Abstract
Aims
Renal inflammation, leading to fibrosis and impaired function is a major contributor to the development of hypertension. The NLRP3 inflammasome mediates inflammation in several chronic diseases by processing the cytokines pro-interleukin (IL)-1β and pro-IL-18. In this study, we investigated whether MCC950, a recently-identified inhibitor of NLRP3 activity, reduces blood pressure (BP), renal inflammation, fibrosis and dysfunction in mice with established hypertension.
Methods and results
C57BL6/J mice were made hypertensive by uninephrectomy and treatment with deoxycorticosterone acetate (2.4 mg/day, s.c.) and 0.9% NaCl in the drinking water (1K/DOCA/salt). Normotensive controls were uninephrectomized and received normal drinking water. Ten days later, mice were treated with MCC950 (10 mg/kg/day, s.c.) or vehicle (saline, s.c.) for up to 25 days. BP was monitored by tail-cuff or radiotelemetry; renal function by biochemical analysis of 24-h urine collections; and kidney inflammation/pathology was assessed by real-time PCR for inflammatory gene expression, flow cytometry for leucocyte influx, and Picrosirius red histology for collagen. Over the 10 days post-surgery, 1K/DOCA/salt-treated mice became hypertensive, developed impaired renal function, and displayed elevated renal levels of inflammatory markers, collagen and immune cells. MCC950 treatment from day 10 attenuated 1K/DOCA/salt-induced increases in renal expression of inflammasome subunits (NLRP3, ASC, pro-caspase-1) and inflammatory/injury markers (pro-IL-18, pro-IL-1β, IL-17A, TNF-α, osteopontin, ICAM-1, VCAM-1, CCL2, vimentin), each by 25–40%. MCC950 reduced interstitial collagen and accumulation of certain leucocyte subsets in kidneys of 1K/DOCA/salt-treated mice, including CD206+ (M2-like) macrophages and interferon-gamma-producing T cells. Finally, MCC950 partially reversed 1K/DOCA/salt-induced elevations in BP, urine output, osmolality, [Na+], and albuminuria (each by 20–25%). None of the above parameters were altered by MCC950 in normotensive mice.
Conclusion
MCC950 was effective at reducing BP and limiting renal inflammation, fibrosis and dysfunction in mice with established hypertension. This study provides proof-of-concept that pharmacological inhibition of the NLRP3 inflammasome is a viable anti-hypertensive strategy.
Keywords: Hypertension , NLRP3 inflammasome , MCC950 , Renal inflammation , Renal fibrosis
1. Introduction
Through baroreceptor-mediated detection of changes in blood pressure (BP), and subsequent regulation of Na+/H2O re-uptake, the kidneys play a major role in BP homeostasis.1,2 However, chronic exposure to excessive salt, as occurs with a western diet, impairs the ability of the kidneys to maintain the pressure-natriuresis relationship, resulting in hypertension.3,4 Although there is debate around the precise mechanisms by which high salt promotes renal dysfunction, there is a growing body of evidence to suggest that inflammation is an important factor. In experimental models, salt-sensitive hypertension is associated with increased renal expression of pro-inflammatory molecules including cytokines, chemokines, and adhesion molecules.5–7 This leads to the accumulation of macrophages and T cells in the tubulointerstitium, which in turn promote tissue damage, fibrosis and dysregulation of Na+ transport.5–8 Therefore, further understanding of the mechanisms that cause the immune system to become activated during salt-sensitive hypertension may lead to novel. pharmacological treatment options and better management of the condition in the clinic
Inflammasomes are cytosolic signalling complexes that sense danger signals emanating from pathogens or damaged host cells and then respond by initiating an inflammatory cascade.7,9 Of the inflammasomes identified to date, the NLRP3 inflammasome is the best characterized, consisting of a pattern recognition receptor, NLRP3; an adaptor protein, ASC; and the effector molecule, caspase-1.7,9 The NLRP3 inflammasome recognizes a diverse range of pathogen- and host-derived danger-associated molecular patterns (PAMPs and DAMPs, respectively) including bacterial lipopolysaccharides, reactive oxygen species, microcrystals, and high concentrations of salt.10,11 Following detection of PAMPs or DAMPs, the subunits of the NLRP3 inflammasome oligomerize, resulting in auto-cleavage, and activation of caspase-1. Caspase-1 then proteolytically cleaves pro-interleukin (IL)-1β and pro-IL-18 into their active forms, which are released from the cell of origin and target neighbouring cells to propagate an inflammatory response.9
Inflammasomes are well established as crucial mediators of inflammation in several chronic inflammatory diseases such as rheumatoid arthritis,12 gout,13 Alzheimer’s Disease,14 and atherosclerosis.15 More recently, studies by our group and others using a variety of experimental models have shown that the development of hypertension and its associated renal inflammation is at least partially dependent on the presence of a functional NLRP3 inflammasome complex.7,16,17 This implies that inflammasomes could represent a novel target for therapies to ameliorate renal dysfunction and BP in hypertension.
MCC950 is a diarylsulfonylurea-containing compound that was shown in vitro to potently (i.e. at nanomolar concentrations) and selectively inhibit the oligomerization and activation of the NLRP3 inflammasome in response to canonical and non-canonical stimuli.18 The precise molecular mechanism by which MCC950 inhibits inflammasome assembly is unclear but it does not appear to involve blocking potassium efflux from the cell, inhibiting calcium signalling, or directly interfering with NLRP3-NLRP3 or NLRP3-ASC protein–protein interactions.18 MCC950 is also effective at inhibiting inflammasome activation in vivo. Coll et al.18 showed that treatment with MCC950 had protective effects in two experimental models of NLRP3-associated diseases; experimental autoimmune encephalitis and Muckle–Wells syndrome. Moreover, in a pilot study, we provided preliminary evidence that MCC950 was effective at lowering tail cuff BP and a limited selection of pro-inflammatory cytokines in the kidneys of mice with one-kidney, deoxycorticosterone acetate and salt (1K/DOCA/salt)-induced hypertension.7 Thus, in this study, we sought to extend these findings and gain insight into the physiological processes underlying the anti-hypertensive effects of MCC950 by comprehensively examining its effects on multiple haemodynamic parameters (e.g. mean arterial pressure, diastolic BP, heart rate), immune cell activation, renal pathology and function.
2. Methods
Refer to Supplementary material online for an expanded Methods.
2.1 Animals
Male C57BL/6J mice, aged 10–12 weeks and weighing 25–30 g were used. All animal procedures were performed with institutional ethics approval (Ethics number: MARP/2013/043) in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (8th Edition, 2013).
