Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by the deterioration of motor neurons. However, this complex disease extends beyond the boundaries of the central nervous system, with metabolic alterations being observed at the systemic and cellular level. While the number of studies that assess the role and impact of metabolic perturbations in ALS is rapidly increasing, the use of metabolism biomarkers in ALS remains largely underinvestigated. In this review, we discuss current and potential metabolism biomarkers in the context of ALS. Of those for which data does exist, there is limited insight provided by individual markers, with specificity for disease, and lack of reproducibility and efficacy in informing prognosis being the largest drawbacks. However, given the array of metabolic markers available, the potential exists for a panel of metabolism biomarkers, which may complement other current biomarkers (including neurophysiology, imaging, as well as CSF, blood and urine markers) to overturn these limitations and give rise to new diagnostic and prognostic indicators.
Keywords: amyotrophic lateral sclerosis, ALS, metabolism, biomarker, motor neurone disease
Overview
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease caused by the death of motor neurons in the brain and spinal cord. The loss of neuronal input leads to progressive paralysis and patient mortality within 2–5 years from diagnosis (1). ALS likely arises from a combination of genetic susceptibility and environmental exposures (2, 3), although it is recognized that ALS is a complex, multi-system disease (4, 5).
Given the complex and heterogeneous nature of ALS, diagnosis and tracking of prognosis remains difficult. Current diagnostic criteria typically follow tests to rule out other pathological causes of symptoms and include: indicators of upper and lower motor neuron involvement, nerve conduction tests, electromyography and “watchful waiting” (4). As a result, researchers have attempted to utilize a wide range of biomarkers—observable biological measurements that confirm the presence or progression of a change in body status, as a means of diagnosing and following disease progression. While the current range of biomarkers in ALS offer some diagnostic and prognostic benefit, there is a need to identify a biomarker that satisfies the following six attributes: specificity to disease; reproducibility; appearance early in the disease; stability across the diurnal period; independence of dietary status and behavior; and a notable change during disease progression. By meeting these criteria, a biomarker can be used to reliably identify and track disease progression, in a manner that can easily be reproduced in a clinical setting.
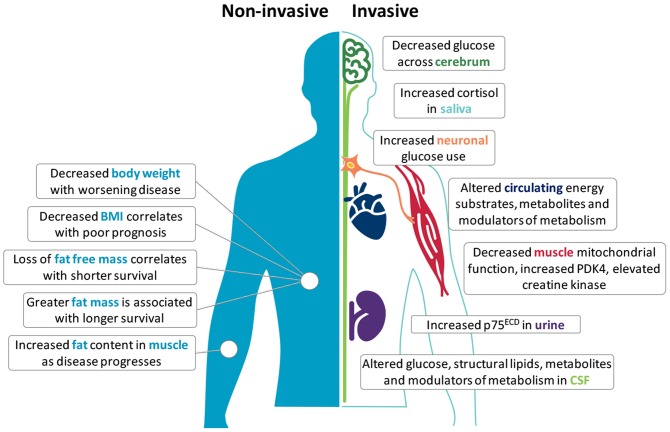
Metabolic perturbations occur in ALS patients and in mouse models of the disease; both at the systemic and cellular level (6, 7). Clinically, an increase in resting energy expenditure (REE) and decline in body mass index (BMI) is linked to worse outcome (8–10), suggesting prognostic potential in metabolic biomarkers. Given that changes in metabolic status are generally reflected in overall body weight, body composition, and tissue/cellular metabolic function, metabolic changes at the anthropometric, tissue and cellular levels may represent appreciable metabolism biomarkers of ALS onset, progression, and/or severity (Figure 1). A list of the potential biomarkers of metabolism in ALS, and their quality relative to the aforementioned identifying attributes are summarized in Table 1.
Figure 1.
Potential metabolism biomarkers in amyotrophic lateral sclerosis (ALS). Metabolic alterations in ALS offer opportunities to use metabolism biomarkers for the diagnosis, categorization, and tracking of disease. Non-invasive anthropometric measures include body weight, body mass index (BMI), fat free mass, fat mass, and fat distribution. Invasive measures include the use of F18-PET to assess glucose metabolism in the central nervous system, or require the sampling of saliva, blood, cerebrospinal fluid (CSF), muscle tissue, and urine. Although few independent markers are specific, reproducible or able to track disease in ALS, used together with complementary biomarkers (including neurophysiology and imaging), these markers may provide deeper insights into metabolic perturbations that are potentially involved in the onset and progression of disease.
Table 1.
Classification of potential biomarkers of metabolism in amyotrophic lateral sclerosis (ALS).
