Abstract
Genomic amplification at 9p24.1, including the loci for JAK2, PD-L1, and PD-L2, has recently been described as a mechanism of resistance in postchemotherapy, triple-negative breast cancer. This genomic signature holds significant promise as a prognostic biomarker and has implications for targeted therapy with JAK2 inhibitors, as well as with immunotherapy. To guide future screening strategies, the frequency of these alterations was determined. A total of 5399 cases were included in the study. This encompassed 2890 institutional cases tested by the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets assay and 2509 cases from The Cancer Genome Atlas (TCGA). The combined incidence of 9p24.1 amplifications in both the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets and TCGA cohorts was 1.0% (56/5399 cases) and showed a >10-fold higher incidence in triple-negative breast cancer (triple-negative: 5.1%; non–triple-negative: 0.5%). Tumor mutation burden and stromal tumor infiltrating lymphocytes, parameters used to assess response to immunotherapy, were not significantly higher for these cases. The significance of genomic losses at 9p24.1 is unclear, and further studies are needed. Herein, we studied the spectrum of copy number alterations in breast cancer cases within our institutional clinical sequencing cohort and those profiled by TCGA to determine the frequency of genomic alterations that may predict response or resistance to JAK2 inhibitors and/or immunotherapy.
CME Accreditation Statement: This activity (“JMD 2019 CME Program in Molecular Diagnostics”) has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“JMD 2019 CME Program in Molecular Diagnostics”) for a maximum of 18.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Genomic amplifications at chromosome 9p24.1 were initially described in and are frequently seen in classical Hodgkin lymphoma and mediastinal large B-cell lymphoma.1 Recently, similar amplifications have been described for a subset of solid tumors including Epstein-Barr virus–positive gastric adenocarcinoma, oral cavity squamous cell carcinoma, and small cell lung carcinoma.2, 3, 4 As amplifications at this locus involve Janus kinase 2 (JAK2), programmed cell death 1 ligand 1 (PD-L1; alias CD274/PDCD1LG1) and programmed cell death 1 ligand 2 (PD-L2; alias CD273/PDCD1LG2), this has significant implications for therapy with both JAK2 kinase inhibitors and immune checkpoint inhibitors.
JAK2 is one of the four JAK-domain–containing tyrosine kinase genes (JAK1, JAK2, JAK3, and TYK2), and its downstream signaling is mediated through the STAT proteins (encoded by STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6) to promote oncogenesis through diverse mechanisms.5 Known mechanisms of activating JAK2 signaling include direct ligand binding, activating mutations (JAK2 p.V617F, exon12 mutations), phosphorylation of wild-type JAK2 by mutant thrombopoietin receptors (MPL p.W515L/K), as well as genomic amplification events that up-regulate JAK2 expression itself.6, 7
PD-L1/PD-L2 play an important role within the tumor microenvironment by binding the programmed cell death protein 1 (PD-1) receptor on immune cells, and down-regulating the host immune response to tumor cells.8 Immune checkpoint inhibitors restore this immune response, and their use is gaining popularity as a therapeutic modality in multiple tumor types; however, their use in breast cancer is limited.8, 9 Parameters used to predict the success of immune checkpoint inhibitors in other tumor types, such as tumor mutation burden (TMB), which is a surrogate metric for assessing tumor immunogenicity by determining the load of neoepitopes, shows only modest changes in breast cancer, whereas the evaluation of tumor-infiltrating lymphocytes (TILs), which is a surrogate for the adaptive immune response, has proven to be more promising.9, 10, 11 Constitutive PD-L1/PD-L2 expression secondary to amplifications at 9p24.1, on the other hand, holds the potential to predict response to immunotherapy independent of these metrics as has been shown in classical Hodgkin lymphoma.12
A recent study involving comprehensive molecular analysis of neoadjuvant chemotherapy-resistant triple-negative breast cancer (TNBC) revealed JAK2 amplifications at 9p24.1 as a potentially targetable mechanism of resistance.13 Follow-up studies confirmed a higher incidence of JAK2/PD-L1/PD-L2 amplifications at 9p24.1 in TNBC, which was correlated with adverse clinical outcomes.7, 14 As JAK2 signaling is implicated in up-regulating PD-L1 expression, coamplification of JAK2/PD-L1 leads to constitutive PD-L1 overexpression on the tumor cell surface, and this may have direct implications for immunotherapy in these tumors.1, 8 Furthermore, in vitro and patient-derived xenograft-based studies suggest that JAK2 itself may be a targetable alteration using selective JAK2 inhibitors.7 Of note, selective JAK2 inhibitors have been used in the management of myeloproliferative neoplasms with no unexpected increased incidence of adverse events over long duration of therapy, and several of these drugs are in clinical trials for the management of solid tumors.15, 16 Given the implications of 9p24.1 amplification in clinical management, recent studies have focused on the development of fluorescent in situ hybridization (FISH)-based strategies to identify these cases in day-to-day clinical practice.17
To date, the overall incidence of these alterations in breast cancer remains undefined because prior studies have interrogated limited clinical cohorts.7, 14 For instance, studies by Barrett et al14 and Balko et al7 identified this signature in 10% (7/68) to 29% (12/41) of TNBC cases. Despite the limited size of the cohorts, taken together, both studies suggest that JAK2/PD-L1/PD-L2 coamplifications are enriched in TNBC.7, 14
Here, we specifically studied the spectrum of copy number alterations (CNA) in all breast cancer cases within our institutional clinical sequencing cohort that were profiled using a next-generation sequencing (NGS)-based assay and those profiled by The Cancer Genome Atlas (TCGA), to determine the frequency of genomic alterations that would potentially predict response or resistance to JAK2 inhibitors and/or immunotherapy.18, 19
Materials and Methods
Patient Specimens, Evaluation of ER/PR/HER2 Status by IHC/FISH, and Tumor Purity Estimation
This study was authorized by the institutional review board and involved analysis of molecular profiling data for all breast cancer cases profiled by a NGS-based assay [Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT)] as part of an institutional clinical cancer genomics initiative, as well as a medical chart review for relevant clinicopathologic parameters such as hormone receptor status and receipt of chemotherapy and/or immunotherapy.20, 21, 22
Estrogen receptor (ER) and progesterone receptor (PR) status was determined on the basis of immunohistochemistry (IHC; ER: clone 6F11, PR: clone 16; Leica, Buffalo Grove, IL), whereas human epidermal growth factor receptor 2 (HER2) amplification status was determined using a combination of both IHC and FISH in accordance with guideline recommendations and the standard practice at our institution.23, 24, 25 Specifically, HER2 amplification was determined using IHC [PATHWAY anti-HER2/neu (4B5), Ventana, Tucson, AZ; HercepTest, Dako, Carpinteria, CA] and amplification status in equivocal cases was resolved using FISH (HER2 IQFISH pharmDx, Dako; PathVysion HER-2 DNA Probe Kit, Vysis, Downers Grove, IL). ERBB2 amplification status was also assessed using MSK-IMPACT as previously described.23
Tumor purity was estimated using semiquantitative evaluation of hematoxylin and eosin–stained sections by a pathologist (S.G.), which allowed for a morphology-based estimate.23 In prior validation studies of ERBB2 amplification status using MSK-IMPACT, copy number gains at a 1.6-fold change (FC) were successfully detected for cases with a 4.2- and 2.4-fold amplification by FISH, at a 12.5% and 50% dilution, respectively.23
IHC: PD-L1
IHC was performed for PD-L1 (clone E1L3N, 1:400 dilution; Cell Signaling Technology, Danvers, MA). Of note, IHC with the PD-L1 antibody (E1L3N clone from Cell Signaling Technology) was clinically validated against the PD-L1 22C3 clone from Dako, and found to be comparable.
