Abstract
Purpose:
To assess tolerability and efficacy following a switch from benzalkonium chloride–latanoprost to preservative-free latanoprost in patients with glaucoma or ocular hypertension.
Methods:
A total of 140 patients with glaucoma or ocular hypertension controlled with benzalkonium chloride-latanoprost for at least 3 months were switched to treatment with preservative-free latanoprost. Assessments were made on days 15, 45, and 90 (D15, D45, and D90) and included best-corrected visual acuity, intraocular pressure, slit lamp examination, fluorescein staining, tear film break-up time, patient symptom evaluation, and subjective estimation of tolerability.
Results:
Mean best-corrected visual acuity remained unchanged during the study. Mean intraocular pressure compared with baseline (D0) remained stable throughout the study (D0, 15.9 mmHg (standard deviation = 2.6); D90, 15.3 mmHg (standard deviation = 2.4); p < 0.006). Tear film break-up time improved or remained unchanged relative to baseline in 92% of patients at D45 and in 93% at D90. Moderate-to-severe conjunctival hyperemia was seen in 56.8% of patients at D0, but this figure decreased to 13.7%, 2.2%, and 1.6% at D15, D45, and D90, respectively. Subjective assessment of tolerability (0–10 scale) indicated improvement with change of therapy (mean score: 5.3 (standard deviation = 2.2) at D0 versus 1.9 (standard deviation = 1.7) at D90; p < 0.0001).
Conclusion:
Preservative-free latanoprost has at least the same intraocular pressure-lowering efficacy as benzalkonium chloride–latanoprost, with a better tolerability profile. This may translate into greater control of treatment and improved quality of life.
Keywords: Benzalkonium chloride, glaucoma treatment, latanoprost, preservatives, tolerability
Introduction
Due to the effective reduction of intraocular pressure (IOP) provided by prostaglandin analogs (PGAs) and their established safety profile, these agents form the mainstay of treatment for ocular hypertension (OHT) and primary open-angle glaucoma (POAG),1 and are approved as first-line glaucoma therapy by the European Glaucoma Society (EGS).2 Latanoprost, an ester prodrug of prostaglandin F2α, offers a favorable balance in terms of IOP-lowering efficacy and tolerability.3 However, although, like other PGAs, latanoprost treatment produces no severe systemic adverse events (AEs), local AEs such as conjunctival hyperemia, increased eyelash growth, and iris pigmentation are common.1 Furthermore, redness of eyes, stinging, and foreign body sensation are common in patients undergoing long-term, topical antiglaucoma treatment.4 These undesirable effects have a significant influence on patients’ quality of life (QoL) and are an important factor limiting adherence to, and persistence with, therapy.
Benzalkonium chloride (BAK) is a commonly used preservative in ophthalmic formulations but has been shown to contribute significantly to ocular surface damage.5–7 Inflammatory and toxic effects of BAK have been extensively documented in in vitro, in vivo, and clinical studies.7,8 Long-term use of preservatives has been shown to lead to apoptosis of conjunctival cells and chronic conjunctival inflammation, which can, for example, negatively influence the outcome of glaucoma surgery.9,10
The use of BAK-free PGA formulations significantly reduces the incidence of ocular signs and symptoms, such as irritation, pain, discomfort, and dry eyes, compared with BAK-containing treatment.11 Hence, a formulation of preservative-free latanoprost (PF-latanoprost; Monoprost®; Laboratoires Théa, Clermont-Ferrand, France) was developed to avoid the detrimental effects of preservatives, particularly BAK, on the ocular surface. Non-inferiority of this PF-latanoprost formulation, in terms of efficacy and improved local tolerability compared with the BAK-preserved formulation (BAK-latanoprost), was demonstrated in a randomised multicentre phase 3 clinical study by Rouland et al.12 The purpose of this study (the RELIEF study) was to assess the impact of eliminating preservatives on the tolerability and efficacy of latanoprost in the treatment of glaucoma or OHT.
Methods
This was a prospective, longitudinal, open-label, multicenter study conducted in eight glaucoma centers in Poland. The study was conducted in accordance with the tenets of the Declaration of Helsinki and Good Clinical Practice and was approved by the local Ethics Committees at each participating center. All participants provided written informed consent.