| Marker | Observation | Utility as a biomarker in ALS | Biomarker Score | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Specific to ALS | Reproducible | Pre-diagnostic | Diurnal stability | Independence | Change with progression | ||||
| ANTHROPOMETRIC MARKERS | |||||||||
| Body mass index (BMI) | Lower BMI is an indicator of poor prognosis. U-shaped association; lower BMI is associated with increased risk and faster progression whereas BMI in the range of morbid obesity is associated with shorter survival. Degree of premorbid loss of BMI predicts risk of ALS | N | N | Y | Y | N | Variable | 2.5 | (8, 11–22) |
| Body weight | Weight loss correlates with faster disease progression; weight loss suggested as a risk factor for ALS | N | N | N | Y | N | Variable | 1.5 | (14, 21, 23–27) |
| Fat mass | Fat mass at diagnosis is not a determinant of survival. Increased fat mass is correlated with longer survival | N | N | Insufficient data | Y | N | Y | 2 | (14, 28) |
| Fat free mass | Fat free mass at diagnosis is not a determinant of survival. Loss of fat free mass is associated with shorter survival | N | Y | Insufficient data | Y | N | Y | 3 | (14, 23) |
| Fat distribution | Redistribution and increased deposition of fat in muscle | N | Insufficient data | Insufficient data | Y | N | Insufficient data | 1 | (29) |
| IMAGING MARKERS | |||||||||
| Brain glucose use | Hypometabolism specific to select brain regions; varies between studies | N | Insufficient data | Insufficient data | Likely | Likely | Y | 2 | (30–33) |
| Spinal cord glucose use | Hypermetabolism; changes in glucose metabolism correlates with disease progression | N | Insufficient data | Insufficient data | Likely | Likely | Variable | 1.5 | (34, 35) |
| MUSCLE MARKERS | |||||||||
| Creatine kinase | Increased in blood; variability in correlation with disease progression/survival. Greater increase observed in male subjects and limb-onset ALS | N | Y | Y | Likely | Likely | Variable | 3.5 | (36–43) |
| Mitochondrial function | Decreased activity of complex I and IV. Activity also declines over course of disease | N | Insufficient data | Insufficient data | Y | Y | Variable | 2.5 | (44, 45) |
| PDK4 levels | Increase in pyruvate dehydrogenase kinase 4 (PDK4) correlated with increased denervation and fuel switch | N | Insufficient data | Insufficient data | Y | Y | Likely | 2.5 | (46) |
| Glucose | Increased | N | N | Insufficient data | N | N | N | 0 | (47) |
| Sphingolipids | Increased | N | Insufficient data | Insufficient data | N | N | Y | 1 | (48) |
| Phosphatidylcholine | Increased | N | Insufficient data | Insufficient data | N | N | N | 0 | (48) |
| Cholesterol + Carriers | Increased | N | Insufficient data | Insufficient data | N | N | N | 0 | (49) |
| Lactate | Increased | N | Insufficient data | Insufficient data | N | N | Insufficient data | 0 | (47, 50) |
| CEREBROSPINAL FLUID (CSF) MARKERS | |||||||||
| Pyruvate | Increased | N | Insufficient data | Insufficient data | N | N | Insufficient data | 0 | (51) |
| Insulin | Decreased | N | Insufficient data | Insufficient data | N | N | N | 0 | (52) |
| Growth hormone | Decreased | N | Insufficient data | Insufficient data | N | N | N | 0 | (52) |
| CIRCULATING MARKERS (BLOOD, PLASMA AND SERUM) | |||||||||
| Glucose | Increased (33% of patients achieve World Health Organization (WHO) criteria for impaired glucose tolerance) | N | N | Insufficient data | N | N | N | 0 | (53) |
| Mannose | Increased | N | N | Insufficient data | N | N | Insufficient data | 0 | (54) |
| Free fatty acids | Increased | N | N | Insufficient data | N | N | N | 0 | (53) |
| Sphingolipids | Increased | N | N | Insufficient data | N | N | N | 0 | (54) |
| Cholesterol + Carriers | Major variations and contradictory reports mask any specific trend | N | N | Insufficient data | N | N | Variable | 0.5 | (53, 55–62) |
| β-hydroxy-butyrate | Increased | N | N | Insufficient data | N | N | Insufficient data | 0 | (63) |
| 2-hydroxy-butyrate | Increased | N | N | Insufficient data | N | N | Insufficient data | 0 | (54) |
| α-ketoglutarate | Increased | N | N | Insufficient data | N | N | Insufficient data | 0 | (54) |
| Acetate | Increased | N | N | Insufficient data | N | N | Insufficient data | 0 | (63) |
| Adiponectin | Increased | N | N | Insufficient data | N | N | N | 0 | (64) |
| Cortisol | Increased | N | N | Insufficient data | N | N | N | 0 | (65) |
| Cortisol (morning peak) | Decreased | N | N | Insufficient data | N | N | N | 0 | (65) |
| Insulin | Decreased | N | N | Insufficient data | N | N | N | 0 | (52, 64) |
| Gastric inhibitory peptide | Decreased | N | N | Insufficient data | N | N | N | 0 | (64) |
| Ghrelin | Decreased | N | N | Insufficient data | N | N | N | 0 | (64, 66) |
| SALIVA MARKERS | |||||||||
| Cortisol (night-time) | Increased | N | Insufficient data | Insufficient data | N | Likely | Insufficient data | 0.5 | (67) |
| Cortisol (Stress-induced) | Decreased | N | Insufficient data | Insufficient data | N | Likely | Insufficient data | 0.5 | (67) |
| Cortisol (circadian rhythm) | Decreased | N | Insufficient data | Insufficient data | N | Likely | Insufficient data | 0.5 | (67) |
| URINE MARKERS | |||||||||
| p75 neurotrophin receptor extracellular domain | Increased | N | Likely | Insufficient data | Y | Y | Y | 3.5 | (68) |
The strength of proposed biomarkers are scored relative to their potential to serve as markers that are specific to ALS, and that conform to the requirements as detailed in text.
Specific to ALS refers to uniqueness of the marker to ALS over other diseases, reproducible refers to whether the indicated change is reproducible across patient cohorts, pre-diagnostic indicates where changes are apparent prior to symptom onset, diurnal stability refers to the consistency of the marker throughout the day, independence indicates the ability of the marker to remain stable regardless of changes in food intake or behavior, change with progression identifies whether the marker changes as disease progresses. For each potential biomarker, a score out of 6 was determined (biomarker score, indicated in bold), where Y (Yes) = 1 point, N (No) = 0 points, Variable = 0.5 points, Likely (supported by animal or statistical modeling studies) = 0.5 points, and Insufficient data = 0 points.
Anthropometric Body Measures
Lower premorbid BMI is associated with increased risk for ALS (11–13), and the degree of decline in premorbid BMI predicts ALS risk and survival (14, 15). Lower BMI, or a decline in BMI following diagnosis correlates with worse survival (16, 17), although this association is not always observed (18, 19, 23, 24). Rather, the mortality risk for ALS relative to BMI exists as a U-shaped curve, in which mortality decreases with increasing BMI, until BMI levels indicate premorbid obesity. Thereafter, mortality risk increases again (8, 20). This seemingly complex association could be explained by changes in body composition throughout disease progression.
BMI is often used as an indirect measure of fatness. However, conventional anthropometric measures of BMI and body adiposity index (BAI) do not always accurately reflect changes in fat and/or fat free mass (FFM) in ALS (69). In this regard, fat mass (FM) and FFM at diagnosis are not associated with survival risk (14), yet redistribution of adipose tissue does occur in ALS (29), and visceral fat is correlated with functional status and survival (28). Moreover, serial assessment of body FM indicates that increases in FM are associated with longer survival (14). While a decrease in FFM serves as an independent prognostic factor for shorter survival in ALS (23), we did not identify any studies that document progressive changes in muscle mass as a potential marker of disease progression in ALS. As a hallmark of ALS, however, there is potential to use the loss of FFM as a marker of disease progression. Such measures must consider the technical difficulties associated with assessing FFM in patients who experience significant and progressive disability, while also accounting for whole body and regional changes in FFM, which differ greatly between patients.
