Abstract
Purpose: To determine if stent placement across the renal vein inflow affects kidney function and renal vein patency. Methods: Between June 2008 and September 2016, 93 patients (mean age 39 years, range 15–70; 54 women) with iliocaval occlusion underwent venous stent placement and were retrospectively reviewed. For this analysis, the patients were separated into treatment and control groups: 51 (55%) patients had suprarenal and infrarenal iliocaval venous disease requiring inferior vena cava stent reconstruction across the renal vein inflow (treatment group) and 42 (45%) patients had iliac vein stenting sparing the renal veins (control group). Treatment group patients received Wallstents (n=15), Gianturco Z-stents (n=24), or suprarenal and infrarenal Wallstents such that the renal veins were bracketed with a “renal gap” (n=12). Stenting technical success, stent type, glomerular filtration rate (GFR), and creatinine before and after stent placement were recorded, along with renal vein patency and complications. Results: All procedures were technically successful. In the 51-patient treatment group, 15 (29%) patients received Wallstents and 24 (47%) received Gianturco Z-stents across the renal veins, while 12 (24%) were given a “renal gap” with no stent placement directly across the renal vein inflow. In the control group, 42 patients received iliac vein Wallstents only. Mean prestent GFR was 59±1.8 mL/min/1.73 m2 and mean prestent creatinine was 0.8±0.2 mg/dL for the entire cohort. Mean prestent GFR and creatinine values in the Wallstent, Gianturco Z-stent, and “renal gap” subgroups did not differ from the iliac vein stent group. Mean poststent GFR and creatinine values were 59±3.3 mL/min/1.73 m2 and 0.8±0.3 mg/dL, respectively. There were no differences between mean pre- and poststent GFR (p=0.32) or creatinine (p=0.41) values when considering all patients or when comparing the treatment subgroups and the control group. There were no differences in the poststent mean GFR or creatinine values between the Wallstent (p=0.21 and p=0.34, respectively) and Gianturco Z-stent (p=0.43 and p=0.41, respectively) groups and the “renal gap” group. One patient with a Wallstent across the renal veins developed right renal vein thrombosis 7 days after the procedure. Conclusion: Stent placement across the renal vein inflow did not compromise renal function. A very small risk of renal vein thrombosis was seen.
Keywords: endovascular therapy, iliocaval stent reconstruction, inferior vena cava, renal function, renal vein confluence, venous occlusive disease, venous disease, Wallstent, Z-stent
Introduction
Iliocaval occlusion may result from idiopathic causes, hypercoagulability, infection or inflammatory etiologies, and malignant disease. Such occlusions may cause debilitating symptoms including lower extremity edema, venous ulcers, and phlegmasia, as well as postthrombotic syndrome. Traditionally, iliocaval occlusion was treated with open surgical reconstruction, but endovascular treatment with pharmacomechanical thrombolysis, balloon angioplasty, and stent placement is now the standard of care for iliocaval occlusion.1–6 Clinical outcomes and patency of iliocaval stent reconstruction correlate with restoration of adequate inflow and outflow through the diseased venous segments.4,7 In instances of iliocaval occlusion with concomitant suprarenal, juxtarenal, and infrarenal venous disease, stent placement across the renal vein inflow is often necessary, but there are limited reports1,8–11 regarding the effects of this treatment with regard to renal function and renal vein patency. The study was designed to investigate these outcomes after stent placement across the renal vein inflow in patients undergoing iliocaval stent reconstruction.
Methods
Study Design and Patient Selection
All patients with iliocaval occlusion who underwent balloon angioplasty and venous stent placement at a university-based hospital were considered for inclusion in this study. Patients were identified from a search of the electronic medical records from June 2008 until September 2016. Inclusion required documentation of normal baseline renal function, including glomerular filtration rate (GFR; 100–130 mL/min/1.73 m2 in men and 90–120 mL/min/1.73 m2 in women) and creatinine (0.6–1.2 mg/dL in men and 0.5–1.1 mg/dL in women). Inclusion also required patent renal veins observed indirectly with venography and intravascular ultrasound evaluation.