2.2 Induction of hypertension and treatment of mice with MCC950
A salt-sensitive model of hypertension was used in this study wherein mice were uninephrectomized (1K) and treated with deoxycorticosterone acetate (DOCA; 2.4 mg/day, s.c.; Innovative Research of America, USA) and 0.9% saline (p.o.).7 Normotensive control mice were uninephrectomized and maintained on normal drinking water post-surgery (1K/placebo). Ten days after induction of hypertension, mice were randomly assigned to commence treatment for up to 28 days with either MCC950 (10 mg/kg/day, s.c.) or vehicle (0.9% saline, 0.5 µL/h, s.c.) via implantation of micro-osmotic minipumps (Alzet, USA). Note, in pilot studies (n = 3–8 per group), mice with established 1K/DOCA/salt-induced hypertension were treated for 11 days with three different doses of MCC950 (2, 5, and 10 mg/kg/day) to establish an optimal antihypertensive dose. The 10 mg/kg/day dose, which is equivalent to that used in previous studies to inhibit NLRP3-mediated processes in mice,19,20 caused a steady state blood concentration of MCC950 of 6.3 ± 0.3 μM (n = 8) and was the only dose effective at reducing BP (data not shown). Moreover, there were no apparent signs of toxicity, which was later confirmed via a Kaplan–Meier comparison of survival of the MCC950- vs. vehicle-treated mice included in the main study (see Supplementary material online, Figure S2).
2.3 Monitoring of blood pressure
BP was monitored either via tail-cuff plethysmography or radiotelemetry.7,21
2.4 Gene expression in the kidney
Following treatment, mice were killed via CO2 asphyxiation and the right kidney was excised and cut in half along the coronal plane. One half of the kidney was used immediately for flow cytometric analysis, while the other half was either snap-frozen in liquid N2 and stored at −80°C for RNA extraction or fixed in 10% formalin and stored at −20°C for immunohistochemistry. Upon removal from −80°C storage, kidney halves were pulverized and RNA extracted using the RNeasy Mini Kit (Qiagen, Germany). RNA was reverse transcribed to cDNA (Applied Biosystems, USA) and used as a template in real-time PCR with commercially-available predesigned TaqMan® primer/probe sets (Life Technologies, USA). GAPDH was used as a house-keeping gene.7 Real-time PCR was performed in a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, USA) and the comparative Ct method was used to calculate the fold-change in mRNA expression relative to the control samples i.e. 1K/placebo + vehicle-treated mice.22
2.5 Flow cytometry
Flow cytometry was performed on cell suspensions derived from freshly isolated kidney halves that had undergone a combination of manual and enzymatic digestion, and Percoll gradient centrifugation to isolate mononuclear cells. Cells were stained for 15 min at 4°C with Live/Dead Aqua Stain (Life Technologies, USA), followed by the antibody cocktail listed in Supplementary material online, Table S1. Cells were then re-suspended in flow cytometry buffer (1% bovine serum albumin in PBS) containing 1% formalin. For detection of interferon-gamma (IFN-γ)-producing T cells, kidney mononuclear cells were stimulated with phorbol myristate acetate (PMA; 50 ng/mL) and ionomycin (20 ng/mL) for 5 h in the presence of Golgiplug/Golgistop (BD Biosciences, USA). Cells were then stained for surface markers as described above, before being fixed, permeabilized, and incubated with an anti-IFN-γ antibody at room temperature for 20 min. All samples were analysed using a Fortessa X-20 instrument controlled by FlowDiva software (BD Biosciences). Data were analysed using FlowJo software v10 (FlowJo, USA; see Supplementary material online for the gating strategy).
2.6 Histopathology staining
Fixed, paraffin-embedded kidney sections (4 or 10 μm) were incubated with Celestine blue to stain for nuclei, and counterstained with haematoxylin (Amber Scientific, Australia) and either 0.3% Picrosirius red solution (Polysciences Inc., USA) or eosin (Amber Scientific, Australia). Sections were imaged using a bright-field (Leica Biosystems, Germany) or polarized microscope (Olympus, Japan) and analysed for percentage collagen content by ImageJ. Changes in renal tubular structure were assessed using a four-point scoring system as follows: 0 = no damage; 1 = mild damage (<25% tubules affected); 2 = moderate damage (25–50% of tubules affected); and 3 = severe damage (>50% of tubules affected). Quantification/scoring of Picrosirius red staining and renal histopathology parameters was performed by investigators who were blinded to the in vivo treatment corresponding to each sample.
2.7 Assessment of kidney function using metabolic cages
Mice were housed individually in metabolic cages for 24 h intervals on three separate occasions: day −1 to obtain baseline parameters; day 9 to assess the impact of 1K/DOCA/salt treatment on kidney function; and day 20 to assess the impact of MCC950 vs. vehicle treatment. On each occasion the volume of water/saline intake and urine output was measured, as was urine osmolality (Advanced Osmometer 2020; Advanced Instruments, USA), Na+ concentration (RAPIDChem744, Siemens, Germany) and albuminuria (Albuwell M, Exocell, USA).
2.8 Statistical analysis
Unless otherwise stated, results are expressed as mean ± standard error of mean (SEM). Data were analysed either by Student’s unpaired t-test or by one- or two-way analysis of variance (ANOVA). Post hoc analyses included Newman–Keul’s tests (for parametric data) or Kruskal–Wallis tests (for non-parametric data). P < 0.05 was considered to be statistically significant.
3. Results
3.1 Intervention with MCC950 reduces BP in mice with established hypertension
Consistent with our previous report,7 1K/DOCA/salt treatment in mice caused a rapid increase in systolic BP (measured by tail cuff) which reached a plateau of approximately 30–35 mmHg above baseline within 10 days post-surgery (Supplementary material online, Figure S3A). In mice that were subsequently treated with the vehicle for MCC950, systolic BP continued to rise gradually over the following 11 days (Supplementary material online, Figure S3A). In contrast, in mice that received 10 mg/kg/day of MCC950, systolic BP gradually decreased such that after 11 days, BP was ∼20 mmHg lower in MCC950- than in vehicle-treated animals (Supplementary material online, Figure S3A). A common consequence of high BP is cardiac hypertrophy.23 1K/DOCA/salt-induced hypertension was found to increase the ratio of heart weight to body weight compared to normotensive mice (Supplementary material online, Figure S3B). Importantly, hypertensive mice treated with MCC950 displayed blunted cardiac hypertrophy (Supplementary material online, Figure S3B). In normotensive animals, systolic BP remained unchanged during the first 10 days after surgery, and both systolic BP and heart weight to body weight ratio were not further altered by MCC950 (Supplementary material online, Figure S3A and B).