Despite BMI and BAI being poor predictors of body composition in ALS, changes in BMI may offer reliable measures for progressive changes in the overall nutritional status of the patient, and by proxy, disease progression. As documented by Kasarskis et al. a progressive decline in body weight is commonly observed in ALS patients in the months prior to death, and this reduction in body weight or BMI likely reflects a state of undernutrition (25). In recent years, lower BMI has been found to be associated with lower ALSFRS-R scores (70), and a loss of body weight (14, 21, 23, 24, 26, 27, 71) and BMI (14, 17, 22, 24) throughout disease course is consistently associated with shorter survival. Not surprisingly, these observations, while serving as markers for disease progression, have resulted in the adoption of interventions aimed at slowing weight loss in ALS (72).
Skeletal Muscle Pathology
With findings suggesting that FFM is a prognostic factor in ALS (23), analysis of skeletal muscle, the primary component of FFM, may offer insights into tissue-specific metabolism biomarkers. Assessment of cellular metabolic changes in skeletal muscle can be challenging, especially when weighing the clinical benefit against that of an invasive procedure on a patient undergoing significant muscle wasting. Furthermore, heterogeneity in site of disease onset leads to variable muscle pathophysiology between patients (73).
Despite these limitations, creatine kinase, an enzyme that is linked with muscle damage and deterioration, has been studied intensely in ALS. While not strictly a metabolic marker, creatine kinase can be considered as an important modulator of body composition (74). As such, it may indirectly influence systemic metabolic processes. Numerous reports of increased creatine kinase in ALS (36–43), and particularly in limb-onset patients (38, 43), highlight the potential for its use as a marker of disease. However, contradictory observations of associations between creatine kinase and clinical parameters of disease, and disease progression and survival attest to the need for further investigations into determining the utility of creatine kinase as a biomarker in ALS.
Mitochondrial Dysfunction
In human ALS muscle, mitochondrial defects including dysregulation of respiratory complex I (44), decreased respiratory complex I and IV activity (45, 75), decreased muscle mitochondrial protein expression (75) and upregulation of muscular mitochondrial uncoupling protein 3 (76) indicate that impairments in mitochondrial function could serve as a metabolic marker of ALS. It should be noted, however, that these studies were unable to correlate mitochondrial defects with functional parameters of disease progression, despite studies in animal models reporting a strong relationship between the two (77–79). Therefore, while there is clear evidence of mitochondrial defects in ALS, mitochondrial defects per se cannot currently be used as a biomarker due to the difficulty in both easily observing these defects in a clinical setting, and linking such defects to a marker of disease progression and/or survival. Instead, emphasis could be placed on the assessment of the more easily detectable metabolites that drive mitochondrial function.
Glucose Metabolism
Glucose use in the brain of ALS patients has been evaluated using fluorodeoxyglucose F18 positron emission tomography (F18-PET) (30–33). These studies have identified decreased glucose use in the primary motor cortex of ALS patients, suggesting that this brain region is hypometabolic (32). Other studies have reported a decrease in the use of glucose across other brain regions (31, 33); although this may reflect the differences in experimental cohorts. In this regard, Claassen et al. investigated a cohort of patients with primary lateral sclerosis, while the study by Ludolph et al. evaluated ALS patients with both upper and lower motor symptoms. Given that the degree of cerebral hypometabolism in ALS is correlated with the duration of clinically-identified symptoms (30), the ability of the motor cortex to utilize glucose may allow for monitoring of disease progression. However, since brain glucose hypometabolism is not specific to ALS (80), its use as a diagnostic/prognostic marker is limited.
F18-PET has also been used to assess the uptake and utilization of glucose in the cervical spinal cords of ALS patients (34, 35, 81). Overall, observations of spinal cord glucose hypermetabolism (34, 35, 81) is congruent with increased levels of glucose in the CSF of ALS patients (47). In a study by Yamashita et al. glucose hypermetabolism on the ipsilateral side to the patient's symptoms was found to be positively correlated with ALSFRS-R, suggesting that changes in spinal cord glucose metabolism are specific to the affected corticospinal tract and the degree of disease severity (35). By contrast, the study by Marini et al. reported spinal cord glucose hypermetabolism independent of disease duration and functional impairment (34). As such, the degree of glucose use in the spinal cord may present some use for diagnostic testing, but provides limited insights for evaluation of disease progression and prognosis. Indeed, glucose hypermetabolism in the spinal cord extends to other neurological conditions (82, 83), thereby limiting its use as a specific biomarker for ALS. Finally, as the reproducibility of F18-PET in both the brain and spinal cord is low (84), more rigorous testing is required to determine if results are consistent across a heterogeneous ALS population.
Alterations in glucose metabolism in ALS extend beyond the central nervous system (CNS). Glucose tolerance tests conducted by Pradat et al. indicate that ALS patients have a significant increase in blood glucose levels following the provision of a glucose load when compared to age- and sex-matched controls. Within ALS patients, a degree of heterogeneity was observed, with 33% of participants meeting World Health Organization criteria for impaired glucose tolerance (53). Impaired glucose tolerance is in line with reports of insulin resistance in ALS (85), and could explain observations of increased expression of pyruvate dehydrogenase kinase 4 (PDK4) in skeletal muscle of ALS patients (46). Similarly, mannose, an epimer of glucose that has recently been shown to be a predictor of insulin resistance (86), has been reported to be significantly increased in the plasma of ALS patients (54). While the assessment of glucose tolerance and insulin resistance is relatively straightforward, these tests lack reproducibility and specificity to ALS (87–89). Therefore, although glucose metabolism is altered in ALS, it cannot be used as an independent biomarker for ALS diagnosis and prognosis.
Fatty Acids and Ketones
In patients with ALS, the resting level of circulating free fatty acids (FFAs) is significantly increased (53). While higher levels of FFAs has been linked to impaired glucose tolerance in ALS, it has not been shown to be correlated with any markers of disease progression or severity. Ketones, including β-hydroxy-butyrate (63) and 2-hydroxy-butyrate and α-ketoglutarate (54), which are produced through fatty acid metabolism under fasting conditions, are also significantly increased in ALS. Similar to FFAs, no correlations have been observed between disease status and the expression of ketones. Thus, FFAs and ketones cannot currently be considered as reliable biomarkers for ALS, and the lack of specificity for ALS-centric pathology indicate that they may not present as particularly valuable diagnostic markers individually.