Data collected from selected patients included age; gender; presenting symptoms; prothrombotic risk factors; presence of hypertension, diabetes, or multiple myeloma; stenting technical success; stent type; GFR and creatinine before and after stent placement; renal vein patency on follow-up imaging; and complications.
In all, 93 patients (mean age 39 years, range 15–70; 54 women) having iliocaval occlusion and stent placement met the inclusion criteria. Patient characteristics are presented in Table 1. Patients presented with lower extremity pain, swelling, or ulcers (48, 52%), deep venous thrombosis (42, 45%), or mass compressing the inferior vena cava (IVC; 3, 3%). Prothrombotic risk factors were evident in 53 (57%) patients and included obesity, smoking, factor V Leiden, malignancy, paraplegia or quadriplegia, nonthrombotic iliac vein compression, antiphospholipid syndrome, lupus erythematosus, oral contraceptives, protein C deficiency, methylene tetrahydrofolate reductase deficiency, prothrombin mutation, protein S deficiency, heparin-induced thrombocytopenia, primary thrombocytopenia, Behcet’s disease, and an undefined hypercoagulable state. Ten (11%) patients had hypertension and 11 (12%) had diabetes.
Table 1.
Patient Characteristics for the Entire Sample and for the Treatment and Control Groups.a
| Variable | Total Cohort (n=93) | Treatment Group (n=51) | Control Group (n=42) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 39±15 | 43±14 | 38±17 | 0.61 |
| Women | 54 (58) | 25 (49) | 29 (69) | 0.71 |
| Presenting indications | ||||
| Lower extremity pain, swelling, or ulcers | 48 (52) | 26 (51) | 22 (52) | 0.32 |
| Deep vein thrombosis | 42 (45) | 23 (45) | 19 (45) | 0.39 |
| Mass compressing IVC | 3 (3) | 2 (4) | 1 (3) | 0.24 |
| Risk factors | ||||
| Prothrombotic | 53 (57) | 26 (51) | 27 (64) | 0.15 |
| Hypertension | 10 (11) | 5 (10) | 5 (12) | 0.24 |
| Diabetes | 11 (12) | 6 (12) | 5 (12) | 0.29 |
Abbreviation: IVC, inferior vena cava.
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number (percentage).
For this analysis, the patients were separated into treatment and control groups: 51 (55%) patients had suprarenal and infrarenal iliocaval venous disease requiring IVC stent reconstruction across the renal vein inflow (treatment group) and 42 (45%) patients had iliac vein stenting sparing the renal veins (control group). Twenty-six (51%) patients in the treatment group and 27 (64%) patients in the control group had prothrombotic risk factors at baseline. Eleven (22%) patients in the treatment group and 10 (24%) patients in the control group had hypertension or diabetes at baseline. No patients in either group had known multiple myeloma.
This study was approved by the University of Michigan Institutional Review Board (No. HUM00107111) and the Inova Institutional Review Board (No. 18-3199) and complied with the Health Insurance Portability and Accountability Act. Informed consent was not required for this retrospective study.
Iliocaval Stent Reconstruction Technique
All patients were seen by an attending interventional radiologist either in clinic or during inpatient consultation prior to the procedure. The procedures were performed using moderate sedation with intravenous midazolam (Roche; Basel, Switzerland) and fentanyl (Akorn Pharmaceuticals; Lake Forest, IL, USA) or general anesthesia administered by a certified registered nurse anesthetist or attending anesthesiologist.
