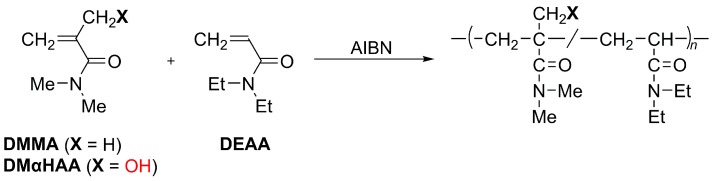
Abstract
α-Functionalized acrylamides have not been considered as an effective monomer design due to their poor polymerizability, although the analogues, α-functionalized acrylates, are attractive monomers of which polymers exhibit characteristic properties. In this article, we report the first example of radical polymerization of α-functionalized N,N-disubstituted acrylamide affording thermo-responsive hydrophilic polymers. N,N-dimethyl-α-(hydroxymethyl)acrylamide (DMαHAA) was (co)polymerized with N,N-diethylacrylamide (DEAA). Although the homopolymerization did not afford a polymeric product, the copolymerizations with various feed ratios yielded a series of the copolymers containing 0%–65% of DMαHAA units. The obtained copolymers exhibited a lower critical solution temperature (LCST) in water; the cloud points (Tcs) were linearly elevated as the contents of DMαHAA units from 32 to 64 °C, indicating that DMαHAA functioned as a more hydrophilic monomer than DEAA. The linear relationship between Tc and DMαHAA content suggests that the homopolymer, poly(DMαHAA), should have Tc at ca. 80 °C, although it is not available by direct radical homopolymerization.
Keywords: α-functionalized acrylamide, temperature-responsive polymer, lower critical solution temperature, hydrophilic monomer, radical polymerization
1. Introduction
As acrylamides have tunable hydrophilicity/hydrophobicity by one or two N-substituents, various thermo-responsive polymers based on acrylamides such as N-isopropylacrylamide [1,2,3,4,5] and N,N-diethylacrylamide (DEAA) [6,7,8] have been developed. On the other hand, N,N-disubstituted methacrylamides are poorly polymerizable both in radical and anionic polymerization [9,10,11,12,13], although they have acrylamide skeletons, where the vinylidene groups should be activated with the electron-withdrawing carbonyl groups. A difference between their typical monomer, N,N-dimethylmethacrylamide (DMMA), and the polymerizable analogue, N,N-dimethylacrylamide (DMAA), is the existence of an α-methyl group. Therefore, the α-methyl group is attributed to the poor polymerizability. In fact, spectral analyses [14,15] and modified neglect of differential overlap (MNDO) calculations [15] of DMMA suggested a twisted conformation between the vinyl and carbonyl groups; if they are in the same plane, the α-methyl group causes the steric repulsion against the N-methyl group in the cisoid form, while the N-substituent creates a similar repulsion to the vinylic hydrogen atom in the transoid form. Therefore, DMMA and other N,N-disubstituted methacrylamides had been categorized as unconjugated vinyl monomers.
Recently, we have been interested in the polymerization chemistry of α-functionalized acrylates, because the α-functionality affects both the polymerization behavior and the properties of the resulting polymer [16,17,18,19,20]. For example, ethyl α-(aminomethyl)acrylate forms an intramolecular hydrogen bond with the carbonyl group, which reduces the nucleophilicity of the amino group and stabilizes the monomer against the ester-amide exchange reaction [19]. The radical polymerization takes place through the unique mechanism accompanying the in situ modification of the pendant amino groups. The resulting polymer exhibits pH/temperature responsivity in acidic aqueous media.
Along the extended line of this concept, we have an interest in the polymerization of α-functionalized acrylamides. Unfortunately, however, the syntheses of α-functionalized N-alkylacrylamides are difficult due to the presence of the acidic amide proton. On the other hand, the preparations of α-functionalized N,N-dialkylacrylamides are relatively facile to prepare [21]. As previously mentioned, N,N-dialkylmethacrylamides are not polymerizable, and thus there are no reports on the polymerization of α-functionalized N,N-dialkylacrylamides, although the α functionality may affect the polymerizability. Herein, we report the polymerization of the hydroxy-functionalized monomer N,N-dimethyl-α-(hydroxymethyl)acrylamide (DMαHAA), and the thermo-responsive properties of the resulting polymer.