Downstream Metabolites
Metabolites, the downstream indicators of metabolic function, are also impacted in ALS. While not specific to ALS, altered expression of metabolites may offer a potential avenue for biomarker discovery. In line with disease heterogeneity, reported levels of metabolites in the blood and CSF are variable. Notably, the levels of lactate (47, 50) and pyruvate (51) in the CNS are increased, potentially reflecting an increase in metabolic output, or increased release of metabolites into the CSF following neuronal deterioration. Given that mitochondrial dysfunction is observed in ALS, further evaluation of the ratio between these metabolites may hold significant informative value in ALS due to the diagnostic value of this test for mitochondrial disorders (90).
Blood levels of acetate are increased in ALS (63), although this is not readily observed in the CSF (47, 51). Acetate is a key metabolite in the oxidation of fatty acids. As acetate synthesis precedes the formation of citric acid in the Krebs cycle, changes in circulating acetate may occur due to excess production via an increase in fatty acid oxidation, increased release from deteriorating muscle cells, or other disruptions to mitochondrial membrane integrity (e.g., due to the presence of free radicals). Such potential mechanisms align with ALS pathology. As a whole, downstream metabolites hold promise as potential biomarkers, and further work that can interrogate relationships between metabolites and clinical parameters of disease would add merit to their use as metabolic biomarkers of disease.
Endocrine Modulators of Metabolism
Insulin is an anabolic hormone that has been reported to be decreased in the blood (64) and CSF (52) of ALS patients. By contrast, other studies have reported no significant differences in plasma insulin levels in ALS patients (91, 92). Other anabolic hormones that have been found to be decreased in ALS include growth hormone (in CSF and blood) (52, 92–94) and gastric inhibitory peptide in blood (64). Conversely, hormones that promote catabolism, such as cortisol (65, 67), and adiponectin (64) are increased or dysregulated in saliva and blood of patients with ALS. Furthermore, ghrelin, an important modulator of appetite, is also reduced in the plasma/blood of ALS patients (64, 66). Given that alterations in these hormones are likely to be symbolic of a change in metabolic function/homeostasis, studies that confirm a link between endocrine markers of metabolism and clinical markers of disease offer potential for their development as prognostic biomarkers.
Metabolism of Structural Lipids
While fatty acids and their derivatives serve as energy substrates through mitochondrial respiration, they also play an essential role in maintaining cellular integrity. Phospholipids, particularly phosphatidylcholine, are significantly increased in the CSF of ALS patients (48). Sphingolipids, such as stearoyl sphingomyelin and ceramide, are also increased in patient blood (48, 54). Interestingly, in the study by Blasco et al. predictions of clinical measurements, such as ALSFRS-R, were found to be correlated to CSF sphingomyelins and triglycerides with long-chain fatty acids (48). Such findings are favorable for the development of biomarker assays, but further tests are required to confirm the reliability of predictive models, before use as a prognostic biomarker.
An increase in cholesterol esters has been observed in ALS patient spinal cord (95). However, cholesterol and its carriers prove to be more difficult to characterize, with variable levels of HDL and LDL cholesterol being reported in ALS. In a population-based longitudinal study, a positive association was found between LDL cholesterol and ALS risk (55), however, there was no indication of the impact of LDL on disease progression or mortality. Nonetheless, this could serve as a diagnostic biomarker for ALS risk. Previously, higher levels of cholesterol, LDL, as well as an elevated LDL/HDL ratio in ALS patient blood have been correlated with increased survival (56–58). Conversely, similar increases in total cholesterol, LDL, and HDL cholesterol in ALS patient blood (59, 60) and CSF (49) have not been found to be correlated with disease progression. Furthermore, a small number of studies contradict these findings, reporting that cholesterol, LDL, and HDL levels do not vary between ALS patients and controls (53, 61, 62), although lower levels of serum lipids may correlate with worse respiratory function (61). Based on these contradictory observations, the validity of cholesterol as a biomarker remains uncertain. Further studies that address these disparate data are required.
Novel Metabolism-associated Biomarkers
p75 neurotrophin receptor (NTR) belongs to the tumor necrosis factor family of receptors. It is a transmembrane receptor which binds neurotrophins and pro-neurotrophins (96). p75NTR has been implicated in processes of energy expenditure (97), glucose uptake, and insulin sensitivity (98). In ALS, the secretion of the extracellular domain of p75NTR (p75ECD) in urine was recently established as a biomarker for disease progression and prognosis (68, 99). Urinary p75ECD increases as disease progresses, and an elevation of urinary p75ECD is observed alongside a decrease in ALSFRS-R scores (68). While it is not clear if increases in urinary p75ECD in ALS match metabolic derangements that accompany disease progression (such as changes in energy metabolism, glucose uptake and insulin sensitivity), the introduction of p75ECD as a fluid biomarker in ALS provides an opportunity for the evaluation and possible co-development of metabolism-associated biomarkers.
Conclusion
The complexity and heterogeneity of disease between patients limits the scope for the use of a single reliable biomarker of ALS. Significant changes in metabolism seen in ALS may represent a potential avenue for biomarker development. As documented in this review, a range of markers might be relevant (Figure 1). However, as investigations into the cause for metabolic derangements in ALS are ongoing, and little emphasis has been placed on the development of metabolism biomarkers as diagnostic or prognostic indicators, few reliable metabolism biomarkers exist (Table 1). Moreover, because metabolic alterations in ALS likely arise from the dysregulation of a number of processes, the utility of biomarkers for assessing early or progressive changes in the metabolic state of ALS patients would necessitate the development of a panel that captures the spectrum of metabolic changes that occur at the systemic and cellular level.
As there is no single biomarker for ALS that sufficiently meets the six major attributes of a biomarker, it is clear that the assessment of biomarkers that cover multiple dimensions of the disease is needed in order to generate a comprehensive view of the state of disease. The complementary assessment of metabolism markers alongside other biomarkers including neurophysiology, imaging, as well as CSF, blood, and urine markers may form a more convincing and reliable diagnostic/prognostic platform, while providing insights into the multifactorial nature of disease.