Iliocaval stent reconstruction has been previously described.1,7,12–16 Iliocaval reconstruction was performed via bilateral accesses to the great saphenous veins (or femoral veins) or single access to the common femoral vein and right internal jugular vein. A vertebral tip catheter (Terumo, Tokyo, Japan) and straight stiff guidewire (Terumo) were used for blunt recanalization. If blunt techniques were unsuccessful, sharp recanalization using an 18-F BRK transseptal needle (Abbott Vascular, Redwood City, CA, USA) and loop snare was employed.12–16
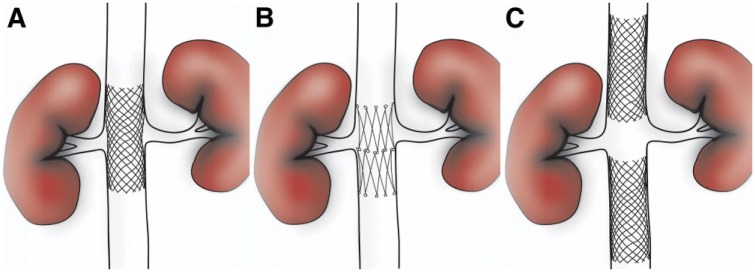
Balloon dilation of the IVC was performed to 18 mm, while the common iliac veins were dilated to 16 mm and the external iliac veins to 14 mm. Stent reconstruction of the IVC (Figure 1), including type and placement of stents, was guided by fluoroscopy, venography, and intravascular ultrasound at the discretion of the operator. Self-expanding stents [18- to 24-mm Wallstents (Boston Scientific, Marlborough, MA, USA) or Gianturco Z-stent (Cook Medical, Bloomington, IN, USA)] were deployed in the IVC. The decision to place Wallstents (Figure 2A-C) or Gianturco Z-stents (Figure 2D-F) across the renal vein inflow was made by the operator at the time of the procedure. In cases where preoperative computed tomography (CT) showed patent renal veins, there was a preference toward placing Gianturco Z-stents; both Wallstents and Gianturco Z-stents were used in patients with large retroperitoneal collaterals communicating with renal hilar veins. Some patients were given a “renal gap,” with Wallstents in the suprarenal and infrarenal IVC bracketing the renal vein inflow but with no stent directly across the confluence (Figure 2G-I). Self-expanding stents (10- to 16-mm) were deployed in the caudal IVC stent to the common femoral veins, with common iliac vein stents deployed such that the square of the stent diameter was half the square of the stented IVC diameter, rounded up to the next available size. Poststent venography and intravascular ultrasound were performed.
Figure 1.
Stent reconstruction across or in the region of the renal vein inflow with (A) Wallstents, (B) Gianturco Z-stents, or (C) a “renal gap” with Wallstents in the suprarenal and infrarenal inferior vena cava bracketing the renal vein inflow but with no stent directly across the confluence.
Figure 2.
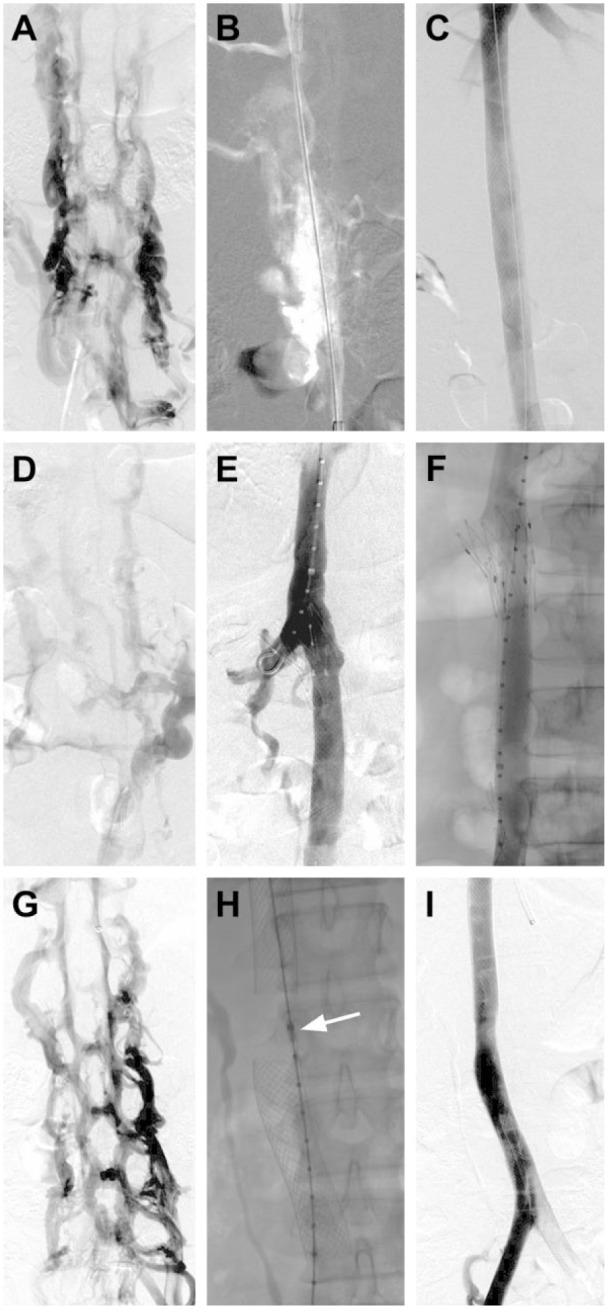