2. Experimental Section
2.1. Materials
DEAA and DMAA were kind gifts from KJ Chemicals Co., Ltd. (Tokyo, Japan). These monomers were used after distillation under reduced pressure. 2,2′-Azobis(isobutyronitrile) (AIBN) was recrystallized in hexane before use. Wakogel C-400HG (Wako Pure Chemical Industries, Osaka, Japan) was used as a silica gel for chromatography. Other solvents and reagents were used as received.
2.2. Instruments
1H NMR spectra were recorded in CDCl3 (Nacalai Tesque, Kyoto, Japan) on an AVANCE 400 (Bruker, Billerica, MA, USA) spectrometer. Chemical shifts in 1H NMR spectra were referred to the signal of tetramethylsilane (TMS). Molecular weight and its distributions were determined at 40 °C by size-exclusion chromatography (SEC) on an EXTREMA chromatograph (JASCO Co., Tokyo, Japan) equipped with two SEC columns (PL-gel, Mixed C (300 mm_7.5 mm), Polymer Laboratories), using tetrahydrofuran (THF) as an eluent (flow rate = 0.8 mL·min−1), and calibrated against standard polystyrene (PS) samples (TSK-gel oligomer kit, Tosoh, Tokyo, Japan, Mn: 1.03 × 106, 3.89 × 105, 1.82 × 105, 3.68 × 104, 1.36 × 104, 5.32 × 103, 3.03 × 103, 8.73 × 102) and detected with UV (UV-4070, JASCO Co., Tokyo, Japan) and RI (RI-4030, JASCO Co., Tokyo, Japan) detectors. Purities of monomers were determined from the gas chromatogram (GC) recorded on a GC-2014 (Shimadzu, Kyoto, Japan) equipped with an SH-Rtx-5 capillary column (Shimadzu, Kyoto, Japan).
2.3. Synthesis of N,N-Dimethyl-α-(hydroxymethyl)acrylamide [21]
To a solution of diazabicyclo[2.2.2]octane (DABCO, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan, 18.2 g, 0.162 mmol) in water (28 mL) was added a solution of phenol (7.67 g, 81.5 mmol) in tert-butanol (12 mL). DMAA (67.0 mL, 0.650 mol) was added, and the reaction mixture was heated at 65 °C for five days. After concentration, acetone-hexane (v/v = 4/1) cosolvent and silica gel (130 g) was added and the reaction mixture was stirred for several hours and then filtered. The filtrate was concentrated, and the residual product was purified by distillation under reduced pressure (132 °C/56.0 Pa–135 °C/61.0 Pa) to afford colorless oil (12.2 g). DMαHAA: yield 14.5%; purity 99.7%, 1H NMR spectrum: δ/ppm 5.53–5.52 (m, 1H, CHH=), 5.23 (dd, J1 = J2 = 8.8 Hz, 1H, CHH=), 4.30 (s, 2H, allylic proton), 3.10 (s, 3H, N–CH3), 3.01 (s, 3H, N–CH3), 2.91 (br, 1H, OH).
2.4. Radical Polymerization
A typical example (Table 1, Run 5): In a glass test tube, a mixture of DMαHAA (210 mg, 1.65 mmol), DEAA (190 mg, 1.53 mmol) and AIBN (5.1 mg, 31 μmol) was dissolved in 1,4–dioxane (3.0 mL). The reaction mixture degassed using a freeze–pump–thaw cycle three times. Nitrogen gas was then introduced into the test tube. The reaction mixture was heated at 65 °C for 12 h. A small portion of the reaction mixture was sampled to estimate the monomer conversion. The reaction mixture was then poured into hexane (200 mL) and the precipitate was collected, washed with hexane, and dried in vacuo to give a copolymer (311 mg), as a white solid.
Table 1.
Radical (co)polymerizations of DMMA (N,N-dimethylmethacrylamide) and DMαHAA (N,N-dimethyl-α-(hydroxymethyl)acrylamide) with DEAA (N,N-diethylacrylamide).