Author Contributions
SEK, TJT, FJS, and STN conducted the literature search and wrote the manuscript. FJS produced all artwork. STN critically revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. TJT is supported by an Australian Postgraduate Award from the University of Queensland, and a Postgraduate top-up grant from the Motor Neurone Disease Research Institute of Australia. STN is supported by the Scott Sullivan MND Research Fellowship (MND and Me Foundation, The Royal Brisbane and Women's Hospital Foundation, and the Queensland Brain Institute) and the Australian Institute for Bioengineering and Nanotechnology.
References
- 1.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. (2007) 369:2031–41. 10.1016/S0140-6736(07)60944-1 [DOI] [PubMed] [Google Scholar]
- 2.Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. (2014) 13:1108–13. 10.1016/S1474-4422(14)70219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chio A, Mazzini L, D'Alfonso S, Corrado L, Canosa A, Moglia C, et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology. (2018) 91:e635–42. 10.1212/WNL.0000000000005996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. (2011) 377:942–55. 10.1016/S0140-6736(10)61156-7 [DOI] [PubMed] [Google Scholar]
- 5.Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. (2017) 377:162–72. 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- 6.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA. (2004) 101:11159–64. 10.1073/pnas.0402026101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. (2011) 10:75–82. 10.1016/S1474-4422(10)70224-6 [DOI] [PubMed] [Google Scholar]
- 8.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. (2011) 44:20–4. 10.1002/mus.22114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jesus P, Fayemendy P, Nicol M, Lautrette G, Sourisseau H, Preux PM, et al. Hypermetabolism is a deleterious prognostic factor in patients with amyotrophic lateral sclerosis. Eur J Neurol. (2018) 25:97–104. 10.1111/ene.13468 [DOI] [PubMed] [Google Scholar]
- 10.Steyn FJ, Ioannides ZA, van Eijk RPA, Heggie S, Thorpe KA, Ceslis A, et al. Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J Neurol Neurosurg Psychiatry. (2018) 89:1016–23. 10.1136/jnnp-2017-317887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. (2002) 59:773–5. 10.1212/WNL.59.5.773 [DOI] [PubMed] [Google Scholar]
- 12.Doyle P, Brown A, Beral V, Reeves G, Green J. Incidence of and risk factors for Motor Neurone Disease in UK women: a prospective study. BMC Neurol. (2012) 12:25. 10.1186/1471-2377-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Reilly EJ, Wang H, Weisskopf MG, Fitzgerald KC, Falcone G, McCullough ML, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2013) 14:205–11. 10.3109/21678421.2012.735240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry. (2011) 82:628–34. 10.1136/jnnp.2010.211474 [DOI] [PubMed] [Google Scholar]
- 15.Mariosa D, Beard JD, Umbach DM, Bellocco R, Keller J, Peters TL, et al. Body mass index and amyotrophic lateral sclerosis: a study of US military veterans. Am J Epidemiol. (2017) 185:362–71. 10.1093/aje/kww140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) 1:91–6. 10.1080/14660820050515386 [DOI] [PubMed] [Google Scholar]
- 17.Jawaid A, Murthy SB, Wilson AM, Qureshi SU, Amro MJ, Wheaton M, et al. A decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALS. Amyotroph Lateral Scler. (2010) 11:542–8. 10.3109/17482968.2010.482592 [DOI] [PubMed] [Google Scholar]
- 18.Wolf J, Safer A, Wohrle JC, Palm F, Nix WA, Maschke M, et al. Factors predicting one-year mortality in amyotrophic lateral sclerosis patients—data from a population-based registry. BMC Neurol. (2014) 14:197. 10.1186/s12883-014-0197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf J, Safer A, Wohrle JC, Palm F, Nix WA, Maschke M, et al. Factors predicting survival in ALS patients—data from a population-based registry in Rhineland-Palatinate, Germany. Neuroepidemiology. (2015) 44:149–55. 10.1159/000381625 [DOI] [PubMed] [Google Scholar]
- 20.Reich-Slotky R, Andrews J, Cheng B, Buchsbaum R, Levy D, Kaufmann P, et al. Body mass index (BMI) as predictor of ALSFRS-R score decline in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. (2013) 14:212–6. 10.3109/21678421.2013.770028 [DOI] [PubMed] [Google Scholar]
- 21.Peter RS, Rosenbohm A, Dupuis L, Brehme T, Kassubek J, Rothenbacher D, et al. Life course body mass index and risk and prognosis of amyotrophic lateral sclerosis: results from the ALS registry Swabia. Eur J Epidemiol. (2017) 32:901–8. 10.1007/s10654-017-0318-z [DOI] [PubMed] [Google Scholar]
- 22.Shimizu T, Nagaoka U, Nakayama Y, Kawata A, Kugimoto C, Kuroiwa Y, et al. Reduction rate of body mass index predicts prognosis for survival in amyotrophic lateral sclerosis: a multicenter study in Japan. Amyotroph Lateral Scler. (2012) 13:363–6. 10.3109/17482968.2012.678366 [DOI] [PubMed] [Google Scholar]
- 23.Roubeau V, Blasco H, Maillot F, Corcia P, Praline J. Nutritional assessment of amyotrophic lateral sclerosis in routine practice: value of weighing and bioelectrical impedance analysis. Muscle Nerve. (2015) 51:479–84. 10.1002/mus.24419 [DOI] [PubMed] [Google Scholar]
- 24.Fasano A, Fini N, Ferraro D, Ferri L, Vinceti M, Errals, et al. Percutaneous endoscopic gastrostomy, body weight loss and survival in amyotrophic lateral sclerosis: a population-based registry study. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:233–42. 10.1080/21678421.2016.1270325 [DOI] [PubMed] [Google Scholar]
- 25.Kasarskis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr. (1996) 63:130–7. 10.1093/ajcn/63.1.130 [DOI] [PubMed] [Google Scholar]