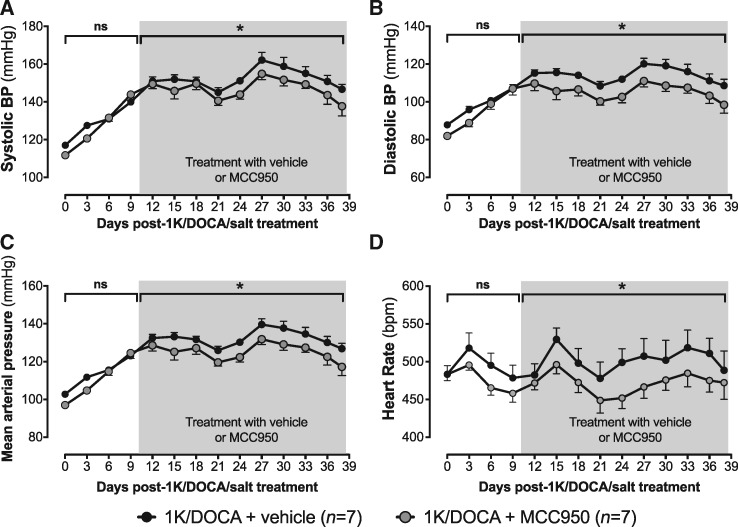
Radiotelemetry was performed in a subset of mice to further characterize the effects of MCC950 on haemodynamic parameters during 1K/DOCA/salt-dependent hypertension. Similar to that observed using tail cuff plethysmography, 1K/DOCA/salt treatment caused a 30–40 mmHg increase in systolic BP over the first 10 days (Figure 1A, Supplementary material online, Figure S4A). Similar increases were observed for both diastolic BP and MAP (Figure 1B and C, Supplementary material online, Figure S4B and C). Also consistent with findings from tail cuff studies, the MCC950 intervention afforded protection against 1K/DOCA/salt-induced hypertension, such that by the end of the 28 day treatment period, all three BP parameters (i.e. systolic BP, diastolic BP and MAP) were approximately 10–12 mmHg lower in MCC950- vs. vehicle-treated mice (Figure 1A–C). Heart rate was not significantly different between the MCC950- and vehicle-treated groups, before or after the drug intervention period (Figure 1D, Supplementary material online, Figure S4D).
Figure 1.
MCC950 reduces BP in mice with 1K/DOCA/salt-induced hypertension. Effects of 1K/DOCA/salt and MCC950 on systolic BP (A), diastolic BP (B), mean arterial pressure (C), and heart rate (D) measured by radiotelemetry at 10 min-intervals and plotted as an average over a 3-day period. All values are expressed as mean ± SEM. *P < 0.05; ns, not significant for one-way ANOVA or two-way repeated-measures ANOVA followed by Newman–Keuls post hoc test as appropriate.
3.2 MCC950 reduces expression of inflammatory markers and leucocyte infiltration in kidneys of 1K/DOCA/salt-treated mice
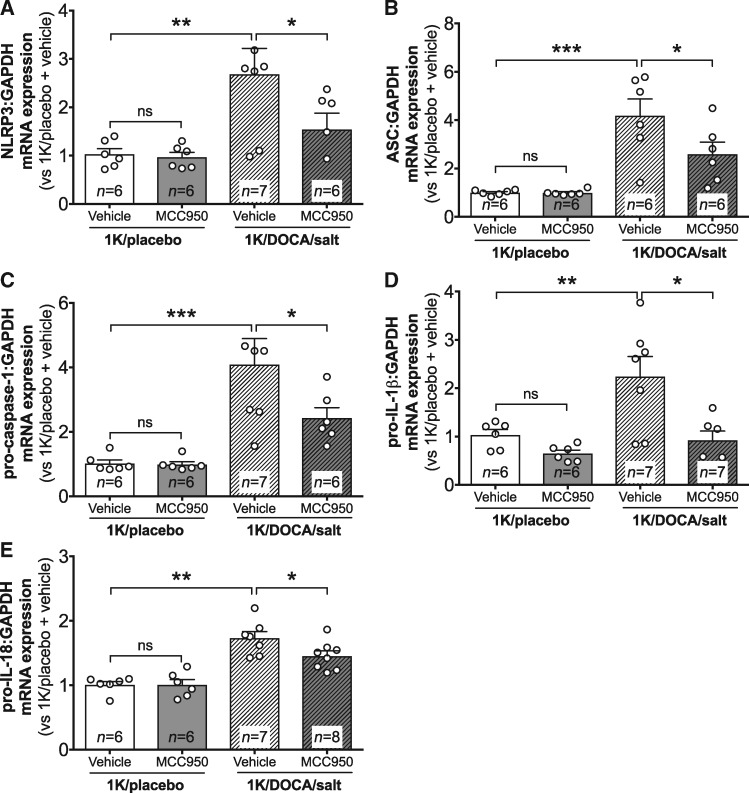
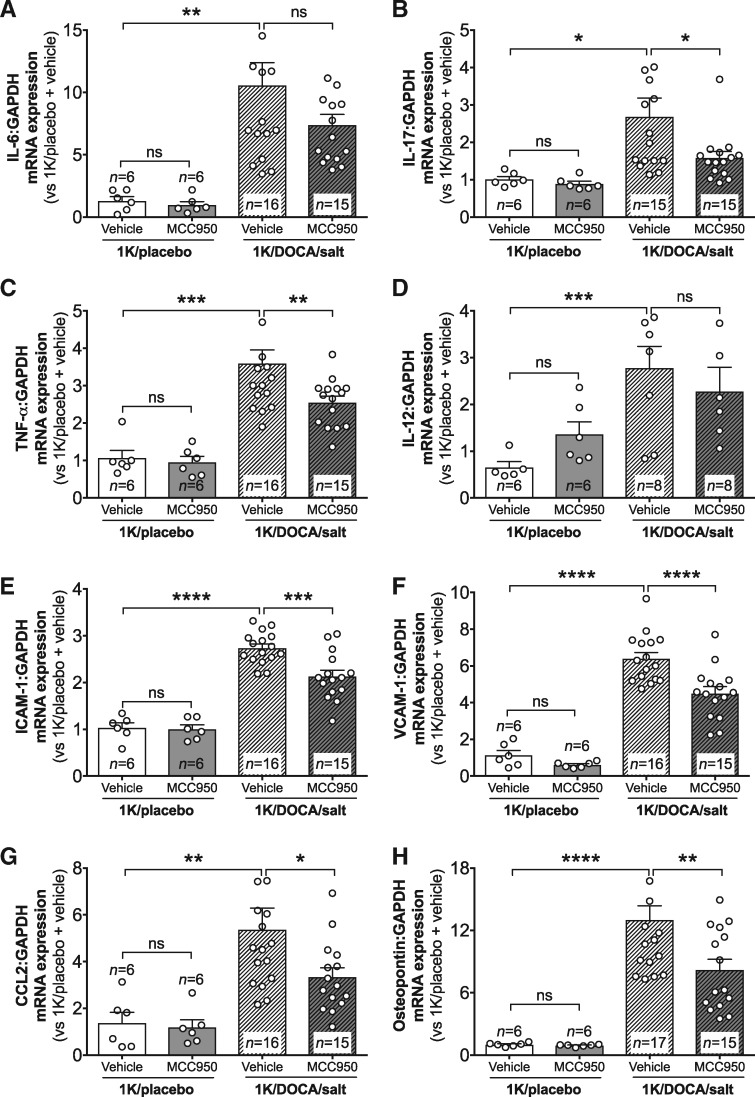
Real-time PCR revealed that 1K/DOCA/salt-induced hypertension was associated with increased renal mRNA expression of NLRP3, ASC, pro-caspase-1, pro-IL-1β, and pro-IL-18 (Figure 2A–E), confirming that this model of hypertension is associated with priming of the inflammasome/IL-1β/IL-18 signalling system in the kidneys. Likewise, increases in expression of several additional pro-inflammatory genes were also observed including the cytokines IL-6, IL-17A, tumour necrosis factor-alpha, and IL-12 (Figure 3A–D); the adhesion molecules, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) (Figure 3E and F); the chemokine, CC-motif chemokine ligand 2 (CCL2; Figure 3G); and a marker of tubular damage, osteopontin (Figure 3H). Treatment of hypertensive mice with MCC950 reduced the expression of most of these genes by 25–40% (Figures 2 and 3), with the exceptions being IL-6 (for which the trend towards a 30% reduction was not statistically significant) and IL-12. Notably, MCC950 had no effect on basal levels of expression of any of these genes in normotensive mice (Figures 2 and 3).