| Run 1 | M1 (mmol) | DEAA (mmol) | Solvent | Yield (%) | Composition 2 | Mn 3 | Mw/Mn 3 | |
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | |||||||
| 1 | DMMA (1.90) | - | - (bulk) | 0 | No polymeric products | |||
| 2 | DMαHAA (2.00) | - | - (bulk) | 0 | No polymeric products | |||
| 3 | - | (3.12) | 1,4-dioxane | 78 | 0 | 100 | 25,000 | 1.71 |
| 4 | DMMA (1.60) | (1.49) | 1,4-dioxane | 35 | 23 | 77 | 5,500 | 1.44 |
| 5 | DMαHAA (1.65) | (1.53) | 1,4-dioxane | 57 | 20 | 80 | 1,400 | 2.90 |
| 6 | DMαHAA (1.50) | (1.57) | CHCl3 | 48 | 24 | 76 | 3,400 | 1.57 |
| 7 | DMαHAA (1.57) | (1.50) | DMF | 71 | 25 | 75 | 4,600 | 1.87 |
| 8 | DMαHAA (1.51) | (1.49) | Ethanol | 0 | No polymeric products | |||
| 9 4 | DMαHAA (1.56) | (1.55) | H2O | 33.1 | 4 | 96 | 25,000 | 1.77 |
1 Polymerization was initiated with AIBN (1 mol %) at 60 °C for 16 h in bulk or solvent (3.0 mL); 2 Determined by 1H NMR (400 MHz, CDCl3, 25 °C); 3 Determined by SEC (PS standards, THF, 40 °C); 4 Initiated by 2,2′-Azobis(4-methoxy-2,4-dimethylvaleronitrile).
2.5. Observation of Cluod Point
A typical example: The polymer obtained in Run 2 (5 mg) was dissolved in distilled water (1 mL) and the solution was heated on hot-plate with a rate of temperature increase as 1 °C·min−1. The cloud point was visually examined as the temperature where solution became cloudy.
2.6. Density Functional Theory (DFT) Calculations
All DFT calculations were performed using Spartan’16 (Wavefunction Inc., Irvine, CA, USA) on a laptop PC (Inspiron 15, DELL, Round Rock, TX, USA) equipped with an Intel core i5-5200U processer (2.20 GHz) with an 8.00 GB RAM. The most stable conformation was searched and optimized with molecular mechanics (MM) based on MVFF94 force field before DFT calculation. B3LYP/6-31G* were used for geometry optimization and the subsequent single point calculation on DFT calculation.
3. Results and Discussion
3.1. Attempted Homopolymerization of DMαHAA
DMαHAA was prepared through the Baylis-Hillman reaction between DMAA and formaldehyde according to the literature [21]. The radical polymerization of DMαHAA was conducted in 1,4-dioxane with AIBN at 65 °C (Scheme 1, Table 1, Run 2). For the control, DMMA and DEAA were polymerized in a similar manner (Runs 1 and 3) in order to examine the effects of the hydroxy group and the α-substituent on polymerization. DMαHAA and DMMA did not yield polymeric products, whereas DEAA afforded the respective polymer.
Scheme 1.
Radical (co)polymerization of DMMA and DMαHAA with DEAA.
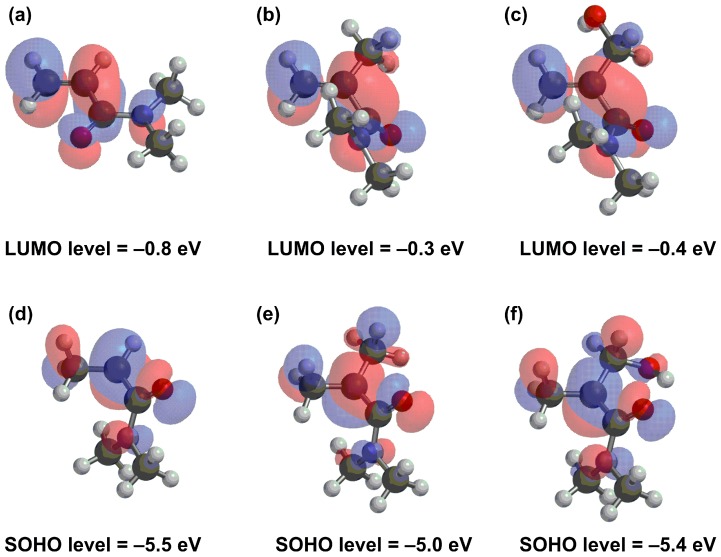