Next Generation Sequencing Assay (MSK-IMPACT)
Details of the MSK-IMPACT assay have been previously reported.20, 21, 22, 23 In brief, the MSK-IMPACT assay involves hybridization capture–based library preparation, followed by deep sequencing of 6614 protein-coding exons of 468 genes, select noncoding regions including 70 introns of commonly rearranged genes, the TERT gene promoter, microsatellite sites, and several single nucleotide polymorphisms at intergenic and intronic sites. The capture probes target approximately 1.5 megabases of the human genome. Specifically, the single nucleotide polymorphism tiling probes are homogenously distributed across the genome, and this allows for accurate genome-wide copy number assessment.23
DNA for this assay was extracted from formalin-fixed, paraffin-embedded material after macrodissection, if necessary, and DNA input ranged between 50 and 200 ng, as measured by fluorometric methods.23 Sequencing was performed on a Illumina HiSeq2500 (Illumina, San Diego, CA) and subjected to the bioinformatics pipeline, where the sequencing reads were aligned to the human genome (hg19) using Burrows-Wheeler Aligner software version 0.7.5a.23 A matched nontumor sample for each tumor (peripheral blood, EDTA tube) was sequenced in parallel to remove germline alterations through the bioinformatics pipeline. This assay is currently FDA authorized as a class II in vitro diagnostic test.
9p24.1 Copy Number Alterations: Bioinformatics
The bioinformatics pipeline used to identify CNA has been described previously.20, 23 Briefly, the average coverage corresponding to every exonic and noncoding capture probe is calculated, and reads with a low mapping quality (<20) are discarded using the Genome Analysis Toolkit Depth of Coverage tool.26 Variables used to normalize these coverage data include overall coverage for each sample, as well as the GC content of each target region.23 False positives are avoided by the exclusion of regions with coverage values in the lowest fifth percentile in >20% of normal specimens.23 A key step in the identification of CNA involves normalization to a diploid normal genome for each probe-region, and this is followed by log-transformation of the corresponding data. These log values are segmented using a circular binary segmentation algorithm, and probe clusters with mean log ratios closest to zero are used to determine a null distribution for statistical analysis; the Benjamin-Hochberg procedure is used to correct for a false discovery rate.23
Based on previously reported criteria, amplifications were defined as a FC ≥2.0.23 In this study, deletions were defined as FC ≤−2.0, borderline deletions/losses were defined as FC >−2.0 and ≤−1.8, and borderline amplifications/gains were defined as FC ≥1.6 and <2.0.
Analysis of TCGA Datasets
The publicly available cBioPortal.32e34 platform was used to analyze data from The Cancer Genome Atlas (TCGA) project related to 2509 cases of invasive ductal carcinomas of the breast, and paired data regarding hormone receptor status were available for 78.9% (1980/2509) of these cases.18, 19
Tumor Mutation Burden Estimation and Microsatellite Instability Status
The total number of nonsynonymous mutations as a fraction of the total genomic target region for which mutations were reported using MSK-IMPACT, for each case, have been reported herein as a metric of TMB.22 In addition, the average TMB for all breast carcinoma cases within the MSK-IMPACT clinical sequencing cohort has been reported for comparison. Of the 34 cases with 9p24.1 CNA identified in the MSK-IMPACT cohort, 20 cases were sequenced using a 468-gene panel, whereas 14 cases were sequenced using prior versions of the assay with fewer genes (9 cases: 410-gene panel, and 5 cases: 341-gene panel). The results have been reported as mutations per megabase (mt/MB). Microsatellite instability (MSI) status was determined as previously described.27
Assessment of Tumor-Infiltrating Lymphocytes
The quantification of TILs was performed based on standardized recommendations issued by the international TILs working group using ImageJ software version 1.47 (NIH, Bethesda, MD; http://imagej.nih.gov/ij).10, 28 Specifically, only the stromal component of primary tumors, after excluding areas of desmoplastic reaction, was evaluated for TILs.10 Important image analysis steps included conversion of representative, raw image files into an 8-bit grayscale format, signal-to-noise threshold adjustments, creation of appropriate masks, and delineation of watershed areas, followed by the manual selection of regions of interest, and finally quantification of involvement by TILs as a percentage of the total stromal area analyzed.
Results
Concordance of a Clinically Validated, NGS-Based Assessment of ERBB2 Copy Number Status with IHC/FISH in the Cohort of Breast Cancer Cases with 9p24.1 CNA
In prior clinical validations of a NGS-based assessment of copy number status using MSK-IMPACT, variables found to affect copy number calls included depth of coverage and tumor purity.23 These variables have been listed in Table 1 for breast cancer specimens, which were found to have significant 9p24.1 CNA. These cases were subclassified based on 9p24.1 amplification/deletion status, as well as into TNBC (ER/PR/HER2 negative) and non-TNBC categories. Importantly, the minimum mean depth of sequencing was ≥654.8× for every category. Mean tumor purity, estimated based on morphologic assessment, was ≥43% for all categories (range, 20% to 80%). Copy number status was assessed for three genes (JAK2/PD-L1/PD-L2) at 9p24.1. The minimum FC for selecting (9p24.1) amplified cases was +1.6, and the minimum FC for selecting cases with copy number losses was −1.8. For the 11 (of 33) cases that were HER2-positive based on IHC/FISH, the mean ERBB2 FC on MSK-IMPACT was 7.1 (interquartile range, 2.7 to 9.5) compared with HER2-negative cases (mean, −0.6, interquartile range, −1.2 to 0), and there was no discordance between IHC/FISH- and NGS-based assessment (Table 2), consistent with our prior clinical validation.23
Table 1.