- 26.Stambler N, Charatan M, Cedarbaum JM. Prognostic indicators of survival in ALS. ALS CNTF Treatment Study Group Neurology. (1998) 50:66–72. 10.1212/WNL.50.1.66 [DOI] [PubMed] [Google Scholar]
- 27.Clavelou P, Blanquet M, Peyrol F, Ouchchane L, Gerbaud L. Rates of progression of weight and forced vital capacity as relevant measurement to adapt Amyotrophic Lateral Sclerosis management for patient result of a French multicentre cohort survey. J Neurol Sci. (2013) 331:126–31. 10.1016/j.jns.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Lindauer E, Dupuis L, Muller HP, Neumann H, Ludolph AC, Kassubek J. Adipose tissue distribution predicts survival in amyotrophic lateral sclerosis. PLoS ONE. (2013) 8:e67783. 10.1371/journal.pone.0067783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerevini S, Agosta F, Riva N, Spinelli EG, Pagani E, Caliendo G, et al. MR imaging of brachial plexus and limb-girdle muscles in patients with amyotrophic lateral sclerosis. Radiology. (2016) 279:553–61. 10.1148/radiol.2015150559 [DOI] [PubMed] [Google Scholar]
- 30.Dalakas MC, Hatazawa J, Brooks RA, Di Chiro G. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann Neurol. (1987) 22:580–6. 10.1002/ana.410220504 [DOI] [PubMed] [Google Scholar]
- 31.Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand. (1992) 85:81–9. 10.1111/j.1600-0404.1992.tb04003.x [DOI] [PubMed] [Google Scholar]
- 32.Claassen DO, Josephs KA, Peller PJ. The stripe of primary lateral sclerosis: focal primary motor cortex hypometabolism seen on fluorodeoxyglucose F18 positron emission tomography. Arch Neurol. (2010) 67:122–5. 10.1001/archneurol.2009.298 [DOI] [PubMed] [Google Scholar]
- 33.Pagani M, Chio A, Valentini MC, Oberg J, Nobili F, Calvo A, et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. (2014) 83:1067–74. 10.1212/WNL.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 34.Marini C, Cistaro A, Campi C, Calvo A, Caponnetto C, Nobili FM, et al. A PET/CT approach to spinal cord metabolism in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging. (2016) 43:2061–71. 10.1007/s00259-016-3440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita T, Hatakeyama T, Sato K, Fukui Y, Hishikawa N, Ohta Y, et al. Flow-metabolism uncoupling in the cervical spinal cord of ALS patients. Neurol Sci. (2017) 38:659–65. 10.1007/s10072-017-2823-y [DOI] [PubMed] [Google Scholar]
- 36.Williams ER, Bruford A. Creatine phosphokinase in motor neurone disease. Clin Chim Acta. (1970) 27:53–6. 10.1016/0009-8981(70)90373-6 [DOI] [PubMed] [Google Scholar]
- 37.Sinaki M, Mulder DW. Amyotrophic-lateral-sclerosis—relationship between serum creatine-kinase level and patient survival. Arch Phys Med Rehabil. (1986) 67:169–71. 10.1016/0003-9993(86)90064-X [DOI] [PubMed] [Google Scholar]
- 38.Felice KJ, North WA. Creatine kinase values in amyotrophic lateral sclerosis. J Neurol Sci. (1998) 160:S30–2. 10.1016/S0022-510X(98)00195-6 [DOI] [PubMed] [Google Scholar]
- 39.Ilzecka J, Stelmasiak Z. Creatine kinase activity in amyotrophic lateral sclerosis patients. Neurol Sci. (2003) 24:286–7. 10.1007/s10072-003-0158-3 [DOI] [PubMed] [Google Scholar]
- 40.Lima AF, Evangelista T, de Carvalho M. Increased creatine kinase and spontaneous activity on electromyography, in amyotrophic lateral sclerosis. Electromyogr Clin Neurophysiol. (2003) 43:189–92. [PubMed] [Google Scholar]
- 41.Gibson SB, Kasarskis EJ, Hu N, Pulst SM, Mendiondo MS, Matthews DE, et al. Relationship of creatine kinase to body composition, disease state, and longevity in ALS. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:473–7. 10.3109/21678421.2015.1062516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafiq MK, Lee E, Bradburn M, McDermott CJ, Shaw PJ. Creatine kinase enzyme level correlates positively with serum creatinine and lean body mass, and is a prognostic factor for survival in amyotrophic lateral sclerosis. Eur J Neurol. (2016) 23:1071–8. 10.1111/ene.12995 [DOI] [PubMed] [Google Scholar]
- 43.Tai HF, Cui LY, Guan YZ, Liu MS, Li XG, Shen DC, et al. Correlation of creatine kinase levels with clinical features and survival in amyotrophic lateral sclerosis. Front Neurol. (2017) 8:322. 10.3389/fneur.2017.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiedemann FR, Winkler K, Kuznetsov AV, Bartels C, Vielhaber S, Feistner H, et al. Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis. J Neurol Sci. (1998) 156:65–72. 10.1016/S0022-510X(98)00008-2 [DOI] [PubMed] [Google Scholar]
- 45.Echaniz-Laguna A, Zoll J, Ponsot E, N'Guessan B, Tranchant C, Loeffler JP, et al. Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man. Exp Neurol. (2006) 198:25–30. 10.1016/j.expneurol.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 46.Palamiuc L, Schlagowski A, Ngo ST, Vernay A, Grosch S, Henriques A, et al. A metabolic switch towards lipid use in glycolytic muscle is an early pathologic event in a mouse model of Amyotrophic Lateral Sclerosis. EMBO Mol Med. (2015) 7:526–46. 10.15252/emmm.201404433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toczylowska B, Jamrozik Z, Liebert A, Kwiecinski H. NMR-based metabonomics of cerebrospinal fluid applied to amyotrophic lateral sclerosis. Biocybernet Biomed Eng. (2013) 33:21–32. 10.1016/S0208-5216(13)70053-6 [DOI] [Google Scholar]