As mentioned in the introduction, N,N-disubstituted methacrylamides are not polymerizable due to the twisted acrylamide skeletons. Consequently, these results are reasonable. In fact, the optimized structures of both DMαHAA and DMMA by density functional theory (DFT) calculations exhibit twisted, non-planar acrylamide skeletons (Figure 1b,c). Nevertheless, importantly, the lowest unoccupied molecular orbitals (LUMOs) of DMαHAA and DMMA spread over the acrylamide skeleton similarly to that of DMAA, a simplified model monomer of DEAA (Figure 1a). This suggests that these monomers have capacities to accept the radical attack to yield the respective monomer-end radicals, despite the non-planar acrylamide skeletons described in the classical discussion. It should be noted that DMAA has a lower LUMO level than others, and thus it should have higher reactivity than the other two monomers. The singly occupied highest orbitals (SOHOs) of the model molecules of resulting radicals were also calculated (Figure 1d–f). These three monomers have similar orbital shapes. For the respective monomers, the effects of the steric repulsion of substituents on the planarity of acrylamide skeletons had been discussed previously. On the other hand, the radicals have sp3 carbons and thus the steric repulsions of substituents are not significant. The presence of the α-substituent, of course, may influence the propagation rate and entropy of polymerization, but these calculation results implied that DMαHAA and DMMA should intrinsically have the potential to polymerize. Presumably, the loss of polymerizability might be caused by the short lifetime of the respective radicals or the large entropy of polymerization. Nevertheless, the results of the DFT calculation suggest that these monomers may undergo copolymerization with appropriate polymerizable monomers.
Figure 1.
LUMOs of (a) DMAA, (b) DMMA and (c) DMαHAA, and SOHOs of the respective radicals to (d) DMAA, (e) DMMA and (f) DMαHAA, by DFT calculation (B3LYP/6-31G*).
3.2. Copolymerization of DMαHAA with DEAA
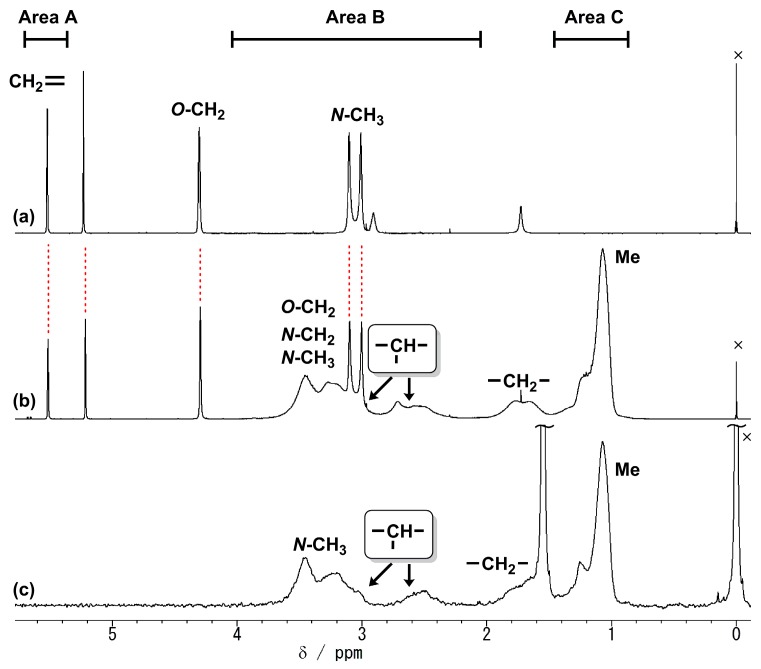
The copolymerizations of DMMA and DMαHAA with an equimolar amount of DEAA were conducted in a similar way to the homopolymerization. In both cases (Runs 4 and 5), the respective copolymers were obtained as expected. Figure 2b shows the 1H NMR spectra of the obtained copolymer of DMαHAA and DEAA, accompanying the monomer (DMαHAA) and poly(DEAA). Since the polymer included the unreacted monomer and the 1H NMR signals of each monomer unit were overlapped, the polymer composition was determined as follows:
Area A (5.75–5.35 ppm) includes the vinylidene signal of the unreacted monomer.
-
Area B (4.05–2.05 ppm) includes the N–CH2 and main-chain CH signals in DEAA units, the N–CH3 and O–CH2 signals in DMαHAA units, and the N–CH3 signals of unreacted DMαHAA.