(A, B, C) Iliocaval reconstruction with Wallstents across the renal veins. (A) Digital subtraction venography revealing complete occlusion of the inferior vena cava (IVC) with filling of the paraspinal veins. (B) Through-and-through wire access was ultimately obtained from a saphenous and jugular venous approach. (C) After balloon angioplasty and reconstruction of the entire IVC and iliac veins with Wallstents, repeat digital subtraction venography demonstrated a widely patent iliocaval system. (D, E, and F) Iliocaval reconstruction using the Gianturco Z-stent technique. (D) Digital subtraction venography showing complete occlusion of the IVC. The iliocaval system was reconstructed with placement of a Z-stent across the renal vein inflow and Wallstents in the IVC. (E) Frontal subtracted venography showing a pigtail catheter in the right renal vein through the stent interstices. Contrast is injected simultaneously through the sheath in the common femoral vein and the pigtail in the right renal vein, showing a patent right renal vein and IVC. (F) Frontal unsubtracted venography showing a widely patent IVC. (G, H, and I) Iliocaval reconstruction using the “renal gap” technique. (H) Digital subtraction venography demonstrating complete occlusion of the IVC with filling of paraspinal veins. (H) An intravascular ultrasound catheter (arrow) was used to identify the level of the renal veins. Wallstents were placed above and below this level to create a “renal gap.” (I) Final frontal digital subtraction venography showing a widely patent iliocaval system with a 2.5-cm stent gap at the level of the renal veins.
Anticoagulation and Follow-up
After the procedure, the majority (>95%) of patients were prescribed enoxaparin (1 mg/kg twice daily), clopidogrel (75 mg daily), and aspirin (81 mg daily). Patients were evaluated in the interventional radiology clinic 2 weeks after the procedure when enoxaparin was transitioned to warfarin or another other novel oral anticoagulant. Clopidogrel was discontinued after 2 months. Aspirin was continued for life unless contraindicated. Discontinuation of the anticoagulant after 6 months was considered on a case-by-case basis. Patients with preexisting hypercoagulability were continued on anticoagulation indefinitely at the discretion of their interventional radiologist. Clinical response was assessed in the interventional radiology clinic at 2 weeks and at 6 months.
Definitions and Outcomes
Stenting technical success was defined as successful stent placement with restoration of inline venous flow. Renal function was based on GFR and creatinine values before and after treatment. If multiple laboratory values were present, values immediately prior to stenting and immediately after stenting were selected. For GFRs >60 mL/min/1.73 m2, 60 was used.