Adult (⩾18 years) patients were eligible for inclusion in the study if they had a diagnosis of glaucoma or OHT that had been controlled by monotherapy (stable IOP < 19 mmHg) with a reference product of BAK-latanoprost for at least 3 months, a stable visual field (based on at least two reliable visual field tests performed within the last 6 months) and central corneal thickness within the range 500−580 µm. Patients were excluded if their best-corrected visual acuity (BCVA) was 5/50 or lower, if they had undergone any intraocular surgery (other than filtration surgery performed at least 6 months before screening) or if they had any ocular surface abnormality preventing accurate IOP measurement.
At the baseline visit (D0), patients were switched from BAK-latanoprost to the new formulation of PF-latanoprost (Monoprost®). Patients were instructed to instill one drop of medication once daily in the evening. If both eyes were being treated, the eye with the more advanced visual field defect (according to the mean defect value at baseline) was regarded as the study eye. Patients were advised to continue with their existing regimen of moisturizing drops during the study.
Follow-up assessments were performed after 15, 45, and 90 days (D15, D45, and D90, respectively) of PF-latanoprost treatment. IOP, BCVA, conjunctival hyperemia, blepharitis, and conjunctival and corneal fluorescein staining were evaluated objectively at each visit. IOP was measured using Goldmann applanation tonometry. Tear film break-up time (TBUT) was measured at D0, D45, and D90 by slit lamp examination using a cobalt blue light. Conjunctival hyperemia was assessed by means of a photographic scale derived from the McMonnies score:13 scores of 0, 1, 2, or 3 indicated no, mild, moderate, or severe conjunctival hyperemia, respectively. Ocular surface epithelial staining was evaluated according to the modified Oxford grading system,14 whereby scores of 0, 1, 2 or 3 represented no, mild, moderate, or severe ocular surface staining, respectively. In addition, any other signs of ocular surface disease, such as palpebral edema, folliculo-papillary conjunctivitis or chemosis, were evaluated during the slit lamp examination.
The study also included patient-reported assessment of symptoms and subjective assessments of treatment tolerability at all visits. A Visual Analog Scale (VAS) was used for the subjective evaluation of drug tolerability, in which 0 indicated very good tolerability and 10 indicated very poor tolerability. In addition, patients were asked at each visit to evaluate individual symptoms of eye redness, burning/stinging after instillation, burning/stinging during the day, and foreign body sensation, using a 5-point scale (0 = none and 4 = very disturbing).
Statistical methods
Changes in efficacy and tolerability measures from baseline were analyzed using the Wilcoxon signed-rank test at a 5% level of significance. Calculations were performed using Stata, version 10 software (Stata Statistical Software, College Station, Texas, USA).
Results
A total of 140 patients (100 females and 40 males) were included in the study. Their mean age was 62.5 years (standard deviation (SD) = 13.8), and the mean duration of previous glaucoma treatment was 5.8 years (SD = 5.5). Diagnoses and glaucoma severity staging are presented in Table 1. Up to D0 all the patients were treated with commercially available 0.005% ophthalmic solution of latanoprost containing 0.02% of BAK. The majority (74.6%) of patients used Xalatan (Xalatan®; Pfizer Europe MA EEIG, Kent, United Kingdom) and the remaining (25.4%) patients used different generic BAK-latanoprost products.
Table 1.
Diagnoses and glaucoma severity staging.
| Patients, n (%) | |
|---|---|
| Diagnosis (n = 140) | |
| Ocular hypertension | 18 (12.9) |
| POAG | 102 (72.8) |
| PACG | 12 (8.6) |
| Secondary glaucoma | 8 (5.7) |
| Staging (n = 136) | |
| Ocular hypertension | 18 (13.2) |
| Early glaucoma (MD, <6 dB) | 89 (65.5) |
| Moderate glaucoma (MD, 6–12 dB) | 18 (13.2) |
| Advanced glaucoma (MD, >12 dB) | 11 (8.1) |
MD: mean defect; PACG: primary angle-closure glaucoma; POAG: primary open-angle glaucoma.
IOP reduction
Mean IOP remained stable throughout the study. There was a trend for a reduction of mean IOP at all visits compared with baseline. By D90, mean IOP had decreased to 15.3 mmHg (SD = 2.4), compared with a mean value of 15.9 mmHg (SD = 2.6) at D0, but the difference was not statistically significant (p ⩽ 0.006; Figure 1).
Figure 1.