If we set the content of DMαHAA units as x (%), the following equation can be established:[Area B] − 6 × [Area A] (N-CH3 of unreacted DMαHAA) = 4x(N-CH2) + x(CH) + 6 × (100 − x) (N-CH3) + 2 × (100 – x) (O-CH2) (1) - Area C (1.45–0.85 ppm) includes the N–CH2CH3 signal in DEAA units, and thus the following equation can be established:
[Area C] = 6x(N-CH2CH3) (2)
Figure 2.
1H NMR spectra of (a) DMαHAA; (b) poly(DMαHAA-co-DEAA) obtained from Run 5; and (c) poly(DEAA) (400 MHz, CDCl3, 25 °C). ×: Tetramethylsilane.
The intensity integrations of each area were inserted in Equations (1) and (2), and the simultaneous equation was solved to give the content of DMαHAA units (x = 20%).
The resulting polymers of the copolymerization of DMMA and DEAA had similar compositions, i.e., 23% of DMAA units against 50% of the feed ratio. Therefore, the effect of the hydroxy group on the polymerization behavior was not significant.
The polymerizations were also examined in other solvents such as CHCl3 (Run 6), DMF (Run 7), ethanol (Run 8), and water (Run 9). While the polymerizations in aprotic solvents afforded similar results with ca. 20% of DMαHAA unit contents, those in protonic solvents such as water and ethanol resulted in significantly different results. In ethanol, no polymeric product was obtained, whereas the homopolymerization of DEAA almost took place in water. Careful discussions are required to explain these results, but presumably the hydrogen bond formation of the protonic solvent molecules to the hydroxy groups of DMαHAA might increase the steric hindrance around the vinylidene group to prevent the polymerization of DMαHAA.
3.3. Syntheses of Thermo-Responsive Polymers with Tunable Cloud Points
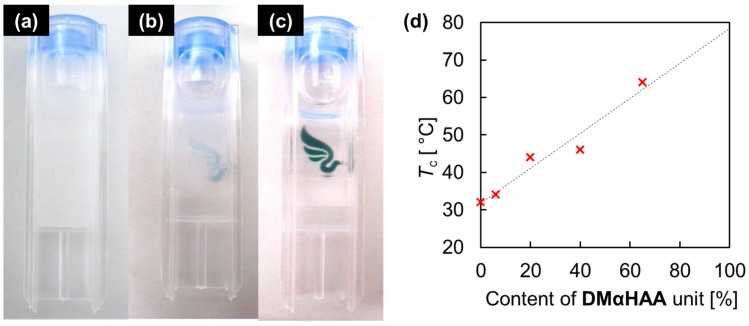
As is well known, poly(DEAA) exhibited a lower critical solution temperature (LCST) around 32 °C. Similarly, the copolymer of DEAA and DMαHAA obtained in Run 4 displayed the cloud point (Tc) at 44 °C, as shown in Figure 3b and Video S1 (available as Supplementary Materials on web), indicating that the incorporation of the hydrophilic hydroxy group increased the Tc. In order to tune the Tc, a series of copolymers with different compositions was prepared (Table 2, Runs 3, 5, 10–12). The molecular weights of the resulting copolymers became lower as the feed ratio of the DMαHAA increased, probably because of the poor homopolymerizablity. It should be noted that the content of the DMαHAA units reached 65% in Run 12, suggesting the existence of the homosequence of DMαHAA-DMαHAA. This means that DMαHAA has slight homopolymerizablity, although the homopolymerization did not yield a polymeric product, probably due to the fast termination.
Figure 3.
Photographs of poly(DMαHAA-co-DEAA), obtained in Run 5, in water (1 wt %): (a) at 50 °C; (b) at 44 °C; and (c) at 25 °C; (d) Plots of content of DMαHAA unit versus Tc.
Table 2.
Radical (co)polymerizations of DMαHAA with DEAA and the cloud points of the resulting polymer.
| Run 1 | DMαHAA (mmol) | DEAA (mmol) | Yield (%) | Composition 2 | Mn 3 | Mw/Mn 3 | Tc (°C) | |
|---|---|---|---|---|---|---|---|---|
| DMαHAA | DEAA | |||||||
| 3 | - | 3.12 | 78 | 0 | 100 | 25,000 | 1.71 | 32 |
| 10 | 1.57 | 6.40 | 99 | 6 | 94 | 23,000 | 1.89 | 34 |
| 5 | 1.65 | 1.53 | 57 | 20 | 80 | 1400 | 2.90 | 44 |
| 11 | 3.12 | 1.60 | 55 | 40 | 60 | 4800 | 1.77 | 46 |
| 12 | 6.43 | 1.63 | 57 | 65 | 35 | 1700 | 2.63 | 64 |
1 Polymerization was initiated with AIBN (1 mol %) at 60 °C in 1,4-dioxane (3.0 mL) for 16 h; 2 Determined by 1H NMR (400 MHz, CDCl3, 25 °C); 3 Determined by SEC (PS standards, THF, 40 °C).