Figure 2.
MCC950 reduces inflammasome priming in the kidneys of mice with 1K/DOCA/salt-induced hypertension. Effect of MCC950 on renal mRNA expression of NLRP3 (A), ASC (B), pro-caspase-1 (C), pro-interleukin (IL)-1β (D), and pro-IL-18 (E) in mice treated with either 1K/DOCA/salt or 1K/placebo. Messenger RNA expression was measured using the comparative Ct method against GAPDH expression. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant for one-way ANOVA followed by Newman–Keuls post hoc test.
Figure 3.
MCC950 reduces the expression of renal inflammatory markers in mice with 1K/DOCA/salt-induced hypertension. Effect of MCC950 on renal mRNA expression of IL-6 (A), IL-17A (B), tumour necrosis factor-α (TNF-α; C), IL-12 (D), intercellular adhesion molecule-1 (ICAM-1; E), vascular cell adhesion molecule-1 (VCAM-1; F), chemokine C-C motif ligand 2 (CCL2; G), and osteopontin (H). Messenger RNA expression was measured using the comparative Ct method against GAPDH expression. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant for one-way ANOVA followed by Newman–Keuls post hoc test.
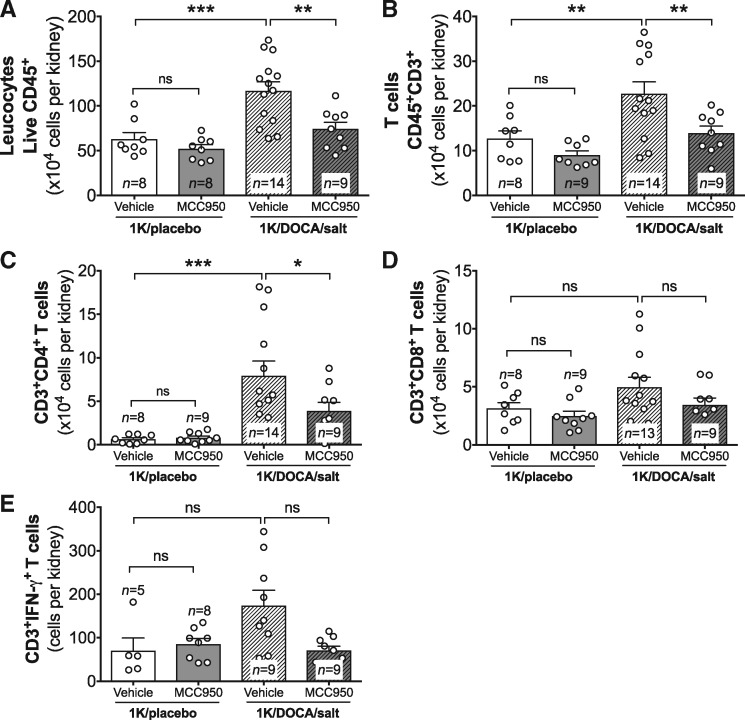
Chemokines and adhesion molecules are important mediators of leucocyte trafficking into tissues. Consistent with its effects on CCL2, ICAM-1, and VCAM-1 expression, 1K/DOCA/salt-treatment caused an accumulation of leucocytes in the kidney (Figure 4A). This included an increase in CD3+ T cells, and in particular the CD4+ subset, with no significant change in CD8+ T cells (Figure 4B and D). Previous studies have shown that IL-1β and IL-18 can act in concert with IL-12 to promote the production of the T cell-derived pro-inflammatory cytokine interferon-gamma (IFN-γ).24 Given that expression of IL-1β, IL-18, and IL-12 were all up-regulated in kidneys of mice with 1K/DOCA/salt-induced hypertension, we investigated whether there might also be an increase in T cell-dependent production of IFN-γ. Although overall expression levels of IFN-γ were not significantly altered in whole kidney homogenates from 1K/DOCA/salt-treated mice (data not shown), there was a three-fold increase in IFN-γ-producing T cells (Figure 4E). Of note, MCC950 markedly inhibited the accumulation of total leucocytes and T cells in the kidneys of 1K/DOCA/salt-treated mice (Figure 4A–D), including those that produced IFN-γ (Figure 4E).
Figure 4.
MCC950 reduces T cell accumulation in the kidneys of mice with 1K/DOCA/salt-induced hypertension. Flow cytometric analysis showing the effect of MCC950 on accumulation of total CD45+ leucocytes (A), total CD3+ T cells (B), CD4+ T cells (C), CD8+ T cells (D), and interferon-gamma+ (IFN-γ+) T cells (E) in the kidneys of mice treated with either 1K/DOCA/salt or 1K/placebo. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant for one-way ANOVA followed by Newman–Keuls post hoc test.
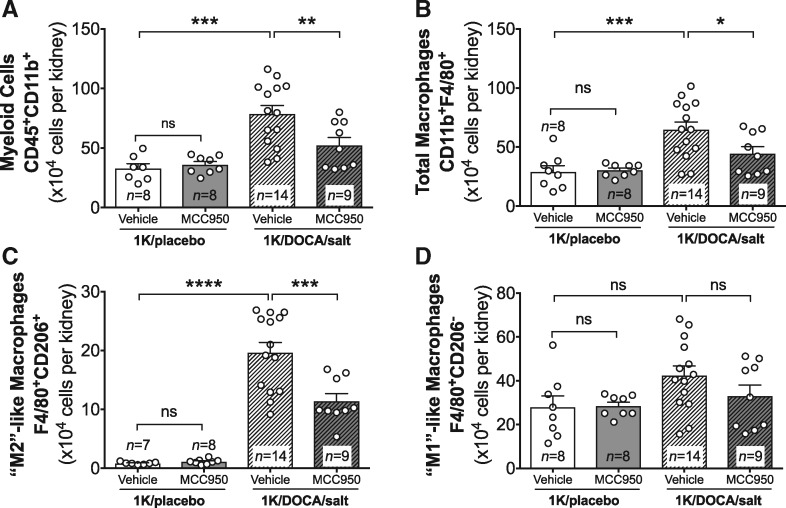
In addition to the accumulation of T cells, 1K/DOCA/salt-induced hypertension in mice was associated with marked increases in numbers of myeloid lineage cells (CD45+CD11b+) and macrophages (CD45+CD11b+F4/80+) in the kidneys, with further analysis of the macrophage subsets revealing that there was a significant increase in the ‘M2’-(F4/80+CD206+) but not the ‘M1’-like (F4/80+CD206−) phenotype (Figure 5A–D). Treatment of 1K/DOCA/salt hypertensive mice with MCC950 mice reduced total myeloid cell and macrophage numbers in the kidneys (Figure 5A and B). More specifically, MCC950 treatment appeared to have its largest effects on the M2-like macrophage population (Figure 5C). Again, MCC950 had no effect on baseline kidney numbers of any of these leucocyte subsets in normotensive mice (Figures 4 and 5).