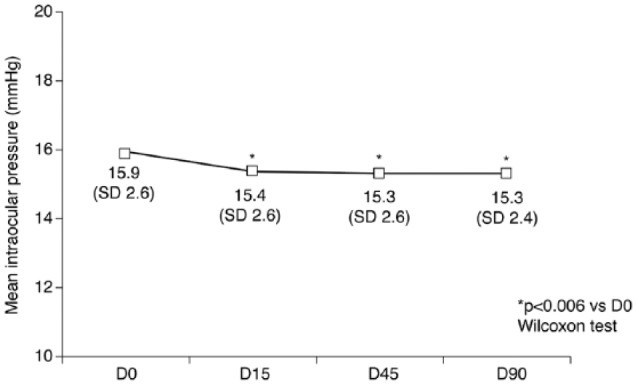
Mean (SD) intraocular pressure at each study visit in patients treated with preservative-free latanoprost. D0: baseline; D15: 15 days after baseline; D45: 45 days after baseline; D90: 90 days after baseline; SD: standard deviation.
Safety and tolerability
No serious AEs were reported. One systemic AE (loss of hair) occurred, which related to an endocrine disorder; this was not considered to be related to the study drug, and the patient continued treatment with PF-latanoprost. Only four topical AEs were reported (one case each of stinging, foreign body sensation, palpebral edema, and increased dry eye sensation), all of which were of mild-to-moderate intensity.
Objective assessments
Mean BCVA remained unchanged during the study.
At baseline (D0), TBUT was >10 s in 32.6% of patients, 5–10 s in 55%, and <5 s in 12.4%. The corresponding figures at D90 were 54.8%, 40.9%, and 4.3%, respectively (Figure 2). TBUT was significantly improved, compared with D0, in 23.4% of patients at D45 and in 30.7% of patients at D90 (p = 0.0023 and p < 0.0001, respectively). TBUT remained unchanged in 68.6% and 62.3% of patients at D45 and D90, respectively, but had deteriorated in 8.0% and 7.0%, respectively.
Figure 2.
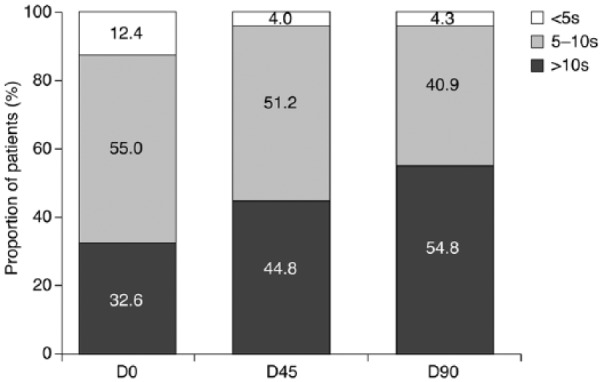
Proportion of patients with tear film break-up time (TBUT) >10 s, 5–10 s and <5 s at each study visit following switch to preservative-free latanoprost. D0: baseline (n = 129); D45: 45 days after baseline (n = 125); D90: 90 days after baseline (n = 115).
Moderate-to-severe conjunctival hyperemia, assessed using the modified McMonnies scale, was present in 56.8% of patients at D0. Following the change to PF-latanoprost, there was a progressive decrease in the prevalence of moderate-to-severe conjunctival hyperemia, to 13.7% of patients at D15, 2.2% at D45 and 1.6% at D90 (p < 0.0001).
A statistically significant decrease in the occurrence of blepharitis was observed at all visits, compared with baseline (p < 0.0001). Moderate-to-severe blepharitis was present in 31.7% of patients at D0, and this figure decreased to 0.8% at D90 (p < 0.0001). Furthermore, the proportion of patients with no signs of blepharitis increased from 27.3% at D0 to 81.7% after 90 days of PF-latanoprost treatment (p < 0.0001; Figure 3).
Figure 3.
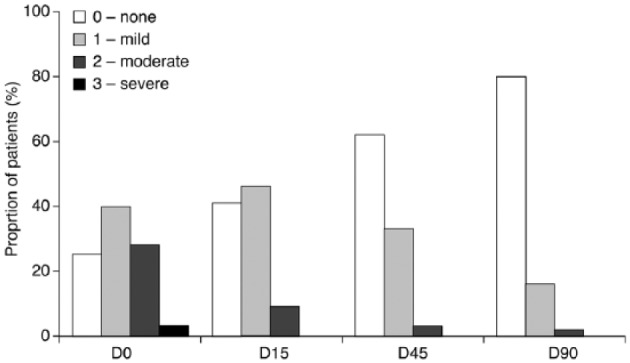
Proportion of patients with blepharitis symptoms at each study visit following switch to preservative-free latanoprost. D0: baseline; D15: 15 days after baseline; D45: 45 days after baseline; D90: 90 days after baseline.