Copy Number Alteration Determination by MSK-IMPACT
| Variable | 9p24.1 (JAK2/PD-L1/PD-L2) amp (non-TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) amp (TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) del (non-TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) del (TNBC) |
|---|---|---|---|---|
| Coverage, mean (range) | 842.6× (309×–2610×) | 754.9× (405×–1135×) | 654.8× (227×–994×) | 852.3× (488×–1300×) |
| Tumor purity, histology, mean (range) | 48% (20%–70%) | 43% (20%–70%) | 56.4% (40%–80%) | 53.3% (30%–80%) |
| Minimum copy number gain | +1.6 FC | +2.0 FC | NA | NA |
| Minimum copy number loss | NA | NA | −1.8 FC | −1.8 FC |
Amp, amplification; Del, deletion; FC, fold change; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling Of Actionable Cancer Targets; NA, not applicable; TNBC, triple-negative breast cancer.
Table 2.
Patient Characteristics: MSK-IMPACT
| Characteristic | 9p24.1 (JAK2/PD-L1/PD-L2) amp (non-TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) amp (TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) del (non-TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) del (TNBC) |
|---|---|---|---|---|
| Total cases | 10 | 10 | 11 | 3 |
| Age in years, mean (range) | 55.1 (32–65) | 63.2 (38–75) | 53.7 (32–75) | 57.7 (53–62) |
| ER+, IHC | 6/9 (66.6%)∗ | 0/10 (0%) | 8/11 (72.7%) | 0/3 (0%) |
| PR+, IHC | 0/9 (0%)∗ | 0/10 (0%) | 4/11 (36.4%) | 0/3 (0%) |
| HER2+, IHC/FISH | 6/10 (60%) | 0/10 (0%) | 6/11 (54.5%) | 0/3 (0%) |
| ERBB2 FC, IMPACT, mean (IQ range) | +4.4 (−0.6 to +5.0) | −0.5 (−1.2 to +0.5) | +3.4 (+1.0–+7.4) | −1.2 (−1.3 to −1.1) |
| JAK2 FC, IMPACT, mean (IQ range) | +3.8 (+2.2–+3.3) | +3.3 (+2.5–+4.1) | −2.4 (−2.5 to −2.0) | −2.0 (−2.2 to −1.9) |
| PD-L1 FC, IMPACT, mean (IQ range) | +2.3 (+2.0–+2.8) | +3.3 (+2.5–+4.1) | −2.0 (−2.4 to −1.6) | −2.0 (−2.2 to −1.9) |
| PD-L2 FC, IMPACT, mean (IQ range) | +2.3 (+1.7–+3.2) | +3.2 (+2.5–+3.8) | −1.7 (−2.0 to −1.4) | −2.1 (−2.2 to −1.9) |
| Primary versus metastasis | P: 2/10 (20%) M: 8/10 (80%) |
P: 6/10 (60%) M: 4/10 (40%) |
P: 2/11 (18.2%) M: 9/11 (81.8%) |
P: 1/3 (33.3%) M: 2/3 (66.6%) |
| Primary with prior NAC | 1/2 (50%) | 2/6 (33.3%) | 2/2 (100%) | 1/1 (100%) |
| Metastasis with prior chemotherapy | 5/8 (62.5%) | 2/4 (50%) | 7/9 (77.7%) | 2/2 (100%) |
| Documented immunotherapy, yes/no | 1/10 (10%) | 1/10 (10%) | 0/11 (0%) | 0/3 (0%) |
Amp, amplification; Del, deletion; FC, fold change; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; IQ range, interquartile range; M, metastasis; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets; NAC, neoadjuvant chemotherapy; P, primary; PR, progesterone receptor; TNBC, triple-negative breast cancer; ER, estrogen receptor.
No ER/PR status was documented for one specimen.
Clinicopathologic Characteristics of Breast Cancer Patients Profiled by MSK-IMPACT with 9p24.1 CNA
For cases with 9p24.1 CNA identified by MSK-IMPACT, prognostic biomarkers such as ER/PR status (based on IHC) and HER2 status (based on IHC/FISH) are shown in Table 2. Relevant clinical parameters such as mean age at the time of specimen collection, type of specimen (primary versus metastasis), and prior chemotherapy status are shown, as well. Importantly, only a single case in each of the 9p24.1 amplified TNBC and non-TNBC cohorts received immunotherapy, whereas none of the 9p24.1 deleted cases received immunotherapy.
9p24.1 (JAK2/PD-L1/PD-L2) CNA in the MSK Clinical Sequencing Cohort and TCGA
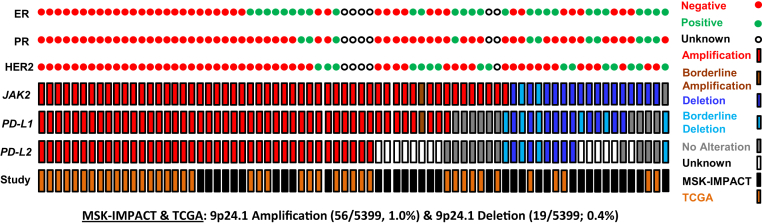
The 9p24.1 amplified (MSK-IMPACT) cohort had a mean FC of 3.6 for JAK2, 2.8 for PD-L1, and 2.7 for PD-L2, whereas the cohort with the 9p24.1 losses had a mean FC of −2.3 for JAK2, −2.0 for PD-L1, and −1.8 for PD-L2 (Table 2). Further substratification of these cases on the basis of hormone receptor status did not show any statistically significant changes. Although a comprehensive assessment of PD-L1 IHC was beyond the scope of the present study, representative images for ER, PR, HER2, and PD-L1 staining in a TNBC with a 4.4-fold amplification have been depicted in Figure 1.
Figure 1.
Immunohistochemistry. Representative hematoxylin and eosin–stained images (A and B) of a triple-negative breast cancer along with corresponding estrogen receptor (C), progesterone receptor (D), human epidermal growth factor receptor 2 (E), and programmed cell death 1 ligand 1 (PD-L1) status (F). PD-L1 shows a diffuse membranous pattern of expression secondary to gene amplification. Original magnification: ×40 (A); ×200 (B–F).
Within the MSK-IMPACT cohort, hormone receptor status was documented for 33.6% (972/2890) of cases, and 13.2% (128/972) of these cases represented TNBC. In all, cases with 9p24.1 copy number gains and losses in the MSK-IMPACT cohort accounted for 1.2% of all cases (34/2890) and approximately a third of these cases represented TNBC (13/34, 38%).
