin GG, et al. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc. 2014;3:e000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guidelines on the Management of Patients With Lower Extremity Peripheral Artery Disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aboyans V, Ricco J-B, Bartelink M-LEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by the European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 6. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 7. Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56:1524–1533. [DOI] [PubMed] [Google Scholar]
- 8. Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–1651.e3. [DOI] [PubMed] [Google Scholar]
- 9. Conte SM, Vale PR. Peripheral arterial disease. Heart Lung Circ. 2018;27:427–432. [DOI] [PubMed] [Google Scholar]
- 10. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. [DOI] [PubMed] [Google Scholar]
- 11. Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Burrows NR, Gregg EW, et al. Declining rates of hospitalization for nontraumatic lower-extremity amputation in the diabetic population aged 40 years or older: U.S., 1988-2008. Diabetes Care. 2012;35:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mustapha J, Martinsen BJ, Igyarto Z. LIBERTY 360° Study presentation at AMP 2016 reveals hope for Rutherford-6 CLI patients. Cath Lab Digest. 2016;24(10). http://www.cathlabdigest.com/article/LIBERTY-360%C2%B0-Study-Presentation-AMP-2016-Reveals-Hope-Rutherford-6-CLI-Patients Accessed April 19, 2017. [Google Scholar]
- 14. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5-S67. [DOI] [PubMed] [Google Scholar]
- 15. Henry AJ, Hevelone ND, Belkin M, et al. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53:330–339.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olin JW, White CJ, Armstrong EJ, et al. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol. 2016;67:1338–1357. [DOI] [PubMed] [Google Scholar]
- 18. Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol. 2016;68:2002–2015. [DOI] [PubMed] [Google Scholar]
- 19. Kullo IJ, Rooke TW. Clinical practice. peripheral artery disease. N Engl J Med. 2016;374:861–871. [DOI] [PubMed] [Google Scholar]
- 20. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 21. Fowkes FGR, Aboyans V, Fowkes FJI, et al. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. [DOI] [PubMed] [Google Scholar]
- 22. Nehler MR, Duval S, Diao L, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695.e2. [DOI] [PubMed] [Google Scholar]
- 23. Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016;151:1070–1077. [DOI] [PubMed] [Google Scholar]
- 24. Aronow HD, Beckman JA. Parsing atherosclerosis: the unnatural history of peripheral artery disease. Circulation. 2016;134:438–440. [DOI] [PubMed] [Google Scholar]
- 25. Jones WS, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J. 2014;167:489–498.e7. [DOI] [PubMed] [Google Scholar]
- 26. Jones WS, Schmit KM, Vemulapalli S, et al. Treatment Strategies for Patients With Peripheral Artery Disease. Rockville, MD: Agency for Healthcare Research and Quality; 2013. http://www.ncbi.nlm.nih.gov/books/NBK148574/ Accessed October 7, 2015. [PubMed] [Google Scholar]
- 27. Swaminathan A, Vemulapalli S, Patel MR, et al. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vasc Health Risk Manag. 2014;10:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katib N, Thomas SD, Lennox AF, et al. An endovascular-first approach to the treatment of critical limb ischemia results in superior limb salvage rates. J Endovasc Ther. 2015;22:473–481. [DOI] [PubMed] [Google Scholar]
- 29. Adams GL, Mustapha J, Gray W, et al. The LIBERTY study: design of a prospective, observational, multicenter trial to evaluate the acute and long-term clinical and economic outcomes of real-world endovascular device interventions in treating peripheral artery disease. Am Heart J. 2016;174:14–21. [DOI] [PubMed] [Google Scholar]
- 30. Patel MR, Conte MS, Cutlip DE, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015;65:931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mustapha JA, Fisher BT, Rizzo JA, et al. Explaining racial disparities in amputation rates for the treatment of peripheral artery disease (PAD) using decomposition methods. J Racial Ethn Health Disparities. 2017;4:784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thukkani AK, Kinlay S. Endovascular intervention for peripheral artery disease. Circ Res. 2015;116:1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones WS, Krucoff MW, Morales P, et al. Registry Assessment of Peripheral Interventional Devices (RAPID): Registry assessment of peripheral interventional devices core data elements. J Vasc Surg. 2018;67:637–644.e30. [DOI] [PubMed] [Google Scholar]
- 34. Whatley E, Malone M. Current considerations on real-world evidence use in FDA regulatory submissions. Endovascular Today. 2017;16:106-108. http://evtoday.com/2017/10/current-considerations-on-real-world-evidence-use-in-fda-regulatory-submissions/ Accessed February 22, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 18-0395_supplementary_tables for One-Year Results of the LIBERTY 360 Study: Evaluation of Acute and Midterm Clinical Outcomes of Peripheral Endovascular Device Interventions by Jihad Mustapha, William Gray, Brad J. Martinsen, Ryan W. Bolduan, George L. Adams, Gary Ansel and Michael R. Jaff in Journal of Endovascular Therapy