Conjunctival fluorescein staining showed gradual but marked improvement over the course of the study. The proportion of patients with no conjunctival staining increased from 35.2% at D0 to 68.5% at D90 (p < 0.0001). Conjunctival staining graded as moderate to severe was seen in 20.9% of patients at D0, compared with 3.2% at D90 (p < 0.0001).
The proportion of patients with no corneal fluorescein staining was significantly higher at D90 than at D0 (90.3% vs 64.0%, respectively, p = 0.0001). Corneal staining graded as mild was seen in 30.2% of cases at D0, compared with 9.7% at D90. Moderate-to-severe corneal staining was noted in 5.8% of patients at D0 but was not observed in any patient after D15 (p = 0.0001).
Subjective assessments
Patients’ subjective assessments of tolerability improved significantly over the study, decreasing from a mean score of 5.3 (SD = 2.2) on the 0–10 VAS at baseline to 1.9 (SD = 1.7) at D90 (p < 0.0001; Figure 4). The mean subjective ocular symptom scores for the individual symptoms of eye redness, burning or stinging after instillation or during the day and foreign body sensation are presented in Table 2. Significant reductions in subjective ocular symptom scores, compared with baseline were observed during PF-latanoprost treatment (p < 0.0001).
Figure 4.
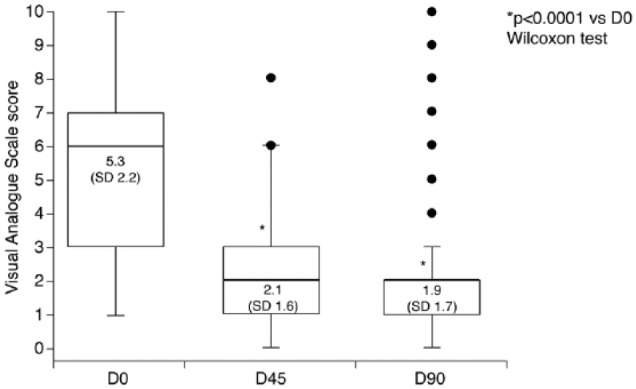
Subjective assessment of tolerability of preservative-free latanoprost by patients; higher scores indicate worse symptoms. Data are presented as mean (SD). Boxes represent the 25th−75th percentile range of results, with the thick horizontal line marking the 50th percentile. Whiskers indicate the non-outlier minimum and maximum values. Outliers are marked by the circles. D0: baseline; D45: 45 days after baseline; D90: 90 days after baseline; SD: standard deviation.
Table 2.
Subjective ocular symptom scores.
| Visit | Eye redness | Burning/stinging after instillation | Burning/stinging during the day | Foreign body sensation |
|---|---|---|---|---|
| D0 | 1.47 (1.32–1.62) | 1.41 (1.27–1.56) | 1.2 (1.02–1.39) | 1.24 (1.08–1.40) |
| D15 | 0.78 (0.65–0.90) | 0.45 (0.34–0.57) | 0.49 (0.37–0.60) | 0.38 (0.25–0.51) |
| D45 | 0.57 (0.47–0.67) | 0.22 (0.14–0.30) | 0.20 (0.12–0.28) | 0.19 (0.10–0.27) |
| D90 | 0.65 (0.48–0.82) | 0.12 (0.06–0.18) | 0.17 (0.10–0.25) | 0.11 (0.04–0.18) |
Symptoms were graded by the patient according to the following scale: 0: none; 1: present but not disturbing; 2: disturbing; 3: very disturbing. D0: baseline; D15: 15 days after baseline; D45: 45 days after baseline; D90: 90 days after baseline. Results are presented as mean (95% confidence interval).
Patients’ self-reported tolerance of PF-latanoprost, compared with prior treatment with BAK-latanoprost was better or significantly better in 87.4% of patients at D45 and 90.8% at D90. In addition, at D90, 64.1% of patients claimed PF-latanoprost was easier or significantly easier to use than BAK-latanoprost, whereas 30% reported no difference in ease of use between the two formulations.
