Abstract
Aims
The use of biologic therapy has increased over the past decade well beyond primary autoimmune diseases. Indeed, a recent trial using an anti-IL-1beta antibody reduced second myocardial infarction (MI) in those who have had MI. Psoriasis is a chronic inflammatory disease often treated with biologics when severe, is associated with increased risk of MI, in part driven by high-risk coronary plaque phenotypes by coronary computed tomography angiography (CCTA). We hypothesized that we would observe a reduction in inflammatory-driven phenotypes of coronary plaque, including non-calcified coronary plaque burden and lipid-rich necrotic core in those treated with biologic therapy after one-year compared with non-biologic therapy.
Methods and results
In a prospective, observational study, 290 participants were recruited from 1 January 2013 through 31 October 2018 with 215 completing one-year follow-up. Of the 238, 121 consecutive participants who were biologic treatment naïve at baseline were included. A blinded reader (blinded to patient demographics, visit and treatment) quantified total coronary plaque burden and plaque subcomponents (calcified and non-calcified) in the three main coronary vessels >2 mm using dedicated software (QAngio, Medis, Netherlands). Psoriasis patients were middle-aged [mean (standard deviation) age, 50.5 (12.1) years], mostly male (n = 70, 58%) with low cardiovascular risk by Framingham score [median (interquartile range, IQR), 3 (1–6)] and had moderate to severe skin disease at baseline [median (IQR) Psoriasis Area Severity Index, PASI, 8.6 (5.3–14.0)]. Biologic therapy was associated with a 6% reduction in non-calcified plaque burden (P = 0.005) reduction in necrotic core (P = 0.03), with no effect on fibrous burden (P = 0.71). Decrease in non-calcified plaque burden in the biologic treated group was significant compared with slow plaque progression in non-biologic treated (Δ, −0.07 mm2 vs. 0.06 mm2; P = 0.02) and associated with biologic treatment beyond adjustment for traditional cardiovascular risk factors (β = 0.20, P = 0.02).
Conclusion
In this observational study, we demonstrate that biologic therapy in severe psoriasis was associated with favourable modulation of coronary plaque indices by CCTA. These findings highlight the importance of systemic inflammation in coronary artery disease and support the conduct of larger, randomized trials.
Keywords: Psoriasis, CCTA, Coronary artery disease, Biologic therapy, Coronary plaque characteristics
1. Introduction
Cardiovascular disease remains the leading cause of death, with residual risk due to inflammation being an emerging critical target.1,2 In a recent study of patients with myocardial infarction (MI) and high residual inflammatory risk (high sensitivity C-reactive protein >2 mg/L), canakinumab, a monoclonal antibody targeting interleukin-1β (IL-1β), decreased the rate of non-fatal MI, non-fatal stroke, and cardiovascular death without affecting cholesterol levels.3 Findings from the study support the need to expand our understanding of potential effects of biologic therapies on coronary vasculature.
Psoriasis is a chronic inflammatory skin disease associated with accelerated atherosclerosis affecting about 3% of the population. Severe psoriasis is associated with early MI risk by over 50%,4 with rates of coronary artery disease being similar to Type 2 diabetes.5 The inflammatory milieu of psoriatic skin harbours cytokines critical to early atherogenesis and plaque rupture, with derangements in pro-inflammatory and pro-atherogenic cytokines, such as IL-1β, interleukin-17 (IL-17), and tumour necrosis factor-α (TNF-α).
Psoriasis, when severe, is treated with biologic therapy. This provides a reliable model to study inflammatory atherogenesis and the longitudinal impact of modulating specific cytokines on vascular behaviour, while treating the primary skin disease with FDA approved biologic therapies.6 In this context, the aim of this study was to perform coronary artery plaque characterization before and after biological therapy in an open-label, one-year follow up study. We hypothesized that these inflammatory driven phenotypes of coronary plaque, including lipid-rich, non-calcified coronary plaque burden, and lipid-rich necrotic core, would decrease following biological therapy compared with patients not treated with biologic therapy after one-year.
2. Methods
2.1 Study design and population
In a prospective, observational study, 290 participants participating in an ongoing cohort study to understand the association between psoriasis and cardiometabolic disease under the Psoriasis, Atherosclerosis and Cardiometabolic Disease Initiative were recruited from 1 January 2013 through 31 October 2018 with 238 completing one-year follow-up and 121 consecutive patients meeting the inclusion criteria (Supplementary material online, Figure S1). Detailed inclusion and exclusion criteria of the study have been previously reported.7 A study provider confirmed the onset and duration of psoriasis and assessed psoriasis severity using the Psoriasis Area and Severity Index (PASI) score, which combines the severity of lesions and the area affected into a single score, considering erythema, induration, and desquamation within each lesion. Previous psoriasis literature has established that the degree of PASI score response defined as greater than 50% improvement is clinically significant and denotes meaningful improvement.8 For this study, only participants who were naïve to biologic or systemic therapies at baseline were included and followed for one year with clinical and laboratory data as well as serial coronary computed tomography angiography (CCTA). Biologic therapy initiation was performed one day to one month after the initial visit whereby an independent dermatologist started treatment. Treatment agents included TNF-α inhibitors (adalimumab, etanercept), interleukin-12/23 inhibitor (ustekinumab), and interleukin-17 inhibitors (secukinumab, ixekizumab). Participants who elected to not receive biologic therapy at their follow-up visit were used as a referent group and were treated with topical and/or light therapies only. Individuals on systemic therapies or started on statin treatment over the course of the one-year study period were excluded. Study protocols were approved by the institutional review board at the National Institutes of Health and all participants provided written informed consent. The study was in accordance with the Declaration of Helsinki. Strengthening the reporting of observational studies in epidemiology guidelines were followed for reporting the findings.9
2.2 Sample size calculations
Modulation of the primary outcome, non-calcified plaque burden, was assessed on a per-artery basis, yielding 363 total arteries in a cohort of 121 study subjects based on prior published methods.10,11 We hypothesized a 15% difference in non-calcified plaque burden with a standard deviation (SD) of 0.5 between treatment groups. Thus, the evaluation of 182 arteries was required for a study with 90% power.