Renal vein patency, specifically renal vein dilation or thrombosis, was assessed via venography, CT, or both. CT was performed using venous phase imaging with 5-mm slices and 60-second scan delay from the time of injection. Complications were categorized according to the Quality Improvement Guidelines for the Reporting and Archiving of Interventional Radiology Procedures.17
Statistical Analysis
Continuous data are presented as the mean ± standard deviation; categorical data are given as the number (percentage). Categorical variables were compared using the Fisher exact test, and continuous variables were compared with a Student t test. The threshold of statistical significance was p<0.05. Calculations of percentages, means, and ranges were performed on the data using spreadsheet software (Excel 2017; Microsoft, Redmond, WA, USA). Statistical analyses were conducted in SPSS software (version 24; IBM Corporation; Armonk, NY, USA) and JMP (version 12.2.0; SAS Institute, Cary, NC, USA).
Results
All procedures were technically successful. In the 51-patient treatment group, 15 (29%) patients received Wallstents and 24 (47%) received Gianturco Z-stents across the renal veins and 12 (24%) were given a “renal gap” with no stent placement directly across the renal vein inflow. In the control group, 42 patients received iliac vein Wallstents only.
Table 2 presents the renal function assessments pre- and poststenting. Renal function parameters were assessed at a mean 12±15 days (range 0–62) prior to stent placement. Mean prestent GFR was 59±1.8 mL/min/1.73 m2 and mean prestent creatinine was 0.8±0.2 mg/dL for the entire cohort. Mean pre-stent GFR and creatinine values in the Wallstent, Gianturco Z-stent, and “renal gap” subgroups (Table 2) did not differ vs the control iliac vein stent group.
Table 2.
Renal Function Before and After Stent Placement.
| Treatment Groupa |
|||||
|---|---|---|---|---|---|
| Variable | Wallstent (n=15) | Z-stent (n=24) | “Renal Gap”c (n=12) | Control (n=42) | Total Cohortb |
| GFR, mL/min/1.73 m2 | |||||
| Prestent | 60±0.2 (p=0.21) | 59±3.2 (p=0.19) | 60±0.4 (p=0.24) | 60±1.2 | 59±1.8 (51–60) |
| Poststent | 60±0.2 (p=0.23) | 60±6.4 (p=0.18) | 60±0.3 (p=0.25) | 59±1.7 | 59±3.3 (42–60) |
| Creatinine, mg/dL | |||||
| Prestent | 0.8±0.2 (p=0.4) | 0.9±0.2 (p=0.24) | 0.7±0.1 (p=0.09) | 0.8±0.2 | 0.8±0.2 (0.4–1.2) |
| Poststent | 0.9±0.1 (p=0.27) | 0.8±0.4 (p=0.32) | 0.7±0.1 (p=0.15) | 0.8±0.2 | 0.8±0.3 (0.5–1.7) |
Data are presented as the mean ± standard deviation (p vs control group).
Data are presented as the mean (range).
Stents on either side of the renal vein inflow but not crossing the confluence.
Renal function was assessed at a mean 167±204 days after balloon angioplasty and stent placement (range 1–932). Mean post-stent GFR and creatinine were 59±3.3 mL/min/1.73 m2 (range 42–60) and 0.8±0.3 mg/dL (range 0.5–1.7), respectively. There was no difference between mean pre- and post-stent GFR (p=0.32) or creatinine (p=0.41) values when considering all patients or when comparing the Wallstent, Gianturco Z-stent, and “renal gap” groups vs the control iliac vein stent group. There were no differences in the poststent mean GFR or creatinine values between the Wallstent (p=0.21 and p=0.34, respectively) and Gianturco Z-stent (p=0.43 and p=0.41, respectively) and the “renal gap” group.
Thirty (77%) of the 39 patients in the treatment group [15 Wallstents and 24 Gianturo Z-stents placed directly across the renal vein inflow (excluding the “renal gap” cohort)] underwent follow-up imaging with conventional or CT venography at a mean 11.0 months (range 0.1–21.7). Twenty-nine (97%) of 30 had patent renal veins.