The Tcs were linearly increased as the contents of the DMαHAA units, as shown Figure 3d. If the linear relationship is maintained in the extended region, the homopolymer poly(DMαHAA) should have a Tc around 80 °C, although it could not be prepared by the direct polymerization of DMαHAA.
4. Conclusions
Although N,N-disubstituted acrylamides had been categorized as unconjugated monomers due to their twisted conformation and thus as not polymerizable, the DFT calculations show the expectation of the potential polymerizability. In fact, DMαHAA did not yield the homopolymer but allowed radical copolymerization with DEAA. The hydrophilicity of the hydroxy group increased the Tc, and the linear relationship between the content of the DMαHAA unit and Tc provides the presumption of the Tc of poly(DMαHAA) around 80 °C. Importantly, the analogue, poly(N,N-dimethylacrylamide), is a water-soluble polymer and displays no Tc. Although the hydroxy group is hydrophilic, the α-substitution did not simply enhance the hydrophilicity to the polymer but gave the thermo-responsivity. This fact clearly shows the importance of α-functionalization. Now the authors are attempting controlled polymerization of these α-functionalized N,N-disubstituted acrylamides, as they can accept a radical addition if the radical has a long lifetime. The results will be reported elsewhere soon.
Acknowledgments
The authors appreciate KJ Chemicals Co., Ltd. for the kind gifts of acrylamide monomers. This research was financially supported by JSPS KAKENHI Grant Number 26810069.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4360/8/10/374/s1, Video S1: Thermo-response of poly(DMαHAA-co-DEAA), obtained in Run 5, in water (1 wt %).
Author Contributions
Yasuhiro Kohsaka designed and supervised the overall research, performed the DFT calculation, characterized the chemicals, and wrote the paper. Yoshiaki Tanimoto performed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Aoshima S., Kanaoka S. Synthesis of stimuli-responsive polymers by living polymerization: Poly(N-isopropylacrylamide) and poly(vinyl ether)s. Adv. Polym. Sci. 2008;210:169–208. [Google Scholar]
- 2.Rimmer S., Soutar I., Swanson L. Switching the conformational behaviour of poly(N-isopropyl acrylamide. Polym. Int. 2009;58:273–278. doi: 10.1002/pi.2537. [DOI] [Google Scholar]
- 3.Tada T., Hirano T., Ute K., Katsumoto Y., Asoh T., Shoji T., Kitamura N., Tsuboi Y. Effects of syndiotacticity on the dynamic and static phase separation properties of poly(N-isopropylacrylamide) in aqueous solution. J. Phys. Chem. B. 2016;120:7724–7730. doi: 10.1021/acs.jpcb.6b03200. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig K., Boerner H.G., Wischerhoff E., Lutz J.-F., Jaeger M.S., Laschewsky A., Duschl C. On the interaction of adherent cells with thermoresponsive polymer coatings. Polymers. 2014;6:1164–1177. doi: 10.3390/polym6041164. [DOI] [Google Scholar]
- 5.Chen W., Ma Y., Pan J., Meng Z., Pan G., Sellergren B. Molecularly imprinted polymers with stimuli-responsive affinity: Progress and perspectives. Polymers. 2015;7:1689–1715. doi: 10.3390/polym7091478. [DOI] [Google Scholar]
- 6.Katsumoto Y., Etoh Y., Shimoda N. Phase diagrams of stereocontrolled poly(N,N-diethylacrylamide) in water. Macromolecules. 2010;43:3120–3121. doi: 10.1021/ma902673z. [DOI] [Google Scholar]