2.3 Coronary computed tomography angiography
2.3.1 Acquisition
All patients underwent CCTA on the same day as blood draw, using the same CT scanner (320-detector row Aquilion ONE ViSION, Toshiba, Japan). Guidelines implemented by the NIH Radiation Exposure Committee were followed. Scans were performed with prospective EKG gating, 100 or 120 kV tube potential, tube current of 100–850 mA adjusted to the patient’s body size, with a gantry rotation time of 275 ms. Images were acquired at a slice thickness of 0.5 mm with a slice increment of 0.25 mm.
2.3.2 Analysis
All scans were read in a blinded fashion to patient characteristics, visit date, and treatment. Coronary plaque characteristics were analysed across each of the main coronary arteries >2 mm using dedicated software (QAngio CT, Medis; The Netherlands).10,11 Automated longitudinal contouring of the inner lumen and outer wall was performed and results were manually adjusted when clear deviations were present.12 Results of the automated contouring were also reviewed on transverse reconstructed cross-sections of the artery on a section-by-section basis at 0.25-mm increments. Lumen attenuation was adaptively corrected on an individual scan basis using gradient filters and intensity values within the artery. Intra-rater reliability was high, with intra-class correlation coefficient = 0.900, 95% CI (0.903–0.919).
To account for variable coronary artery lengths, plaque volume (in cubic millimetres) was divided by the corresponding segment length (in millimetres), yielding a plaque index.9 Total plaque burden was defined as the sum of calcified plaque burden and non-calcified plaque burden. Non-calcified plaque subcomponents were obtained after adaptively correcting for lumen attenuation and depicted based on Hounsfield Units derived by the software.
2.4 Clinical data and laboratory measurements
Upon recruitment of participants, initial contact with investigators involved a comprehensive medical history, physical examination, medication evaluation, and anthropometric measurements. Blood samples were collected after an overnight fast. Samples were analysed for basic chemistry, complete lipid panel, insulin, and high sensitivity C-reactive protein at the NIH Clinical Center. Cholesterol efflux capacity was measured using a validated ex vivo assay of J774 cholesterol-loaded macrophages as previously published.11 Blood inflammatory markers including interferon-γ, TNF-α, and cytokines were quantified using multiplex ELISA assays (Mesoscale Diagnostics, Gaithersburg, MD, USA).
2.5 Statistical analysis
Skewness and kurtosis measures were considered to assess normality. Data were reported as mean with SD for parametric variables, median with interquartile range (IQR) for non-parametric variables and as percentages for categorical variables. Parametric variables were compared between two groups using paired t-test. Non-parametric variables were compared using Wilcoxon signed-rank test and Pearson’s χ2 test was performed for categorical variables between two groups. We conducted Spearman’s rank order correlation coefficient to evaluate the relationship between non-calcified plaque burden and different cardiometabolic risk factors.
To understand the change in various coronary parameters over one-year follow up, we used paired t-test for parametric variables, Wilcoxon signed-rank test for non-parametric variables and Pearson’s χ2 test for categorical variables. The change in coronary parameters over one-year was compared between groups using paired t-test. We performed multi-variable linear regression analysis and adjusted for Framingham risk score, body mass index, and statin use. A two-tailed P-value <0.05 was considered statistically significant. Statistical analysis was performed using STATA-12 software (STATA Inc., College Station, TX, USA).
3. Results
3.1 Baseline characteristics of study groups
Study participants were middle-aged [mean (SD) age, 50.4 (12.1) years], mostly male (n = 70, 58%) with low cardiovascular risk by Framingham score [median (IQR), 3 (1–6)] and had moderate to severe skin disease at baseline [median (IQR) PASI, 8.6 (5.3–14.0)] (Table 1). There were no significant differences in demographic characteristics, in baseline medication use or laboratory values between the two groups along with no biologic or systemic therapy use at baseline in either group.
Table 1.