In TCGA cohort of invasive ductal carcinomas, hormone receptor status was known for 78.9% (1980/2509) of these cases, with TNBC accounting for 16.6% (320/1980). In this cohort, 9p24.1 copy number gains and losses were identified in 1.4% of cases (36/2509), and approximately half of these cases represented TNBC (20/36, 56%).18, 19
The incidence of 9p24.1 CNA was assessed among combined MSK-IMPACT and TCGA cases for which ER/PR/HER2 status was known (TNBC: 448, non-TNBC: 2504). Overall, the incidence of 9p24.1 amplification events was significantly higher for TNBC (TNBC: 5.1% versus non-TNBC: 0.5%, P < 0.00001) (Figure 2, Table 3). 9p24.1 deletion events, on the other hand, were only identified in the non-TNBC cohort (MSK-IMPACT and TCGA: 0.4%) (Table 3).
Figure 2.
Incidence of 9p24.1 copy number alterations, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) and The Cancer Genome Atlas (TCGA). The incidences of 9p24.1 amplifications and deletions for breast cancer cases profiled by the MSK-IMPACT and TCGA cohorts combined and corresponding hormone receptor status.18, 19 CNA, copy number alteration; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Table 3.
Incidence of 9p24.1 (JAK2/PD-L1/PD-L2) Copy Number Alterations
| Patient cohorts | 9p24.1 (JAK2/PD-L1/PD-L2) amp (non-TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) amp (TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) del (non-TNBC) | 9p24.1 (JAK2/PD-L1/PD-L2) del (TNBC) |
|---|---|---|---|---|
| MSK-IMPACT and TCGA18, 19 | 13/2504 (0.5%)∗ | 23/448 (5.1%)∗ | 11/2504 (0.4%) | 0/448 (0%) |
Data analysis platform: cBioPortal.
Amp, amplification; Del, deletion; MSK-IMPACT, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets; TCGA, The Cancer Genome Atlas; TNBC, triple-negative breast cancer.
P < 0.00001 (χ2 test).
Alternate Predictive Markers of Response to Immunotherapy in Breast Cancer Patients with 9p24.1 Amplifications
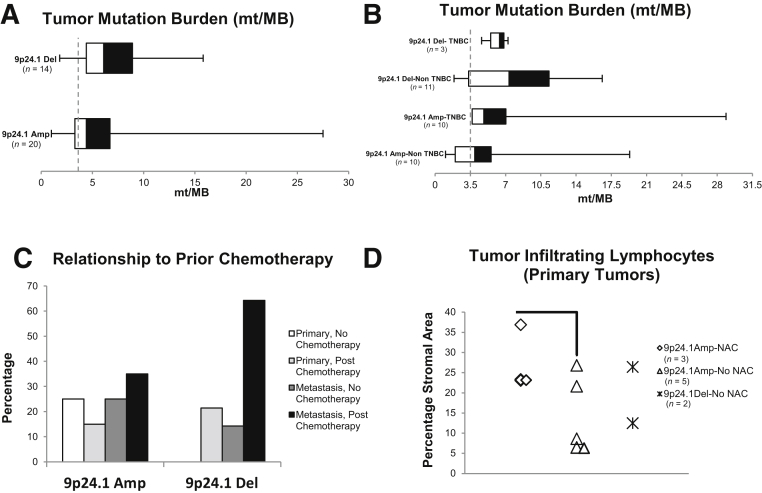
Because a high TMB has been correlated with response to immunotherapy, TMB was assessed in the MSK-IMPACT cohort of breast cancer cases with 9p24.1 CNA (Figure 3, A and B).29 The mean TMB (reported as mutations per megabase, mt/MB) was not found to be statistically different between amplified cases (6.3, interquartile range, 3.3 to 6.7) when compared with deleted cases (mean, 7.0, interquartile range: 4.4 to 8.9) and was similar to the mean TMB of all breast cancer cases profiled by MSK-IMPACT (3.5 mt/MB, 2890 cases). Finally, stratification of these cases into TNBC and non-TNBC did not reveal any statistically significant trends (Figure 3B). Furthermore, MSI status has been used as an alternative NGS metric for determining response to immunotherapy, and this was determined using the MSIsensor algorithm as part of the MSK-IMPACT assay.27 However, the mean MSIsensor score for 9p24.1 amplified (1.96, n = 20) and 9p24.1 deleted tumors (2.29, n = 14) was stable (MSI-high defined as MSIsensor score ≥10) and not significantly different between both these groups (P = 0.46).
Figure 3.
Prognostic factors affecting response to immunotherapy in breast cancer cases with 9p24.1 copy number alterations. A: Tumor mutation burden (x axis) for breast cancers with 9p24.1 copy number alterations. B: No statistically significant trends are observed when cases with 9p24.1 amplifications/deletions are subclassified based on triple-negative status. The dotted vertical line represents the mean tumor mutation burden (TMB) for all breast cancer cases profiled by Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) [3.5 mutations per megabase (mt/MB)]. C: The percentage of cases with 9p24.1 amplifications/deletions identified by MSK-IMPACT in primary or metastatic tumors, before or after chemotherapy. D: The percentage of tumor infiltrating lymphocytes morphologically involving the stromal area (y axis) for primary tumors with 9p24.1 amplifications/deletions. These cases were further subclassified based on whether they received neoadjuvant chemotherapy. Data are expressed as means ± SEM. n = 2890 cases (B); n = 7/10 (D, TNBC cases: n = 2/3 9p24.1Amp-NAC, n = 4/5 9p24.1Amp-No NAC, n = 1/2 9p24.1Del-No NAC). P = 0.08 (D). Amp, amplification; Del, deletion; NAC, neoadjuvant chemotherapy; TNBC, triple-negative breast cancer.
Of interest, fewer cases with 9p24.1 amplifications occurred in the postchemotherapy setting (10/20, 50%; primary: 3/8, 37.5%, metastatic: 7/12, 58.3%), compared with cases with 9p24.1 deletions (12/14, 85.7%, P = 0.03; primary: 3/3, 100%; metastatic: 9/11, 81.8%) (Table 2, Figure 3C).
A standardized evaluation of TILs was conducted in the cohort of breast cancer cases with 9p24.1 CNA given the increasing interest in the use of this parameter as an important prognostic marker, particularly for TNBC.10, 30 All metastatic tumors were excluded from this assessment, and cases were subclassified based on prior therapy. Evaluation of TILs in this limited cohort did not reveal any marked differences between cases with 9p24.1 amplifications or deletions; however, a marginally higher mean infiltration with TILs in 9p24.1 amplified cases that had been treated with prior neoadjuvant chemotherapy was noted [neoadjuvant chemotherapy (n = 3): 27.8%, no neoadjuvant chemotherapy: 14% (n = 5)] (Figure 3D).