- 48.Blasco H, Veyrat-Durebex C, Bocca C, Patin F, Vourc'h P, Nzoughet JK, et al. Lipidomics reveals cerebrospinal-fluid signatures of ALS. Sci Rep. (2017) 7:17652. 10.1038/s41598-017-17389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdel-Khalik J, Yutuc E, Crick PJ, Gustafsson JA, Warner M, Roman G, et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J Lipid Res. (2017) 58:267–78. 10.1194/jlr.P071639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray E, Larkin JR, Claridge TDW, Talbot K, Sibson NR, Turner MR. The longitudinal cerebrospinal fluid metabolomic profile of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:456–63. 10.3109/21678421.2015.1053490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blasco H, Corcia P, Moreau C, Veau S, Fournier C, Vourc'h P, et al. H-1-NMR-based metabolomic profiling of CSF in early amyotrophic lateral sclerosis. PLoS ONE. (2010) 5:e13223. 10.1371/journal.pone.0013223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilic E, Rudan I, Kusec V, Zurak N, Delimar D, Zagar M. Comparison of the growth hormone, IGF-1 and insulin in cerebrospinal fluid and serum between patients with motor neuron disease and healthy controls. Eur J Neurol. (2006) 13:1340–5. 10.1111/j.1468-1331.2006.01503.x [DOI] [PubMed] [Google Scholar]
- 53.Pradat PF, Bruneteau G, Gordon PH, Dupuis L, Bonnefont-Rousselot D, Simon D, et al. Impaired glucose tolerance in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. (2010) 11:166–71. 10.3109/17482960902822960 [DOI] [PubMed] [Google Scholar]
- 54.Lawton KA, Cudkowicz ME, Brown MV, Alexander D, Caffrey R, Wulff JE, et al. Biochemical alterations associated with ALS. Amyotroph Lateral Scler. (2012) 13:110–8. 10.3109/17482968.2011.619197 [DOI] [PubMed] [Google Scholar]
- 55.Mariosa D, Hammar N, Malmstrom H, Ingre C, Jungner I, Ye WM, et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: a more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol. (2017) 81:718–28. 10.1002/ana.24936 [DOI] [PubMed] [Google Scholar]
- 56.Dupuis L, Corcia P, Fergani A, Gonzalez De Aguilar JL, Bonnefont-Rousselot D, Bittar R, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. (2008) 70:1004–9. 10.1212/01.wnl.0000285080.70324.27 [DOI] [PubMed] [Google Scholar]
- 57.Dorst J, Kuhnlein P, Hendrich C, Kassubek J, Sperfeld AD, Ludolph AC. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol. (2011) 258:613–7. 10.1007/s00415-010-5805-z [DOI] [PubMed] [Google Scholar]
- 58.Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. (2012) 51:1501–8. 10.2169/internalmedicine.51.7465 [DOI] [PubMed] [Google Scholar]
- 59.Rafiq MK, Lee E, Bradburn M, McDermott CJ, Shaw PJ. Effect of lipid profile on prognosis in the patients with amyotrophic lateral sclerosis: insights from the olesoxime clinical trial. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:478–84. 10.3109/21678421.2015.1062517 [DOI] [PubMed] [Google Scholar]
- 60.Delaye JB, Patin F, Piver E, Bruno C, Vasse M, Vourc'h P, et al. Low IDL-B and high LDL-1 subfraction levels in serum of ALS patients. J Neurol Sci. (2017) 380:124–7. 10.1016/j.jns.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 61.Chio A, Calvo A, Ilardi A, Cavallo E, Moglia C, Mutani R, et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology. (2009) 73:1681–5. 10.1212/WNL.0b013e3181c1df1e [DOI] [PubMed] [Google Scholar]
- 62.Huang R, Guo X, Chen X, Zheng Z, Wei Q, Cao B, et al. The serum lipid profiles of amyotrophic lateral sclerosis patients: a study from south-west China and a meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener. (2015) 16:359–65. 10.3109/21678421.2015.1047454 [DOI] [PubMed] [Google Scholar]
- 63.Kumar A, Bala L, Kalita J, Misra UK, Singh RL, Khetrapal CL, et al. Metabolomic analysis of serum by 1H NMR spectroscopy in amyotrophic lateral sclerosis. Clin Chim Acta. (2010) 411:563–7. 10.1016/j.cca.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 64.Ngo ST, Steyn FJ, Huang L, Mantovani S, Pfluger CMM, Woodruff TM, et al. Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J Neurol Sci. (2015) 357:22–7. 10.1016/j.jns.2015.06.053 [DOI] [PubMed] [Google Scholar]
- 65.Spataro R, Volanti P, Vitale F, Meli F, Colletti T, Di Natale A, et al. Plasma cortisol level in amyotrophic lateral sclerosis. J Neurol Sci. (2015) 358:282–6. 10.1016/j.jns.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 66.Czell D, Baldinger R, Schneider U, Neuwirth C, Weber M. The role of the SenseWear device and ghrelin for metabolism in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2016) 17:295–6. 10.3109/21678421.2015.1113299 [DOI] [PubMed] [Google Scholar]
- 67.Patacchioli FR, Monnazzi P, Scontrini A, Tremante E, Caridi I, Brunetti E, et al. Adrenal dysregulation in amyotrophic lateral sclerosis. J Endocrinol Invest. (2003) 26:Rc23–5. 10.1007/BF03349149 [DOI] [PubMed] [Google Scholar]
- 68.Shepheard SR, Wuu J, Cardoso M, Wiklendt L, Dinning PG, Chataway T, et al. Urinary p75(ECD) A prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology. (2017) 88:1137–43. 10.1212/WNL.0000000000003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ioannides ZA, Steyn FJ, Henderson RD, McCombe PA, Ngo ST. Anthropometric measures are not accurate predictors of fat mass in ALS. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:486–91. 10.1080/21678421.2017.1317811 [DOI] [PubMed] [Google Scholar]
- 70.Park Y, Park J, Kim Y, Baek H, Kim SH. Association between nutritional status and disease severity using the amyotrophic lateral sclerosis (ALS) functional rating scale in ALS patients. Nutrition. (2015) 31:1362–7. 10.1016/j.nut.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 71.Moglia C, Calvo A, Grassano M, Canosa A, Manera U, D'Ovidio F, et al. Early weight loss in amyotrophic lateral sclerosis: outcome relevance and clinical correlates in a population-based cohort. J Neurol Neurosurg Psychiatry. (2019). [Epub ahead of print]. 10.1136/jnnp-2018-319611 [DOI] [PubMed] [Google Scholar]