Baseline and one-year follow-up characteristics of patients with psoriasis
| Parameters | Biologic treated (n = 89) |
Non-biologic treated (n = 32) |
At baselinea | ||||
|---|---|---|---|---|---|---|---|
| Baseline | One-year | P-value | Baseline | One-year | P-value | P-value | |
| Demographics and medical history | |||||||
| Age (years) | 49.1 ± 12.2 | 50.2 ± 12.2 | – | 51.2 ± 12.0 | 53.1 ± 12.3 | – | 0.35 |
| Males | 50 (56) | 50 (56) | – | 20 (63) | 20 (63) | – | 0.65 |
| Body mass index | 29.9 ± 6.0 | 29.6 ± 6.1 | 0.18 | 29.4 ± 5.6 | 29.0 ± 5.4 | 0.06 | 0.57 |
| Hypertension | 27 (30) | 26 (29) | 0.71 | 10 (31) | 9 (28) | 0.32 | 0.63 |
| Hyperlipidaemia | 32 (36) | 30 (34) | 0.76 | 14 (44) | 14 (44) | 1.00 | 0.46 |
| Statin treatment | 25 (28) | 22 (25) | 0.71 | 10 (31) | 9 (28) | 0.32 | 0.92 |
| Type-2 diabetes mellitus | 8 (9) | 6 (7) | 0.16 | 2 (6) | 3 (9) | 0.32 | 0.60 |
| Current smoker | 9 (10) | 7 (8) | 0.18 | 4 (13) | 4 (13) | 1.00 | 0.59 |
| Clinical and laboratory data | |||||||
| Total cholesterol (mg/dL) | 181.3 ± 33.3 | 181.2 ± 36.0 | 0.49 | 184.4 ± 38.5 | 183.4 ± 41.2 | 0.43 | 0.50 |
| HDL cholesterol (mg/dL) | 53.8 ± 14.2 | 54.7 ± 16.2 | 0.17 | 53.4 ± 16.2 | 55.8 ± 19.5 | 0.16 | 0.79 |
| LDL cholesterol (mg/dL) | 105.6 ± 28.0 | 102.4 ± 33.0 | 0.19 | 103.1 ± 28.2 | 98.8 ± 35.9 | 0.25 | 0.51 |
| Framingham risk score | 3 (1–6) | 2 (1–5) | 0.15 | 3 (2–7) | 4 (1–7) | 0.65 | 0.42 |
| C-reactive protein | 2.0 (0.8–5.0) | 1.4 (0.7–3.6) | <0.001 | 2.3 (0.6–4.5) | 1.8 (0.7–3.8) | 0.21 | 0.71 |
| HOMA-IR | 3.1 (2.0–5.6) | 2.9 (1.9–4.9) | 0.11 | 2.6 (1.6–4.9) | 2.7 (1.8–5.3) | 0.57 | 0.59 |
| Psoriasis characterization | |||||||
| PASI score | 9.0 (5.6–15.0) | 3.2 (1.8–5.7) | <0.001 | 8.1 (5.0–12.0) | 7.0 (4.0–9.9) | 0.08 | 0.64 |
| Disease duration | 23.0 ± 14.4 | 24.0 ± 14.7 | – | 20.3 ± 14.6 | 21.3 ± 17.1 | – | 0.48 |
| Topical therapy | 56 (63) | 39 (44) | 0.03 | 22 (69) | 25 (78) | 0.32 | 0.62 |
| Light therapy | 16 (18) | 11 (12) | 0.25 | 9 (28) | 10 (31) | 0.66 | 0.08 |
| Systemic therapy | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 1.00 |
Values are reported as mean ± SD or median (IQR) for continuous data and N (%) for categorical data. Two-tailed P-values less than 0.05 deemed significant (bold values).
HOMA-IR, homeostatic model assessment of insulin resistance; PASI, Psoriasis Area and Severity Index.
Comparison between groups at baseline.
3.2 Relationship of non-calcified plaque burden with risk factors
Table 2 demonstrates associations between non-calcified plaque burden and cardiometabolic risk factors. Non-calcified plaque burden was correlated with traditional cardiovascular risk factors, including male gender (β = 0.37; P < 0.001), body mass index, (β = 0.52; P < 0.001), hypertension (β = 0.24; P < 0.001), hyperlipidaemia (β = 0.10; P = 0.02), HDL cholesterol (β = −0.30; P < 0.001), Framingham risk score (β = 0.29; P < 0.001), C-reactive protein (β = 0.11, P = 0.005), and homeostatic model assessment insulin resistance score (HOMA-IR, β = 0.19; P < 0.001). Additionally, non-calcified plaque burden was correlated with skin disease severity as assessed by PASI score (β = 0.20; P < 0.001), which remained significant after adjustment for traditional cardiovascular risk factors and high sensitivity C-reactive protein (hsCRP) (β = 0.13; P < 0.001).
Table 2.
Multivariable linear regressions for the associations between non-calcified coronary plaque burden and cardiovascular risk factors and psoriasis characterization
| Parameters | Non-calcified plaque burden (mm2) (n = 121) |
|---|---|
| Demographics and medical history | β (P-value) |
| Age (years) | 0.04 (0.40) |
| Males | 0.37 (<0.001) |
| Body mass index | 0.52 (<0.001) |
| Hypertension | 0.24 (<0.001) |
| Hyperlipidaemia | 0.10 (0.02) |
| Type-2 diabetes mellitus | 0.05 (0.19) |
| Current smoker | 0.10 (0.03) |
| Clinical and laboratory data | |
| Total cholesterol (mg/dL) | −0.05 (0.45) |
| HDL cholesterol (mg/dL) | −0.30 (<0.001) |
| LDL cholesterol (mg/dL) | −0.01 (0.72) |
| Framingham risk score | 0.29 (<0.001) |
| C-reactive protein | 0.11 (0.005) |
| HOMA-IR | 0.19 (<0.001) |
| Psoriasis characterization | |
| PASI score | 0.20 (<0.001) |
| Disease duration | 0.05 (0.24) |
All data in the table is expressed as standardized β (P-value). Two-tailed P-values less than 0.05 deemed significant (bold values).