One major and 3 minor complications (self-limiting hematomas) occurred. One patient with placement of a Wallstent across the renal veins developed right renal vein thrombosis 7 days after the procedure and required thrombolysis, sharp recanalization through the stent interstices, and right renal vein stent placement.
Discussion
Stent placement across the renal vein inflow has been described during iliocaval stent reconstruction in patients with venous disease extending into the juxtarenal and suprarenal IVC.1,8–10 In a series of 97 patients with iliocaval stent reconstruction, Raju et al1 described 18 patients in whom Wallstent placement was necessary across the renal vein inflow. Although pre- and postprocedure renal function and renal vein patency were not detailed, the authors reported no detectable renal dysfunction in these patients. Of note, Raju et al1 initiated anticoagulation therapy in addition to an antiplatelet regimen in all patients with stent placement across the renal veins, similar to the postprocedure anticoagulation management in the present study. In another study of 60 patients with endovascular treatment of chronic IVC occlusion, Murphy et al8 reported Wallstent placement across the renal vein inflow in 20 patients. Although this study also did not report postprocedure renal function, the authors reported no adverse outcome of stent placement across the renal veins.
Only 2 published studies, an animal model10 and a clinical study,11 have directly investigated renal vein overstenting during IVC reconstruction and its impact on renal function and renal vein patency. Kim et al10 placed self-expanding stents across the renal vein inflow in 12 rabbits and found no impact on blood urea nitrogen and creatinine or on renal perfusion and excretion during radionuclide scans up to 3 months after the procedure. Interestingly, Gianturco Z-stents, which have larger interstices than Wallstents, were used in this study and may have been less likely to impede blood flow from the renal veins. While this animal study is commendable, the results may not be generalizable to patients with preexisting venous thrombotic disease, many of whom have underlying prothrombotic conditions, including thrombophilia and malignancy. In a small human case series, O’Sullivan et al11 studied renal function and renal vein patency in 4 patients with malignant IVC obstruction requiring Wallstent placement across the renal veins. All patients in this study had patent renal veins and no change in creatinine during follow-up of at least 3 months, further supporting the safety of stent placement across the renal veins.
The present 93-patient study is the largest to date designed to evaluate the impact of IVC stent placement across the renal veins. There was no difference in renal function and in renal vein patency between the treatment and control groups, supporting existing data and suggesting that stent placement across the renal veins is safe. Furthermore, there was no difference in pre- and postintervention renal function in patients treated with Wallstents and Gianturco Z-stents across the renal veins or in patients with stents bracketing the renal vein inflow (“renal gap” group). Of note, 1 patient experienced renal vein thrombosis after placement of a Wallstent across the renal vein inflow. As a result, whenever possible, the authors recommend using Gianturco Z-stents across the renal veins. The majority of available studies utilize Wallstents rather than Gianturco Z-stents across the renal veins; however, they suggest that renal vein thrombosis is an uncommon complication overall.1,8,9,11 Larger studies are necessary to determine if stent type and size may affect outcomes.
Limitations
This was a retrospective study performed at a single institution. The decision to place Wallstents or Gianturco Z-stents or to create a “renal gap” across the renal vein inflow was made by the operator at the time of the procedure and thus may have introduced selection bias. In cases where preoperative CT showed patent renal veins, there was a preference toward placing Gianturco Z-stents; both Wallstents and Gianturco Z-stents were used in patients with large retroperitoneal collaterals communicating with renal hilar veins. Hydration status of patents was rarely available. Additionally, iodinated contrast volumes were not reported consistently. Finally, patients in the present study were relatively young and had normal baseline GFR and creatinine. As such, study results may not be generalizable to older patients with preexisting renal disease or risk factors for renal disease.