- 72.Ngo ST, Mi JD, Henderson RD, McCombe PA, Steyn FJ. Exploring targets and therapies for amyotrophic lateral sclerosis: current insights into dietary interventions. Degener Neurol Neuromuscular Dis. (2017) 7:95–108. 10.2147/DNND.S120607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. (2009) 4:3. 10.1186/1750-1172-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saks VA, Ventura-Clapier R, Aliev MK. Metabolic control and metabolic capacity: two aspects of creatine kinase functioning in the cells. Biochim Biophys Acta. (1996) 1274:81–8. 10.1016/0005-2728(96)00011-4 [DOI] [PubMed] [Google Scholar]
- 75.Vielhaber S, Kunz D, Winkler K, Wiedemann FR, Kirches E, Feistner H, et al. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain. (2000) 123:1339–48. 10.1093/brain/123.7.1339 [DOI] [PubMed] [Google Scholar]
- 76.Dupuis L, di Scala F, Rene F, de Tapia M, Oudart H, Pradat PF, et al. Up-regulation of mitochondrial uncoupling protein 3 reveals an early muscular metabolic defect in amyotrophic lateral sclerosis. FASEB J. (2003) 17:2091–3. 10.1096/fj.02-1182fje [DOI] [PubMed] [Google Scholar]
- 77.Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. (2008) 8:425–36. 10.1016/j.cmet.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 78.Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, et al. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS ONE. (2009) 4:e5390. 10.1371/journal.pone.0005390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. (2010) 19:2284–302. 10.1093/hmg/ddq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: ways and mechanisms of metabolic correction. J Neurosci Res. (2017) 95:2217–35. 10.1002/jnr.24064 [DOI] [PubMed] [Google Scholar]
- 81.Marini C, Morbelli S, Cistaro A, Campi C, Caponnetto C, Bauckneht M, et al. Interplay between spinal cord and cerebral cortex metabolism in amyotrophic lateral sclerosis. Brain. (2018) 141:2272–9. 10.1093/brain/awy152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dubey N, Miletich RS, Wasay M, Mechtler LL, Bakshi R. Role of fluorodeoxyglucose positron emission tomography in the diagnosis of neurosarcoidosis. J Neurol Sci. (2002) 205:77–81. 10.1016/S0022-510X(02)00225-3 [DOI] [PubMed] [Google Scholar]
- 83.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res. (2017) 95:943–72. 10.1002/jnr.23777 [DOI] [PubMed] [Google Scholar]
- 84.Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. (1979) 6:371–88. 10.1002/ana.410060502 [DOI] [PubMed] [Google Scholar]
- 85.Reyes ET, Perurena OH, Festoff BW, Jorgensen R, Moore WV. Insulin resistance in amyotrophic lateral sclerosis. J Neurol Sci. (1984) 63:317–24. 10.1016/0022-510X(84)90154-0 [DOI] [PubMed] [Google Scholar]
- 86.Lee S, Zhang C, Kilicarslan M, Piening BD, Bjornson E, Hallstrom BM, et al. Integrated network analysis reveals an association between plasma mannose levels and insulin resistance. Cell Metab. (2016) 24:172–84. 10.1016/j.cmet.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. (2002) 359:2140–4. 10.1016/S0140-6736(02)09089-X [DOI] [PubMed] [Google Scholar]
- 88.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. (2007) 30:753–9. 10.2337/dc07-9920 [DOI] [PubMed] [Google Scholar]
- 89.de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer's disease. Drugs. (2017) 77:47–65. 10.1007/s40265-016-0674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Pinieux G, Chariot P, Ammi-saïd M, Louarn F, Lejonc JL, Astier A, et al. Lipid-lowering drugs and mitochondrial function: effects of HMG-CoA reductase inhibitors on serum ubiquinone and blood lactate/pyruvate ratio. Br J Clin Pharmacol. (1996) 42:333–7. 10.1046/j.1365-125.1996.04178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perurena OH, Festoff BW. Reduction in insulin-receptors in amyotrophic-lateral-sclerosis correlates with reduced insulin sensitivity. Neurology. (1987) 37:1375–9. 10.1212/WNL.37.8.1375 [DOI] [PubMed] [Google Scholar]
- 92.Pellecchia MT, Pivonello R, Monsurro MR, Trojsi F, Longo K, Piccirillo G, et al. The GH-IGF system in amyotrophic lateral sclerosis: correlations between pituitary GH secretion capacity, insulin-like growth factors and clinical features. Eur J Neurol. (2010) 17:666–71. 10.1111/j.1468-1331.2009.02896.x [DOI] [PubMed] [Google Scholar]
- 93.Morselli LL, Bongioanni P, Genovesi M, Licitra R, Rossi B, Murri L, et al. Growth hormone secretion is impaired in amyotrophic lateral sclerosis. Clin Endocrinol. (2006) 65:385–8. 10.1111/j.1365-2265.2006.02609.x [DOI] [PubMed] [Google Scholar]
- 94.Sacca F, Quarantelli M, Rinaldi C, Tucci T, Piro R, Perrotta G, et al. A randomized controlled clinical trial of growth hormone in amyotrophic lateral sclerosis: clinical, neuroimaging, and hormonal results. J Neurol. (2011) 259:132–8. 10.1007/s00415-011-6146-2 [DOI] [PubMed] [Google Scholar]
- 95.Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. (2002) 52:448–57. 10.1002/ana.10312 [DOI] [PubMed] [Google Scholar]
- 96.Chao MV. The p75 neurotrophin receptor. J Neurobiol. (1994) 25:1373–85. 10.1002/neu.480251106 [DOI] [PubMed] [Google Scholar]
- 97.Baeza-Raja B, Sachs BD, Li PP, Christian F, Vagena E, Davalos D, et al. p75 Neurotrophin receptor regulates energy balance in obesity. Cell Rep. (2016) 14:255–68. 10.1016/j.celrep.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baeza-Raja B, Li P, Le Moan N, Sachs BD, Schachtrup C, Davalos D, et al. p75 neurotrophin receptor regulates glucose homeostasis and insulin sensitivity. Proc Natl Acad Sci USA. (2012) 109:5838–43. 10.1073/pnas.1103638109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shepheard SR, Chataway T, Schultz DW, Rush RA, Rogers ML. The extracellular domain of neurotrophin receptor p75 as a candidate biomarker for amyotrophic lateral sclerosis. PLoS ONE. (2014) 9:e87398. 10.1371/journal.pone.0087398 [DOI] [PMC free article] [PubMed] [Google Scholar]