HOMA-IR, homeostasis model assessment of insulin resistance; PASI, Psoriasis Area and Severity Index.
3.3 Modulation of coronary plaque characteristic following treatment
At one-year follow-up (Table 1), we observed a significant improvement in psoriasis by PASI score in the biologic treated group (64% improvement, P < 0.001) and not in the non-biologic treated group [median (IQR), 8.1 (5.0–12.0) vs. 7 (4.0–9.9); P = 0.08]. There were no significant effects on body mass index, lipids, or glucose. A reduction in hsCRP was seen only in the biologic treated group [median (IQR), 2.0 mg/L (0.8–5.0) vs. 1.4 mg/L (0.7–3.6); P < 0.001]. No participants in either group started a new lipid lowering therapy during the study period.
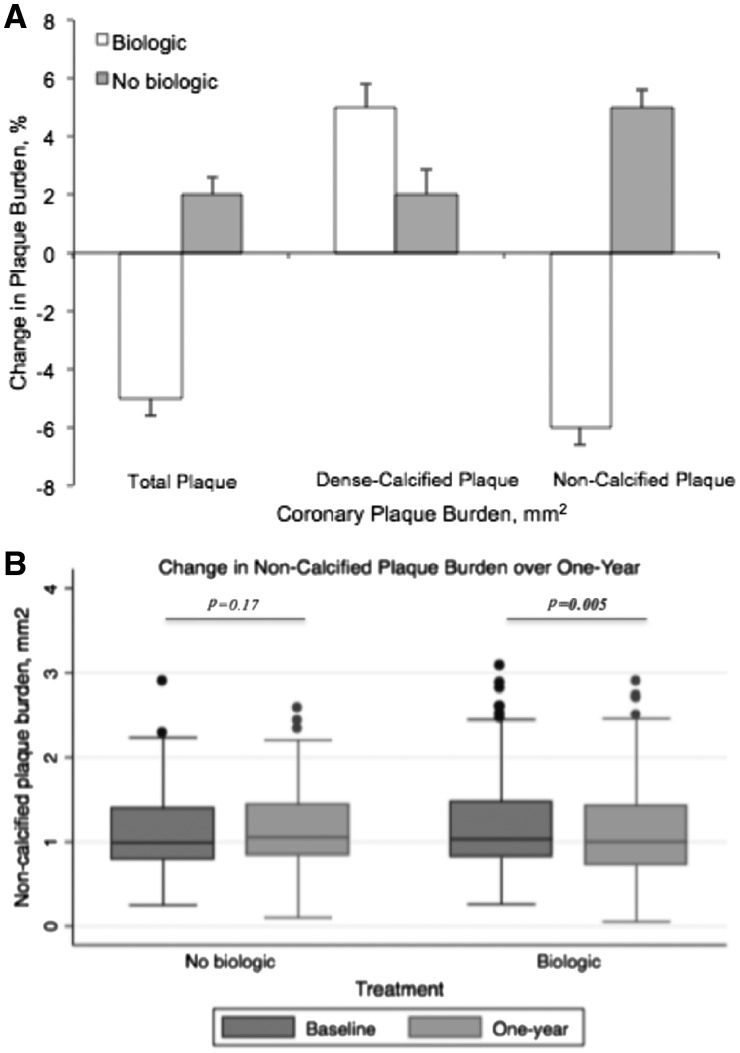
Table 3 summarizes measures of coronary artery disease burden as determined by CCTA. In those receiving biologic therapy, there was a 5% reduction in total coronary plaque burden [mean (SD), 1.30 mm2 (0.60) vs. 1.24 mm2 (0.60); P = 0.009], primarily driven by a reduction in non-calcified plaque burden [mean (SD), 1.22 mm2 (0.59) vs. 1.15 mm2 (0.60); P = 0.005] (Figure 1A and B). We observed no change in fibrous burden (P = 0.71), and there was a significant reduction in both fibro-fatty burden [mean (SD), 0.22 mm2 (0.19) vs. 0.10 mm2 (0.14); P = 0.004] and necrotic burden [mean (SD), 0.07 mm2 (0.19) vs. 0.03 mm2 (0.09); P = 0.03]. On the contrary, in those not receiving systemic or biologic therapy over one-year, there were no significant changes in total plaque burden [mean (SD), 1.28 mm2 (0.53) vs. 1.31 (0.48); P = 0.22] and non-calcified plaque burden [mean (SD), 1.19 mm2 (0.41) vs. 1.25 (0.10); P = 0.17] with no change in fibrous burden (4% decrease, P = 0.22), a significant increase in fibro-fatty burden (38% increase, P = 0.004) and non-significant increase in necrotic core (33% increase, P = 0.27) (Figure 2A and B).
Table 3.