Discussion
Monoprost®, a PF, unidose formulation of latanoprost, was developed to eliminate the detrimental influence of BAK on the ocular surface15 and improve local tolerability and is approved in Europe for the treatment of patients with POAG and OHT. In an initial preclinical in vitro study of this formulation, the viability of human corneal epithelial cells after 24 h of treatment was slightly reduced, to 83% of cells treated with phosphate-buffered saline (PBS), but this did not reach statistical significance; by contrast, BAK-latanoprost or 0.02% BAK induced a significant decrease in cellular viability (p < 0.05 versus PBS).8 Furthermore, in an animal corneo-conjunctival surface model, PBS and PF-latanoprost showed the lowest toxicities, whereas BAK-latanoprost and 0.02% BAK induced significant toxic and inflammatory responses.8 Another animal model study of PF-latanoprost demonstrated the same effectiveness for IOP reduction, but a better tolerability profile, compared with BAK-latanoprost; indeed, the incidence of conjunctival hyperemia was reduced by 42% with PF-latanoprost.16
These preclinical findings have subsequently been confirmed in a prospective, international, multicenter, randomized, parallel-group, phase III study (T2345 Study Group Trial) of PF-latanoprost (Monoprost®) versus BAK-latanoprost in patients with OHT or POAG, which showed that PF-latanoprost was non-inferior in terms of IOP-lowering efficacy.12 Similarly, an indirect comparison meta-analysis of randomized clinical trials of monotherapy with currently available PGAs (preserved and unpreserved) showed no statistically significant differences in mean IOP at 3 months between PF-latanoprost and most other PGAs, except for BAK-tafluprost, which was inferior to PF-latanoprost in this respect.17
To our knowledge, our study is the first prospective study in which patients have been switched from BAK-preserved latanoprost to PF-latanoprost: the study by Rouland et al.12 was a parallel-group non-inferiority study comparing the two preparations. The results indicate that PF-latanoprost has at least the same IOP-lowering efficacy as BAK-preserved latanoprost: mean IOP remained stable throughout the study. We have observed a trend for mean IOP reduction at all visits compared to baseline: however, the difference was not statistically significant. This is in agreement with the suggestion that optimization of the ocular surface in patients with glaucoma and ocular surface disease improves IOP control.18 Moreover, our findings show that the IOP-lowering efficacy of latanoprost is not dependent upon the presence of BAK, as has been suggested by some groups.19,20 Indeed, our findings are consistent with those of recent studies of PF-latanoprost12 and PF-travoprost,21,22 which have also shown similar efficacies of BAK-preserved and PF glaucoma medications.
This study also showed that the local tolerability of latanoprost, in terms of both subjective symptoms and conjunctival hyperemia, improved significantly after switching from the BAK-preserved formulation to the PF formulation. Both the incidence and severity of conjunctival hyperemia were significantly reduced with PF-latanoprost, compared with BAK-latanoprost, at both D42 (p = 0.003) and D84 (p = 0.019). Moreover, the incidence of moderate-to-severe hyperemia decreased over time in patients receiving PF-latanoprost, but increased in patients treated with the BAK-latanoprost formulation. These findings are consistent with those of the meta-analysis by Cucherat et al.,17 which found that the risk of hyperemia is substantially lower with PF-latanoprost treatment than with all other PGAs.
This study showed significant improvements in all objective measures of safety and tolerability (TBUT, conjunctival hyperemia, blepharitis, and corneal and conjunctival epithelial fluorescein staining) following the switch from BAK-latanoprost to PF-latanoprost. In addition, patients’ subjective ratings of ocular symptoms, such as eye redness, burning or stinging after instillation or during the day, and foreign body sensation tended to be lower with PF-latanoprost than with the BAK-preserved formulation. The statistically significant improvement in VAS score after 3 months of PF-latanoprost treatment (p < 0.0001) demonstrates the extent of improvement of symptoms of ocular surface disease and increased patient satisfaction with treatment. A further study, currently reported in abstract form only, showed a significant reduction in anterior chamber flare 1 month after switching from BAK-latanoprost to PF-latanoprost in 22 patients with POAG treatment (Kestelyn PG, De Bacquer D, Stevens A. Switch from BAK-preserved to PF-latanoprost decreases anterior chamber flare in POAG patients. Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, 30 April 2014, 55:547, A0183). This finding supports the hypothesis that BAK induces low-grade inflammation in the anterior chamber. Switching studies with other PGAs have also shown improved local tolerance and fewer adverse effects after switching to a PF medication.11,23
An important aspect of our study was the assessment of ocular surface signs and symptoms over time after switching to PF-latanoprost. The main limitation of the study, however, was the open-label design, which may have influenced the investigators’ evaluations and the patients’ subjective assessments of tolerability, adherence, and satisfaction.