- 7.Hufendiek A., Trouillet V., Meier M.A.R., Barner-Kowollik C. Temperature responsive cellulose-graft-copolymers via cellulose functionalization in an ionic liquid and RAFT polymerization. Biomacromolecules. 2014;15:2563–2572. doi: 10.1021/bm500416m. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda K., Enomoto R., Ishihara K., Morishima Y., Yusa S. Thermo-responsive and biocompatible diblock copolymers prepared via reversible addition-fragmentation chain transfer (RAFT) radical polymerization. Polymers. 2014;6:846–859. doi: 10.3390/polym6030846. [DOI] [Google Scholar]
- 9.Ishizone T., Kitazawa K., Suzuki T., Kawauchi S. Anionic polymerization behavior of α-methylene-N-methylpyrrolidone. Macromol. Symp. 2013;323:86–91. doi: 10.1002/masy.201100095. [DOI] [Google Scholar]
- 10.Butler G.B., Myers G.R. The fundamental basis for cyclopolymerization. IV. Radiation initiated solid-state polymerization of certain dimethacrylamides. J. Macromol. Sci. Chem. 1971;A5:135–166. doi: 10.1080/00222337108061030. [DOI] [Google Scholar]
- 11.Kodaira T., Aoyama F. Cyclopolymerization. II. Mechanism of the free-radical polymerization of N-n-propyldimethacrylamide. J. Polym. Sci. Polym. Chem. Ed. 1974;12:897–910. doi: 10.1002/pol.1974.170120419. [DOI] [Google Scholar]
- 12.Otsu T., Yamada B., Mori T., Inoue M. Ceiling temperatures in radical polymerizations of N-phenyl and N-n-butyl methacrylamide. J. Polym. Sci. Polym. Lett. Ed. 1976;14:283. doi: 10.1002/pol.1976.130140506. [DOI] [Google Scholar]
- 13.Okamoto Y., Yuki H. Anionic polymerization of N-methacryloylaziridine. J. Polym. Sci. Polym. Chem. Ed. 1981;19:2647–2650. doi: 10.1002/pol.1981.170191023. [DOI] [Google Scholar]
- 14.Xie X., Hogen-Esch T.E. Anionic synthesis of narrow molecular weight distribution water-soluble poly(N,N-dimethylacrylamide) and poly(N-acryloyl-N′-methylpiperazine) Macromolecules. 1996;29:1746–1752. doi: 10.1021/ma950688d. [DOI] [Google Scholar]
- 15.Kodaira T., Tanahashi H., Hara K. Cyclopolymerization XVII. Anionic cyclopolymerization tendency of N-methyldiacrylamide and N-substituted dimethacrylamides. Polym. J. 1990;22:649–659. doi: 10.1295/polymj.22.649. [DOI] [Google Scholar]
- 16.Kohsaka Y. Polymerization chemistry of α-substituted acrylates toward precise polymerization and functional materials. Kobunshi Ronbunshu. 2016;73:412–427. doi: 10.1295/koron.2016-0024. [DOI] [Google Scholar]
- 17.Kohsaka Y., Kurata T., Kitayama T. End-functional stereoregular poly(methyl methacrylate) with clickable C=C bonds: Facile synthesis and thiol-ene reaction. Polym. Chem. 2013;4:5043–5047. doi: 10.1039/c3py00799e. [DOI] [Google Scholar]
- 18.Kohsaka Y., Yamaguchi E., Kitayama T. Anionic alternating copolymerization of alpha-arylacrylates with methyl methacrylate: Effect of monomer sequence on fluorescence. J. Polym. Sci. Part A Polym. Chem. 2014;52:2806–2814. doi: 10.1002/pola.27305. [DOI] [Google Scholar]
- 19.Kohsaka Y., Matsumoto Y., Kitayama T. α-(Aminomethyl)acrylate: Polymerization and spontaneous post-polymerization modification of β-amino acid ester for a pH/temperature-responsive material. Polym. Chem. 2015;6:5026–5029. doi: 10.1039/C5PY00723B. [DOI] [Google Scholar]
- 20.Kohsaka Y., Matsumoto Y., Zhang T., Matsuhashi Y., Kitayama T. α-Exomethylene lactone possessing acetal-ester linkage: Polymerization and postpolymerization modification for water-soluble polymer. J. Polym. Sci. Part A Polym. Chem. 2016;54:955–961. doi: 10.1002/pola.27931. [DOI] [Google Scholar]
- 21.Davoust M., Brière J.-F., Metzner P. A diastereoselective and concise synthesis of functionalised vinyl epoxides with a Morita-Baylis-Hillman backbone. Org. Biomol. Chem. 2006;4:3048–3051. doi: 10.1039/B607040J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.