Coronary artery parameters by artery at baseline and one-year follow-up
| Coronary characterization | Biologic treated (n = 267 arteries) |
Non-biologic treated (n = 96 arteries) |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | One-year | Change (%) | P-value | Baseline | One-year | Change (%) | P-value | |
| Total plaque burden (mm2) | 1.30 ± 0.60 | 1.24 ± 0.60 | −0.06 (−5) | 0.009 | 1.28 ± 0.53 | 1.31 ± 0.48 | 0.03 (2) | 0.22 |
| Dense-calcified plaque burden (mm2) | 0.064 ± 0.12 | 0.067 ± 0.14 | 0.003 (5) | 0.36 | 0.082 ± 0.17 | 0.084 ± 0.15 | 0.002 (2) | 0.48 |
| Non-calcified plaque burden (mm2) | 1.22 ± 0.59 | 1.15 ± 0.60 | −0.07 (−6) | 0.005 | 1.19 ± 0.41 | 1.25 ± 0.41 | 0.06 (5) | 0.17 |
| Plaque morphology index | ||||||||
| Fibrous burden (mm2) | 0.99 ± 0.45 | 0.98 ± 0.51 | −0.01 (−1) | 0.71 | 0.98 ± 0.32 | 0.94 ± .31 | −0.04 (−4) | 0.22 |
| Fibro-fatty burden (mm2) | 0.22 ± 0.19 | 0.10 ± 0.14 | −0.12 (−55) | 0.004 | 0.16 ± 0.15 | 0.22 ± 0.14 | 0.06 (38) | 0.004 |
| Necrotic burden (mm2) | 0.07 ± 0.19 | 0.03 ± 0.09 | −0.04 (−57) | 0.03 | 0.06 ± 0.08 | 0.08 ± 0.22 | 0.02 (33) | 0.27 |
Values are reported as mean ± SD for continuous data. Two-tailed P-values less than 0.05 deemed significant (bold values).
Figure 1.
Change in coronary plaque burden components over one-year by treatment. (A) Percent change in coronary plaque burden components over one-year by treatment. (B) Change in non-calcified plaque burden over one-year by treatment.
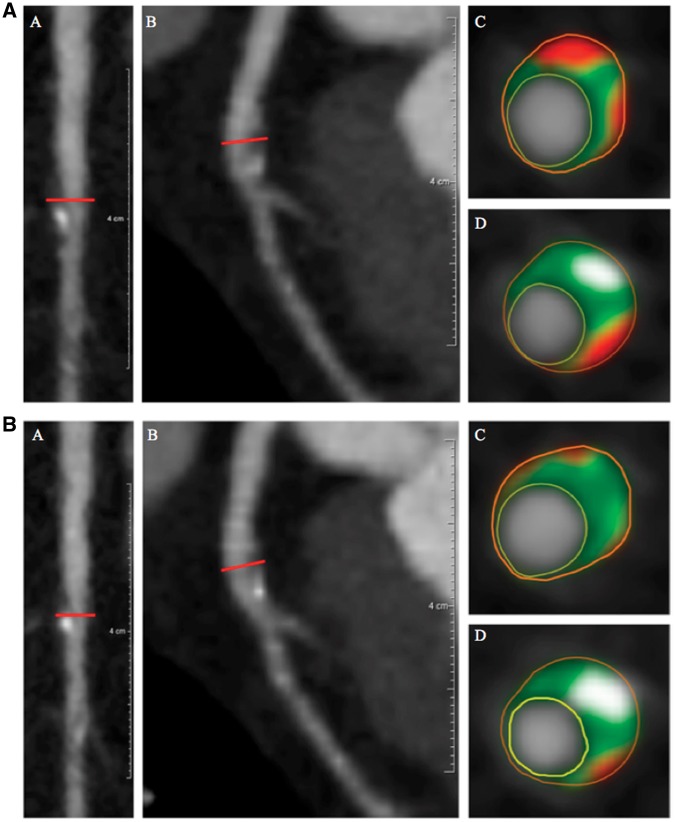
Figure 2.
Left anterior descending artery plaque identified before (2A) and after (2B) biologic therapy. (A) (a) Longitudinal planar and (b) curved planar reformat. (c and d) Representative cross-sectional views with colour overlay for plaque subcomponents. Lumen is encircled in yellow, vessel wall in orange with subcomponents in between, including fibrous (dark green), fibro-fatty (light green), necrotic (red), and dense-calcified (white). Non-calcified plaque burden = 1.03 mm2 and total atheroma volume = 99.2 mm3. (B) (a) Longitudinal planar and (b) curved planar reformat. (c and d) Representative cross-sectional views with colour overlay for plaque subcomponents. Lumen is encircled in yellow, vessel wall in orange with subcomponents in between, including fibrous (dark green), fibro-fatty (light green), necrotic (red), and dense-calcified (white). Non-calcified plaque burden = 0.85 mm2 and total atheroma volume = 80.6 mm3.
When comparing change in plaque characteristics between groups over one-year, the decrease in non-calcified plaque burden in the biologic treated group was significant compared with non-biologic treated (Δ, −0.07 mm2 vs. 0.06 mm2; P = 0.03) and associated with biologic therapy even after adjustment for traditional cardiovascular risk factors, including Framingham risk score, body mass index, and statin use (β = 0.20, P = 0.02).