In conclusion, PF-latanoprost has at least the same IOP-lowering efficacy as BAK-preserved latanoprost but is better tolerated, particularly in patients who have signs and symptoms of ocular surface disease on BAK-latanoprost therapy. PF-latanoprost improves the ocular surface status, as assessed both objectively and subjectively. As this improvement might translate into better QoL for the patient, it could greatly contribute to adherence to therapy and to better control of long-term glaucoma treatment.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided in the form of an unrestricted research grant from Théa Poland, which is gratefully acknowledged. D.R. is a consultant to Théa Poland. Editorial support during the writing of this paper, funded by Laboratoires Théa), was provided by Dr Michael Shaw (Anagram Communications, UK).
References
- 1. Orme M, Collins S, Dakin H, et al. Mixed treatment comparison and meta-regression of the efficacy and safety of prostaglandin analogues and comparators for primary open-angle glaucoma and ocular hypertension. Curr Med Res Opin 2010; 26: 511–528. [DOI] [PubMed] [Google Scholar]
- 2. European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed. Savona: Publicomm srl, 2014. [Google Scholar]
- 3. Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol 2014; 26: 1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valente C, Iester M, Corsi E, et al. Symptoms and signs of tear film dysfunction in glaucomatous patients. J Ocul Pharmacol Ther 2011; 27: 281–285. [DOI] [PubMed] [Google Scholar]
- 5. Wilson LA. To preserve or not to preserve, is that the question? Br J Ophthalmol 1996; 80: 583–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 2002; 86: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baudouin C, Labbé A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res 2010; 29: 312–334. [DOI] [PubMed] [Google Scholar]
- 8. Pauly A, Roubeix C, Liang H, et al. In vitro and in vivo comparative toxicological study of a new preservative-free latanoprost formulation. Invest Ophthalmol Vis Sci 2012; 53: 8172–8180. [DOI] [PubMed] [Google Scholar]
- 9. Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol 1994; 112: 1446–1454. [DOI] [PubMed] [Google Scholar]
- 10. Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma 2013; 22: 730–735. [DOI] [PubMed] [Google Scholar]
- 11. Uusitalo H, Chen E, Pfeiffer N, et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol 2010; 88: 329–336. [DOI] [PubMed] [Google Scholar]
- 12. Rouland JF, Traverso CE, Stalmans I, et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol 2013; 97: 196–200. [DOI] [PubMed] [Google Scholar]
- 13. McMonnies CW, Chapman-Davies A. Assessment of conjunctival hyperemia in contact lens wearers. Part I. Am J Optom Physiol Opt 1987; 64: 246–250. [DOI] [PubMed] [Google Scholar]
- 14. Bron A, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003; 22: 640–650. [DOI] [PubMed] [Google Scholar]
- 15. Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol 2007; 18: 134–139. [DOI] [PubMed] [Google Scholar]
- 16. Daull P, Buggage R, Lambert G, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther 2012; 28: 515–523. [DOI] [PubMed] [Google Scholar]
- 17. Cucherat M, Stalmans I, Rouland JF. Relative efficacy and safety of preservative-free latanoprost (T2345) for the treatment of open-angle glaucoma and ocular hypertension: an adjusted indirect comparison meta-analysis of randomized clinical trials. J Glaucoma 2014; 23: e69–e75. [DOI] [PubMed] [Google Scholar]
- 18. Batra R, Tailor R, Mohamed S. Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma 2014; 23: 56–60. [DOI] [PubMed] [Google Scholar]
- 19. Okabe K, Kimura H, Okabe J, et al. Effect of benzalkonium chloride on transscleral drug delivery. Invest Ophthalmol Vis Sci 2005; 46: 703–708. [DOI] [PubMed] [Google Scholar]
- 20. Majumdar S, Hippalgaonkar K, Repka MA. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir cross isolated rabbit cornea. Int J Pharm 2008; 348: 175–178. [DOI] [PubMed] [Google Scholar]
- 21. Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma 2007; 16: 98–103. [DOI] [PubMed] [Google Scholar]
- 22. Gross RL, Peace JH, Smith SE, et al. Duration of IOP reduction with travoprost BAK-free solution. J Glaucoma 2008; 17: 217–222. [DOI] [PubMed] [Google Scholar]
- 23. Iester M, Telani S, Frezzotti P, et al. Ocular surface changes in glaucomatous patients treated with and without preservatives beta-blockers. J Ocul Pharmacol Ther 2014; 30: 476–481. [DOI] [PubMed] [Google Scholar]