Copy Number Assessment after Immunotherapy in a Breast Cancer Patient with 9p24.1 Amplification Using MSK-IMPACT
Of the two patients treated with immunotherapy, the first patient was a 50-year–old woman with pathogenic germline alterations in BRCA1 (c.68_69delAG and c.5266dupC) who had bone metastasis at the time of molecular testing and was found to have a ER+/PR−/HER2− tumor with a 3.3-fold amplification of 9p24.1 (JAK2/PD-L1/PD-L2).31 This patient was then treated with atezolizumab (a monoclonal antibody against PD-L1), which was discontinued at 4 months of follow-up due to persistence of disease. At the time this article was accepted for publication, she was currently alive with disease at 47 months of follow-up and has been managed with drugs including a nucleoside analog prodrug (sapacitabine), a PARP inhibitor (olaparib), eribulin as well as a phase I immunomodulatory fucosylation inhibitor.
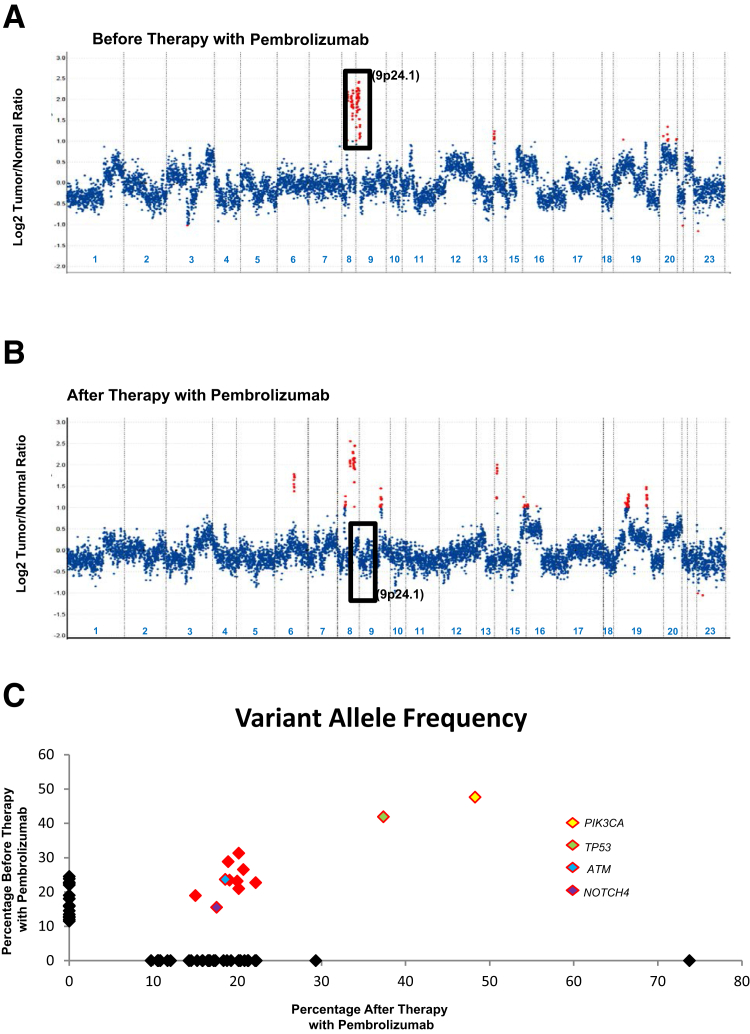
The second patient was a 62-year–old woman with TNBC, who presented with metastatic disease involving axillary lymph nodes and bone, and had a 4.1-fold amplification of 9p24.1 (JAK2/PD-L1) (Figure 4A). This patient was then started on pembrolizumab (a monoclonal antibody against PD-1); however, this therapy was discontinued after 2.5 months due to hepatotoxicity. Of interest, MSK-IMPACT tumor profiling of a subpectoral chest wall mass at 20 months after initial presentation revealed the recurrent tumor to be clonally related but without 9p24.1 amplification (Table 4, Figure 4, B and C).
Figure 4.
Copy number assessment before and after immunotherapy in a breast cancer patient with 9p24.1 amplification. Copy number plot before (A) and after therapy with pembrolizumab (B) has been shown for the same patient. Relative (Log2) tumor/normal ratios (y axis) and corresponding chromosomes (x axis) are displayed, with each blue dot representing an individual probe region. Amplified regions are shown in red. Black rectangles show probes targeting the 9p24.1 locus to demonstrate amplification that is present only in A and not in B. C: The variant allele frequencies in the specimens before (y axis) and after (x axis) therapy with pembrolizumab. The shared alterations including those involving PIK3CA, TP53, ATM, and NOTCH4 are depicted in red.
Table 4.
Molecular Profile Before and After Immunotherapy in a Breast Cancer Patient with 9p24.1 Amplification Using MSK-IMPACT
| Shared alteration | Before pembrolizumab | After pembrolizumab |
|---|---|---|
| Site | Metastasis, axillary lymph node | Sub-pectoral, chest wall mass |
| Tumor purity, morphology | 60% | 50% |
| Coverage | 1086× | 961× |
| Total number of shared mutations | 12 | 12 |
| ATM p.D1758N, c.5272 G>A | 23% | 19% |
| CDKN2Ap16INK4A p.R138T, c.413 G>C | 21% | 20% |
| ERCC5 p.E713K, c.2137 G>A | 29% | 19% |
| HLA-A p.V189M, c.565 G>A | 31% | 20% |
| MGA p.S300,* c.899C>G | 23% | 22% |
| MSH6 p.W50C, c.150 G>C | 19% | 19% |
| NOTCH4 p.E1836Q, c.5506 G>C | 16% | 18% |
| PIK3CA p.H1047R, c.3140A>G | 48% | 48% |
| RUNX1 p.D123fs, c.367dupG | 23% | 20% |
| SPEN p.E2260,* c.6778 G>T | 27% | 21% |
| SPEN p.E3305Q, c.9913 G>C | 24% | 19% |
| TP53 p.N29fs, c.86dupA | 42% | 37% |
| CNA, TCEB1, 8q21.1 | FC: 4.2 | FC: 4.4 |
| CNA, RAD21, 8q24.1 | FC: 3.3 | FC: 3.6 |
| CNA, MYC, 8q24.21 | FC: 3.3 | FC: 3.6 |
| CNA, NFKBIA, 14q13.2 | FC: 2 | FC: 3.1 |
Variant allele frequencies for alterations listed in column 1 are shown in columns 2 and 3.
CNA, copy number alteration; FC, fold change.
Finally, none of the cases in our cohort showed activating/inactivating JAK2 mutations.