Figure 5.
MCC950 reduces macrophage accumulation in the kidneys of mice with 1K/DOCA/salt-induced hypertension. Flow cytometric analysis showing the effect of MCC950 on accumulation of total CD45+CD11+ myeloid lineage cells (A), total F4/80+ macrophages (B), CD206+ M2-like macrophages (C), and CD206 M1-like macrophages (D) in the kidneys of mice treated with either 1K/DOCA/salt or 1K/placebo. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant for one-way ANOVA followed by Newman–Keuls post hoc test.
3.3 MCC950 reduces the accumulation of collagen in the kidneys of 1K/DOCA/salt-treated mice
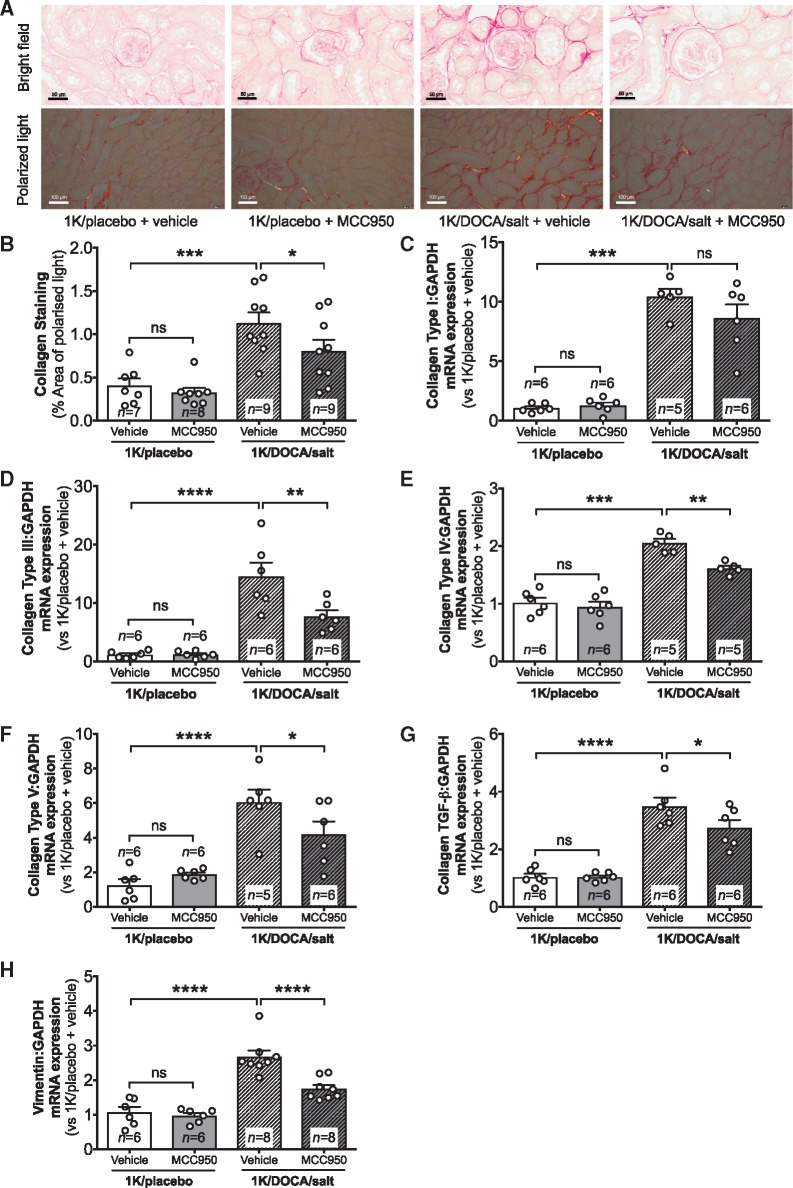
Kidney sections from 1K/DOCA/salt-treated mice displayed an approximately three-fold increase in renal interstitial collagen protein expression compared with normotensive mice, whether assessed by bright field or polarized microscopy (Figure 6A and B). Treatment with MCC950 reduced collagen deposition by approximately 30% in mice with 1K/DOCA/salt-induced hypertension but had no effect on the amount of collagen protein in kidneys of normotensive mice (Figure 6A and B).
Figure 6.
MCC950 reduces renal interstitial fibrosis in mice with 1K/DOCA/salt-induced hypertension. Effect of MCC950 on interstitial collagen deposition (A and B) and mRNA expression levels of the collagen α-subunits Type I (C), Type III (D), Type IV (E), and Type V (F), transforming growth factor-beta (TGF-β; G), and vimentin (VIM; H) in kidneys of mice treated with either 1K/DOCA/salt or 1K/placebo. Representative bright-field picrosirius red stained images are shown at 40× magnification (scale = 50 µm; A). Polarized picrosirius red stained images are shown at 20× magnification (scale = 50 µm). Messenger RNA expression was measured using the comparative Ct method against GAPDH expression. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant for one-way ANOVA followed by Newman–Keuls post hoc test.
The increase in collagen protein in kidneys of 1K/DOCA/salt-treated mice was reflected at the gene level with mRNA expression of four of the predominant renal collagen subtypes (I, III, IV, and V) elevated compared with kidneys from 1K/placebo-treated mice (Figure 6C–F). Renal mRNA expression of the pro-fibrotic cytokine, transforming growth factor-beta (TGF-β), was also significantly increased by 1K/DOCA/salt-treatment, as was that of the marker of epithelial-mesenchymal transition (EMT), vimentin (Figure 6G and H). Treatment with MCC950 reduced mRNA expression of TGF-β and vimentin, along with collagen III, IV, and V (Figure 6C–H).
Analysis of haematoxylin and eosin-stained sections from 1K/DOCA/salt-treated mice also revealed significant damage to the tubular architecture as evidenced by tubular dilatation, tubular atrophy, and a loss of epithelial brush borders on the luminal surface of proximal tubules (Supplementary material online, Figure S5). While there was a trend for MCC950 to reduce at least some of these markers of tubular epithelial damage in 1K/DOCA/salt-treated mice, these effects were not statistically significant (Supplementary material online, Figure S5).