We performed an exploratory analysis in the biologic treated group by stratifying by treatment agents (anti-TNF, anti-IL12/23, and anti-IL17). There were no significant differences in demographic characteristics, in baseline medication use, or laboratory values between the three treatment groups (Supplementary material online, Table S1). After one-year of therapy, an improvement in hsCRP was observed in the anti-IL12/23 and anti-IL17 treated groups (P = 0.02 and P = 0.01; respectively). An improvement in HDL cholesterol was observed only in the anti-IL17 treated patients [mean (SD), 54.2 (19.9) vs. 61.2 (28.6); P = 0.03]. At baseline, there were no differences in coronary characteristics between the three groups. After one-year of therapy, we observed the following: a 5% reduction in non-calcified plaque burden on anti-TNF therapy (P = 0.06), a 2% reduction on anti-IL12/23 therapy (P = 0.36), and a 12% reduction on anti-IL17 (P = <0.001) (Table 4, Supplementary material online, Table S2). The reduction in coronary plaque burden observed on anti-IL17 therapy was significantly greater than that observed on anti-IL12/23 and no biologic treatment. Reduction in non-calcified coronary plaque burden on anti-TNF therapy was only significant when compared with non-biologic treated patients (P < 0.01).
Table 4.
Change in non-calcified coronary plaque burden over one-year between treatment groups
| Treatments | Change over one-year (mm2) (%) | P-value | |
|---|---|---|---|
| Anti-TNF therapy (n = 48) | −0.06 (−5) | – | |
| vs. Anti-IL12/23 | – | −0.02 (−2) | 0.27 |
| vs. Anti-IL17 | – | −0.15 (−12) | 0.08 |
| vs. NBT | – | 0.06 (5) | 0.009 |
| Anti-IL12/23 therapy (n = 19) | −0.02 (−2) | – | |
| vs. Anti-IL17 | – | −0.15 (−12) | 0.01 |
| vs. NBT | – | 0.06 (5) | 0.09 |
| Anti-IL17 therapy (n = 22) | −0.15 (−12) | – | |
| vs. NBT | – | 0.06 (5) | 0.005 |
Values are reported as Mean (% change) for continuous data. Two-tailed P-values less than 0.05 significant (bold values).
IL, interleukin; NBT, non-biologic treated.
Finally, we explored the modulation of inflammatory blood markers (IFN-γ, TNF-α, IL-6, IL1-β) with treatment of skin disease. PASI score was associated with IFN-γ (β = 0.12; P = 0.003), TNF-alpha (β = 0.08; P = 0.05), and IL-6 (β = 0.10; P = 0.02) at baseline. After one-year of biologic therapy, there were significant reductions in interferon-γ [median (IQR), 10.7 (5.6–28.0) vs. 10.2 (5.2–20.3); P = 0.02], TNF-α [median (IQR), 1.7 (1.0–3.5) vs. 1.3 (0.6–2.5); P = 0.04], and interleukin-6 [median (IQR), 1.4 (0.9–3.0) vs. 1.2 (0.7–2.1); P = 0.01]. These findings were not observed in the non-biologic treated group (Supplementary material online, Table S3).
4. Discussion
Herein, this observational study demonstrated favourable modulation in coronary artery plaque disease indices by CCTA in a consecutive sample of severe psoriasis patients treated with commonly used biological classes of drugs: anti-TNF, anti-IL12/23, and anti-IL17, compared with those not treated with biologic therapy. Despite not knowing the specific progression rate of subclinical atherosclerosis on CCTA in psoriasis, we used those with similar disease patterns choosing to receive topical or light therapy only as our reference. In this referent group, we observed progression of coronary artery disease with conversion of fibrous burden to fibro-fatty burden, suggestive of lipid infiltration within the coronary plaque. In those treated with biological therapy, we found that inflammatory driven phenotypes including lipid-rich plaque and the necrotic core decreased following therapy. Taken together, these data provide preliminary evidence that treatment with biologic modulates coronary artery plaque in psoriasis.
Inflammation is causal in atherosclerosis and still accounts for approximately 20–30% of residual risk for cardiovascular events in the population.13 Those with inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, and psoriasis have a disproportional rate of cardiovascular events compared with age and gender matched counterparts. Therefore, these populations serve as a unique vehicle to understand inflammatory atherogenesis. We recently showed that this increase in MI in psoriasis might be due to early, increased coronary artery disease that is equivocal to individuals who are on average 10 years older with diagnosed hyperlipidaemia.11 Whether these burdens modulate with skin disease treatment has been the topic of intense investigation. Furthermore, when a sample of psoriasis patients was followed for one year after any treatment, a 6% decrease in aortic vascular inflammation was observed6; however, the coronary arteries were not analysed in that study.
CCTA has long been utilized for characterization of coronary plaque burden beyond X-ray angiography and has been extensively compared with and validated against intravascular ultrasound.14 CCTA provides characterization of not only lumen stenosis and arterial remodelling, but also plaque subcomponents, including calcified, non-calcified, and high-risk features.15 Studies have shown that there is an increase in non-calcified plaque volumes in acute coronary syndrome patients,16 obese diabetics,9 and also undergoes modulation in response to statin therapy.17,18 Recently, when CCTA plaque features are accounted for, patients with widespread non-obstructive coronary artery disease had similar event rates when compared with patients with localized obstructive disease,19 suggesting that plaque characteristics are important in defining accurate cardiovascular risk beyond obstructive stenosis.