Discussion
TNBC are often managed with neoadjuvant chemotherapy, and many patients show either a partial response or a lack of response before progressing to metastatic disease.7 Prior studies have suggested genomic amplifications at the 9p24.1 locus as a mechanism contributing to resistance to chemotherapy and as having both prognostic and therapeutic relevance.7, 13 Specifically, a recent study demonstrated an inferior response to neoadjuvant chemotherapy in 9p24.1 amplified tumors based on Miller-Payne scores, as well as poorer recurrence-free and overall survival rates.7 Currently, in the absence of specific histopathologic correlates, there is a clinical need to identify similar copy number–based predictive markers that determine response to therapy, and comprehensive molecular testing platforms such as MSK-IMPACT provide an effective solution. This is especially true in the case of 9p24.1 amplifications where the shortest region of amplification overlap spans approximately 777 kb including loci for JAK2, PD-L1, and PD-L2, because IHC for markers such as PD-L1 have shown inferior sensitivity when compared with global copy number assessment for the identification of such cases.14, 17 FISH-based methodologies may also represent an effective screening strategy for these tumors because hybridization probes spanning >300 kb may include both the loci for JAK2 as well as PD-L1.17 However, this approach provides less detailed information regarding multiple genes at the same locus and cannot distinguish focal 9p24.1 gains from larger segmental gains, and does not document other clinically relevant molecular alterations.
Prior studies have demonstrated that PD-L1 can be directly induced by JAK/STAT signaling, and in this context, tumors with 9p24.1 amplifications are likely to have significantly high levels of PD-L1 expression.1 Because in vitro studies using JAK2 inhibitors have demonstrated a dose-dependent decrease in JAK2 activation and PD-L1 expression, JAK2 inhibitors, therefore, have the potential to have a dual therapeutic effect.1 Agents such as ruxolitinib have proven to be efficacious in hematologic neoplasia such as myelofibrosis.15 However, their use in solid tumors has mostly been restricted to phase I/II clinical trials.16 For instance, a phase I/II trial of ruxolitinib in combination with trastuzumab in metastatic HER2-positive breast cancer is currently open (ClinicalTrials.gov, https://clinicaltrials.gov, identifier NCT02066532, last accessed June 5, 2018).16
Immune checkpoint inhibitors, such as the PD-1–blocking antibody nivolumab, have shown therapeutic efficacy in hematolymphoid neoplasms characterized by JAK2/PD-L1/PD-L2 coamplifications at 9p24.1, including classical Hodgkin lymphoma, primary central nervous system lymphoma, and primary testicular lymphoma.32, 33, 34 Importantly, this included patients with Hodgkin lymphoma with low-level copy number gains/polysomy, as well.34 Although there is a paucity of similar studies in solid tumors with 9p24.1 amplifications, it could be hypothesized that solid tumors with constitutive PD-L1 expression due to copy number gains might be promising candidates for immunotherapy. For instance, a recent study of 9p24.1 amplifications in solid tumors documented that six of nine patients (66.7%) with 9p24.1 amplified tumors had objective responses to immune checkpoint blockade administration.35 Ongoing clinical trials of immune checkpoint inhibitors for breast cancer in the neoadjuvant setting have reported promising initial results; however, these trials have not specifically screened cases for 9p24.1 amplification (ClinicalTrials.gov, https://clinicaltrials.gov, identifier NCT01042379, last accessed June 5, 2018).11, 36, 37, 38 In addition, a trial of combination therapy with ruxolitinib and the immunotherapeutic agent pembrolizumab for patients with stage IV triple-negative breast cancer is currently underway (ClinicalTrials.gov, https://clinicaltrials.gov, identifier NCT03012230, last accessed August 24, 2018).
The MSK-IMPACT NGS assay, which has been clinically validated to determine CNA for multiple genes including ERBB2, was used to determine 9p24.1 copy number status.23 Important variables that affect copy number calls include depth of sequencing, tumor purity, as well as intratumoral heterogeneity.23 Cases with 9p24.1 CNA were all sequenced to a relatively high mean depth of >650×, similar to the prior study.23 Furthermore, in contrast to estimates of tumor purity for the initial ERBB2 clinical validation study, which ranged from 10% to 60%, the minimum tumor purity in the current study was ≥20% based on morphologic estimates.23 Because the initial validation of a log2 tumor/normal fold-change cutoff of >1.5 had a 100% specificity and a 95% sensitivity for identifying ERBB2 amplifications, a more stringent cutoff of ≥1.6 was used to select cases with 9p24.1 amplifications for the current study, whereas a cutoff of ≤−1.8 was used to identify cases with genomic losses.23 Importantly, these prevalidated criteria were concordant with ERBB2 amplification status based on a combination of IHC/FISH for all cases with 9p24.1 CNA.
In a prior study, the analysis of 9p24.1 copy number gains in a limited number of primary tumors before and after neoadjuvant chemotherapy, along with paired metastases, had revealed progressive genomic amplifications at this locus, suggesting that this was a mechanism of resistance to chemotherapy.7 Although this signature appears to be relevant to disease progression, the frequency of these events is unclear. Estimates range from 0% in non-TNBC to 10% to 29% for TNBC; however, these studies are limited by the relatively small number of cases that were analyzed (64 to 68 total cases).7, 14
The incidence of 9p24.1 amplification events for breast cancer in the combined MSK-IMPACT and TCGA cohorts was 1.0% (56/5399 total cases).18, 19 This is similar to what has been reported by Goodman et al,35 who recently reported their incidence of 9p24.1 amplifications in 118,187 solid tumors. In their study, the incidence of these alterations was 1.9% (111/5838 total cases). Taken together both studies suggest a combined frequency of approximately 1.5% (167/11,237 cases).
Within the combined MSK-IMPACT and TCGA cohorts, ER/PR/HER2 status was available for a subset of cases (2952 cases), and further analysis revealed that 9p24.1 amplifications were significantly enriched in TNBC compared with non-TNBC (5.1% vs 0.5%). These results not only confirm the higher incidence of 9p24.1 amplification in TNBC, but also significantly revise the incidence of these events. It should, however, be noted that unlike the TCGA cohort, which was composed entirely of invasive ductal carcinoma, the MSK-IMPACT cohort included multiple subtypes, predominantly invasive ductal carcinoma, and this potentially accounts for any differences between the two groups.