3.4 MCC950 improves kidney function in 1K/DOCA/salt-treated mice
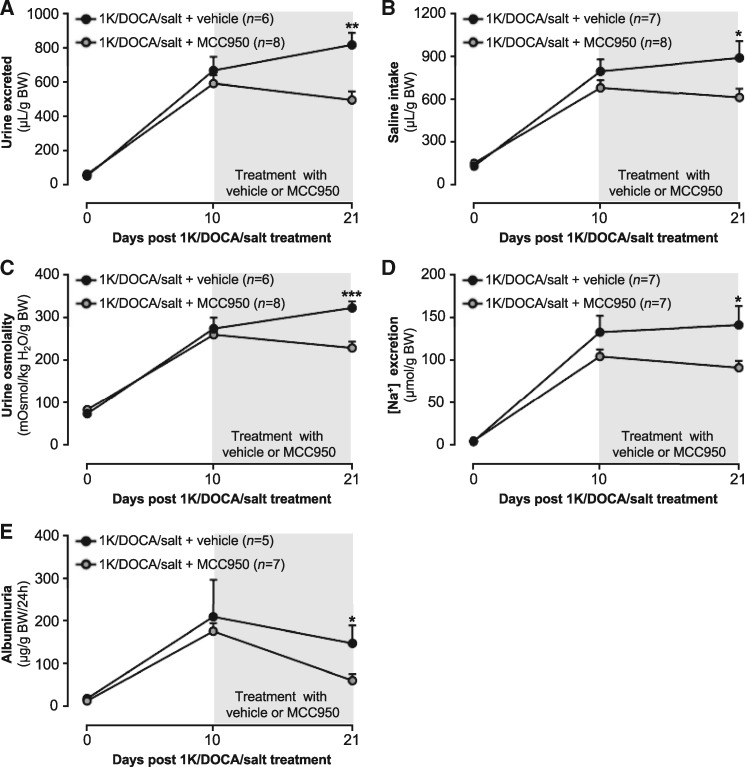
Inflammation and fibrosis of the kidneys are associated with impaired function and a shift in the pressure-natriuresis relationship. Metabolic cage studies were performed to assess the impact of 1K/DOCA/salt-induced hypertension on kidney function, and to determine if treatment with MCC950 protects against functional impairment. Ten days of 1K/DOCA/salt treatment resulted in a marked increase in the volume of urine excreted (11-fold) and in the amount of saline consumed (five-fold;Figure 7A and B). The osmolality of the urine was approximately 3.5 times higher following 10 days of 1K/DOCA/salt-treatment than it was prior to the induction of hypertension, including a 27-fold increase in urinary [Na+] (Figure 7C and D). Excessive amounts of albumin leakage into the urine is a clinically relevant sign of kidney injury and dysfunction, and in mice treated with 1K/DOCA/salt it was found that albuminuria levels were increased by 18-fold (Figure 8E). In hypertensive mice that were subsequently treated with vehicle for a further 11 days, these parameters remained unchanged (Figure 7A–E). In contrast, MCC950-treatment reduced urine volume, saline intake, urine osmolality, urine [Na+], and albuminuria, such that after 11 days all of these parameters were lower than in the vehicle-treated animals (Figure 7A–E).
Figure 7.
MCC950 improves kidney function in mice with 1K/DOCA/salt-induced hypertension. Effect of MCC950 on volume of urine excreted (A), volume of saline consumed (B), urine osmolality (C), urine [Na+] (D), and albuminuria (E) in mice treated with either 1K/DOCA/salt or 1K/placebo. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 for two-way repeated measures ANOVA followed by Newman–Keuls post hoc test.
4. Discussion
The major new findings from this study are that MCC950, a selective small-molecule NLRP3 inflammasome inhibitor, is highly effective at reducing renal inflammation and fibrosis, and improving renal function in mice, even when administered 10 days after the establishment of 1K/DOCA/salt-induced hypertension. Moreover, these protective effects of MCC950 on the kidneys were associated with a modest reduction in BP and blunted cardiac hypertrophy. Hence, together with earlier reports of BP-lowering and renal anti-inflammatory effects of ASC-deficiency and IL-1R antagonism,7,21 this study highlights the NLRP3 inflammasome as a promising target for therapies aimed at reducing BP and the end-organ damage associated with hypertension.
It is well established that hypertension is associated with increased expression of adhesion molecules and pro-inflammatory cytokines, and the accumulation of inflammatory T cells and macrophages in the kidneys.5–7 Moreover, these inflammatory events are thought to contribute to the renal fibrosis and damage that disrupts pressure-natriuresis and re-sets BP at a chronically elevated level.6–8 Using transgenic mouse models, we and others have shown that NLRP3 inflammasome activity is essential for the development of renal inflammation and elevated BP in response to a variety of hypertensive stimuli including 1K/DOCA/salt and angiotensin II.7,17 While these findings implied that the NLRP3 inflammasome is a promising target for future anti-hypertensive therapies, it remained to be determined (in a more clinically relevant context) whether inflammasome inhibition could reverse BP and limit renal inflammation and dysfunction after hypertension is established. Indeed, administration of MCC950 to mice, 10 days after induction of hypertension with 1K/DOCA/salt, profoundly reduced renal inflammasome priming, expression of adhesion molecules, chemokines and pro-inflammatory cytokines, and accumulation of T cells and macrophages. Intervention with MCC950 also suppressed renal interstitial collagen deposition and the pro-fibrotic cytokine, TGF-β. Importantly, these effects culminated in improved renal function, in terms of Na+ and electrolyte handling and less albumin leakage into the urine. Hence, our findings provide proof-of-concept that pharmacological modulation of inflammasome activity may be a viable therapeutic strategy for limiting renal damage, even in patients with existing disease.
Macrophages, in particular those of an M2 phenotype, are an important source of TGF-β and other factors involved in extracellular matrix remodelling25 and can promote fibrosis of other tissues; for example, in the heart after myocardial infarction,26 the lungs after bleomycin treatment,27 and the skin in experimental scleroderma.28 Furthermore, in an alternative model of hypertension (i.e. angiotensin II-dependent) we showed that M2-like macrophages are major contributors to fibrosis in the aortic adventitia.23 Here, we demonstrated that 1K/DOCA/salt-dependent hypertension involves MCC950-sensitive accumulation of M2-like macrophages in the kidneys, consistent with the possibilities that M2 macrophages are drivers of renal fibrosis during hypertension and renal protection by inflammasome inhibition involves preventing the accumulation of these cells.
Another important driver of fibrosis in the kidneys is EMT, where polarized tubular epithelial cells assume a mesenchymal cell phenotype, enabling them to detach from the basement membrane, migrate through tissues, and produce extracellular matrix components.29 Vimentin is a commonly used marker of EMT30 and we showed that 1K/DOCA/salt hypertension was associated with vimentin up-regulation. Furthermore, treatment of hypertensive mice with MCC950 reduced vimentin expression suggesting that it afforded protection against EMT. Tubular epithelial cells are known to express an NLRP3 inflammasome31 and activation of the enzyme in this cell type has been implicated in renal injury in response to a number of stimuli including aldosterone and angiotensin II.7,16,17 Furthermore, a recent study demonstrated that NLRP3 is a key mediator of EMT in the kidneys in response to hyperuricaemia, and that this involves a direct molecular interaction between NLRP3 and Smad2/3.32 Our present findings are consistent with such a role for NLRP3 during 1K/DOCA/salt-induced hypertension, and highlight MCC950 as an effective pharmacological tool for inhibiting EMT.