The use of biologic therapy for psoriasis has rapidly increased over the past decade given its remarkable success in early clearance of psoriatic plaque. First, we observed reduction in non-calcified plaque across all three classes of biologic agents in the study with varying degrees, suggesting that clearance of psoriasis itself is important in the context of vascular disease. Anti-TNF therapy is commonly accepted as the first line biologic agent for the management of psoriasis; however, some of the patients on this treatment do not have adequate response.20 In psoriasis, TNF inhibitors have also been linked to worsening of cardiometabolic risk factors, including weight gain and a shift in apolipoprotein B,21 and recently were shown to not reduce vascular inflammation.22 In that study, however, important inflammatory biomarkers, including TNF-α, interleukin-6, hsCRP, and glycoprotein acetylation, all decreased following anti-TNF therapy. Observational studies have shown that biological therapy, more specifically TNF inhibitors, reduces MI,23 suggesting that longer term observed benefit may relate to coronary plaque modulation over time. The cardiovascular effects of newer, cytokine specific biologic agents have yet to be extensively studied in psoriasis. In a meta-analysis studying the association of major adverse cardiovascular events with the use of anti-IL12/23 agents, the potential of these agents to further increase cardiovascular morbidity could not be excluded.24,25 However, previous literature in animal studies has been conflicting regarding whether IL17 is pro- vs. anti-atherogenic.26 While some studies have suggested the pro-atherogenic effects of IL17,27,28 a recent athero-protective pathway has been proposed through regulation of IFN-γ producing Th1 cells.29 In our present study, we observed the greatest percent reduction of non-calcified plaque burden in patients on anti-IL17 therapy with a reduction in necrotic core suggesting a potential role for IL17 in atherosclerotic pathways. It has been implicated in literature that IL17 is a central mediator in lipoprotein entrapment, leading to vascular stiffness and promoting early atherosclerosis.30 Mouse studies have also suggested that IL17 increases monocyte adhesion to the vascular walls, promoting inflammatory cytokine production and endothelial dysfunction,31 with this phenomenon being normalized by IL17A blockade. Moreover, the potential role of IL17 in linking skin disease and vascular disease in psoriasis was expanded on in another mice study whereby clearance of psoriatic skin manifestations by IL17 blockade was shown to diminish peripheral oxidative stress and vascular dysfunction.32 Taken together, these data provide evidence to support further investigation of IL17 blockade on coronary disease in humans.
Our study does have important limitations. This was an observational study, and therefore, is subjected to potential for confounders compared with a randomized clinical trial. Moreover, the use of biologic agents was open-label, non-randomized, in a small sample and with a short duration of follow-up. However, this is the largest consecutive sample of psoriasis patients followed over time using CCTA. Furthermore, the biologic treated groups had variability in baseline coronary parameters due to a small sample size. Finally, we have not studied hard, cardiovascular events, but instead used coronary artery plaque indices by CCTA to understand modulation on cardiovascular disease risk.
5. Conclusions
In conclusion, we demonstrate that treatment of psoriasis with biologic therapy is associated with a reduction of non-calcified coronary plaque and improvement in plaque morphology compared with those not treated with biologic therapy. These findings highlight the potential role of quelling residual inflammation in cardiovascular disease and risk reduction. These findings support the conduct of larger randomized trials of biologic therapy on cardiovascular disease in psoriasis and potentially other inflammatory diseases.
Authors’ contributions
Integrity of the data: Y.A.E. and N.N.M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Y.A.E. and N.N.M. conceived the study concept and the study design was by N.N.M. Acquisition, analysis, or interpretation of data: J.R., Y.A.E., M.Y.C. and N.N.M. acquired and analysed the data. Drafting of the manuscript: Y.A.E. drafted the manuscript. Critical revision of the manuscript for important intellectual content: All co-authors provided critical revisions of the manuscript. Statistical analysis: Y.A.E. and A.K.D. performed analyses. Administrative, technical, or material support: N.N.M. provided technical guidance to Y.A.E. and A.K.D. during the study. Study supervision: The study was conducted under the supervision of N.N.M.
Acknowledgements
We would like to acknowledge and thank the NIH Clinical Center outpatient clinic-7 nurses for their invaluable contribution to the process of patient recruitment.
Conflict of interest: N.N.M. is a full-time US Government Employee and receives research grants to the NHLBI from AbbVie, Janssen, Celgene and Novartis. J.M.G. in the past 12 months has served as a consultant for Coherus (DSMB), Dermira, Janssen Biologics, Merck (DSMB), Novartis Corp, Regeneron, Dr. Reddy’s labs, Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly and Abbvie. J.M.G. is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. All other authors declared no conflict of interest.