A smaller cohort of cases showed copy number losses at this locus, including 0.4% of non-TNBC, and it is unclear whether these alterations are likely to underlie resistance to JAK2 inhibitors/immunotherapy; further studies are needed to address this hypothesis. Some of the better characterized genomic alterations associated with resistance to immunotherapy include loss-of-function mutations of JAK1/JAK2, which are known to abrogate responsiveness to cytokines such as interferon gamma, as well as truncating mutations of beta-2-microglobulin that adversely affect antigen presentation.39, 40, 41
The incidence of 9p24.1 amplifications was not significantly higher for postchemotherapy specimens in our cohort, contrary to what might be expected for a genomic signature for a chemotherapy resistance mechanism. However, our analysis in this regard is limited by a lack of paired pre- and post-treatment specimens. Of interest, 9p24.1 losses seemed to occur preferentially in a postchemotherapy setting and this signature should be evaluated in future studies as a potential mechanism of resistance. This is a particularly intriguing possibility, given our observation of a loss of genomic amplification at 9p24.1 in a clonally related tumor following therapy with pembrolizumab, a PD-1–specific antibody.
Given the emergence of immunotherapy as a potential therapeutic option for breast cancer, it is important to identify potential predictive markers of response to therapy. Although this study did not address the immune microenvironment, other parameters that were assessed included TMB and MSIsensor, as well as TILs. Cases with 9p24.1 CNA did not exhibit a significantly higher TMB compared with the average TMB for all breast cancer specimens profiled by MSK-IMPACT (2890 cases), or MSI. Similarly, the percentage stromal involvement by TILs for all primary tumors, regardless of prior chemotherapy, was less than the 50% to 60% cutoff used to define “lymphocyte-predominant breast cancer.”8, 10,p.267
In summary, genomic 9p24.1 (JAK2/PD-L1/PD-L2) amplifications are enriched in TNBC, and identification of these cases using global copy number assessment–based strategies may help identify patients who might benefit most from alternative treatment options, including immunotherapy or JAK2 inhibitors.
Footnotes
Supported in part through NIH/NCI Cancer Center Support grant P30 CA008748 and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Disclosures: None declared.
References
- 1.Green M.R., Monti S., Rodig S.J., Juszczynski P., Currie T., O'Donnell E., Chapuy B., Takeyama K., Neuberg D., Golub T.R., Kutok J.L., Shipp M.A. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straub M., Drecoll E., Pfarr N., Weichert W., Langer R., Hapfelmeier A., Gotz C., Wolff K.D., Kolk A., Specht K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. 2016;7:12024–12034. doi: 10.18632/oncotarget.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George J., Saito M., Tsuta K., Iwakawa R., Shiraishi K., Scheel A.H., Uchida S., Watanabe S.I., Nishikawa R., Noguchi M., Peifer M., Jang S.J., Petersen I., Buttner R., Harris C.C., Yokota J., Thomas R.K., Kohno T. Genomic amplification of CD274 (PD-L1) in small-cell lung cancer. Clin Cancer Res. 2017;23:1220–1226. doi: 10.1158/1078-0432.CCR-16-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuai K., Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 6.Levine R.L., Pardanani A., Tefferi A., Gilliland D.G. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 7.Balko J.M., Schwarz L.J., Luo N., Estrada M.V., Giltnane J.M., Davila-Gonzalez D., Wang K., Sanchez V., Dean P.T., Combs S.E., Hicks D., Pinto J.A., Landis M.D., Doimi F.D., Yelensky R., Miller V.A., Stephens P.J., Rimm D.L., Gomez H., Chang J.C., Sanders M.E., Cook R.S., Arteaga C.L. Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci Transl Med. 2016;8:334ra353. doi: 10.1126/scitranslmed.aad3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube J.M., Galon J., Sholl L.M., Rodig S.J., Cottrell T.R., Giraldo N.A., Baras A.S., Patel S.S., Anders R.A., Rimm D.L., Cimino-Mathews A. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31:214–234. doi: 10.1038/modpathol.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonderheide R.H., Domchek S.M., Clark A.S. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res. 2017;23:2640–2646. doi: 10.1158/1078-0432.CCR-16-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., Van den Eynden G., Baehner F.L., Penault-Llorca F., Perez E.A., Thompson E.A., Symmans W.F., Richardson A.L., Brock J., Criscitiello C., Bailey H., Ignatiadis M., Floris G., Sparano J., Kos Z., Nielsen T., Rimm D.L., Allison K.H., Reis-Filho J.S., Loibl S., Sotiriou C., Viale G., Badve S., Adams S., Willard-Gallo K., Loi S., International TILs Working Group 2014 The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burugu S., Asleh-Aburaya K., Nielsen T.O. Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer. 2017;24:3–15. doi: 10.1007/s12282-016-0698-z. [DOI] [PubMed] [Google Scholar]
- 12.Merryman R.W., Armand P., Wright K.T., Rodig S.J. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1:2643–2654. doi: 10.1182/bloodadvances.2017012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balko J.M., Giltnane J.M., Wang K., Schwarz L.J., Young C.D., Cook R.S., Owens P., Sanders M.E., Kuba M.G., Sanchez V., Kurupi R., Moore P.D., Pinto J.A., Doimi F.D., Gomez H., Horiuchi D., Goga A., Lehmann B.D., Bauer J.A., Pietenpol J.A., Ross J.S., Palmer G.A., Yelensky R., Cronin M., Miller V.A., Stephens P.J., Arteaga C.L. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett M.T., Anderson K.S., Lenkiewicz E., Andreozzi M., Cunliffe H.E., Klassen C.L., Dueck A.C., McCullough A.E., Reddy S.K., Ramanathan R.K., Northfelt D.W., Pockaj B.A. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget. 2015;6:26483–26493. doi: 10.18632/oncotarget.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison C.N., Vannucchi A.M., Kiladjian J.J., Al-Ali H.K., Gisslinger H., Knoops L., Cervantes F., Jones M.M., Sun K., McQuitty M., Stalbovskaya V., Gopalakrishna P., Barbui T. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701–1707. doi: 10.1038/leu.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchert M., Burns C.J., Ernst M. Targeting JAK kinase in solid tumors: emerging opportunities and challenges. Oncogene. 2016;35:939–951. doi: 10.1038/onc.2015.150. [DOI] [PubMed] [Google Scholar]