In addition to increasing macrophage numbers in the kidneys, 1K/DOCA/salt promoted the accumulation of CD4+ T cells. Furthermore, T cells were shown to be a source of the pro-inflammatory cytokine IFN-γ. Previous studies have shown that IL-18 can act in concert with IL-12 to promote the production of IFN-γ by T cells.33 Indeed, we found that both of these cytokines were increased in the kidneys of 1K/DOCA/salt-treated mice, and that MCC950 reduced expression of IL-18 and the accumulation of IFN-γ-producing T cells. Mice deficient in IFN-γ are protected from angiotensin II-dependent hypertension and the associated renal inflammation.34 Kamat et al.34 suggested that IFN-γ promotes hypertension by increasing the abundance of the phosphorylated forms of the Na-K-2Cl cotransporter, Na-Cl cotransporter, and Ste20/SPS-1-related proline-alanine-rich kinase in tubular epithelial cells, thereby increasing Na+ reuptake. Hence, our findings imply that IFN-γ may be a common mediator of renal damage in hypertension irrespective of the stimulus and that inhibition of the NLRP3 inflammasome effectively reverses activation of this pro-hypertensive pathway.
A novel finding was that MCC950 partially reversed albuminuria in 1K/DOCA/salt-treated mice, consistent with improved glomerular filtration. Curiously, while the intervention with MCC950 reduced urine output, osmolality and [Na+], it also reduced Na+/H2O intake. This highlights a limitation of the current model in that salt was provided to mice ad libitum via the drinking water making it difficult to interpret whether the reduced urine output drove the reduced saline intake, or vice versa. A future approach to directly investigate if MCC950 alters Na+/electrolyte handling might involve challenging treated and untreated animals with equal amounts of salt and volume via an intraperitoneal bolus.35
The intervention with MCC950 reduced hypertension by approximately 10–15 mmHg (i.e. 25% of the 1K/DOCA/salt-induced pressor effect), with reductions in both systolic and diastolic BP. This magnitude of effect is clinically meaningful because in humans, the level of cardiovascular risk halves with every 10 mmHg reduction in diastolic BP.36 Nevertheless, the magnitude of its anti-hypertensive actions were modest relative to the profound effects of MCC950 on renal inflammation and fibrosis. Others have also found only an indirect relationship between renal inflammation and BP following 1K/DOCA/salt-treatment. For example, Liang et al.37 demonstrated that 1K/DOCA/salt-treated mice genetically deficient in the chemokine, CXCL16, were markedly protected against renal fibrosis, albuminuria, and macrophage and T cell infiltration, but not increased BP. Conversely, using a similar intervention protocol, we found that the IL-1 receptor antagonist (IL-1Ra), anakinra, was comparable to MCC950 in its ability to reduce 1K/DOCA/salt-induced elevations in BP, yet had little impact on renal inflammation or leucocyte accumulation.21 A unifying explanation for these findings might be that the two major inflammasome-derived cytokines—IL-1β and IL-18—exert largely separate non-renal vs. renal actions, respectively. Indeed, in support of a predominantly extra-renal role, IL-1β increases endothelial superoxide production and impairs endothelium-dependent relaxation in resistance-like arteries.38 Moreover, IL-1R−/− mice are fully protected against the endothelial dysfunction which usually accompanies aldosterone administration,39 whereas IL-1β−/− mice are not protected against ischaemic renal injury.40 Thus, future work to directly compare the impact of inhibition of IL-1β vs. IL-18 on 1K/DOCA/salt-induced hypertension is warranted.
In addition to regulating the production of IL-1 family cytokines, inflammasome-mediated activation of caspase-1 and subsequent cleavage of gasdermin D can lead to a form of programmed cell death known as pyroptosis.41 Previous studies have reported the association between elevated caspase-1/IL-1β expression and tubular epithelial cell death in models of acute kidney injury including ischaemia–reperfusion and unilateral ureteral obstruction and have suggested that this may be evidence for a role of pyroptosis.42,43 Although we did not investigate directly whether 1K/DOCA/salt-treatment is associated with tubular epithelial cell death (or death of other inflammasome-expressing cells such as macrophages), we did find evidence of both tubular atrophy and damage to the epithelial brush border, both of which are indicative of epithelial cell death. Nonetheless, the fact that these processes were not prevented by MCC950, suggests either that pyroptosis is not involved, or that it occurs independently of NLRP3, possibly downstream of an alternative inflammasome isoform.
It remains unknown which stimuli activate the NLRP3 inflammasome in the kidneys during 1K/DOCA/salt hypertension, but both high salt and aldosterone—each of which are key components of the 1K/DOCA/salt model—can act as danger signals. For example, hyperosmotic stress induced by high [Na+] is detected as a danger signal by cultured macrophages and results in oligomerization of both NLRP3 and NLRC4 inflammasomes, caspase-1 activation and IL-1β production.44 Further, stimulation of isolated macrophages with aldosterone induces caspase-1 activation and IL-18 production, and is attenuated by eplerenone, an antagonist of the mineralocorticoid receptor.16 Interestingly, recent evidence suggests that mineralocorticoid receptor antagonists, including eplerenone and spironolactone, are effective in the treatment of many patients with resistant hypertension.45 Thus, it would be interesting to determine whether inflammasome inhibition contributes to the beneficial effects of mineralocorticoid receptor antagonists, and whether drugs that directly target the inflammasome (e.g. MCC950) or its cytokine products may be effective therapies for resistant hypertension. On this latter point, canakinumab, a therapeutic monoclonal antibody against IL-1β reduced cardiovascular events by 15% in patients with previous myocardial infarction and evidence of systemic inflammation.46 The protective effects of canakinumab occurred independently of any actions on plasma lipids, but it was not reported whether IL-1β inhibition lowered BP in these patients. It will thus be interesting to determine whether an anti-hypertensive action of canakinumab contributed to its protective actions against cardiovascular events in high risk patients.
5. Conclusion
In conclusion, this study demonstrates that pharmacological inhibition of NLRP3 with MCC950 protects mice from the renal dysfunction, fibrosis and inflammation associated with the development of 1K/DOCA/salt-induced hypertension. Although several questions remain about the mechanisms that contribute to these protective actions of MCC950, our findings support the concept that drugs targeting inflammasome activity hold potential for treatment of hypertension and renal damage. Indeed, the favourable pharmacodynamic (IC50 for NLRP3 < 10 nM) and pharmacokinetic (oral bioavailability of 68%)18 properties of MCC950 highlight it as a promising lead for the development of such drugs.
Conflict of interest: M.A.C. currently holds a fractional Professorial Research Fellow appointment at the University of Queensland with his remaining time as CEO of Inflazome Ltd., a company headquartered in Dublin, Ireland that is developing drugs to address clinical unmet needs in inflammatory disease by targeting the inflammasome. E.L. is a shareholder in IFM Therapeutics, a company that works to improve the lives of patients with serious diseases by developing medicines that target the innate immune system.
Funding
This work was supported by the National Health and Medical Research Council of Australia (APP1143674 to G.R.D., A.M., C.S., and A.V.; APP1062721 to G.R.D., C.S., A.M. and E.L.; APP1006017 to G.R.D.; APP1079467 to C.G.S.; APP1079492 to K.P.; and APP1041766 to C.S.S.), the European Research Council InflammAct (616777 to E.L.) and the TRR57 grant by the Deutsche Forschungsgemeinschaft (to E.L.).
Supplementary Material
Footnotes
Time for primary review: 27 days
This manuscript was handled by Consulting Editor, Giuseppe Lembo.