Funding
This work was supported by the National Heart, Lung and Blood Institute (NHLBI) Intramural Research Program (HL006193-02). This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
Supplementary Material
Footnotes
Time for primary review: 18 days
References
- 1. Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E.. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1998;98:839–844. [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–369. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 4. Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, Troxel AB.. The risk of stroke in patients with psoriasis. J Invest Dermatol 2009;129:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mansouri B, Kivelevitch D, Natarajan B, Joshi AA, Ryan C, Benjegerdes K, Schussler JM, Rader DJ, Reilly MP, Menter A, Mehta NN.. Comparison of coronary artery calcium scores between patients with psoriasis and type 2 diabetes. JAMA Dermatol 2016;152:1244–1253. [DOI] [PubMed] [Google Scholar]
- 6. Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, Teague HL, Harrington CL, Rivers JP, Chung JH, Kabbany MT, Natarajan B, Silverman JI, Ng Q, Sanda GE, Sorokin AV, Baumer Y, Gerson E, Prussick RB, Ehrlich A, Green LJ, Lockshin BN, Ahlman MA, Playford MP, Gelfand JM, Mehta NN.. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrington CL, Dey AK, Yunus R, Joshi AA, Mehta NN.. Psoriasis as a human model of disease to study inflammatory atherogenesis. Am J Physiol Heart Circ Physiol 2017;312:H867–H873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG.. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol 2004;50:859–866. [DOI] [PubMed] [Google Scholar]
- 9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP.. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 10. Kwan AC, May HT, Cater G, Sibley CT, Rosen BD, Lima JAC, Rodriguez K, Lappe DL, Muhlestein JB, Anderson JL, Bluemke DA.. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology 2014;272:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lerman JB, Joshi AA, Chaturvedi A, Aberra TM, Dey AK, Rodante JA, Salahuddin T, Chung JH, Rana A, Teague HL, Wu JJ, Playford MP, Lockshin BA, Chen MY, Sandfort V, Bluemke DA, Mehta NN.. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation 2017;136:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salahuddin T, Natarajan B, Playford MP, Joshi AA, Teague H, Masmoudi Y, Selwaness M, Chen MY, Bluemke DA, Mehta NN.. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J 2015;36:2662–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrington RA. Targeting inflammation in coronary artery disease. N Engl J Med 2017;377:1197–1198. [DOI] [PubMed] [Google Scholar]
- 14. Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC.. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 2013;7:256–266. [DOI] [PubMed] [Google Scholar]
- 15. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J.. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015;66:337–346. [DOI] [PubMed] [Google Scholar]
- 16. Dey D, Achenbach S, Schuhbaeck A, Pflederer T, Nakazato R, Slomka PJ, Berman DS, Marwan M.. Comparison of quantitative atherosclerotic plaque burden from coronary CT angiography in patients with first acute coronary syndrome and stable coronary artery disease. J Cardiovasc Comput Tomogr 2014;8:368–374. [DOI] [PubMed] [Google Scholar]
- 17. Sandfort V, Lima JA, Bluemke DA.. Noninvasive imaging of atherosclerotic plaque progression: status of coronary computed tomography angiography. Circ Cardiovasc Imaging 2015;8:e003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK.. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, Nasir K, Gowdak LHW, Hainer J, Brady TJ, Di Carli MF, Hoffmann U, Abbara S, Blankstein R.. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–291. [DOI] [PubMed] [Google Scholar]
- 20. Boehncke WH, Menter A.. Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol 2013;14:377–388. [DOI] [PubMed] [Google Scholar]
- 21. Sattar N, Crompton P, Cherry L, Kane D, Lowe G, McInnes IB.. Effects of tumor necrosis factor blockade on cardiovascular risk factors in psoriatic arthritis: a double-blind, placebo-controlled study. Arthritis Rheum 2007;56:831–839. [DOI] [PubMed] [Google Scholar]
- 22. Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, Fuxench ZC, Harrington CL, Hubbard RA, Kalb RE, Menter A, Rader DJ, Reilly MP, Simpson EL, Takeshita J, Torigian DA, Werner TJ, Troxel AB, Tyring SK, Vanderbeek SB, Van Voorhees AS, Playford MP, Ahlman MA, Alavi A, Jm G.. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging 2018;11:e007394.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu JJ, Poon KY, Channual JC, Shen AY.. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol 2012;148:1244–1250. [DOI] [PubMed] [Google Scholar]
- 24. Tzellos T, Kyrgidis A, Trigoni A, Zouboulis CC.. Association of ustekinumab and briakinumab with major adverse cardiovascular events: an appraisal of meta-analyses and industry sponsored pooled analyses to date. Dermatoendocrinol 2012;4:320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reich K, Langley RG, Lebwohl M, Szapary P, Guzzo C, Yeilding N, Li S, Hsu MC, Griffiths CE.. Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br J Dermatol 2011;164:862–872. [DOI] [PubMed] [Google Scholar]
- 26. Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res 2012;110:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ.. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol 2009;183:8167–8175. [DOI] [PubMed] [Google Scholar]
- 28. Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG.. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol 2011;31:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T.. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2012;32:273–280. [DOI] [PubMed] [Google Scholar]
- 30. Huang LH, Zinselmeyer BH, Chang CH, Saunders BT, Elvington A, Baba O, Broekelmann TJ, Qi L, Rueve JS, Swartz MA, Kim BS, Mecham RP, Wiig H, Thomas MJ, Sorci-Thomas MG, Randolph GJ.. Interleukin-17 drives interstitial entrapment of tissue lipoproteins in experimental psoriasis. Cell Metabol 2018;doi:10.1016/j.cmet.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nordlohne J, Helmke A, Ge S, Rong S, Chen R, Waisman A, Haller H, von Vietinghoff S.. Aggravated atherosclerosis and vascular inflammation with reduced kidney function depend on interleukin-17 receptor A and are normalized by inhibition of interleukin-17A. JACC Basic Transl Sci 2018;3:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schuler R, Brand A, Klebow S, Wild J, Protasio Veras F, Ullmann E, Roohani S, Kolbinger F, Kossmann S, Wohn C, Daiber A, Munzel T, Wenzel P, Waisman A, Clausen BE, Karbach S.. Antagonization of IL-17A attenuates skin inflammation and vascular dysfunction in mouse models of psoriasis. J Invest Dermatol 2018;doi:10.1016/j.jid.2018.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.