Conclusion
Stent placement across the renal vein inflow in patients undergoing iliocaval reconstruction, with stent choice based on operator assessment of central renal vein anatomy, did not compromise renal function. A very small risk of renal vein thrombosis was seen.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jeffrey Forris Beecham Chick  https://orcid.org/0000-0001-9626-6142
https://orcid.org/0000-0001-9626-6142
Ravi N. Srinivasa  https://orcid.org/0000-0003-0984-3664
https://orcid.org/0000-0003-0984-3664
References
- 1. Raju S, Hollis K, Neglen P, et al. Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg. 2006;44:820–827. [DOI] [PubMed] [Google Scholar]
- 2. Hartung O, Loundou AD, Barthelemy P, et al. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38:118–124. [DOI] [PubMed] [Google Scholar]
- 3. Neglén P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. [DOI] [PubMed] [Google Scholar]
- 4. Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg. 2015;28:47–53. [DOI] [PubMed] [Google Scholar]
- 5. Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–645. [DOI] [PubMed] [Google Scholar]
- 6. Hartung O, Otero A, Boufi M, et al. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138–1144. [DOI] [PubMed] [Google Scholar]
- 7. Williams DM. Iliocaval reconstruction in chronic deep vein thrombosis. Tech Vasc Interv Radiol. 2014;17:109–113. [DOI] [PubMed] [Google Scholar]
- 8. Murphy EH, Johns B, Varney E, et al. Endovascular management of chronic total occlusions of the inferior vena cava and iliac veins. J Vasc Surg Venous Lymphat Disord. 2017;5:47–59. [DOI] [PubMed] [Google Scholar]
- 9. Arabi M, Krishnamurthy V, Cwikiel W, et al. Endovascular treatment of thrombosed inferior vena cava filters: techniques and short-term outcomes. Indian J Radiol Imaging. 2015;25:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JK, Park SJ, Kim YH, et al. Experimental study of self-expandable metallic inferior vena caval stent crossing the renal vein in rabbits. Radiologic-pathologic correlation. Invest Radiol. 1996;31:311–315. [DOI] [PubMed] [Google Scholar]
- 11. O’Sullivan GJ, Lohan DA, Cronin CG, et al. Stent implantation across the ostia of the renal veins does not necessarily cause renal impairment when treating inferior vena cava occlusion. J Vasc Interv Radiol. 2007;18:905–908. [DOI] [PubMed] [Google Scholar]
- 12. Chick JFB, Jo A, Meadows JM, et al. Endovascular iliocaval stent reconstruction for inferior vena cava filter–associated iliocaval thrombosis: approach, technical success, safety, and two-year outcomes in 120 patients. J Vasc Interv Radiol. 2017;28:933–939. [DOI] [PubMed] [Google Scholar]
- 13. Hage AN, Srinivasa RN, Abramowitz SD, et al. Endovascular iliocaval stent reconstruction for iliocaval thrombosis: a multi-institutional international practice pattern survey. Ann Vasc Surg. 2018;49:64–74. [DOI] [PubMed] [Google Scholar]
- 14. Hage AN, Srinivasa RN, Abramowitz SD, et al. Endovascular iliocaval reconstruction for the treatment of iliocaval thrombosis: from imaging to intervention. Vasc Med. 2018;23:267–275. [DOI] [PubMed] [Google Scholar]
- 15. Cooper KJ, Chick JFB, Khaja MS, et al. Total endovascular iliocaval reconstruction using polytetrafluoroethylene stent-graft placement for the treatment of inferior vena cava resection. Cardiovasc Intervent Radiol. 2018;41:1116–1120. [DOI] [PubMed] [Google Scholar]
- 16. Chick JFB, Srinivasa RN, Cooper KJ, et al. Endovascular iliocaval reconstruction for chronic iliocaval thrombosis: the data, where we are, and how it is done. Tech Vasc Interv Radiol. 2018;21:92–104. [DOI] [PubMed] [Google Scholar]
- 17. Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2002;13:879–881. [DOI] [PubMed] [Google Scholar]