References
- 1. Wadei HM, Textor SC.. The role of the kidney in regulating arterial blood pressure. Nat Rev Nephrol 2012;8:602–609. [DOI] [PubMed] [Google Scholar]
- 2. Ivy JR, Bailey MA.. Pressure natriuresis and the renal control of arterial blood pressure. J Physiol 2014;592:3955–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayer G. An update on the relationship between the kidney, salt and hypertension. Wien Med Wochenschr 2008;158:365–369. [DOI] [PubMed] [Google Scholar]
- 4. Luzardo L, Noboa O, Boggia J.. Mechanisms of salt-sensitive hypertension. Curr Hypertens Rev 2015;11:14–21. [DOI] [PubMed] [Google Scholar]
- 5. Gu J-W, Tian N, Shparago M, Tan W, Bailey AP, Manning RD.. Renal NF-kappaB activation and TNF-alpha upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2006;291:R1817–R1824. [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ.. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol 2012;39:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, Arumugam TV, Hewitson TD, Kemp-Harper BK, Robertson AAB, Cooper MA, Latz E, Mansell A, Sobey CG, Drummond GR.. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 2016;173:752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blasi ER, Rocha R, Rudolph AE, Blomme EAG, Polly ML, McMahon EG.. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 2003;63:1791–1800. [DOI] [PubMed] [Google Scholar]
- 9. Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 2010;22:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LRG, Elias RM, Barreto CR, Silva-Cunha C, Hyane MI, Gonçalves GM, Brum PC, Fujihara C, Zatz R, Pacheco-Silva A, Zamboni DS, Camara NOS.. Pivotal role of toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS One 2011;6:e29004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS.. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 2013;84:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Eyre S, Churchman SM, Wilson AG, Isaacs JD, Hyrich K, Barton A, Plant D, Savic S, Cook GP, Sarzi-Puttini P, Emery P, Barrett JH, Morgan AW, McDermott MF.. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); Genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis 2014;73:1202–1210. [DOI] [PubMed] [Google Scholar]
- 13. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J.. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–241. [DOI] [PubMed] [Google Scholar]
- 14. Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT.. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 2008;9:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E.. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kadoya H, Satoh M, Sasaki T, Taniguchi SNI, Takahashi M, Kashihara N.. Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J 2015;29:3899–3910. [DOI] [PubMed] [Google Scholar]
- 17. Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S, Takahashi M.. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology 2015;156:4281–4292. [DOI] [PubMed] [Google Scholar]
- 18. Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KHG, Masters SL, Schroder K, Cooper MA, O'Neill LAJ.. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015;21:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coates BM, Staricha KL, Ravindran N, Koch CM, Cheng Y, Davis JM, Shumaker DK, Ridge KM.. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza a virus infection. Front Immunol 2017;8:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heijden T, Van Der Kritikou E, Venema W, Duijn J, Van Santbrink PJ, Van Slütter B, Foks AC, Bot I, Kuiper J.. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice—brief report. Arterioscler Thromb Vasc Biol 2017;37:1457–1461. [DOI] [PubMed] [Google Scholar]
- 21. Ling YH, Krishnan SM, Chan CT, Diep H, Ferens D, Chin-Dusting J, Kemp-Harper BK, Samuel CS, Hewitson TD, Latz E, Mansell A, Sobey CG, Drummond GR.. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacol Res 2017;116:77–86. [DOI] [PubMed] [Google Scholar]
- 22. Schmittgen TD, Livak KJ.. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 23. Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR.. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss and elevated blood pressure. Am J Physiol Heart Circ Physiol 2015;309:H9069–H9017. [DOI] [PubMed] [Google Scholar]
- 24. Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K.. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol 1998;161:3400–3407. [PubMed] [Google Scholar]
- 25. Wang L, Li YL, Zhang CC, Cui W, Wang X, Xia Y, Du J, Li HH.. Inhibition of toll-like receptor 2 reduces cardiac fibrosis by attenuating macrophage-mediated inflammation. Cardiovasc Res 2014;101:383–392. [DOI] [PubMed] [Google Scholar]
- 26. Carlson S, Helterline D, Asbe L, Dupras S, Minami E, Farris S, Stempien-Otero A.. Cardiac macrophages adopt profibrotic/M2 phenotype in infarcted hearts: role of urokinase plasminogen activator. J Mol Cell Cardiol 2017;108:42–49. [DOI] [PubMed] [Google Scholar]
- 27. Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, Rooijen N, Van Elias JA, Hogaboam CM, Herzog EL.. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol 2011;43:154–162. [DOI] [PubMed] [Google Scholar]
- 28. Maier C, Ramming A, Bergmann C, Weinkam R, Kittan N, Schett G, Distler JHW, Beyer C.. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis 2017;76:1133–1141. [DOI] [PubMed] [Google Scholar]
- 29. Border WA, Noble NA.. Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994;331:1286–1292. [DOI] [PubMed] [Google Scholar]
- 30. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442–454. [DOI] [PubMed] [Google Scholar]
- 31. Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA.. Inflammasome-independent NLRP3 augments TGF-signaling in kidney epithelium. J Immunol 2013;190:1239–1249. [DOI] [PubMed] [Google Scholar]
- 32. Romero CA, Remor A, Latini A, Paul AL, De Torres AI, Mukdsi JH.. Uric acid activates NRLP3 inflammasome in an in-vivo model of epithelial to mesenchymal transition in the kidney. J Mol Histol 2017;48:209–218. [DOI] [PubMed] [Google Scholar]
- 33. Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K.. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol 2000;12:151–160. [DOI] [PubMed] [Google Scholar]
- 34. Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, Mcdonough AA.. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ-/-and interleukin-17A-/-mice. Hypertension 2015;65:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG.. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 2014;64:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Law M, Morris J, Wald N.. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang H, Ma Z, Peng H, He L, Hu Z, Wang Y.. CXCL16 deficiency attenuates renal injury and fibrosis in salt-sensitive hypertension. Sci Rep 2016;6:28715.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jimenez-Altayo F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E.. Increased superoxide anion production by interleukin-1beta impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther 2006;316:42–52. [DOI] [PubMed] [Google Scholar]
- 39. Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, Neves KB, Alves-Lopes R, Campos E, Silva CAA, Fazan R, Carlos D, Mestriner FL, Prado D, Pereira FV, Braga T, Luiz JPM, Cau SB, Elias PC, Moreira AC, Câmara NO, Zamboni DS, Alves-Filho JC, Tostes RC.. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation 2016;134:1866–1880. [DOI] [PubMed] [Google Scholar]
- 40. Haq M, Norman J, Saba SR, Ramirez G, Rabb H.. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol 1998;9:614–619. [DOI] [PubMed] [Google Scholar]
- 41. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J.. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016;535:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung SD, Lai TY, Chien CT, Yu HJ.. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One 2012;7:e47299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang J-R, Yao F-H, Zhang J-G, Ji Z-Y, Li K-L, Zhan J, Tong Y-N, Lin L-R, He Y-N.. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol 2014;306:F75–F84. [DOI] [PubMed] [Google Scholar]
- 44. Ip WKE, Medzhitov R.. Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun 2015;6:6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N, Russell RP.. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens 2015;28:1376–1385. [DOI] [PubMed] [Google Scholar]
- 46. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.