- 17.Chen M., Andreozzi M., Pockaj B., Barrett M.T., Ocal I.T., McCullough A.E., Linnaus M.E., Chang J.M., Yearley J.H., Annamalai L., Anderson K.S. Development and validation of a novel clinical fluorescence in situ hybridization assay to detect JAK2 and PD-L1 amplification: a fluorescence in situ hybridization assay for JAK2 and PD-L1 amplification. Mod Pathol. 2017;30:1516–1526. doi: 10.1038/modpathol.2017.86. [DOI] [PubMed] [Google Scholar]
- 18.Curtis C., Shah S.P., Chin S.F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., Graf S., Ha G., Haffari G., Bashashati A., Russell R., McKinney S., Group M., Langerod A., Green A., Provenzano E., Wishart G., Pinder S., Watson P., Markowetz F., Murphy L., Ellis I., Purushotham A., Borresen-Dale A.L., Brenton J.D., Tavare S., Caldas C., Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira B., Chin S.F., Rueda O.M., Vollan H.K., Provenzano E., Bardwell H.A., Pugh M., Jones L., Russell R., Sammut S.J., Tsui D.W., Liu B., Dawson S.J., Abraham J., Northen H., Peden J.F., Mukherjee A., Turashvili G., Green A.R., McKinney S., Oloumi A., Shah S., Rosenfeld N., Murphy L., Bentley D.R., Ellis I.O., Purushotham A., Pinder S.E., Borresen-Dale A.L., Earl H.M., Pharoah P.D., Ross M.T., Aparicio S., Caldas C. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng D.T., Mitchell T.N., Zehir A., Shah R.H., Benayed R., Syed A., Chandramohan R., Liu Z.Y., Won H.H., Scott S.N., Brannon A.R., O'Reilly C., Sadowska J., Casanova J., Yannes A., Hechtman J.F., Yao J., Song W., Ross D.S., Oultache A., Dogan S., Borsu L., Hameed M., Nafa K., Arcila M.E., Ladanyi M., Berger M.F. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman D.M., Solit D.B., Arcila M.E., Cheng D.T., Sabbatini P., Baselga J., Berger M.F., Ladanyi M. Precision medicine at Memorial Sloan Kettering Cancer Center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today. 2015;20:1422–1428. doi: 10.1016/j.drudis.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross D.S., Zehir A., Cheng D.T., Benayed R., Nafa K., Hechtman J.F., Janjigian Y.Y., Weigelt B., Razavi P., Hyman D.M., Baselga J., Berger M.F., Ladanyi M., Arcila M.E. Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J Mol Diagn. 2017;19:244–254. doi: 10.1016/j.jmoldx.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff A.C., Hammond M.E., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M., Bilous M., Fitzgibbons P., Hanna W., Jenkins R.B., Mangu P.B., Paik S., Perez E.A., Press M.F., Spears P.A., Vance G.H., Viale G., Hayes D.F., American Society of Clinical Oncology, College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 25.Hammond M.E., Hayes D.F., Dowsett M., Allred D.C., Hagerty K.L., Badve S., Fitzgibbons P.L., Francis G., Goldstein N.S., Hayes M., Hicks D.G., Lester S., Love R., Mangu P.B., McShane L., Miller K., Osborne C.K., Paik S., Perlmutter J., Rhodes A., Sasano H., Schwartz J.N., Sweep F.C., Taube S., Torlakovic E.E., Valenstein P., Viale G., Visscher D., Wheeler T., Williams R.B., Wittliff J.L., Wolff A.C. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hechtman J.F., Middha S., Stadler Z.K., Zehir A., Berger M.F., Vakiani E., Weiser M.R., Ladanyi M., Saltz L.B., Klimstra D.S., Shia J. Universal screening for microsatellite instability in colorectal cancer in the clinical genomics era: new recommendations, methods, and considerations. Fam Cancer. 2017;16:525–529. doi: 10.1007/s10689-017-9993-x. [DOI] [PubMed] [Google Scholar]
- 28.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hida A.I., Sagara Y., Yotsumoto D., Kanemitsu S., Kawano J., Baba S., Rai Y., Oshiro Y., Aogi K., Sagara Y., Ohi Y. Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat. 2016;158:1–9. doi: 10.1007/s10549-016-3848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abeliovich D., Kaduri L., Lerer I., Weinberg N., Amir G., Sagi M., Zlotogora J., Heching N., Peretz T. The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet. 1997;60:505–514. [PMC free article] [PubMed] [Google Scholar]
- 32.Younes A., Santoro A., Shipp M., Zinzani P.L., Timmerman J.M., Ansell S., Armand P., Fanale M., Ratanatharathorn V., Kuruvilla J., Cohen J.B., Collins G., Savage K.J., Trneny M., Kato K., Farsaci B., Parker S.M., Rodig S., Roemer M.G., Ligon A.H., Engert A. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayak L., Iwamoto F.M., LaCasce A., Mukundan S., Roemer M.G.M., Chapuy B., Armand P., Rodig S.J., Shipp M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., Rodig S.J., Chapuy B., Ligon A.H., Zhu L., Grosso J.F., Kim S.Y., Timmerman J.M., Shipp M.A., Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman A.M., Piccioni D., Kato S., Boichard A., Wang H.Y., Frampton G., Lippman S.M., Connelly C., Fabrizio D., Miller V., Sicklick J.K., Kurzrock R. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 2018;4:1237–1244. doi: 10.1001/jamaoncol.2018.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanda R., Chow L.Q., Dees E.C., Berger R., Gupta S., Geva R., Pusztai L., Pathiraja K., Aktan G., Cheng J.D., Karantza V., Buisseret L. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanda R., Liu M.C., Yau C., Asare S., Hylton N., Van't Veer L., Perlmutter J., Wallace A.M., Chien A.J., Forero-Torres A., Ellis E., Han H., Clark A.S., Albain K.S., Boughey J.C., Elias A.D., Berry D.A., Yee D., DeMichele A., Esserman L. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35(15 Suppl):506. [abstract 506] [Google Scholar]
- 38.Emens LA, Braiteh FS, Cassier P, DeLord J-P, Eder JP, Shen X, Xiao Y, Wang Y, Hegde PS, Chen DS, Krop I: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. 37th Annual CTRC-AACR San Antonio Breast Cancer Symposium [abstract PD1-6], Dec. 9–13, 2014, San Antonio, TX. Philadelphia, PA, American Association for Cancer Research, 2015
- 39.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., Saco J., Homet Moreno B., Mezzadra R., Chmielowski B., Ruchalski K., Shintaku I.P., Sanchez P.J., Puig-Saus C., Cherry G., Seja E., Kong X., Pang J., Berent-Maoz B., Comin-Anduix B., Graeber T.G., Tumeh P.C., Schumacher T.N., Lo R.S., Ribas A. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin D.S., Zaretsky J.M., Escuin-Ordinas H., Garcia-Diaz A., Hu-Lieskovan S., Kalbasi A., Grasso C.S., Hugo W., Sandoval S., Torrejon D.Y., Palaskas N., Rodriguez G.A., Parisi G., Azhdam A., Chmielowski B., Cherry G., Seja E., Berent-Maoz B., Shintaku I.P., Le D.T., Pardoll D.M., Diaz L.A., Jr., Tumeh P.C., Graeber T.G., Lo R.S., Comin-Anduix B., Ribas A. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh W., Chen P.L., Reuben A., Spencer C.N., Prieto P.A., Miller J.P. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9:eaah3560. doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]